Abstract
Background
As of May 4, 2020, the coronavirus disease 2019 (COVID-19) pandemic has affected >3.5 million people and touched every inhabited continent. Accordingly, it has stressed health systems worldwide, leading to the cancellation of elective surgical cases and discussions regarding health care resource rationing. It is expected that rationing of surgical resources will continue even after the pandemic peak and may recur with future pandemics, creating a need for a means of triaging patients for emergent and elective spine surgery.
Methods
Using a modified Delphi technique, a cohort of 16 fellowship-trained spine surgeons from 10 academic medical centers constructed a scoring system for the triage and prioritization of emergent and elective spine surgeries. Three separate rounds of videoconferencing and written correspondence were used to reach a final scoring system. Sixteen test cases were used to optimize the scoring system so that it could categorize cases as requiring emergent, urgent, high-priority elective, or low-priority elective scheduling.
Results
The devised scoring system included 8 independent components: neurologic status, underlying spine stability, presentation of a high-risk postoperative complication, patient medical comorbidities, expected hospital course, expected discharge disposition, facility resource limitations, and local disease burden. The resultant calculator was deployed as a freely available Web-based calculator (https://jhuspine3.shinyapps.io/SpineUrgencyCalculator/).
Conclusions
We present the first quantitative urgency scoring system for the triage and prioritizing of spine surgery cases in resource-limited settings. We believe that our scoring system, although not all encompassing, has potential value as a guide for triaging spine surgical cases during the COVID pandemic and post-COVID period.
Key words: COVID-19, Medical ethics, Pandemic, Rationing, Resource allocation, Spine surgery, Triage
Abbreviations and Acronyms: COVID-19, Coronavirus disease 2019
Background
On December 27, 2019, the first case of the novel coronavirus, coronavirus disease 2019 (COVID-19) (SARS-CoV-2) was reported in Wuhan, China as the cause of a new viral pneumonia with the potential to culminate in acute respiratory distress syndrome and/or death.1 , 2 Since that time, it has spread rapidly to affect nearly every country, placing significant stresses on the global health care system.3 To mobilize resources to combat this pandemic, the U.S. Centers for Medicare and Medicaid Services,4 the Centers for Disease Control and Prevention,5 and multiple professional organizations6 , 7 have recommended the cancellation of elective surgical procedures. Despite this recommendation, it was recognized that there were cases, many of them neurosurgical, which required urgent or emergent intervention to minimize patient morbidity and maximize the chances of an optimal outcome.8 In response, several centers have presented frameworks for the management of neurosurgical patients presenting during the COVID-19 pandemic.8, 9, 10, 11 In addition, a triage scoring system has been previously developed in an attempt to guide spine surgery consults.12 , 13 However, there has not been a systematic, multi-institutional scoring system that includes resource availability and disease burden to aid in triaging patients for spine surgery during this crisis. Although certain symptoms referable to chronic spinal conditions may not necessarily be life threatening, these can cause significant pain and disability, prompting the challenge of determining who to operate on and when in times of crises.
Effective triaging of these cases in the post-COVID era will be essential to prevent the health care system from being overwhelmed by the backlog of elective spinal cases that have been deferred because of the COVID-19 pandemic.14, 15, 16 Recently, a scoring system aimed at triaging such cases has been reported in the general surgery literature17; however, no comparable system has been described for spine patients. We present an applicable example of such a system assembled based on input by a multi-institutional collaboration. This scoring system is designed to assist in 2 ways. First, it may assist spine surgeons and administrators with triaging surgical patients during the COVID-19 pandemic. Second, the scoring system may help health systems triage elective cases in the post-COVID crisis, which is likely to also see a relative shortage of surgical resources and has been described by some as a potential collateral pandemic.15
Methods
Scoring System Development
To generate this scoring system, the first author proposed an a priori scale highlighting those elements believed to be pertinent to the triaging of an operative spine patient in the setting of limited resources. The elements applicable to the spine patient included the patient's current neurologic status (rapidity of progressive and severity), the presence of underlying spinal instability, and radiographic evidence of neural element compression. Several general elements were added that could be used to triage any surgical patient, including general patient health/comorbidities, expected resource utilization, current resource availability, and local disease burden. Medical comorbidities were pulled from the Charlson Comorbidity Index18 and from previously reported series describing comorbidities associated with increased symptom severity in patients infected with the SARS-CoV-2 virus.2 , 19, 20, 21, 22, 23 After these elements were identified, weights were initially assigned based on input from surgeons at the lead institution using a modified Delphi approach, which included both neurosurgical and orthopedic spine surgeons. Component weighting of the preliminary scale was tested using 10 example spine patients, testing the assessed urgency of the patient as determined by the scoring system against the consensus opinion of the group of surgeons.
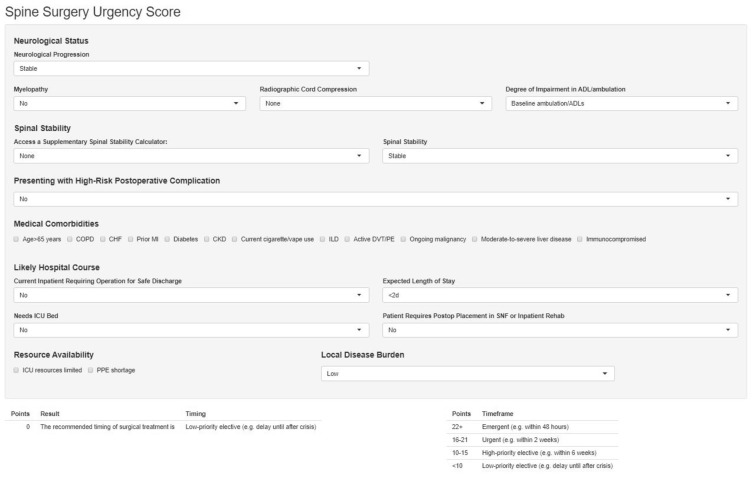
After identifying a preliminary scoring system, a multi-institutional group was convened, including neurosurgical and orthopedic spine surgeons from multiple institutions with varying levels of experience. A modified Delphi approach was again used to alter the weights assigned to the categories to refine the preliminary score. Three rounds of written communication, polling, and electronic teleconferencing sessions were used to solicit input. Example cases were again devised to test the degree of agreement between the scoring system and the consensus opinions regarding the urgency of the hypothetical patient's issue (Supplementary Material). The final scoring system was then deployed as a freely available Web-based calculator (Figure 1 ; https://jhuspine3.shinyapps.io/SpineUrgencyCalculator/).
Figure 1.
Screenshot of Web-based calculator deployed based on scoring system identified (https://jhuspine3.shinyapps.io/SpineUrgencyCalculator/).
Details of the Multi-Institutional Panel
The study group comprised 16 spine surgeons representing 12 institutions in 11 municipalities distributed over the Northeast, Mid-Atlantic, Midwest, South, and West Coast regions, including New York, Baltimore, Boston, Chicago, and San Francisco. All surgeons were fellowship trained and a mean of 12.8 ± 9.3 years out of residency. Eleven surgeons were neurosurgeons and 5 were orthopedic surgeons.
Results
Our modified Delphi approach showed overall agreement with the scoring system in example cases to be 66.3% and 71.5% in the first and last survey rounds, respectively, resulting in the scoring system shown in Table 1 . The score is composed of 8 domains: neurologic status, spinal stability, presentation of a high-risk postoperative complication, medical comorbidities, predicted hospital course, postdischarge placement, resource availability concerns, and local disease burden. Within neurologic status, patients are categorized by their deficit progression, the presence of a radiographic correlate to their neurologic symptoms, and the degree of impairment that their deficit causes in ambulation or the ability to perform activities of daily living.
Table 1.
Spine Surgery Urgency Scoring System
| Neurologic status | |
| Progression of symptoms | |
| Progressive symptoms | See “rapidity of progression” |
| Stable symptoms | 0 |
| Rapidity of progression | |
| <48 hours | 14 |
| 48 hours to 7 days | 10 |
| 1 week to 1 month | 8 |
| >1 month | 4 |
| Myelopathy | 4 |
| With radiographic cord compression | 2 |
| With signal change | 1 |
| Radiographic cord compression without myelopathy | 2 |
| With signal change | 1 |
| Degree of impairment in ADLs or ambulation | |
| Baseline ambulation/ADLs | 0 |
| Newly impaired ambulation/ADLs | 14 |
| New inability to ambulate/perform ADLs | 20 |
| Spinal stability | |
| Stable | 0 |
| Potentially unstable | 6 |
| Chronic instability | 10 |
| Acute instability | 20 |
| High-risk postoperative complications | |
| Deep wound infection requiring surgery∗ | 30 |
| Cerebrospinal fluid leak requiring surgery∗ | 30 |
| New neurologic deficit | 30 |
| Malpositioned hardware with threat to vital structure† | 30 |
| Medical comorbidities‡ | |
| 0–2 | 0 |
| 3–4 | −2 |
| ≥5 | −4 |
| Expected hospital course/discharge | |
| Current inpatient requiring operation for safe discharge | 5 |
| Patient will need ICU bed | −1 |
| Expected stay | |
| Surgery can be performed in ambulatory surgery center or as outpatient surgery | 2 |
| Expected stay <2 days | 0 |
| Expected stay 2–5 days | −1 |
| Expected stay >5 days | −2 |
| Will patient require postoperative placement to skilled nursing facility or inpatient rehabilitation | |
| Yes | −4 |
| Possibly/unknown | −2 |
| No | 0 |
| Resource limitations | |
| No resource limitations | 0 |
| ICU resources limited | −2 |
| Personal protective equipment shortage | −2 |
| Local disease burden | |
| High | −4 |
| Moderate | −2 |
| Low | 0 |
ADL, activity of daily living; ICU, intensive care unit.
Whether the complication requires surgical intervention or can be treated with nonoperative management is decided at the discretion of the attending surgeon.
Vital structures include spinal cord, esophagus, trachea, aorta, and lung.
Medical comorbidities included active malignancy, age >65 years, congestive heart failure, chronic kidney disease, chronic obstructive pulmonary disease, current cigarette or vape use, diabetes mellitus, history of myocardial infarction, interstitial lung disease, moderate to severe liver disease.
The scoring system runs from −19 (lowest priority elective case) to 91 (highest priority emergent case) and classifies cases as “emergent,” “urgent,” “high-priority elective,” or “low-priority elective” as identified in Table 2 . In addition, in Table 2 we surgical time frames for each category. However, these time frames are meant as suggestions and should be no means replace an individual surgeon's clinical judgment.
Table 2.
Proposed Time Frames for Surgical Treatment Based on Urgency Score
| Points | Proposed Surgical Time Frame |
|---|---|
| ≥22 | Emergent (e.g., ≤48 hours) |
| 15–21 | Urgent (e.g., within 2 weeks) |
| 10–14 | High-priority elective (e.g., within 6 weeks) |
| <10 | Low-priority elective (e.g., delay until after COVID-19 crisis) |
Within the scoring system, higher points are assigned to patients with more pressing surgical needs, including more severe neurologic deficits, underlying spinal instability, and the presence of a high-risk postoperative complication. Patients with more extensive comorbidities, longer expected hospitalizations, and a need for postdischarge placement to an inpatient rehabilitation facility or skilled nursing facility are assigned lower points because they are believed to be at highest risk for adverse outcomes when hospitalized during the pandemic. In addition, points are subtracted for patients being treated at facilities in regions with high disease burden and those with shortages of intensive care unit beds or personal protective equipment. We found that this scoring system was able to predict the optimal surgical timing identified by >70% of the surgeon cohort for each of the sample cases.
Discussion
Since the peak of the COVID-19 pandemic, immense pressure has been placed on health care systems worldwide. Various resources, including personal protective equipment, ventilators, intensive care unit beds, and medical staff had been significantly limited and stretched thin.9 , 17 , 24 , 25 In many cases, resources had been stretched so thin that health systems were required to consider how best to allocate their limited resources.3 To address this situation, many hospital systems have curtailed nonurgent surgical procedures, allowing crucial resources to be redeployed for the treatment of patients with COVID-19.11 , 15 Nevertheless, some spinal diseases require urgent or emergent intervention (e.g., cauda equina syndrome) to prevent severe adverse patient outcomes (e.g., death or permanent disability).15 Although previous reports have highlighted which surgical patients qualify for urgent or emergent interventions,8 , 10 , 12 , 13 they have not provided an algorithm for the prioritization of such patients in the setting of potential resource shortages. We present a scoring system devised by a multi-institutional collaboration that aims to assist with these triage issues. The ability to assist with both populations is a strength of this scoring system, which we believe may be a useful tool for health systems both during the COVID pandemic and in the postcrisis period, as they struggle to accommodate the large volume of nonemergent surgical cases. In addition, although we hope that such a need does not arise, the scoring system could also have value in the triaging of patients if a second wave of the coronavirus pandemic occurs, which may lead to further resource limitations.26 Such a wave occurred during the 1918 Spanish influenza pandemic27 and many experts have speculated that a similar phenomenon could occur during the present pandemic.26 , 28 Furthermore, the framework of the proposed scoring system could apply to future pandemics in which health care resources are stretched similarly to the current COVID-19 pandemic.
Previous Examinations of Triaging in Neurosurgery
Several broad descriptions of triage strategies have been presented in the neurosurgical literature,29 , 30 and guidelines from the American College of Surgeons divide surgery into 5 levels based on apparent acuity.11 However. many spinal patients require emergent or urgent addressal29 and fall within the same category of the American College of Surgeons system. Consequently, it is not clear that such a system possesses the granularity necessary to triage patients with surgical issues of grossly similar acuity. Similar limitations are noted for other triaging systems from the trauma surgery literature29 , 31 and for the previous schema in the neurosurgical and orthopedic literature.29 , 30
In addition to a perceived lack of granularity, neurosurgical triage systems reported in the pre-COVID era focused predominately on emergent surgical issues. Triage among nonemergent cases has been largely overlooked. One exception is the Accountability for Reasonableness (A4R) framework described by Ibrahim et al.32 to emphasize scheduling fairness and minimize operating room downtime at an academic center treating a mixture of emergent and elective cases. However, unlike the present scoring system, their framework was purely qualitative: triaging was performed by a single stakeholder without an obvious means by which surgical cases were ranked. Another exception is the Calgary Spine Severity Score proposed by Lwu et al.,12 which assessed spine referrals based on the clinical, pathologic, and radiologic aspects. Although similar to the A4R framework, this score was not intended for implementation in the setting of a crisis or the acute resource shortages that are expected in the post-COVID era.15 , 16
Identifying Surgical Priority in the Setting of COVID
Several institutions have reported their experiences with triaging neurosurgical patients during the COVID-19 pandemic.8 , 10 Burke et al.8 described a multilevel algorithm devised by a multidisciplinary team using a modified Delphi system. Their system included 3 tiers: case urgency, operating room availability, and postoperative bed availability. Assuming that adequate surgical resources were available, patients with emergent surgical issues (e.g., epidural hematoma) were prioritized for operative management regardless of local disease burden. Urgent cases were scheduled if sufficient resources were available and local disease burden was low enough to be managed without assistance from outside institutions. Elective cases were to be deferred unless local disease burden was negligible. Similar to the present system, certain indications were flagged as emergent surgical issues (e.g., intracranial hemorrhage, shunt obstruction, and cauda equina syndrome). However, the investigators only generally identified what constituted an urgent case, namely a surgical issue requiring treatment within 2 weeks that was not identified in the emergency list. Elective cases were similarly identified as all cases that did not fall into these 2 categories. However, unlike the system presented here, no formalized system was identified for the prioritization of cases within the urgent or elective categories.
Eichberg et al.10 similarly recommended that nonurgent cases be deferred. In addition, these investigators suggested that surgeons consider alterations to their surgical practice (e.g., the use of dissolvable suture) to decrease the likelihood that patients would have to return for in-person follow-up, which would increase their COVID-19 exposure risk. Categorizations of surgical emergencies similar to those of Burke et al.8 and Eichberg et al.10 have also been reached by groups at Harvard11 and abroad.9 , 25 In addition, a joint publication by the American Association of Neurological Surgeons, Congress of Neurological Surgeons, and Society for Neuro-Oncology made recommendations to prioritize adjuvant therapies (e.g., chemotherapy and radiotherapy) over earlier surgical intervention for spinal and intracranial malignancies, because this decreases the risks posed by hospitalizing oncologic patients in the same facility as COVID-19–positive patients.33 However, the groups acknowledge that this strategy is not always possible and that care deferral may cause some elective patients to progress to the point of requiring urgent operative management. The European Association for Neurosurgical Societies has attempted to address the question of how to prioritize elective neurosurgical cases through an Adapted Elective Surgery Acuity Scale. Although this scale provides some guidance, the 3 tiers it uses are broad and there are no guidelines for prioritizing cases within a category or a given diagnosis (e.g., “degenerative spinal pathology”).34 Consequently, we believe that the need for a means of triaging both emergent and elective spine cases remains unmet.
Although there have been several general frameworks highlighting those cranial diseases requiring emergent management,8 , 10 , 11 there has been only 1 description of a framework for triaging emergent spine surgery.25 Derived from the experiences at a single Italian center tasked with treating cord compression and spinal instability, the framework of Giorgi et al. is a care pathway intended to expedite the identification, treatment, and safe discharge of patients with spine emergencies. Priority within the system was based on American Spinal Injury Association grade and radiographic evidence of instability. Although good results were described for the 19 patients treated under the framework, the pathway is nonquantitative and seemingly lacks the granularity to prioritize between 2 or more emergent patients. Similarly, it is not equipped to triage nonemergent cases.
A more quantitative approach was described by Jean et al.35 based on nearly 500 respondents to an Internet survey, asking respondents to assign an urgency score to each of 9 hypothetical cases. The investigators found mild to moderate agreement regarding the extent of surgical urgency for each case (range, 22.8%–37.0%); however, their “acuity index” was simplistic in that it was based solely on the perceived case risk and case urgency assigned to it by respondents. Case risk was graded on a 1–4 scale (“no risk” and “cannot postpone”) and case urgency on a 1–5 scale (“leave until after the end of the pandemic” and “case already done”). The scale itself did not incorporate neurologic status, patient comorbidities, or local resource limitations, all of which are likely to influence the timing of operative management. Because of this lack of granularity, it is unclear that this acuity index can be generalized to other case scenarios, thus limiting its potential usefulness relative to the multidimensional scoring system described here.
Limitations
As with scoring systems reported in other domains of neurosurgery, the present scoring system is not intended to be prescriptive in its guidance. Rather, we present it as a potential tool to aid surgeons and health care systems when triaging patients in times of national crisis or global resource shortages. As with the triage frameworks presented to date, the present scoring system is derived from expert opinions. Consequently, the scoring system is limited by the biases of the surgeons recruited and their respective institutions. We attempted to address this by recruiting surgeons at multiple levels of training, at academic centers spread across a large geographic region subjected to varying COVID-19 burdens. Furthermore, by including only surgeons into the decision-making process of the urgency of spine patients, there is potential that additional points from the nonsurgical and administrative personnel could have altered the final scoring system. In addition, in an effort to maximize the usability of the scoring system, it was necessarily simplified and is consequently not all encompassing. For example, the broad term of “new neurologic deficit” was included under the “high-risk postoperative complication” category; however, this policy leaves it up to the treating surgeon whether this new deficit is high risk. Therefore, although the system can assist in determining surgical priority, final disposition should be based on the clinical judgment of the treating surgeon and institution. Nevertheless, we believe that it can be an effective tool for informing clinical stakeholders as to how each patient's case may be triaged at peer institutions. Our scoring system is also limited by the fact that it operates on the assumption that the patient desires surgery at the same time recommended by the treating surgeon. This is not always the case and the timing of surgery must therefore rely on an in-depth discussion between provider and patient. The present scoring system was devised with the COVID-19 pandemic in mind. Consequently, it could be argued that it may not be applicable to other resource-challenging situations, and future pandemics may limit resources in a manner not assessed in the current work. However, we believe that the modular structure used could easily be adapted to other crises that cause a shortage of medical resources. Therefore, the present system may have usefulness beyond the present crisis and any second wave that may arise.
Conclusions
We present a scoring system for the triaging of spine surgery patients during times of crisis and severe resource scarcity. Our system was developed by a multi-institutional panel using a modified Delphi technique and has the potential to assist surgeons, hospital administrators, and other clinical stakeholders in assigning priority to both emergent and nonemergent spine surgery patients. Although not intended to be prescriptive, this scoring system may prove useful as a guide during both the COVID crisis and the post-COVID period to help prioritize patients with the greatest surgical needs, although determining the urgency of an individual procedure should be left to the operating surgeon. In addition, we believe that the modular structure of the scoring system implies that it may be adapted to other crises resulting in an acute shortage of medical resources.
CRediT authorship contribution statement
Daniel M. Sciubba: Conceptualization, Data curation, Writing - review & editing. Jeff Ehresman: Conceptualization, Data curation, Formal analysis, Writing - original draft. Zach Pennington: Conceptualization, Data curation, Writing - original draft. Daniel Lubelski: Conceptualization, Data curation, Writing - original draft. James Feghali: Conceptualization, Data curation, Writing - original draft. Ali Bydon: Data curation, Writing - review & editing. Dean Chou: Data curation, Writing - review & editing. Benjamin D. Elder: Data curation, Writing - review & editing. Aladine A. Elsamadicy: Data curation, Writing - review & editing. C. Rory Goodwin: Data curation, Writing - review & editing. Matthew L. Goodwin: Data curation, Writing - review & editing. James Harrop: Data curation, Writing - review & editing. Eric O. Klineberg: Data curation, Writing - review & editing. Ilya Laufer: Data curation, Writing - review & editing. Sheng-Fu L. Lo: Data curation, Writing - review & editing. Brian J. Neuman: Data curation, Writing - review & editing. Peter G. Passias: Data curation, Writing - review & editing. Themistocles Protopsaltis: Data curation, Writing - review & editing. John H. Shin: Data curation, Writing - review & editing. Nicholas Theodore: Data curation, Writing - review & editing. Timothy F. Witham: Data curation, Writing - review & editing. Edward C. Benzel: Data curation, Writing - review & editing.
Footnotes
Conflict of interest statement: D.M.S. is a consultant for Baxter, DePuy Synthes, Globus Medical, K2M, Medtronic, NuVasive, and Stryker and receives unrelated grant support from Baxter Medical, North American Spine Society, and Stryker. D.C. is a consultant for Globus Medical and Medtronic and receives unrelated royalties from Globus Medical. A.B. is a consultant for K2M. C.R.G. receives unrelated grants support from NIH/NINDSK12NRCDP Physician Scientist Award (2K12NS080223–06) and Robert Wood Johnson Harold Amos Medical Faculty Development Program (RWJ 76238). M.L.G. is a consultant for ROM3 and Augmedics and receives unrelated grant support from Children's Discovery Institute and AOSpine North America. E.O.K. is a consultant for DePuy and Stryker, receives fellowship support from AOSpine, and is a paid speaker for AOSpine and K2M. I.L. is a consultant for SpineWave, DePuy Synthes, Medtronic, Globus, and Brainlab. B.J.N. is a consultant for Medtronic and received unrelated research support from DePuy Synthes. P.G.P. is a consultant for Zimmer Biomet, Medicrea, and SpineWave. T.P. receives royalties from Altus and is a consultant for Globus, Stryker K2, Medicrea, NuVasive, and Innovasis. J.H.S. is a consultant for Carbofix, DePuy Synthes, Medtronic, and NuVasive. N.T. receives royalties from Globus Medical, and DePuy Synthes, owns stock in Globus Medical, is a consultant for Globus Medical, and serves on the Scientific Advisory Board/Other Office for Globus Medical. T.F.W. receives unrelated grants from Ely Lilly and Marylin Macklin Foundation. All other authors declare that the article content was composed in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material Representative Case 1
A 70-year-old man with history of osteoporosis and hypertension who underwent T10-S1 fusion for thoracolumbar deformity 1 month ago who presents with S1 radicular pain for 2 weeks that has been progressing over the past 8 days. Lumbar spine plain films show loosening of several implants with a fracture of his right S1 screw. No evidence of PJK or evidence of new deformity. On examination, he is neurologically intact. Ability to perform activities of daily living and ambulation are preserved. MRI is obtained and shows moderate compression of the right S1 nerve; there is no central compression. His surgery will require a 3-day hospital stay without ICU admission, though ICU resources are available. Local disease burden is moderate. If I were to perform surgery, it would be:
ASAP: 0%
Within 48 hours: 6.25%
Next week: 6.25%
Next month: 62.5% (agreement with the score)
After COVID crisis: 25.0%
Representative Case 2
A 53-year-old man with PMH of chronic obstructive pulmonary disease and diabetes presents after motor vehicle accident with mechanical pain. Neurologically intact and CT of thoracic spine shows fracture of T11 vertebrae with TLICS of 4. MRI shows cord compression without evidence of cord signal change. Patient would likely require a 4- to 5-day stay in the hospital without ICU needs. ICU resources are limited and local disease burden is high. He will not require placement to a rehab facility after discharge. If I were to perform surgery, it would be:
ASAP: 0%
Within 48 hours: 0%
Next week: 12.50%
Next month: 6.25%
After COVID crisis: 75.0% (agreement with score)
Representative Case 3
A 63-year-old woman with PMH of COPD presents with a 3-month history of cervical myelopathy, now with 2 weeks of significant worsening to the point that she requires assistance with ambulation. MRI of her cervical spine demonstrates multilevel cord compression without signal change. There is no history of mechanical pain or evidence of instability on flexion/extension x-rays. She is expected to be hospitalized for 5 days without the need for ICU care, but will require discharge to a subacute rehab facility. ICU beds are available and there are currently no PPE shortages at your facility. Local disease burden is low. If I were to do surgery, it would be:
ASAP: 0%
Within 48 hours: 20%
Next week: 80% (agreement with score)
Next month: 0%
After COVID crisis: 0%
References
- 1.Zhu N., Zhang D., Wang W. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guan W., Ni Z., Hu Y. Clinical Characteristics of Coronavirus Disease 2019 in China [e-pub ahead of print] https://doi.org/10.1056/NEJMoa2002032 N Engl J Med. Accessed February 28, 2020. [DOI] [PMC free article] [PubMed]
- 3.Emanuel E.J., Persad G., Upshur R. Fair Allocation of scarce medical resources in the time of COVID-19 [e-pub ahead of print] https://doi.org/10.1056/NEJMsb2005114 N Engl J Med. Accessed March 23, 2020. [DOI] [PubMed]
- 4.Centers for Medicare & Medicaid Services Non-Emergent, Elective Medical Services, and Treatment Recommendations. Published 2020. https://www.cms.gov/files/document/31820-cms-adult-elective-surgery-and-procedures-recommendations.pdf Available at:
- 5.Centers for Disease Control and Prevention (CDC) Coronavirus Disease 2019 (COVID-19): Healthcare Facility Guidance. Published 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-hcf.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Fhealthcare-facilities%2Fguidance-hcf.html Available at:
- 6.Amarican College of Surgeons COVID-19: Recommendations for Management of Elective Surgical Procedures. ACS: COVID-19 and Surgery. Published 2020. https://www.facs.org/covid-19/clinical-guidance/elective-surgery Available at:
- 7.Bono C.M., Dohring E.J., Finkenberg J.G. NASS Guidance Document on Elective, Emergent, and Urgent Procedures. Published 2020. https://www.spine.org/Portals/0/assets/downloads/Publications/NASSInsider/NASSGuidanceDocument040320.pdf Available at:
- 8.Burke J.F., Chan A.K., Mummaneni V. Letter: The coronavirus disease 2019 global pandemic: a neurosurgical treatment algorithm [e-pub ahead of print] https://doi.org/10.1093/neuros/nyaa116 Neurosurgery. Accessed April 3, 2020. [DOI] [PMC free article] [PubMed]
- 9.Cenzato M., DiMeco F., Fontanella M., Locatelli D., Servadei F. Editorial. Neurosurgery in the storm of COVID-19: suggestions from the Lombardy region, Italy (ex malo bonum) [e-pub ahead of print] https://doi.org/10.3171/2020.3.JNS20960 J Neurosurg. Accessed April 10, 2020. [DOI] [PMC free article] [PubMed]
- 10.Eichberg D.G., Shah A.H., Luther E.M. Letter: Academic neurosurgery department response to COVID-19 pandemic: the University of Miami/Jackson Memorial Hospital Model [e-pub ahead of print] https://doi.org/10.1093/neuros/nyaa118 Neurosurgery. Accessed April 11, 2020. [DOI] [PMC free article] [PubMed]
- 11.Arnaout O., Patel A., Carter B., Chiocca E.A. Letter: Adaptation under fire: two Harvard neurosurgical services during the COVID-19 pandemic [e-pub ahead of print] https://doi.org/10.1093/neuros/nyaa146 Neurosurgery. Accessed April 17, 2020. [DOI] [PMC free article] [PubMed]
- 12.Lwu S., Paolucci E.O., Hurlbert R.J., Thomas K.C. A scoring system for elective triage of referrals: Spine Severity Score. Spine J. 2010;10:697–703. doi: 10.1016/j.spinee.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 13.St-Pierre G.H., Jack A., Thomas K., Hurlbert J.R., Nataraj A. Validation of the Calgary Spine Severity Score. Spine J. 2015;15:2182–2187. doi: 10.1016/j.spinee.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 14.Stahel P.F. How to risk-stratify elective surgery during the COVID-19 pandemic? Patient Saf Surg. 2020;14:8. doi: 10.1186/s13037-020-00235-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galarza M., Gazzeri R. Letter: Collateral pandemic in face of the present COVID-19 pandemic: a neurosurgical perspective [e-pub ahead of print] https://doi.org/10.1093/neuros/nyaa155 Neurosurgery. Accessed April 20, 2020. [DOI] [PMC free article] [PubMed]
- 16.Lee Z.D., Chyi Yeu D.L., Ang B.T., Ng W.H., Seow W.T. Editorial. COVID-19 and its impact on neurosurgery: our early experience in Singapore [e-pub ahead of print] https://doi.org/10.3171/2020.4.JNS201026 J Neurosurg. Accessed April 20, 2020. [DOI] [PMC free article] [PubMed]
- 17.Prachand V.N., Milner R., Angelos P. Medically Necessary, Time-sensitive procedures: scoring system to ethically and efficiently manage resource scarcity and provider risk during the COVID-19 pandemic [e-pub ahead of print] https://doi.org/10.1016/j.jamcollsurg.2020.04.011 J Am Coll Surg. Accessed April 20, 2020. [DOI] [PMC free article] [PubMed]
- 18.Charlson M.E., Pompei P., Ales K.L., MacKenzie C.R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 19.Bhatraju P.K., Ghassemieh B.J., Nichols M. COVID-19 in critically ill patients in the Seattle region–case series [e-pub ahead of print] https://doi.org/10.1056/NEJMoa2004500 N Engl J Med. Accessed March 30, 2020. [DOI] [PMC free article] [PubMed]
- 20.Goyal P., Choi J.J., Pinheiro L.C. Clinical characteristics of covid-19 in New York City [e-pub ahead of print] https://doi.org/10.1056/NEJMc2010419 N Engl J Med. Accessed April 17, 2020. [DOI] [PMC free article] [PubMed]
- 21.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323:1061. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang X., Yu Y., Xu J. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study [e-pub ahead of print] https://doi.org/10.1016/S2213-2600(20)30079-5 Lancet Respir Med. Accessed May 1, 2020. [DOI] [PMC free article] [PubMed]
- 24.Amin-Hanjani S., Bambakidis N.C., Barker F.G. Editorial. COVID-19 and neurosurgical practice: an interim report [e-pub ahead of print] https://doi.org/10.3171/2020.4.JNS201099 J Neurosurg. Accessed May 1, 2020. [DOI] [PMC free article] [PubMed]
- 25.Giorgi P.D., Villa F., Gallazzi E. The management of emergency spinal surgery during the COVID-19 pandemic in Italy [e-pub ahead of print] https://doi.org/10.1302/0301-620X.102B6.BJJ-2020-0537 Bone Joint J. Accessed April 23, 2020. [DOI] [PMC free article] [PubMed]
- 26.Xu S., Li Y. Beware of the second wave of COVID-19. Lancet. 2020;395:1321–1322. doi: 10.1016/S0140-6736(20)30845-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nickol M.E., Kindrachuk J. A year of terror and a century of reflection: perspectives on the great influenza pandemic of 1918–1919. BMC Infect Dis. 2019;19:117. doi: 10.1186/s12879-019-3750-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leung K., Wu J.T., Liu D., Leung G.M. First-wave COVID-19 transmissibility and severity in China outside Hubei after control measures, and second-wave scenario planning: a modelling impact assessment. Lancet. 2020;395:1382–1393. doi: 10.1016/S0140-6736(20)30746-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ehresman J., Ahmed A.K., Lubelski D. Assessment of a triage protocol for emergent neurosurgical cases at a single institution. World Neurosurg. 2020;135:e386–e392. doi: 10.1016/j.wneu.2019.12.005. [DOI] [PubMed] [Google Scholar]
- 30.Ahmed K., Zygourakis C., Kalb S. Protocol for urgent and emergent cases at a large academic level 1 trauma center. Cureus. 2019;11:e3973. doi: 10.7759/cureus.3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chowdhury S., Nicol A.J., Moydien M.R., Navsaria P.H., Montoya-Pelaez L.F. Is case triaging a useful tool for emergency surgeries? A review of 106 trauma surgery cases at a level 1 trauma center in South Africa. World J Emerg Surg. 2018;13:4. doi: 10.1186/s13017-018-0166-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ibrahim G.M., Tymianski M., Bernstein M. Priority setting in neurosurgery as exemplified by an everyday challenge. Can J Neurol Sci. 2013;40:378–383. doi: 10.1017/s0317167100014347. [DOI] [PubMed] [Google Scholar]
- 33.Ramakrishna R., Zadeh G., Sheehan J.P., Aghi M.K. Inpatient and outpatient case prioritization for patients with neuro-oncologic disease amid the COVID-19 pandemic: general guidance for neuro-oncology practitioners from the AANS/CNS Tumor Section and Society for Neuro-Oncology [e-pub ahead of print] https://doi.org/10.1007/s11060-020-03488-7 J Neurooncol. Accessed April 9, 2020. [DOI] [PMC free article] [PubMed]
- 34.European Association of Neurosurgical Societies European Association of Neurosurgical Societies advice: Triaging non-emergent neurosurgical procedures during the COVID-19 outbreak. Published 2020. https://cdn.ymaws.com/www.eans.org/resource/resmgr/documents/corona/eans_advice2020_corona.pdf Available at:
- 35.Jean W.C., Ironside N.T., Sack K.D., Felbaum D.R., Syed H.R. The impact of COVID-19 on neurosurgeons and the strategy for triaging non-emergent operations: a global neurosurgery study [e-pub ahead of print] https://doi.org/10.1007/s00701-020-04342-5 Acta Neurochir (Wien) Accessed April 21, 2020. [DOI] [PMC free article] [PubMed]