Abstract
AMP-activated protein kinase (AMPK) is a cellular energy sensor activated during energy stress to stimulate ATP production pathways and restore homeostasis. AMPK is widely expressed in the kidney and involved in mitochondrial protection and biogenesis upon acute renal ischemia, AMPK activity being blunted in metabolic disease-associated kidney disease. Since little is known about AMPK in the regulation of renal blood flow, the present study aimed to assess the role of AMPK in renal vascular function. Functional responses to the selective AMPK activator A769662 were assessed in intrarenal small arteries isolated from the kidney of renal tumour patients and Wistar rats and mounted in microvascular myographs to perform simultaneous measurements of intracellular calcium [Ca2+]i and tension. Superoxide (O2.-) and hydrogen peroxide (H2O2) production were measured by chemiluminescence and fluorescence and protein expression by Western blot. Activation of AMPK with A769662 increased AMPKα phosphorylation at Thr-172 and induced potent relaxations compared to AICAR in isolated human and rat intrarenal arteries, through both endothelium-dependent mechanisms involving nitric oxide (NO) and intermediate-conductance calcium-activated potassium (IKCa) channels, as well as activation of ATP-sensitive (KATP) channels and sarcoplasmic reticulum Ca2+-ATPase (SERCA) in vascular smooth muscle (VSM). Furthermore, AMPK activator reduced NADPH oxidase 4 (Nox4) and Nox2-derived reactive oxygen species (ROS) production. These results demonstrate that A769662 has potent vasodilator and antioxidant effects in intrarenal arteries. The benefits of AMPK activation in rat kidney are reproduced in human arteries and therefore vascular AMPK activation might be a therapeutic target in the treatment of metabolic disease-associated kidney injury.
Keywords: AMPK activators, Renal arteries, IKCa channels, KATP channels, SERCA, Reactive oxygen species
Graphical abstract
Model of AMPK activation in kidney arteries. Activation of AMPK with the thienopyridine derivative A7669662 stimulates eNOS and NO production and activates IKCa channels at the endothelium, the latter producing endothelial cell (EC) hyperpolarization that spreads to VSM leading to [Ca2+]i reduction and relaxation. At VSM, AMPK activates metabolic KATP channels and SERCA which also reduces [Ca2+]i and produces relaxation. AMPK activation induces a powerful inhibition of ROS generation by suppressing Nox4 and Nox2 activity in renal arteries.
Abbreviations
- AMPK
AMP-activated protein kinase
- ACh
acetylcholine
- BKCa channel
large-conductance calcium-activated K+ channel
- COX
cyclooxygenase
- EC
endothelial cell
- EDH
endothelium-derived-hyperpolarization
- eNOS
endothelial nitric oxide synthase
- H2O2
hydrogen peroxide
- IKCa channel
intermediate-conductance calcium-activated K+ channel
- KATP
ATP-sensitive potassium channels
- NADPH
nicotinamide adenine dinucleotide phosphate
- NO
nitric oxide
- NOS
nitric oxide synthase
- Nox
NADPH oxidase enzymes
- O2.
superoxide
- Phe
phenylephrine
- PSS
physiological saline solution
- ROS
reactive oxygen species
- SERCA
sarcoplasmic reticulum Ca2+-ATPase
- VSM
vascular smooth muscle
1. Introduction
AMPK is an ubiquitous heterotrimeric kinase considered as a master energy sensor/metabolic switch that is activated during energy stress in response to ATP depletion, to preserve cell survival under low-caloric conditions [[1], [2], [3], [4]]. As the ratio of AMP/ATP increases, activation of AMPK turns on ATP-generating pathways (e.g. glucose uptake, glycolysis, fatty acid oxidation) and reduces ATP-consuming pathways (free fatty acid –FFA-, triglyceride and protein synthesis) [1]. AMPK is made up of a catalytic α-subunit (α1 and α2), a structural β-subunit (β1 and β2), and the AMP-binding site containing γ-subunit (γ1, γ2 and γ3). AMPK activation requires phosphorylation of the α-subunit and occurs in response to increased AMP/ATP ratio, as well as through phosphorylation by upstream kinases such as the tumor suppressor liver kinases B1 (LKB1) or the Ca2+/calmodulin-dependent protein kinase kinase β (CAMKKβ) [2].
AMPK is not only involved in metabolism but also in the regulation of physiological processes such as mitochondria biogenesis, cell growth and proliferation, inflammation, oxidative stress and cell autophagy. AMPK also plays a key role in cardiovascular function, in part through phosphorylation and activation of endothelial nitric oxide synthase (eNOS) in endothelial cells and cardiomyocytes [[5], [6], [7], [8], [9]]. By stimulating nitric oxide (NO) production, the AMPK-eNOS endothelial signalling pathways is involved in endothelium-dependent vasodilatation [10] in response to physiological stimuli such as shear stress [7,11,12] or adipokines acting via Gq receptors like adiponectin and leptin [1].
The kidney is an organ with one of the highest energy consumption rates in the body, used for the regulation of fluids and electrolytes homeostasis and for waste excretion, and renal blood flow represents about 25% of cardiac output. AMPK is highly expressed in the kidney and regulates activity of various ion and creatinine transporters in renal tubular cells and reduces podocyte permeability and albuminuria upon kinase activation with adiponectin [13,14]. Acute renal ischemia is a potent activator of AMPK which induces cell survival mechanisms including mitochondrial protection and biogenesis [15], suppression of extracellular matrix proteins [16] and induction of autophagy [17]. Accordingly, AMPK activators have been shown to be protective and attenuate ischemia-reperfusion injury in canine models of renal transplantation and in rat models of kidney ischemia-reperfusion injury [[18], [19], [20]]. On the other hand, AMPK has been reported to exert anti-oxidant effects in the cardiovascular system by inhibiting the expression and activity of NADPH oxidase (Nox) subunits, suggestive of potential crosstalk between AMPK and Nox, despite how AMPK suppresses Nox remains to be clarified [21].
Reduced renal AMPK activity has been associated to impaired renal function and inflammation in the kidney of diabetic patients [22], in obesity-associated nephropathy [23] and in experimental models of chronic kidney disease [24,25], and is involved in renal inflammation and fibrosis [23]. Therefore, AMPK activation has recently been proposed as a therapeutic target of metabolic disease-associated nephropathy and experimental chronic kidney disease. Cardiovascular-protective effects of AMPK are well characterized [26] and many therapeutic agents used for the treatment of diabetes and atherosclerosis, including metformin, thiazolidinediones and statins may exert their cardiovascular protective effects by activation of AMPK [[27], [28], [29]]. However, despite the role of AMPK in kidney function and dysfunction is well stablished, little is known about its role in the regulation of renal vascular function.
The hypothesis of the present study is that activation of AMPK may regulate renal vascular function thus coupling renal blood flow to kidney metabolism. Therefore, we sought to investigate AMPK vasodilator effects in human renal arteries and to identify AMPK targets in kidney arterial tissue. For this, we assessed the contribution of the eNOS-NO endothelium-dependent pathway, as well as vascular smooth muscle (VSM) relaxant mechanisms involving K+ channels and the sarcoplasmic reticulum Ca2+-ATPase (SERCA). Moreover, the interaction between AMPK and Nox-mediating mechanisms generating reactive oxygen species (ROS) in kidney vascular tissues were also investigated.
2. Methods
2.1. Animal model
Male Wistar rats were housed and maintained on standard chow and water ad libitum at the Pharmacy School animal care facility. They were killed by decapitation and exsanguination at 12–14 weeks. The kidneys, heart and mesentery were quickly removed and placed in cold (4 °C) physiological saline solution (PSS) of the following composition (mM): NaCl 119, NaHCO3 25, KCl 4.7, KH2PO4 1.17, MgSO4 1.18, CaCl2 1.5, EDTA 0.027 and glucose 11, continuously gassed with a mixture of 5% CO2/95% O2 to maintain pH at 7.4. Animal care and experimental protocols conformed to the European Union Directive 2010/63/EU on the Protection of Animals Used for Scientific Purposes and were approved by the Animal Care and Use Committee of Complutense University of Madrid. All animal experiments are reported in compliance with the ARRIVE Guidelines.
2.2. Patients
Intrarenal arteries and cortex samples were obtained from the kidney of renal tumour patients who underwent nephrectomy as earlier reported [30]. The investigation with human tissue conformed to the principles outlined in the Declaration of Helsinki. Permission was obtained from the Ethics Committee of the University Hospital Puerta de Hierro-Majadahonda, Spain (Reg. no 5.16) and patients gave their informed consent.
2.3. Dissection and mounting of microvessels
Renal interlobar or arcuate arteries, 2nd- 4th order branches of the main renal artery were carefully dissected by removing the surrounding connective tissue from tumour-free parts of the kidney of human patients, or kidney of Wistar rats. For some experiments, mesenteric resistance arteries and coronary arteries of Wistar rats were dissected. Small samples of both renal arteries and cortex were also dissected out for ROS measurements, as described earlier [30]. Arterial segments were mounted in microvascular myographs (Danish Myotechnology, Denmark) by inserting two 40 μm tungsten wires into the vessel lumen and were equilibrated for 30 min in PSS maintained at 37 °C. The relationship between passive wall tension and internal circumference was determined for each individual artery and from this, the internal circumference, L100, corresponding to a transmural pressure of 100 mmHg for a relaxed vessel in situ was calculated. The arteries were set to an internal diameter I1 (I1 = 0.9 × L100) at which tension development is maximal.
2.4. Experimental procedures for the functional experiments
At the beginning of each experiment, arteries were challenged twice with 120 mM K+ (KPSS) in order to test vessel viability. Concentration-responses curves to the endothelial agonist acetylcholine (ACh) and to the β-adrenergic agonist isoprenaline were performed to assess arterial function on arteries precontracted with phenylephrine (Phe) (0.1–0.5 μM). The effects of the AMPK selective activator A769662 (Tocris Cookson, Bristol, UK) was assessed in intrarenal, coronary and mesenteric arteries precontracted with Phe and responses to A769662 were added in a second concentration-curve in order to test whether the relaxations were reproducible (Fig. S1). The relaxant responses of A769662 were compared to those elicited by the AMPK activator (AICAR) (Tocris Cookson, Bristol, UK) by adding cumulative concentrations of these agents on arteries precontracted with Phe. The AMPK inhibitor (compound C, 0.5 μM) (Tocris Cookson, Bristol, UK) was tested on the relaxations to the AMPK activators and was added to the myograph chamber 30 min before a second concentration-response curve was performed. To evaluate whether the relaxant effect of AMPK was endothelium-dependent, endothelium-intact and endothelium-denuded rat and human renal arterial segments were pre-contracted with Phe and treated with A769662. The endothelium was mechanically removed by inserting a human hair in the vessel lumen and guiding it back and forwards several times. The absence of functional endothelium was confirmed by lack of the relaxation to ACh (10 μM), as earlier described [31]. Functional responses to A769662 were further obtained in the absence and presence of NOS synthase inhibitor (L-NOARG, 100 μM) (Sigma-Aldrich, Spain), inhibitors of intermediate-conductance KCa channels (IKCa, TRAM-34 1 μM) (Tocris Cookson, Bristol, UK), large-conductance KCa channels (BKCa, iberiotoxin 0.3 μM) (Tocris Cookson, Bristol, UK), Kir6 ATP-sensitive K channels (KATP, glibenclamide 1 μM) (Tocris Cookson, Bristol, UK) or the inhibitor of SERCA ATPase, cyclopiazonic acid (CPA, 10 μM) (Tocris Cookson, Bristol, UK) in rat and human renal arteries.
2.5. Measurements of VSM intracellular Ca2+ ([Ca2+]i)
Simultaneous measurements of VSM [Ca2+]i and tension in intact renal interlobar arteries were performed as described ealier [31]. Arterial segments were mounted in a microvascular myograph placed on an inverted microscope (Zeiss Axiovert S100 TV) equipped for dual excitation wavelength microfluorimetry. Arteries were incubated in the dark in PSS with 8 μM Fura 2-acetoxymethyl ester (Fura 2-AM) (Thermo Fisher Scientific, Life Technologies SA, Madrid, Spain) and 0.05% Cremophor EL. After Fura-2-AM loading, arteries were washed for 30 min in PSS and were illuminated with alterning 340 nm and 380 nm light using a monochromator-based system (Deltascan, PTI). Simultaneous measurements of [Ca2+]i and tension by Fura2-AM fluorescence were performed in rat interlobar arteries to further study AMPK-mediated calcium-dependent mechanisms in VSM. The ratio (R) F340/F380 was taken as a measure of [Ca2+]i. Time-response curves to 10 μM of the AMPK selective activator A769662 were performed in renal arteries with intact endothelium pre-contracted with Phe or with 30 mM K+ in the absence and presence of inhibitor of IKCa TRAM-34 (1 μM). In order to restrict Ca2+ measurements to VSM when assessing the role of VSM K+ channels or SERCA ATPase, experiments were performed in renal endothelium-denuded arteries pre-contracted with Phe. Then time-response curves to 10 μM A769662 were performed in the absence and presence of inhibitors of BKCa channels (iberiotoxin, 0.3 nM), KATP channel (glibenclamide, 1 μM) or SERCA ATPase (CPA, 10 μM).
2.6. Measurement of superoxide production by chemiluminescence
Changes in basal and NADPH-stimulated levels of superoxide (O2.-) were measured by lucigenin chemiluminescence in renal arteries and cortex of wistar rats and human samples, as earlier reported [30,31]. Cortex samples and segments of the renal interlobar arteries were dissected and equilibrated in PSS for 30 min at room temperature and then incubated in the absence (basal) and presence of AMPK selective activator A769662 (30 μM) and the NADPH oxidase inhibitor Nox2ds-tat (1 μM) (AnaSpec Seraing, Belgium) for 30 min at 37 °C. Samples were then transferred to microtiter plate wells containing 5 μM bis-N-methylacridinium nitrate (lucigenin) in the absence and presence of different ROS sources inhibitors and then stimulated with NADPH (100 μM) which was added prior to ROS measurements. In another set of experiments, the protein kinase C activator phorbol 12,13-dibutyrate (PDBu, 10 μM) (Tocris Cookson, Bristol, UK) was used instead of NADPH in order to indirectly stimulate the Nox2-derived superoxide production. Chemiluminescence was measured in a luminometer (BMG Fluostar Optima), and for calculation baseline values were subtracted from the counting values under the different experimental conditions and superoxide production was normalized to dry tissue weight.
2.7. Measurement of hydrogen peroxide by Amplex Red
Hydrogen peroxide (H2O2) production was measured by Amplex Red assay Kit (Thermo Fisher Scientific, Life Technologies SA, Madrid, Spain) in renal arteries and cortex of wistar rats [30,31]. Samples were equilibrated in HEPES-physiological saline solution (PSS) for 30 min at room temperature and then incubated in the absence (basal) and presence of AMPK selective activator A769662 (30 μM) for 30 min at 37 °C. Arteries and cortex samples were then transferred to microtiter plate black wells containing 10 mM final concentration (Amplex Red) and 10 U/ml final concentration (horseradish peroxidase) and some samples were stimulated with NADPH (100 μM) just prior to determination. Fluorescence was measured in a fluorimeter (BMG Fluostar Optima), using an excitation filter of 544 nm and an emission filter of 590 nm. Background fluorescence was subtracted from the counting values under the different experimental conditions and H2O2 production was normalized to dry tissue weight.
2.8. Western blot analysis
Renal arterial tissue incubated 30 min with A769662 or vehicle were snap frozen in liquid nitrogen, homogenized and lysed in buffer containing Tris-HCl (pH 7.5) 50 mmol/L, EGTA 1 mmol/L, EDTA 1 mmol/L, Triton X-100 1% vol/vol, sodium orthovanadate 0.1 mmol/L, sodium fluoride 50 mmol/L, sodium pyrophosphate 5 mmol/L, sucrose 0.27 mol/L and protease inhibitor cocktail. The protein concentration was determined by Bradford assay (Bio-Rad Laboratories, S.A, Madrid, Spain). Protein lysates (7 μg) were subjected to 6,5% SDS-PAGE, electrotransferred on a polyvinylidene fluoride membrane and probed with the following primary antibodies: pACC Ser79 (1:1000; ref 3661), pAMPKα Thr172 (1:1000; ref 2535), AMPKα1/2 (1:1000; ref 2532) from Cell Signaling Technology (Leiden, The Netherlands); NADPH oxidase 4 (1:500; ref ab154244) from Abcam (Cambridge, UK); β-actin (1:5000; ref. A5316) from Sigma (St. Louis, MO, USA) [4,32]. The blots were visualized using enhanced chemiluminescence and quantified by densitometry with ImageJ free software. Values were expressed in relation to β-actin protein levels.
2.9. Data presentation and statistical analysis
Results are expressed as either absolute values (units of R (F340/F380) or Nm−1 of tension) or as a percent of the response to either Phe or KPSS in each artery, as means ± SEM of 6–10 arteries from 4 to 12 rats (1–2 artery from each animal) for the functional experiments. For the measurement of O2.- or H2O2 production, results are expressed in counts per minute (cpm) per mg of tissue and relative fluorescence units (RFU) per mg of tissue in arterial segments and cortex samples, respectively, as means ± SEM of 5–7 patients or means ± SEM of 4–10 rats.
Statistical significance was determined by using paired or unpaired Student's t-test where appropriate, or one-way ANOVA followed by Bonferroni's post hoc test for comparisons involving more than two groups. P < 0.05 were considered statistically significant. Calculations were made using a standard software package (GraphPad Prism 5.0, GraphPad Software, Inc., San Diego, CA, United States).
3. Results
3.1. AMPK activation relaxes intrarenal arteries
Normalized internal lumen diameters, l1, vasomotor responses and endothelium-dependent relaxations of human renal arteries are shown in Table 1. Since vascular AMPK has recently been identified as a potent vasodilator in resistance arteries [33], the effects of the AMPK selective activator A769662 (3 μM and 10 μM) were assessed in interlobar arteries from human and rat kidney, as well as in rat mesenteric and coronary arteries, in order to compare the renal vascular effects of A769662 to that in small arteries from systemic and coronary vascular beds. AMPK activation produced potent relaxations of both human and rat intrarenal arteries (Fig. 1 a and c) and also in mesenteric resistance arteries (Fig. 1e). Notably, A769662 did not have significant relaxant effects in precontracted coronary arteries (Fig. 1g). The relaxations elicited by AMPK activation were compared to those evoked by the endothelium-dependent agonist ACh or the β-adrenoceptor agonist isoprenaline in each arterial bed (Fig. 1b, d, f and h). Relaxant responses to A769662 were reproducible in rat and human renal arterial segments (Supplementary Fig. S1).
Table 1.
Vasomotor function of the human intrarenal arteries.
| n | ||
|---|---|---|
| l1 (μm) | 454 ± 28 | 25 |
| KPSS (Nm-1) | 1.97 ± 0.30 | 28 |
| ACh (Relaxation %) | 73.4 ± 4 | 26 |
Values represent mean ± S.E.M. of the number n of individual arteries, 3–4 from each patient (7 patients). l1 is the effective lumen diameter of the arteries.
Fig. 1.
AMPK activation induces vasorelaxation of renal and mesenteric but not coronary arteries
Original recordings showing A769662-induced relaxations of intact renal interlobar arteries from human (a) and rat (c) kidney and rat mesenteric arteries (e), and the lack of effect of AMPK activator in coronary arteries from rat (g). Average vasorelaxant effects of A769662 (3 μM and 10 μM) (a,c,e,g) compared to those of the endothelial agonist acetylcholine (ACh) and the β-adrenoceptor isoprenaline (b,d,f,h) in rat renal, mesenteric and coronary arteries. Results are expressed as percentage of the increases in tension induced by phenylephrine (Phe) or serotonin (5-HT) for coronary arteries. Data are shown as the mean ± SEM of 9–28 arteries from 6 -14 rats (1–2 per animal) and as the mean ± SEM of 20 arteries from 7 patients.
Pharmacological characterization of AMPK activation in intrarenal arteries was performed by constructing concentration-response curves to two different AMPK activators: A769662 that selectively binds the AMPK β1 subunit and AICAR, an unspecific and allosteric AMPK activator (Fig. 2a). A769662-elicited relaxations were about 2.5 orders of magnitude more potent than those produced by AICAR (Table 2), and therefore A769662 was used in the subsequent experiments. Treatment for 30 min with the AMPK inhibitor (compound C, 0.5 μM) inhibited the relaxant responses elicited by both A769662 and AICAR in a second concentration-response curve (Fig. 2b and c; Table 2).
Fig. 2.
A769662-induced potent relaxations compared to AICAR- and phosphorylates AMPK and ACC in renal arteries
Relaxant responses of AMPK activation with A769662 vs AICAR in renal arteries from rats (a) and inhibitory effect of compound C (0.5 μM) (b,c). Data are shown as the mean ± SEM of 6–8 arteries from 6 rats. Western blots analysis for pAMPKα (d), AMPKα (e) and pACCα (f) protein levels in samples of renal arteries from rats with or without acute treatment with A769662 (10 μM). Results were quantified by densitometry and presented as a ratio of density of the protein band vs that of β-actin from the sample. Data are shown as the mean ± SEM of 4–5 animals. Statistical significant differences between means were analyzed by using unpaired Student's t-test *P < 0.05, **P < 0.01 vs control.
Table 2.
Relaxant effects of the AMPK activators AICAR and A769662 and effect of the AMPK inhibitor compound C in rat renal interlobar arteries.
| pEC50 (%) | Emax (%) | n | I1 (μm) | |
|---|---|---|---|---|
| A769662 | 4.92 ± 0.05 | 71.9 ± 5.7 | 6 | 308 ± 16 |
| + Compound C | 3.89 ± 0.15** | 43.5 ± 6** | 6 | 312 ± 16 |
| AICAR | 2.67 ± 0.09††† | 61.8 ± 0,9 | 6 | 316 ± 17 |
| + Compound C | 1.79 ± 0.1* | 33.1 ± 13 | 6 | 326 ± 15 |
Values represent mean ± S.E.M. of the number n of individual arteries, 1–2 per animal. pEC50 is –logEC50, EC50 being the agonist concentration giving half-maximal relaxation; Emax = maximal relaxation (% Phe). Significant differences were analyzed by ANOVA or paired or unpaired Student t-test. *P < 0.05; **P < 0.01 versus control before treatment and †††P < 0.001 versus A769662.
Western blot analysis confirmed that activity of AMPK was augmented by A769662 in intrarenal arteries as depicted by the 3-fold enhancement of AMPKα phosphorylation at Thr-172 after 20 min incubation with A769662 (Fig. 2d and e). In addition, A769662 increased phosphorylation of the AMPK's downstream target acetyl-CoA carboxylase (ACC) in renal arteries (Fig. 2f).
3.2. A769662 induces relaxations by endothelial NO release
AMPK plays a role in cardiovascular function in part through phosphorylation and activation of eNOS in endothelial cells and cardiomyocytes [8]. In order to assess the contribution of the endothelium to the relaxant responses of AMPK, human and rat renal arterial segments with intact endothelium or endothelium-denuded pre-contracted with Phe were stimulated with A769662 (3 and 10 μM). Both mechanical endothelium removal and (Fig. 3 a and c) and inhibition of NOS with L-NOARG (Fig. 3 b and d) markedly reduced A769662-evoked relaxations thus suggesting that the vasodilator mechanism of AMPK is in part dependent on the vascular endothelium and involves the eNOS-NO pathway.
Fig. 3.
Endothelial-derived NO is involved in the relaxations induced by AMPK activation in renal arteries
Original recordings showing the inhibitory effects of mechanical endothelium removal and NOS blockade with L-NOARG (100 μM) on the relaxations induced by AMPK activator A7699662 in intrarenal arteries from human (a,b) and rat (c,d) kidney. Results are expressed as percentage of the increases in tension induced by phenylephrine (Phe). Data are shown as the mean ± SEM of 5–15 rats (1–2 arteries per animal) and as the mean ± SEM of 4–7 patients (2–3 arteries per patient). Statistical significant differences between means were analyzed by paired Student's t-test **P < 0.01, ***P < 0.001.
3.3. A769662 reduces VSM [Ca2+]i and activates endothelial IKca channels and VSM KATP sensitive channels to induce relaxation
Simultaneous measurements of VSM [Ca2+]i and tension were performed in rat interlobar arteries to further investigate the role of AMPK in calcium-dependent mechanisms of human renal vasodilation. Time-response curves to 10 μM of the AMPK selective activator A769662 produced relaxations that were accompanied by simultaneous decreases in VSM [Ca2+]i (Fig. 4a). However, relaxations elicited by A769662 were larger than the corresponding decreases in VSM [Ca2+]i, thus suggesting the involvement of Ca2+desensitization mechanisms (Fig. 4b), as confirmed by tension-[Ca2+]i relationships for A769662 (Fig. 4c).
Fig. 4.
AMPK activation relaxes renal arteries by reducing VSM [Ca2+]iand by calcium-independent mechanisms. Representative traces showing simultaneous recordings of VSM [Ca2+]i and tension and the effect of the AMPK activator A7699662 in endothelium-intact rat renal arteries pre-contracted with phenylephrine (Phe). (a) Average concentration-dependent decreases in [Ca2+]i (upper panel) and relaxations (lower panel) in response to A769662 (b) and [Ca2+]i-tension relationships for the effect of A769662 in renal arteries. (c) AMPK activation-induced decreases in [Ca2+]i and relaxations in arteries precontracted with Phe were abolished when raising extracellular K+ in arteries precontracted with 30 mM K+(d, e) All results are expressed as percentage of the increases in [Ca2+]i or tension induced by Phe or with 30 mM K+. Data are shown as the mean ± SEM of 5 arteries, 1 per animal. Statistical significance was calculated by paired Student's t-test *P < 0.05, **P < 0.01.
A769662-induced relaxations and [Ca2+]i decreases were abolished when raising the extracellular K+ concentration (Fig. 4d and e) which suggests that K+ efflux and hyperpolarization mechanisms are in part responsible for the AMPK relaxant effects in renal arteries. Since KCa channels have recently been involved in AMPK relaxant mechanisms in small arteries [33], the effects of selective KCa inhibitors were assessed in the AMPK responses of endothelium-intact renal arteries. Treatment with the inhibitor of IKCa channels TRAM-34 significantly reduced A769662-evoked relaxations in human renal arteries (Fig. 5a and b), and this inhibition was confirmed in rat intrarenal arteries as shown in Fig. 5e. Moreover, IKCa channel blockade caused a significant reversion of the decreases in [Ca2+]i evoked by A769662 (Fig. 5c and d). In order to determine whether VSM BKCa channels may be involved in the relaxant mechanism of A-769662, as recently reported for small mesenteric arteries [33], the selective inhibitor of BKCa channels iberiotoxin was used in endothelium-denuded rat intrarenal arteries. A769662-elicited relaxations and [Ca2+]i-decreasing effects were not sensitive to iberiotoxin (Fig. 5f and g). Therefore, the fact that A-769662-induced relaxations were sensitive to TRAM-34 in endothelium intact arteries but not to iberiotoxin in endothelium-denuded microvessels, suggests that AMPK activation might have a major effect on IKCa channels expressed in endothelial cells.
Fig. 5.
Intermediate-conductance KCa(IKCa) channels are implicated in AMPK-induced vasodilation of human renal arteries.
Representative traces showing the effect of the inhibitor of intermediate-conductance KCa (IKCa) channels TRAM-34 (1 μM) on the A769662-elicited vasodilation of human renal arteries (a) and the average inhibitory effect of TRAM-34 (b). Data are shown as the mean ± SEM of 10 arteries from 4 patients. Original recordings showing A769662-induced decreases in VSM [Ca2+]i (top) that were blunted by TRAM-34 (bottom) in intact renal interlobar arteries from rat. (c) Average inhibitory effect of TRAM-34 on the decreases in [Ca2+]i(d) and the relaxations to A769662 (e) in renal interlobar arteries. Average inhibitory effect of the inhibitor of high-conductance KCa channels (BKCa), Iberiotoxin (0.03 μM) on the decreases in [Ca2+]i(f) and the relaxations to A769662 (g) in endothelium-denuded renal interlobar arteries. Data are shown as the mean ± SEM of 4–5 rats (1 artery per animal). Statistical significance was calculated by paired Student's t-test *P < 0.05, **P < 0.01.
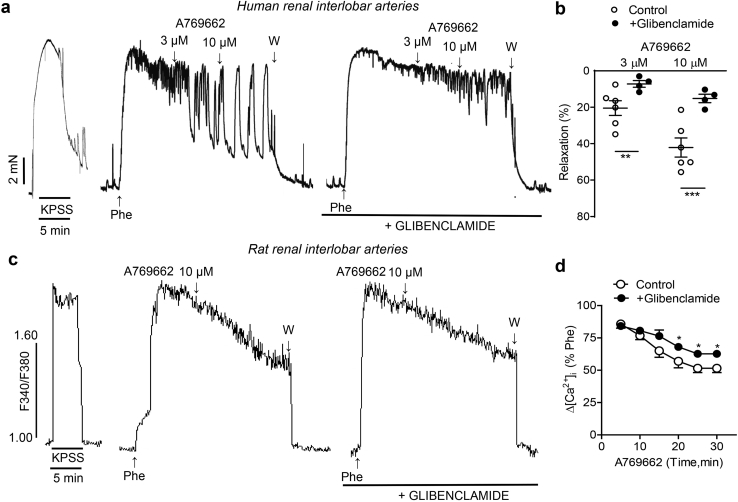
In the kidney, ATP-sensitive potassium channels (KATP) are localized in tubular epithelial cells, glomerular mesangial cells and VSM of renal blood vessels [34]. Activation of arterial smooth muscle KATP channels produces smooth muscle hyperpolarization leading to a reduction in [Ca2+]i and vasodilatation [35].Therefore, we assessed whether KATP channels might be involved in the relaxant responses elicited by AMPK activation in renal small arteries. Treatment with the selective KATP channel inhibitor glibenclamide markedly reduced A769662-evoked relaxations in human renal arteries (Fig. 6a and b) and significantly inhibited decreases in [Ca2+]i induced by A-769662 in rat renal arteries (Fig. 6c and d).
Fig. 6.
A769662 activates ATP-sensitive K+(KATP) channels in VSM of human renal arteries
Representative traces illustrating the inhibition of both A769662-elicited vasorelaxation (top) in human renal arteries (a) and decreases in VSM [Ca2+]i (bottom) in renal arteries from rat (c) induced by the Kir6 KATP channel blocker glibenclamide (1 μM). Arteries were endothelium-denuded. Summarized data showing the average changes in relaxation (b) and [Ca2+]i(d) induced by A769662 in presence of glibenclamide. Values means ± SEM of n = 4–5 rats (1 artery per animal) and the mean ± SEM of from 4 patients (2 arteries per patient).
3.4. Activation of SERCA is involved in the relaxations of A-769662
SERCA is an important and potent modulator of smooth muscle [Ca2+]i and therefore the effects of the selective SERCA inhibitor cyclopiazonic acid (CPA, 10 μM) on changes in VSM [Ca2+]i and relaxation produced by AMPK activation were further explored in endothelium-denuded renal arteries, as shown in Fig. 7. Treatment with CPA induced a marked inhibition of the relaxations of A769662 in renal microvessels (Fig. 7a and b), suggesting that AMPK activates SERCA in VSM to produce vasodilation of human intrarenal arteries. This relaxant mechanism was confirmed in rat intrarenal arteries since treatment with CPA significantly reduced the VSM [Ca2+]i -decreasing effects of A769662 (Fig. 7c and d).
Fig. 7.
SERCA activation in involved in A769662-induced relaxations of human renal arteries
Original recorgings showing that both vasodilation (top) in human renal arteries (a) and decreases in [Ca2+]i (bottom) in rat renal arteries evoked by AMPK activation with A769662 (c) were blunted by the inhibitor of sarcoplasmic reticulum calcium ATPase (SERCA) CPA (10 μM). Experiments wer performed in endothelium-denuded arteries. Summarized data showing the average changes in relaxation (b) and [Ca2+]i(d) induced by A769662 in presence of CPA. Data are shown as means ± SEM of n = 6 rats (1 artery per rat) and as the mean ± SEM from 3 patients (2 arteries per patient). Statistical significant differences between means were analyzed by paired Student's t-test *P < 0.05, **P < 0.01, ***P < 0.001.
3.5. A769662 reduces ROS generation through inhibition of Nox2 and Nox4
AMPK is known to modulate reactive oxygen species (ROS) generation in the vascular endothelium [21] and therefore we explored whether AMPK activation plays a role in the regulation of ROS in the renal vascular wall. Basal O2•− levels were enhanced by NADPH addition and acute treatment with the AMPK activator A769662 induced a powerful inhibition of NADPH-derived O2•− production in samples of arteries and cortex from human and rat kidneys (Fig. 8a and b). Since Nox2 and Nox4 are involved in kidney physiological processes and are a source of vasodilator H2O2 in the renal endothelium [30], we next assessed whether these Nox subunits are targets of A769662 antioxidant action in renal arteries. Basal H2O2 production assessed by Amplex Red fluorescence assay was markedly increased by NADPH addition and blunted by the AMPK activator in both renal interlobar arteries and cortex from rats (Fig. 8c). Furthermore, Western blot analysis showed that protein levels of Nox4 were significantly reduced in renal arteries acutely treated with A769662 (Fig. 8d), thus suggesting that AMPK activation downregulates Nox4-derived arterial H2O2 production.
Fig. 8.
Activation of AMPK dowregulates Nox4-and Nox2-derived ROS generation.
(a,b) Powerful inhibitory effect of AMPK activation on NADPH-stimulated levels O2•− in renal arteries and cortex samples from human (a) and rat (b) kidney measured by lucigenin-enhanced chemiluminescence. (c) Basal and NADPH-stimulated levels of H2O2 in renal arteries and renal cortex from rat measured by Amplex Red fluorescence were reduced by A769662 (30 μM). Results are expressed in counts per minute (cpm) per mg of tissue for chemiluminescence and in relative fluorescence units (RFU) per mg of tissue for fluorescence. Bars represent mean ± SEM of 7 patients (2 samples per patient) and 6–10 animals (1–2 samples per animal). Significant differences were analyzed using one-way ANOVA followed by Bonferroni as a posterio test **P < 0.01, ***P < 0.001 versus control before treatment, ††P < 0.01; †††P < 0.001 versus NADPH-treated. (d) Western blots analysis for Nox4 protein levels in renal arteries and downregulation upon acute in vitro treatment with the AMPK activator A769662 (30 μM). Results were quantified by densitometry and presented as a ratio of density of the NOX4 band vs that of β-actin from the sample. Data are shown as the mean ± SEM of 8–10 rats. Statistical significance was calculated by unpaired Student's t-test *P < 0.05, **P < 0.01.(e) NADPH-stimulated levels O2•− in samples of renal arteries and cortex from rat kidney were reduced by the selective Nox2 inhibitor Nox2ds-tat (1 μM) and by A769662 (30 μM) alone, but there was no further inhibition by combined treatment with Nox2ds-tat plus A769662. (f) Protein kinase C activator phorbol 12,13-dibutyrate PDBu (10 μM) was used to indirectly stimulate Nox2 and O2•− production. Basal and PDBu-stimulated levels O2•− in renal tissues were reduced by the Nox2 inhibitor Nox2ds-tat and by A769662 (f) Results are expressed in counts per minute (cpm) per mg of tissue for chemiluminescence. Bars represent mean ± SEM of 6–10 rats. Significant differences were analyzed using one-way ANOVA followed by Bonferroni as a posterio test **P < 0.01, ***P < 0.001 versus control before treatment, ††P < 0.01; †††P < 0.001 versus NADPH or PDBu-treated.
To assess the involvement of Nox2 in the antioxidant action of AMPK, the effects of A769662 on NADPH-stimulated O2•− production were measured in the absence and presence of the selective Nox2 inhibitor, Nox2ds-tat, that inhibits the assembly of the cytosolic subunit p47phox with Nox2 thus blocking enzyme activity. NADPH-derived O2•− levels were reduced to a similar extent by both Nox2ds-tat and A769662, and combined treatment with the AMPK activator and the Nox2 inhibitor did not further inhibit NADPH-derived O2•− production (Fig. 8e). To confirm that A769662 may have the same pharmacological target as Nox2ds-tat, the PKC activator PDBu was used, since PKC augments assembly of p47phox with Nox2 thus stimulating Nox2 activity and ROS production. Basal O2•− levels were significantly increased by PDBu addition as shown in Fig. 8f. PDBu-stimulated O2•− levels were significantly larger in cortex than in renal arteries and were blunted by Nox2ds-tat and A769662 in both arteries and cortex samples (Fig. 8f), thus suggesting overlapping mechanisms.
4. Discussion
Previous studies have shown that AMPK serves a role in renal physiology by mediating kidney cell survival mechanisms upon activation by acute ischemia and by regulating activity of various tubular cell transporters [[13], [14], [15],20]. Moreover, AMPK activation is protective in metabolic disease-associated nephropathy wherein AMPK activity is blunted [23]. The present findings demonstrate that AMPK might be involved in metabolic regulation of renal blood flow, since activation of this kinase causes vasodilatation of human renal arteries through both endothelium-dependent and direct VSM mechanisms; moreover, our data show that AMPK also modulates Nox-derived ROS production. Specific features of kidney AMPK–mediated arterial vasodilatation such as activation of endothelial IKCa channels and VSM KATP channels are first unveiled.
Pharmacological activation of AMPK with the thienopyridine derivative A7669662 induced potent relaxations of arteries from the rat kidney. Notably, that effect is reproduced in a similar extend in human intrarenal arteries. A7669662 also relaxed mesenteric resistance but not coronary arteries from the same rats, thus suggesting that A-7669662 vasodilator action is vascular tissue-specific. Renal vasodilatation induced by A7669662 was 3 orders of magnitude more potent than that elicited by the pharmaceutical AMPK activator AICAR [10,36,37]. The higher vasodilator potency in the micromolar range found for A-7669662 versus AICAR in intrarenal arteries would confirm its high specificity towards AMPK and is in agreement with that recently reported for other small arteries such as muscular and mesenteric resistance arteries [33].
A7669662 relaxation was found to be associated with phosphorylation of AMPK and of the AMPK downstream target ACC in renal interlobar arteries. A7669662 selectively binds the AMPK β1 catalytic subunit and allosterically increases kinase activity of the ɑ1 catalytic subunit, but this compound can also activate AMPK by inhibiting dephosphorylation of AMPK on Thr-172, like the effects of AMP [38]. Accordingly, acute exposure of renal arteries to A769662 enhanced AMPK Thr-172 phosphorylation and also phosphorylation (and thus inhibition) of the AMPK downstream target ACC, rate-limiting enzyme for fatty acid synthesis, the latter indicating that AMPK vasodilator action accompanies kinase regulatory activity of lipid metabolism and energy storage/mobilization in kidney vascular tissues.
AMPK cardiovascular actions have mainly been attributable to AMPK-activating eNOS in endothelial cells and cardiomyocytes, although AMPK is expressed in both endothelium and VSM and its activation can induce endothelium-independent vasodilation [33,36,39]. In endothelial cells AMPK can be regulated by stimuli affecting cellular ATP levels such as hypoxia, as well as by fluid shear stress, Ca2+-elevating agonists and hormones such as insulin, leptin, adiponectin and thyroid hormones, through upstream kinases including LKB1 or CAMKKβ [2,8]. Activation of AMPK by AICAR in endothelial cells of both large and small resistance arteries has been demonstrated to increase phosphorylation of eNOS at Ser1177 and Ser635, resulting in increased NO production and vasodilatation [[5], [6], [7],10,40]. In agreement with these data, A769662-induced relaxations of both human and rat intrarenal arteries were found to be partially inhibited by mechanical endothelial cell removal and by pharmacological blockade of eNOS, suggesting the involvement of the eNOS/NO pathway in renal vasodilatation induced by AMPK. However, a significant amount of the A769662-induced relaxant response persisted in arteries denuded from endothelium indicating that AMPK activation can directly relax VSM of renal arteries.
VSM expresses AMPK ɑ1-subunit and both β1 and β2 subunits, predominantly β1-AMPK [36,39]. AMPK is activated by metabolic stress in arterial smooth muscle and has been involved in endothelium-independent vasodilation of both large and small arteries [33,36,39] and in the regulation of VSM proliferation and inflammation [9,41]. The present findings demonstrate that AMPK activation causes relaxation of renal arteries mainly by reducing VSM [Ca2+]i and to a lesser extent through Ca2+-independent mechanisms decreasing calcium sensitivity. While recent reports demonstrate that AMPK activators reduce VSM [Ca2+]i of resistance arteries through hyperpolarization mediated by smooth muscle BKCa channels [33], the present findings suggest that stimulation of IKCa rather than BKCa channels is involved in the decrease of smooth muscle [Ca2+]i of renal arteries. Thus, while the inhibitor of BKCa channels iberiotoxin had no effect on A769662 vascular responses, selective pharmacological blockade of IKCa channels with TRAM reduced both relaxation of human renal arteries and the decrease in VSM intracellular Ca2+ elicited by A769662. The differences between our findings and those reported by Schneider et al. (2015) [33] in small muscle and mesenteric arteries support again vascular tissue-specificity of the A769662 action and are likely to be related to the features of the endothelium-dependent hyperpolarization (EDH)-type dilatation of intrarenal arteries. Thus, EDH-elicited response is initiated by stimulation of intermediate- and small conductance KCa channels and hyperpolarization of renal endothelial cells which further spreads through myoendothelial gap junctions to the underlying VSM thus inducing hyperpolarization and vasorelaxation, greatly reduced by blockade of IKCa channels with TRAM both in vitro and in vivo [31,42,43]. The inhibitory effect of TRAM-34 on both decreases in VSM [Ca2+]i and relaxation elicited by A-769662 would be consistent with the AMPK activator stimulating endothelial IKCa channels and further producing VSM relaxation in intrarenal arteries. These findings would be supported by recent studies demonstrating that (EDH)-type dilatation of small arteries is lost after endothelium-specific knock out of the AMPK ɑ1-subunit, thus suggesting that AMPK mediates EDH-type responses of microvessels therefore being involved in blood pressure regulation [44].
Interestingly, our findings first provide evidence that AMPK can also activate VSM KATP channels and thus contribute to renal arterial relaxation, since the sulfonylurea glibenclamide markedly inhibited both vasodilation of human renal artery and the decrease in VSM [Ca2+]i elicited by A-769662. While AMPK is activated in response to metabolic stress usually associated with ATP depletion and increase in the AMP/ATP ratio [1], sarcolemmal KATP channels are likewise stimulated by decrease in intracellular ATP, these channels functioning as metabolic sensors which couple intracellular metabolic state to membrane excitability [45]. In the kidney, KATP channels are functionally expressed in renal arteries and arterioles and KATP activators dilate afferent arterioles in vitro and increase renal blood flow in vivo and in the isolated perfused kidney [[46], [47], [48]]. KATP channels were early involved in metabolic vasodilation of preglomerular arterioles wherein glucose deprivation elicited dilation that was reversed by glibenclamide [46]. Therefore, stimulation of renal arterioles KATP channels by AMPK activation, as shown in the present study, might represent a mechanism to couple renal blood flow and metabolism. Relationship between KATP channels and AMPK activation has been reported in the heart wherein AMPK stimulates sarcolemmal KATP channel activity and recruitment in cardiac myocytes thus contributing to cardioprotection evoked by preconditioning [49]. In the kidney, both AMPK activators and KATP channels openers have independently been reported to provide protection against renal isquemia/reperfusion injury through antioxidant, antiapoptotic and prosurvival actions [17,19,50], while the present findings first show that KATP channels are downstream targets for the AMPK vasodilator action probably implicated in the metabolic regulation of renal blood flow.
Activation of SERCA has been involved in lowering smooth muscle [Ca2+]i and relaxation, and both redox regulation and more recently phosphorylation of SERCA2b, the major SERCA isoenzyme in VSM, increase pump activity to introduce Ca2+ in the SR and therefore to reduce VSM [Ca2+]i and relax arterial smooth muscle [33,[51], [52], [53]]. In our study, pharmacological blockade of SERCA and subsequent SR store depletion by treatment with CPA greatly reduced both relaxation of human renal arteries and the VSM [Ca2+]i decreasing effects induced by AMPK activation, which suggests that SERCA pump is also a target for AMPK phosphorylation in renal VSM accounting for the AMPK-induced vasorelaxation of kidney arteries. This is in agreement with that recently reported for other small arteries wherein AMPK activation augmented VSM calcium transients elicited by SR store depletion with caffeine and inhibition of SERCA reduced the relaxations induced by A769662 [33]. Moreover, in the latter study relaxation induced by A769662 was associated with increased phosphorylation of the SERCA modulator phospholamban at the regulatory T17 site which stimulates SERCA activity [33]. Therefore, the present findings in intrarenal arteries confirm that stimulation of SERCA and reduction of VSM [Ca2+]i is a powerful universal mechanism for the relaxant effects of AMPK activators in small arteries and arterioles.
In the cardiovascular system, AMPK activity can be regulated by oxidative stress and AMPK in turn is able to modulate both mitochondrial ROS generation and gene expression of antioxidant defenses in endothelial cells [21,54]. Hence, pharmacological activation of AMPK in the vascular endothelium has been proposed to be beneficial in metabolic disease not only because of its bioenergetic effects but also due to its ability to counteract oxidative stress [54]. In the present study, we first provide evidence for Nox subunits being targets of AMPK in kidney vascular tissues and demonstrate that AMPK regulates both Nox expression and ROS generating activity. Up-regulation of Nox4 induced by high glucose in podocytes and high fat diet in renal tubular cells has been reported to be inhibited by activation of AMPK both in vitro and in vivo [13,20,55]. Nox4, is highly expressed in the kidney and is a source of endothelial vasodilator H2O2 and protective of vascular function under physiological conditions [30]. In line with this scenario, we have found that acute treatment of renal interlobar arteries with the AMPK activator A-769662 induced a powerful inhibition of NADPH-derived ROS associated to a marked down-regulation of Nox4 expression and reduced H2O2 generation. Nox4 produces H2O2 rather than O2 and is a functional source of ROS in the mitochondria of kidney cortex and renal endothelium [30,56]. Despite AMPK negatively regulates Nox4-dependent oxidative stress and apoptosis in diabetes and obesity [17,55], the present findings suggest that AMPK can also modulate Nox4-dependent H2O2 production and vasoactive actions in the kidney under normal physiological conditions.
On the other hand, AMPK has also been shown to reduce activity and expression of other Nox subunits such as Nox2; thus, AMPKα1 knockout mice displayed increased vascular oxidative stress, Nox2 up-regulation and endothelial dysfunction mediated by enhanced NF-κB activation in endothelial cells, these effects being reversed by AICAR [21,57,58]. The present findings also support a role for AMPK as a physiological regulator of Nox2 activity in intrarenal arteries, since acute exposure to A-769662 did not further inhibited Nox-derived ROS production under conditions of Nox2 blockade, whereas it suppressed O2.- generation stimulated by phorbol esters, which reduces traslocation and phosphorylation of p47phox thus inhibiting Nox2 activation, as reported for cardiac myocytes and human neutrophils [59,60].
In summary, we provide evidence here for new physiological targets of AMPK activation in the kidney leading to renal vasodilation and downregulation of vascular ROS production. These vasorelaxant and antioxidant effects would be consistent with the cardiovascular protective actions of antidiabetic drugs like metformin whose actions have been shown to be mediated by AMPK activation [26,61]. Amelioration of renal fibrosis and inflammation reported for pharmacological AMPK activators [17,25] along with the benefits of vascular AMPK activation suggest that these drugs may be useful therapeutic tools in the treatment of metabolic disease-associated nephropathy and chronic kidney disease.
Sources of support
This work was supported by Ministerio de Ciencia e Innovacion (Spain) co-funded by the FEDER Program of EU (grants nº SAF2016-77526-R and RTI2018-101840-B-I00). Claudia Rodríguez was supported by grant FPU16/04582 from MECD (Spain).
Declaration of competing interest
All authors declare no competing interests.
Acknowledgements
We thank Francisco Puente and Manuel Perales for expert technical assistance.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2020.101575.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Fig. S1.
A769662 showed the same relaxant effect when a second concentration-response curves was performed in human renal arteries (a) and in rat renal arteries with intact endothelium (b) and denuded-endothelium (c). Data are shown as the mean ± SEM of 6 arteries from rat and as the mean ± SEM of 20 arteries from 7 patients.
References
- 1.Hardie D.G., Ross F.A., Hawley S.A. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 2012;13(4):251–262. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ruderman N.B. AMPK, insulin resistance, and the metabolic syndrome. J. Clin. Invest. 2013;123(7):2764–2772. doi: 10.1172/JCI67227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lopez M. Hypothalamic AMPK: a canonical regulator of whole-body energy balance. Nat. Rev. Endocrinol. 2016;12(7):421–432. doi: 10.1038/nrendo.2016.67. [DOI] [PubMed] [Google Scholar]
- 4.Martinez-Sanchez N. Hypothalamic AMPK-ER stress-JNK1 Axis mediates the central actions of thyroid hormones on energy balance. Cell Metabol. 2017;26(1):212–229 e12. doi: 10.1016/j.cmet.2017.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li J. Role of the nitric oxide pathway in AMPK-mediated glucose uptake and GLUT4 translocation in heart muscle. Am. J. Physiol. Endocrinol. Metab. 2004;287(5):E834–E841. doi: 10.1152/ajpendo.00234.2004. [DOI] [PubMed] [Google Scholar]
- 6.Levine Y.C., Li G.K., Michel T. Agonist-modulated regulation of AMP-activated protein kinase (AMPK) in endothelial cells. Evidence for an AMPK -> Rac1 -> Akt -> endothelial nitric-oxide synthase pathway. J. Biol. Chem. 2007;282(28):20351–20364. doi: 10.1074/jbc.M702182200. [DOI] [PubMed] [Google Scholar]
- 7.Chen Z. AMP-activated protein kinase functionally phosphorylates endothelial nitric oxide synthase Ser633. Circ. Res. 2009;104(4):496–505. doi: 10.1161/CIRCRESAHA.108.187567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fisslthaler B., Fleming I. Activation and signaling by the AMP-activated protein kinase in endothelial cells. Circ. Res. 2009;105(2):114–127. doi: 10.1161/CIRCRESAHA.109.201590. [DOI] [PubMed] [Google Scholar]
- 9.Salt I.P., Grahame D. AMP-activated protein kinase-A ubiquitous signalling pathway with key roles in the cardiovascular system. Circ. Res. 2017;120(11):1825–1841. doi: 10.1161/CIRCRESAHA.117.309633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bradley E.A. Activation of AMP-activated protein kinase by 5-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside in the muscle microcirculation increases nitric oxide synthesis and microvascular perfusion. Arterioscler. Thromb. Vasc. Biol. 2010;30(6):1137–1142. doi: 10.1161/ATVBAHA.110.204404. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y. AMP-activated protein kinase is involved in endothelial NO synthase activation in response to shear stress. Arterioscler. Thromb. Vasc. Biol. 2006;26(6):1281–1287. doi: 10.1161/01.ATV.0000221230.08596.98. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Q.J. Endothelial nitric oxide synthase phosphorylation in treadmill-running mice: role of vascular signalling kinases. J. Physiol. 2009;587(Pt 15):3911–3920. doi: 10.1113/jphysiol.2009.172916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharma K. Adiponectin regulates albuminuria and podocyte function in mice. J. Clin. Invest. 2008;118(5):1645–1656. doi: 10.1172/JCI32691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hallows K.R. Role of the energy sensor AMP-activated protein kinase in renal physiology and disease. Rev. Lit. Arts Am. 2010;298(February 2010):F1067–F1077. doi: 10.1152/ajprenal.00005.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xie Z. Upregulation of mitochondrial uncoupling protein-2 by the AMP-activated protein kinase in endothelial cells attenuates oxidative stress in diabetes. Diabetes. 2008;57(12):3222–3230. doi: 10.2337/db08-0610. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Mishra R. AMP-activated protein kinase inhibits transforming growth factor-beta-induced Smad3-dependent transcription and myofibroblast transdifferentiation. J. Biol. Chem. 2008;283(16):10461–10469. doi: 10.1074/jbc.M800902200. [DOI] [PubMed] [Google Scholar]
- 17.Decleves A.E., Sharma K., Satriano J. Beneficial effects of AMP-activated protein kinase agonists in kidney ischemia-reperfusion: autophagy and cellular stress markers. Nephron Exp. Nephrol. 2014;128:98–110. doi: 10.1159/000368932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin A. Attenuation of ischemia-reperfusion injury in a canine model of autologous renal transplantation. Transplantation. 2004;78(5):654–659. doi: 10.1097/01.tp.0000131664.18670.17. [DOI] [PubMed] [Google Scholar]
- 19.Lempiainen J. AMPK activator AICAR ameliorates ischaemia reperfusion injury in the rat kidney. Br. J. Pharmacol. 2012;166(6):1905–1915. doi: 10.1111/j.1476-5381.2012.01895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Decleves A.E. Regulation of lipid accumulation by AMP-activated kinase [corrected] in high fat diet-induced kidney injury. Kidney Int. 2014;85(3):611–623. doi: 10.1038/ki.2013.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song P., Zou M.-H. Regulation of NAD(P)H oxidases by AMPK in cardiovascular systems. Free Radic. Biol. Med. 2012;52(9):1607–1619. doi: 10.1016/j.freeradbiomed.2012.01.025. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dugan L.L. AMPK dysregulation promotes diabetes-related reduction of superoxide and mitochondrial function. J. Clin. Invest. 2013;123(11):4888–4899. doi: 10.1172/JCI66218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Decleves A.E. AMPK mediates the initiation of kidney disease induced by a high-fat diet. J. Am. Soc. Nephrol. 2011;22(10):1846–1855. doi: 10.1681/ASN.2011010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Satriano J. Induction of AMPK activity corrects early pathophysiological alterations in the subtotal nephrectomy model of chronic kidney disease. Am. J. Physiol. Ren. Physiol. 2013;305(5):F727–F733. doi: 10.1152/ajprenal.00293.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kikuchi H. Failure to sense energy depletion may be a novel therapeutic target in chronic kidney disease. Kidney Int. 2018;95(1):123–137. doi: 10.1016/j.kint.2018.08.030. [DOI] [PubMed] [Google Scholar]
- 26.Ewart M.A., Kennedy S. AMPK and vasculoprotection. Pharmacol. Ther. 2011;131(2):242–253. doi: 10.1016/j.pharmthera.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 27.Zhou G. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Invest. 2001;108(8):1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun W. Statins activate AMP-activated protein kinase in vitro and in vivo. Circulation. 2006;114(24):2655–2662. doi: 10.1161/CIRCULATIONAHA.106.630194. [DOI] [PubMed] [Google Scholar]
- 29.Ceolotto G. Rosiglitazone reduces glucose-induced oxidative stress mediated by NAD(P)H oxidase via AMPK-dependent mechanism. Arterioscler. Thromb. Vasc. Biol. 2007;27(12):2627–2633. doi: 10.1161/ATVBAHA.107.155762. [DOI] [PubMed] [Google Scholar]
- 30.Muñoz M. Hydrogen peroxide derived from NADPH oxidase 4- and 2 contributes to the endothelium-dependent vasodilatation of intrarenal arteries. Redox Biol. 2018;19:92–104. doi: 10.1016/j.redox.2018.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muñoz M. CYP epoxygenase-derived H2O2is involved in the endothelium-derived hyperpolarization (EDH) and relaxation of intrarenal arteries. Free Radic. Biol. Med. 2017;106:p168–183. doi: 10.1016/j.freeradbiomed.2017.02.031. [DOI] [PubMed] [Google Scholar]
- 32.Contreras C. Reduction of hypothalamic endoplasmic reticulum stress activates browning of white fat and ameliorates obesity. Diabetes. 2017;66(1):87–99. doi: 10.2337/db15-1547. [DOI] [PubMed] [Google Scholar]
- 33.Schneider H. AMPK dilates resistance arteries via activation of SERCA and BKCa channels in smooth muscle. Hypertension. 2015;66(1):108–116. doi: 10.1161/HYPERTENSIONAHA.115.05514. [DOI] [PubMed] [Google Scholar]
- 34.Zhou M. Expression of ATP sensitive K+ channel subunit Kir6.1 in rat kidney. Eur. J. Histochem. 2007;51(1):43–51. [PubMed] [Google Scholar]
- 35.Tykocki N.R., Boerman E.M., Jackson W.F. Smooth muscle ion channels and regulation of vascular tone in resistance arteries and arterioles. Comp. Physiol. 2017;7(2):485–581. doi: 10.1002/cphy.c160011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goirand F. Activation of AMP kinase α1 subunit induces aortic vasorelaxation in mice. J. Physiol. 2007;581(3):1163–1171. doi: 10.1113/jphysiol.2007.132589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ford R.J. AMP-activated protein kinase activator AICAR acutely lowers blood pressure and relaxes isolated resistance arteries of hypertensive rats. J. Hypertens. 2012;30(4):725–733. doi: 10.1097/HJH.0b013e32835050ca. [DOI] [PubMed] [Google Scholar]
- 38.Sanders M.J. Defining the mechanism of activation of AMP-activated protein kinase by the small molecule A-769662, a member of the thienopyridone family. J. Biol. Chem. 2007;282(45):32539–32548. doi: 10.1074/jbc.M706543200. [DOI] [PubMed] [Google Scholar]
- 39.Rubin L.J. Metabolic activation of AMP kinase in vascular smooth muscle. J. Appl. Physiol. 2005;98(1):296–306. doi: 10.1152/japplphysiol.00075.2004. [DOI] [PubMed] [Google Scholar]
- 40.Morrow V.A. Direct activation of AMP-activated protein kinase stimulates nitric-oxide synthesis in human aortic endothelial cells. J. Biol. Chem. 2003;278(34):31629–31639. doi: 10.1074/jbc.M212831200. [DOI] [PubMed] [Google Scholar]
- 41.Igata M. Adenosine monophosphate-activated protein kinase suppresses vascular smooth muscle cell proliferation through the inhibition of cell cycle progression. Circ. Res. 2005;97(8):837–844. doi: 10.1161/01.RES.0000185823.73556.06. [DOI] [PubMed] [Google Scholar]
- 42.Bussemaker E. Characterization of the endothelium-derived hyperpolarizing factor (EDHF) response in the human interlobar artery. Kidney Int. 2003;63(5):1749–1755. doi: 10.1046/j.1523-1755.2003.00910.x. [DOI] [PubMed] [Google Scholar]
- 43.Rasmussen K.M.B. Contribution of K(+) channels to endothelium-derived hypolarization-induced renal vasodilation in rats in vivo and in vitro. Pflügers Archiv. 2016;468(7):1139–1149. doi: 10.1007/s00424-016-1805-x. [DOI] [PubMed] [Google Scholar]
- 44.Enkhjargal B. Endothelial AMP-activated protein kinase regulates blood pressure and coronary flow responses through hyperpolarization mechanism in mice. Arterioscler. Thromb. Vasc. Biol. 2014;34(7):1505–1513. doi: 10.1161/ATVBAHA.114.303735. [DOI] [PubMed] [Google Scholar]
- 45.Nichols C.G. KATP channels as molecular sensors of cellular metabolism. Nature. 2006;440(7083):470–476. doi: 10.1038/nature04711. [DOI] [PubMed] [Google Scholar]
- 46.Lorenz J.N. Intracellular ATP can regulate afferent arteriolar tone via ATP-sensitive K+ channels in the rabbit. J. Clin. Invest. 1992;90(3):733–740. doi: 10.1172/JCI115945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kawata T. The K(ATP) channel opener nicorandil: effect on renal hemodynamics in spontaneously hypertensive and Wistar Kyoto rats. Kidney Int. Suppl. 1998;67:S231–S233. doi: 10.1046/j.1523-1755.1998.06758.x. [DOI] [PubMed] [Google Scholar]
- 48.Li L., Wu J., Jiang C. Differential expression of Kir6.1 and SUR2B mRNAs in the vasculature of various tissues in rats. J. Membr. Biol. 2003;196(1):61–69. doi: 10.1007/s00232-003-0625-z. [DOI] [PubMed] [Google Scholar]
- 49.Sukhodub A. AMP-activated protein kinase mediates preconditioning in cardiomyocytes by regulating activity and trafficking of sarcolemmal ATP-sensitive K(+) channels. J. Cell. Physiol. 2007;210(1):224–236. doi: 10.1002/jcp.20862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grossini E. Levosimendan protection against kidney ischemia/reperfusion injuries in anesthetized pigs. J. Pharmacol. Exp. Therapeut. 2012;342(2):376–388. doi: 10.1124/jpet.112.193961. [DOI] [PubMed] [Google Scholar]
- 51.Chandrasekera P.C. Determination of apparent calcium affinity for endogenously expressed human sarco(endo)plasmic reticulum calcium-ATPase isoform SERCA3. Am. J. Physiol. Cell Physiol. 2009;296(5):C1105–C1114. doi: 10.1152/ajpcell.00650.2008. [DOI] [PubMed] [Google Scholar]
- 52.Kargacin M.E., Kargacin G.J. Direct measurement of Ca2+ uptake and release by the sarcoplasmic reticulum of saponin permeabilized isolated smooth muscle cells. J. Gen. Physiol. 1995;106(3):467–484. doi: 10.1085/jgp.106.3.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cohen R.A., Adachi T. Nitric-oxide-induced vasodilatation: regulation by physiologic s-glutathiolation and pathologic oxidation of the sarcoplasmic endoplasmic reticulum calcium ATPase. Trends Cardiovasc. Med. 2006;16(4):109–114. doi: 10.1016/j.tcm.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 54.Colombo Sergio L., Moncada S. AMPKα1 regulates the antioxidant status of vascular endothelial cells. Biochem. J. 2009;421(2):163–169. doi: 10.1042/BJ20090613. [DOI] [PubMed] [Google Scholar]
- 55.Eid A.A. AMP-activated Protein Kinase (AMPK) negatively regulates Nox4-dependent activation of p53 and epithelial cell apoptosis in diabetes. J. Biol. Chem. 2010;285(48):37503–37512. doi: 10.1074/jbc.M110.136796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Block K., Gorin Y., Abboud H.E. Subcellular localization of Nox4 and regulation in diabetes. Proc. Natl. Acad. Sci. U. S. A. 2009;106(34):14385–14390. doi: 10.1073/pnas.0906805106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang S. AMPKalpha2 deletion causes aberrant expression and activation of NAD(P)H oxidase and consequent endothelial dysfunction in vivo: role of 26S proteasomes. Circ. Res. 2010;106(6):1117–1128. doi: 10.1161/CIRCRESAHA.109.212530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schuhmacher S. alpha1AMP-activated protein kinase preserves endothelial function during chronic angiotensin II treatment by limiting Nox2 upregulation. Arterioscler. Thromb. Vasc. Biol. 2011;31(3):560–566. doi: 10.1161/ATVBAHA.110.219543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Alba G. Stimulators of AMP-activated protein kinase inhibit the respiratory burst in human neutrophils. FEBS (Fed. Eur. Biochem. Soc.) Lett. 2004;573(1–3):219–225. doi: 10.1016/j.febslet.2004.07.077. [DOI] [PubMed] [Google Scholar]
- 60.Balteau M. AMPK activation by glucagon-like peptide-1 prevents NADPH oxidase activation induced by hyperglycemia in adult cardiomyocytes. Aust. J. Pharm.: Heart Circ. Physiol. 2014;307(8):H1120–H1133. doi: 10.1152/ajpheart.00210.2014. [DOI] [PubMed] [Google Scholar]
- 61.Calvert J.W. Acute metformin therapy confers cardioprotection against myocardial infarction via AMPK-eNOS-mediated signaling. Diabetes. 2008;57(3):696–705. doi: 10.2337/db07-1098. [DOI] [PubMed] [Google Scholar]