Abstract
Stereotactic ablative radiotherapy (SABR/SBRT) is a revolutionary technique for tumor therapy. Its advantages are especially beneficial for the treatment spinal tumors. It has a wide range of indications in radiotherapy alone and in preoperative and postoperative treatments for spinal tumor. The mechanism of stereotactic radiotherapy for spinal tumors is special, and completely different from traditional radiotherapy. Compared with traditional radiotherapy, SBRT creates more DNA double-strand breaks, leads to less DNA damage repair, and also has anti-vascular effects, in situ vaccine effects and abscopal effect. In the present study, the literature regarding SABR for the treatment of spinal tumors is summarized, and we reviewed characteristics of SABR and spinal tumors, as well as the clinical efficacy and toxicity of SABR in treating spinal tumors. In addition, we proposed several issues around the SABR treatment of spinal tumor, the standard of treatment dose, and the post-treatment follow-up. We also made predictions with respect to future management of spinal tumors, SABR development, multi-modality integration between SABR and other treatments, and other future development trends, thereby providing future research directions as a contribution to the field.
Keywords: stereotactic ablative radiotherapy, spinal tumor, efficacy, toxicity, spine
Introduction
The spine is a common site for primary and metastatic cancers. Especially with the recent advancement in tumor targeting treatments and immunotherapy, spinal metastasis is often discussed, and evaluated in cancer treatment. Treatment for spinal tumors is complicated by the vicinity to the major nerve tracts in the spinal cord. The dose of traditional radiotherapy cannot be increased easily in the spinal cord, making it only a palliative treatment rather than definitive (1, 2). Therefore, improvement in radiotherapy for spinal tumors is critical, and SABR has become an uprising trend in radiotherapy for spinal tumors due to its revolutionary advantages, as discussed below.
The Mechanism and Unique Characteristics of SABR
The Mechanism of SABR
The mechanism of stereotactic radiotherapy for spinal tumors is completely different from traditional radiotherapy. Compared with traditional radiotherapy, SABR creates more double-strand breaks in DNA, results in less DNA damage repair, and even has anti-vascular effects, in situ vaccine effects and abscopal effect (3, 4). Therefore, stereotactic radiotherapy is an effective local ablation treatment. In addition, it improves the overall control of the disease through the local control of the disease and through several remote effects (5).
The Characteristics of SABR
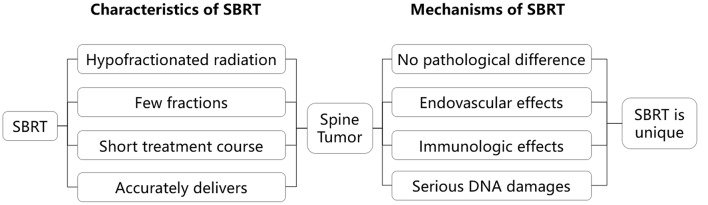
The advantages of SABR are especially helpful in the treatment of spinal tumors. First, primary and metastatic spinal tumors have a variety of pathologies, with some cell types being more resistant to radiation. SABR, compared to traditional radiotherapy, produces high-dose fractions in a short course of irradiation, making it more effective for radioresistant tumors (6). Second, pain is the most common symptom of patients with spinal tumors, and a short course of irradiation with SABR can relieve pain more quickly. Third, SABR methods can ensure the accuracy of the treatment by tracking movements in between radiation (7–10). However, Traditional radiotherapy cannot easily accommodate for movements during treatment sessions. Fourth, because spinal tumors are often close to the spinal cord, a rapid dose drop outside the target is required. SABR can just achieve a rapid dose drop-off from treatment field to outside of treatment field. Moreover, the treatment days of stereotactic radiotherapy is usually shorter than other radiotherapy methods (for example, IMRT), decreasing cost in staffing and maintenance of hospital facilities. The unique advantages of SABR make it an increasingly popular treatment modality for spinal tumors (Figure 1). It is also important to note that SABR may not be the best option for all patients either. For example, for patients with an expected survival <3 months, 30 Gy in 10 fractions, 20 Gy in 5 fractions, or 8 Gy in 1 fraction with external beam radiotherapy are the reasonable alternative.
Figure 1.
The characteristics of SBRT and its effects on spinal tumor. SBRT is unique. It is “completely different” from traditional fractionated radiation, and SBRT is an ablative treatment.
Efficacy of SABR for the Treatment of Spinal Tumors
The drawbacks of traditional radiotherapy make it increasingly incompatible with multimodality treatments for spinal tumors involving new systemic treatments. In comparison, SABR has shown high efficacy and low toxicity for spinal tumors while in conjunction with other treatment modalities. Overall, the application of SABR in spinal tumors include three different ways: primary treatment, repeat treatment after other radiotherapy, and postoperative SABR.
SABR as Primary Treatment
SABR as primary treatment is the most important way that SABR is used for spinal tumors, and also the most important research area for SABR in spinal tumors. A representative study of SABR showed that the local control rate of SABR was >80% (11–28), and local control was even higher (>90%) in other studies (29), greatly improved compared with traditional radiotherapy where recurrence rates are close to 80% (30, 31). Moreover, SABR also shows significant benefit in pain relief. The efficacy of SABR promotes the change in radiotherapy for spinal tumor from palliative treatment with traditional radiotherapy to definitive radiation with SABR (32, 33), improving local control and quality of life for patients with spinal tumors (Table 1).
Table 1.
Selected spine SBRT series for spinal metastases with no prior history of radiation.
| Authors & year | Study type | No.of Tumors/Patients | Histology | Total Dose (Range)/No. of Fractions (Range) | Follow-up time Months (Range) | Local Control | Overall Survival | Pain Response |
|---|---|---|---|---|---|---|---|---|
| Gerszten et al. (11) | Prospective | 156 | Mixed | Mean:20 Gy (12.5–25 Gy)/1f | Median: 21 (3–53) | 90% (crude) | na | 86% reported long-term improvement |
| Yamada et al. (12) | Retrospective | 103/93 | Mixed | Median: 24 Gy (18–24 Gy)/1f | Median: 15 (2–45) | 90% (15 months) | Median: 15 months | na |
| Sahgal et al. (13) | Retrospective | 23/14 | Mixed | Median: 24 Gy (7–40 Gy)/3 (1–5)f | Median: 9 (1–26) | 85%(1 year)/69%(2 years) | 45% (2 years) | na |
| Nguyen et al. (14) | Prospective | na/22a | Renal cell carcinoma | Median: 27 Gy (24–30 Gy)/3 (1–5)f | Median: 13.1(3.3–54.5) | 82% (1 year)c | 72% (1 year)c | BPI:no pain 23%(baseline) to 52% (12 months) |
| Wang et al. (15) | Prospective | 166/149 | Mixed | 27–30 Gy/3f | Median: 15.9(1.0–91.6) | 80.5% (1 year)/72.4%(2 years) | 68.5%(1 year)/46.4%(2 years) | BPI:no pain 26% (baseline) to 54% (6 months) |
| Ahmed et al. (16) | Retrospective | 63/46a | Mixed | Median: 24 Gy (10–40 Gy)/3 (1–5)f | Mean: 8.2 | 91.2% (1 year) | 59% (1 year) | na |
| Thibault et al. (17) | Retrospective | 60/37a | Renal cell carcinoma | Median: 24 Gy (18–30 Gy)/2 (1–5) | Median: 12.3(1.2–55.4) | 83.4% (1 year)/66.2%(2 years) | 64.1%(1 year)/45.6%(2 years) | na |
| Guckenberger et al. (18) | Retrospective | 387/301 | Mixed | Median: 24 Gy (10–60 Gy)/3 (1–20)f | Median: 11.8 (0–105) | 89.9% (1 year)/83.9%(2 years) | 64.9% (1 year)/43.7%(2 years) | na |
| Sohn et al. (19) | Retrospective | 13/13 | Renal cell carcinoma | Mean: 38.0 Gy/median: 4f | na | 85.7% (1 year) | Median: 15 months | 23.1% complete; 53.8% partial |
| Folkert et al. (20) | Retrospective | 108/88a | Sarcoma | Median:24Gy (18–24Gy)/1 or median: 28.5 Gy (24–36 Gy)/3 (3–6) | Median: 12.3(1–80.7) | 87.9% (1 year) | 60.6% (1 year) | na |
| Park et al. (21) | Retrospective | 45/28a | Mixed | Median: 27 Gy (18–35 Gy)/3 (1–5)f | Median: 7.4(1.1–42.5) | 93.2% (1 year)/93.2%(2 years) | 47.4% (1 year)/27.9%(2 years) | VAS:median4(pre-SBRT)to 1(1–3 months post-SBRT) |
| Azad et al. (22) | Retrospective | 25/25 | Mixed | Median: 20 Gy(15–25.5)/2(1–5)f | Median: 18(1–81) | 84.2% (crude) | Median: 28 months | na |
| Bate et al. (23) | Retrospective | 48/36a | Mixed | 16–23 Gy/1 or 20–30 Gy/2–5f | Median: 9.8 | 95.8% (1 year) | 44% (crude) | na |
| Bishop et al. (24) | Retrospective | 332/285f | Mixed | Median (tumor dose): 43 Gy | Median: 19(0–111) | 88% (1 year)/82% (3 years) | 64% (1 year)/33% (3 years) | na |
| Sellin et al. (25) | Retrospective | 40/37 | Renal cell carcinoma | Median: 24 Gy (24–30 Gy)/1 (1–5)f | Median: 49.0(38.2–75.8) | 57% | Median: 16.3 months | VAS: 41.4% improved pain |
| Anand et al. (26) | Retrospective | 76/52e | Mixed | Median: 24 Gy (24–27 Gy)/3 (1–3)f | Median: 8.5(3.0–40.0) | 94% (1 year)/82.6%(2 years) | 68% (1 year)/45.4%(2 years) | 92.3% complete; 5.8% partial |
| Ghia et al. (28) | Prospective | 28/28 | Mixed | 18 or 24 Gy/1f | Median:17 (12.7–21.0) | 89% (1 year) | Median: 28.6 months | na |
| Tseng et al. (27) | Prospective | 279/145 | Mixed | 24Gy/2f | Median:15 (0.1–71.6) | 1-year local failure: 9.7% | 1-year OS:73.1% | na |
SABR Treatment After Prior Radiotherapy
Recurrence after prior radiotherapy is common in the treatment of spinal tumor. Due to the dose limitation on spinal cord, ordinary radiotherapy cannot be repeated at sites that received prior radiotherapy. Therefore, SABR is the only option for repeat radiation. The results (13, 17, 34–39) demonstrated that repeat SABR achieved good efficacy in controlling tumor-related pain (Table 2).
Table 2.
Selected re-irradiation spine SABR series for spinal metastases.
| Authors & Year | Study type | No. of Tumors/No.of Patients | Histology | Prior RT Dose (Range)/No. of Fractions (Range) | Total Dose(Range)/No. of Fractions (Range) | Follow-up in Months(Range) | Local Control | Overall Survival | Pain Response |
|---|---|---|---|---|---|---|---|---|---|
| Sahgal et al. (13) | Retrospective | 37/25 | Mixed | Median: 24 Gy (7–40 Gy)/3 (1–5) | Median: 36 Gy/14 | Median: 7 (1–48) | 92% (1 year) | 45% (2 years)a | na |
| Mahadevan et al. (38) | Retrospective | 81/60 | Mixed | Median: 24 Gy (24–30 Gy)/3 (3–5) | Median: 30 Gy (8–46 Gy)/10 (1–25) | Median: 12 (4–36) | Median: 9 months | Median: 11 months | 4.7% reported pain response; 18% complete response |
| Choi et al. (35) | Retrospective | 51/42 | Mixed | Median: 20 Gy (10–30 Gy)/2 (1–5) | Median: 40 Gy (30–40 Gy)/20 (10–20) | Median: 7 (2–47) | 73% (1 year) | 68% (1 year) | 65% reported pain response |
| Garg et al. (34) | Prospective | 63/59 | Mixed | Median: 27 Gy (20–30 Gy)/3 (3–5) | Median: 30 Gy/na | Median: 13 (0.9–67.5) | 76% (1 year) | 76% (1 year) | na |
| Damast et al. (36) | Retrospective | 97/95 | Mixed | Median: 30 Gy (16–30 Gy)/5 (4–6) | Median: 30 Gy (8–66 Gy)/na | Median: 12.1 (0.2–63.6) | 66% (1 year) | 52–59% (1 year); median: 13.6 months | 77% reported pain response |
| Thibault et al. (17) | Retrospective | 11/37 | Renal cell carcinoma | Median: 24 Gy (18–30 Gy)/2 (1–5) | Median: 30 Gy (8–30 Gy)/10 (1–10) | Median: 12.3 (1.2–55.4) | 83.4% (1 year)/66.2% (2 years) | 64.1% (1 year)/45.6% (2 years) | na |
| Thibault et al. (39) | Retrospective | 56/40 | Mixed | Median: 30 Gy (20–35 Gy)/4 (2–5) | Median (SBRT): 24 Gy(20–35 Gy)/2(1–5); median (cEBRT, n 24):22.5 Gy (20–30 Gy)/5 (5–40) | Median: 6.8 (0.9–39) | 80.6% (1 year)/71.5% (2 years) | 48% (1 year) | na |
| Kawashiro et al. (37) | Retrospective | 23/23 | Mixed | Median: 24.5 Gy (14.7–50 Gy)/5 (3–25) | Median: 30 Gy (30–40 Gy)/10 (10–20) | Median: 10 (1–54) | 88% (1 year)/75% (2 years) | 50% (1 year)/20% (2 years) | 78.9% reported pain relief |
Postoperative SABR
The role of decompressive surgery in patients with symptomatic single-level MESCC was established by Patchell et al. (40). This article demonstrated the effect of surgery and postoperative radiation as a standard in the treatment of MESCC. Moreover, several studies of stereotactic radiotherapy have also confirmed that stereotactic radiotherapy has better advantages for postoperative treatment of spinal tumors. Stereotactic radiotherapy can achieve better local control (Based on the available data, the rate of local control is about 80–90%) and pain relief (17, 22, 41–50), although treatment dose and fraction greatly varied in the published series (Table 3).
Table 3.
Selected postoperative spine SABR series for spinal metastases.
| Study authors (Year) | Study design | No. of Tumors/No.of Patients | Histology | Total dose (Range)/No. of Fractions (Range) | Follow-up in Months(Range) | Local Control | Overall survival | Pain response |
|---|---|---|---|---|---|---|---|---|
| Gerszten et al. (41) | Prospective | 26/26 | Mixed | Mean: 18 Gy to 80% isodose line (16–20 Gy)/1 | Median: 16 (11–24) | na | na | VAS: 92% long-term improvement |
| Rock et al. (42) | Retrospective | 18/18 | Mixed | Mean: 11.4 Gy (6–16)/1 | Median: 7 (4–36) | na | na | na |
| Gerszten et al. (43) | Prospective | 11/11 | Mixed | Mean: 19 Gy (16–22.5 Gy)/1 | Median: 11 (7–44) | na | na | VAS: 100% long-term improvement |
| Moulding et al. (44) | Retrospective | 21/21 | Mixed | Median: 24 Gy (18–24 Gy)/1 | Median: 10.2 (1.2–54.0) | 90.5% (1 year) | Median: 10.2 months | na |
| Massicotte et al. (45) | Retrospective | 10/10 | Mixed | Median: 24 Gy (18–35 Gy)/3 (1–5) | Median: 13 (3–18) | 70% (crude) | na | na |
| Al-Omair et al. (46) | Retrospective | 80/80 | Mixed | Median: 24 Gy (18–40 Gy)/2 (1–5) | Median: 8.3 (0.13–39.1) | 84% (1 year) | 64% (1 year) | na |
| Laufer et al. (47) | Retrospective | 186/186 | Mixed | 24 Gy/1 (21.5%) or 24–30 Gy/3 (19.9%), or 18–36 Gy/5–6 (58.6%) | Median: 7.6 (1.0–66.4) | 83.6% (1 year) | 29.0% (crude); median among patients who died: 6.1 months | na |
| Azad et al. (22) | Retrospective | 21/21 | Mixed | 16–22 Gy/1 or 20–30 Gy/2–5 | Median: 13.7 | 90.5% (1 year) | 44%a (crude) | na |
| Zabi Wardak et al. (48) | Prospective | 29/25 | Mixed | 20 Gy/1 | Median: 9.6 | 92% | na | VAS: 91% significantly improved |
| Redmond et al. (49) | Prospective | 33/35 | Mixed | 30 Gy/5f | na | 90% (1 year) | na | na |
In conclusion, SABR has shown great efficacy in treating spinal tumors as a primary treatment, as salvage treatment after prior radiotherapy and as postoperative radiotherapy. Compared to traditional radiotherapy, SABR for spinal tumors is more effective in symptom relief, tumor control, and potentially improves survival.
Practical Questions When Using SABR for the Treatment of Spinal Tumors
Selection of Equipment of SABR: What Are the Differences Between Different Devices?
SABR could be used with different treatment platforms from different companies. The similarities and differences of these devices are also frequently asked by patients in clinical practice. The accuracy, efficacy, and cost-efficiency of equipment are the main factors for hospitals to decide on equipment. Among all current treatment platforms, some devices use CBCT as the treatment accuracy support equipment, the Cyberknife has a real-time tracking system. This is the advantage of CyberKnife, but there are also a lot of disadvantages, such as no posterior beams, more anterior spillage in the visceral organs as all beam come from the front and side. Further, CyberKnife treatment has long delivery time. There are many top centers using LINAC-based SABR for spine tumors in the world. Cost-efficiency is another crucial criterion for the assessment of the treatment equipment. For example, the cost of Cyberknife treatments is high in china and some countries. Therefore, selecting the appropriate equipment and treatment is an important consideration when SABR for spinal tumors, but different devices have different advantages and disadvantages (27, 51–54). The ideal equipment for clinical needs is a radiotherapy machine with real-time tracking system, full angle radiation field and short time to complete treatment. However, doctors' professional experience, academic level and a good teamwork maybe more important factors than equipment.
Understanding the Balance Between Tumor Control and Radiation-Related Adverse Events in the Treatment Process: Deciding Treatment Dosage
The goal of tumor treatment is to control the tumor and reduce injury to surround tissue. However, in many cases, the tumor cannot be controlled without damaging surrounding tissue. If left uncontrolled, spinal tumors often inevitably lead to spinal cord injury. Therefore, the benefits of radiotherapy for spinal tumors still outweighs its harm. The current standard doses used in radiation for spinal tumors are usually low enough to avoid damaging neurologic structures in the spinal cord (22, 23). Clinical practice in choosing dosage for spinal cord irradiation can be mainly divided into two situations. First, in the case of achieving spinal tumor control without damaging the spinal cord, it is necessary to achieve the two goals at the same time. Second, in the case of tumor control where uncontrolled tumor growth causes spinal cord injury, an optimal dose to control the tumor is critical and the first priority. This phenomenon indicated that SABR dosing selection is the key to improve spinal tumor treatment and requires further research.
Efficacy Evaluation After SABR for Spinal Tumors: How to Study the Efficacy of SABR for Spinal Tumors?
Spinal tumor is different from other solid tumors of other organs. Radiologic changes are sometimes not the best representation of tumor control after radiation treatment. Therefore, the commonly used criterion RECIST does not apply to the evaluation after tumor control after radiotherapy of spinal tumors (55). The spine response assessment In Neuro-Oncology (SPINO) group present the first report on the challenges in standardizing imaging-based assessment of local control and pain for spinal metastases. The ultimate goal of the SPINO group is to report consensus criteria for tumor imaging, clinical assessment, and symptom-based response criteria to help standardize the evaluation (56). The SPINO standard improved the evaluation of spinal tumors after fusion of different clinical factors. However, there are still many clinical puzzles in clinical practice. After all, the evaluation of spinal tumors after radiotherapy is very complicated. Overall, the combination of radiologic changes in the setting of comprehensive consideration for metabolic and functional changes is likely the future direction for evaluating spinal cord tumor control after radiotherapy. First, evaluating tumor control after SABR for spinal tumors requires a combination of multiple radiology modalities: CT is used to observe the bone mass, MRI for morphology, and ECT and PET for metabolic activity. Second, imaging techniques such as functional nuclear magnetics and other new evaluation methods (for example: artificial intelligence) of spinal tumors are being developed, which may play a potentialrole in predicting the prognosis on spinal tumor and in evaluating treatment response after SABR. In conclusion, evaluating treatment response of spinal tumors after SABR is an area for further investigation, with the integration of radiological, functional, and metabolic changes as a novel direction for studying the efficacy of SABR.
In conclusion, as a revolutionary technique for tumor treatment, SABR has several advantages that makes it a good treatment modality for spinal tumors. As a result, SABR has shown excellent efficacy as primary treatment, repeat radiation treatment, and postoperative radiotherapy for spinal tumors. Spinal tumor is one of the best indications for SABR, and SABR is becoming part of the backbone of spinal tumor treatment. With several issues remain regarding the selection of specific equipment and type of SABR, standardization of radiation dose, and evaluation of treatment response, more will come in the future with the development of SABR, further accumulation of clinical data, and integration of SABR into multi-disciplinary cancer treatment.
Author Contributions
HongqZ and JL participated in the idea of the article. HongqZ and NL collected the data. HongqZ and HongxZ wrote the paper. All authors were responsible for the final review of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This Paper was supported by Clinical key project of Peking University Third Hospital (BYSY2017030, BYSY2018007) and National Natural Science Foundation of China (81701648).
References
- 1.Gerszten PC, Mendel E, Yamada Y. Radiotherapy and radiosurgery for metastatic spine disease: what are the options, indications, and outcomes? Spine. (2009) 34(22 Suppl):S78–92. 10.1097/BRS.0b013e3181b8b6f5 [DOI] [PubMed] [Google Scholar]
- 2.Mendel E, Bourekas E, Gerszten P, Golan JD. Percutaneous techniques in the treatment of spine tumors: what are the diagnostic and therapeutic indications and outcomes? Spine. (2009) 34(22 Suppl):S93–S100. 10.1097/BRS.0b013e3181b77895 [DOI] [PubMed] [Google Scholar]
- 3.Brooks ED, Chang JY. Time to abandon single-site irradiation for inducing abscopal effects. Nat Rev Clin Oncol. (2019) 16:123–35. 10.1038/s41571-018-0119-7 [DOI] [PubMed] [Google Scholar]
- 4.Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. (2015) 348:69–74. 10.1126/science.aaa4971 [DOI] [PubMed] [Google Scholar]
- 5.Bernstein MB, Krishnan S1, Hodge JW, Chang JY. Immunotherapy and stereotactic ablative radiotherapy (ISABR): a curative approach? Nat Rev Clin Oncol. (2016) 13:516–24. 10.1038/nrclinonc.2016.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hörner-Rieber J, Bernhardt D, Dern J, König L, Adeberg S, Paul A, et al. Histology of non-small cell lung cancer predicts the response to stereotactic body radiotherapy. Radiother Oncol. (2017) 125:317–24. 10.1016/j.radonc.2017.08.029 [DOI] [PubMed] [Google Scholar]
- 7.Esposito M, Masi L, Zani M, Doro R, Fedele D, Garibaldi C, et al. SBRT planning for spinal metastasis: indications from a large multicentric study. Strahlenther Onkol. (2019) 195:226–35. 10.1007/s00066-018-1383-2 [DOI] [PubMed] [Google Scholar]
- 8.Saenz DL, Crownover R, Stathakis S, Papanikolaou N. A dosimetric analysis of a spine SBRT specific treatment planning system. J Appl Clin Med Phys. (2019) 20:154–9. 10.1002/acm2.12499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rijken J, Jordan B, Crowe S, Kairn T, Trapp J. Improving accuracy for stereotactic body radiotherapy treatments of spinal metastases. J Appl Clin Med Phys. (2018) 19:453–62. 10.1002/acm2.12395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Furuya T, Phua JH, Ruschin M, Tanaka H, Nihei K, Pinnaduwage D, et al. Assessing functionality and benefits of comprehensive dose volume prescriptions: an international, multi-institutional, treatment planning study in spine stereotactic body radiation therapy. Pract Radiat Oncol. (2019) 9:9–15. 10.1016/j.prro.2018.05.006 [DOI] [PubMed] [Google Scholar]
- 11.Gerszten PC, Burton SA, Ozhasoglu C, Welch WC. Radiosurgery for spinal metastases: clinical experience in 500 cases from a single institution. Spine. (2007) 32:193–9. 10.1097/01.brs.0000251863.76595.a2 [DOI] [PubMed] [Google Scholar]
- 12.Yamada Y1, Bilsky MH, Lovelock DM, Venkatraman ES, Toner S, Johnson J, et al. High-dose, single-fraction image-guided intensity-modulated radiotherapy for metastatic spinal lesions. Int J Radiat Oncol Biol Phys. (2008) 71:484–90. 10.1016/j.ijrobp.2007.11.046 [DOI] [PubMed] [Google Scholar]
- 13.Sahgal A, Ames C, Chou D, Ma L, Huang K, Xu W, et al. Stereotactic body radiotherapy is effective salvage therapy for patients with prior radiation of spinal metastases. Int J Radiat Oncol Biol Phys. (2009) 74:723–31. 10.1016/j.ijrobp.2008.09.020 [DOI] [PubMed] [Google Scholar]
- 14.Nguyen QN, Shiu AS, Rhines LD, Wang H, Allen PK, Wang XS, et al. Management of spinal metastases from renal cell carcinoma using stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys. (2010) 76:1185–92. 10.1016/j.ijrobp.2009.03.062 [DOI] [PubMed] [Google Scholar]
- 15.Wang XS, Rhines LD, Shiu AS, Yang JN, Selek U, Gning I, et al. Stereotactic body radiation therapy for management of spinal metastases in patients without spinal cord compression: a phase 1–2 trial. Lancet Oncol. (2012) 13:395–402. 10.1016/S1470-2045(11)70384-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahmed KA, Stauder MC, Miller RC, Bauer HJ, Rose PS, Olivier KR, et al. Stereotactic body radiation therapy in spinal metastases. Int J Radiat Oncol Biol Phys. (2012) 82:e803–9. 10.1016/j.ijrobp.2011.11.036 [DOI] [PubMed] [Google Scholar]
- 17.Thibault I, Al-Omair A, Masucci GL, Masson-Côté L, Lochray F, Korol R, et al. Spine stereotactic body radiotherapy for renal cell cancer spinal metastases: analysis of outcomes and risk of vertebral compression fracture. J Neurosurg Spine. (2014) 21:711–8. 10.3171/2014.7.SPINE13895 [DOI] [PubMed] [Google Scholar]
- 18.Guckenberger M, Mantel F, Gerszten PC, Flickinger JC, Sahgal A, Létourneau D, et al. Safety and efficacy of stereotactic body radiotherapy as primary treatment for vertebral metastases: a multi-institutional analysis. Radiat Oncol. (2014) 9:226. 10.1186/s13014-014-0226-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sohn S, Chung CK, Sohn MJ, Chang UK, Kim SH, Kim J, et al. Stereotactic radiosurgery compared with external radiation therapy as a primary treatment in spine metastasis from renal cell carcinoma: a multicenter, matched-pair study. J Neurooncol. (2014) 119:121–8. 10.1007/s11060-014-1455-9 [DOI] [PubMed] [Google Scholar]
- 20.Folkert MR, Bilsky MH, Tom AK, Oh JH, Alektiar KM, Laufer I, et al. Outcomes and toxicity for hypofractionated and single-fraction image-guided stereotactic radiosurgery for sarcomas metastasizing to the spine. Int J Radiat Oncol Biol Phys. (2014) 88:1085–91. 10.1016/j.ijrobp.2013.12.042 [DOI] [PubMed] [Google Scholar]
- 21.Park HJ, Kim HJ, Won JH, Lee SC, Chang AR. Stereotactic body radiotherapy (SBRT) for spinal metastases: who will benefit the most from SBRT? Technol Cancer Res Treat. (2015) 14:159–67. 10.7785/tcrt.2012.500411 [DOI] [PubMed] [Google Scholar]
- 22.Azad TD, Esparza R, Chaudhary N, Chang SD. Stereotactic radiosurgery for metastasis to the craniovertebral junction preserves spine stability and offers symptomatic relief. J Neurosurg Spine. (2016) 24:241–7. 10.3171/2015.6.SPINE15190 [DOI] [PubMed] [Google Scholar]
- 23.Bate BG, Khan NR, Kimball BY, Gabrick K, Weaver J. Stereotactic radiosurgery for spinal metastases with or without separation surgery. J Neurosurg Spine. (2015) 22:409–15. 10.3171/2014.10.SPINE14252 [DOI] [PubMed] [Google Scholar]
- 24.Bishop AJ, Tao R, Rebueno NC, Christensen EN, Allen PK, Wang XA, et al. Outcomes for spine stereotactic body radiation therapy and an analysis of predictors of local recurrence. Int J Radiat Oncol Biol Phys. (2015) 92:1016–26. 10.1016/j.ijrobp.2015.03.037 [DOI] [PubMed] [Google Scholar]
- 25.Sellin JN, Reichardt W, Bishop AJ, Suki D, Rhines LD, Settle SH, et al. Factors affecting survival in 37 consecutive patients undergoing de novo stereotactic radiosurgery for contiguous sites of vertebral body metastasis from renal cell carcinoma. J Neurosurg Spine. (2015) 22:52–9. 10.3171/2014.9.SPINE1482 [DOI] [PubMed] [Google Scholar]
- 26.Anand AK, Venkadamanickam G, Punnakal AU, Walia BS, Kumar A, Bansal AK, et al. Hypofractionated stereotactic body radiotherapy in spinal metastasis - with or without epidural extension. Clin Oncol. (2015) 27:345–52. 10.1016/j.clon.2015.01.035 [DOI] [PubMed] [Google Scholar]
- 27.Tseng CL, Soliman H, Myrehaug S, Lee YK, Ruschin M, Atenafu EG, et al. Imaging-based outcomes for 24 Gy in 2 daily fractions for patients with de novo spinal metastases treated with spine stereotactic body radiation therapy (SBRT). Int J Radiat Oncol Biol Phys. (2018) 102:499–507. 10.1016/j.ijrobp.2018.06.047 [DOI] [PubMed] [Google Scholar]
- 28.Ghia AJ, Guha-Thakurta N, Hess K, Yang JN, Settle SH, Sharpe HJ, et al. Phase 1 study of spinal cord constraint relaxation with single session spine stereotactic radiosurgery in the primary management of patients with inoperable, previously unirradiated metastatic epidural spinal cord compression. Int J Radiat Oncol Biol Phys. (2018) 102:1481–88. 10.1016/j.ijrobp.2017.06.362 [DOI] [PubMed] [Google Scholar]
- 29.Wang Q, Song Y, Zhuang H, Wang X, Li F, Dong Y, et al. Robotic stereotactic irradiation and reirradiation for spinal metastases: safety and efficacy assessment. Chin Med J. (2014) 127:232–8. 10.3760/cma.j.issn.0366-6999.20120145 [DOI] [PubMed] [Google Scholar]
- 30.Maranzano E, Latini P. Effectiveness of radiation therapy without surgery in metastatic spinal cord compression: final results from a prospective trial. Int J Radiat Oncol Biol Phys. (1995) 32:959–67. 10.1016/0360-3016(95)00572-G [DOI] [PubMed] [Google Scholar]
- 31.Mizumoto M, Harada H, Asakura H, Hashimoto T, Furutani K, Hashii H, et al. Radiotherapy for patients with metastases to the spinal column: a review of 603 patients at Shizuoka Cancer Center Hospital. International journal of radiation oncology, biology, physics. Int J Radiat Oncol Biol Phys. (2011) 79:208–13. 10.1016/j.ijrobp.2009.10.056 [DOI] [PubMed] [Google Scholar]
- 32.Chow E, Harris K, Fan G, Tsao M, Sze WM. Palliative radiotherapy trials for bone metastases: a systematic review. J Clin Oncol. (2007) 25:1423–36. 10.1200/JCO.2006.09.5281 [DOI] [PubMed] [Google Scholar]
- 33.Chow E, Zeng L, Salvo N, Dennis K, Tsao M, Lutz S. Update on the systematic review of palliative radiotherapy trials for bone metastases. Clin Oncol. (2012) 24:112–24. 10.1016/j.clon.2011.11.004 [DOI] [PubMed] [Google Scholar]
- 34.Garg AK, Wang XS, Shiu AS, Allen P, Yang J, McAleer MF, et al. Prospective evaluation of spinal reirradiation by using stereotactic body radiation therapy: the University of Texas MD anderson cancer center experience. Cancer-Am Cancer Soc. (2011) 117:3509–16. 10.1002/cncr.25918 [DOI] [PubMed] [Google Scholar]
- 35.Choi CY, Adler JR, Gibbs IC, Chang SD, Jackson PS, Minn AY, et al. Stereotactic radiosurgery for treatment of spinal metastases recurring in close proximity to previously irradiated spinal cord. Int J Radiat Oncol Biol Phys. (2010) 78:499–506. 10.1016/j.ijrobp.2009.07.1727 [DOI] [PubMed] [Google Scholar]
- 36.Damast S, Wright J, Bilsky M, Hsu M, Zhang Z, Lovelock M, et al. Impact of dose on local failure rates after image-guided reirradiation of recurrent paraspinal metastases. Int J Radiat Oncol Biol Phys. (2011) 81:819–26. 10.1016/j.ijrobp.2010.06.013 [DOI] [PubMed] [Google Scholar]
- 37.Kawashiro S, Harada H, Katagiri H, Asakura H, Ogawa H, Onoe T, et al. Reirradiation of spinal metastases with intensity-modulated radiation therapy: an analysis of 23 patients. J Radiat Res. (2016) 57:150–6. 10.1093/jrr/rrv083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mahadevan A, Floyd S, Wong E, Jeyapalan S, Groff M, Kasper E. Stereotactic body radiotherapy reirradiation for recurrent epidural spinal metastases. Int J Radiat Oncol Biol Phys. (2011) 81:1500–1505. 10.1016/j.ijrobp.2010.08.012 [DOI] [PubMed] [Google Scholar]
- 39.Thibault I, Campbell M, Tseng CL, Atenafu EG, Letourneau D, Yu E, et al. Salvage stereotactic body radiotherapy (SBRT) following in-field failure of initial sbrt for spinal metastases. Int J Radiat Oncol Biol Phys. (2015) 93:353–60. 10.1016/j.ijrobp.2015.03.029 [DOI] [PubMed] [Google Scholar]
- 40.Patchell RA, Tibbs PA, Regine WF, Payne R, Saris S, Kryscio RJ, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet. (2005) 366:643–8. 10.1016/S0140-6736(05)66954-1 [DOI] [PubMed] [Google Scholar]
- 41.Gerszten PC, Germanwala A, Burton SA, Welch WC, Ozhasoglu C, Vogel WJ. Combination kyphoplasty and spinal radiosurgery: a new treatment paradigm for pathological fractures. J Neurosurg Spine. (2005) 3:296–301. 10.3171/spi.2005.3.4.0296 [DOI] [PubMed] [Google Scholar]
- 42.Rock JP, Ryu S, Shukairy MS, Yin FF, Sharif A, Schreiber F, et al. Postoperative radiosurgery for malignant spinal tumors. Neurosurgery. (2006) 58:891–8. 10.1227/01.NEU.0000209913.72761.4F [DOI] [PubMed] [Google Scholar]
- 43.Gerszten PC, Monaco ER. Complete percutaneous treatment of vertebral body tumors causing spinal canal compromise using a transpedicular cavitation, cement augmentation, and radiosurgical technique. Neurosurg Focus. (2009) 27:E9. 10.3171/2009.9.FOCUS09184 [DOI] [PubMed] [Google Scholar]
- 44.Moulding HD, Elder JB, Lis E, Lovelock DM, Zhang Z, Yamada Y, et al. Local disease control after decompressive surgery and adjuvant high-dose single-fraction radiosurgery for spine metastases. J Neurosurg Spine. (2010) 13:87–93. 10.3171/2010.3.SPINE09639 [DOI] [PubMed] [Google Scholar]
- 45.Massicotte E, Foote M, Reddy R, Sahgal A. Minimal access spine surgery (MASS) for decompression and stabilization performed as an out-patient procedure for metastatic spinal tumours followed by spine stereotactic body radiotherapy (SBRT): first report of technique and preliminary outcomes. Technol Cancer Res Treat. (2012) 11:15–25. 10.7785/tcrt.2012.500230 [DOI] [PubMed] [Google Scholar]
- 46.Al-Omair A, Masucci L, Masson-Cote L, Campbell M, Atenafu EG, Parent A, et al. Surgical resection of epidural disease improves local control following postoperative spine stereotactic body radiotherapy. Neuro Oncol. (2013) 15:1413–9. 10.1093/neuonc/not101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Laufer I, Sciubba DM, Madera M, Bydon A, Witham TJ, Gokaslan ZL, et al. Surgical management of metastatic spinal tumors. Cancer Control. (2012) 19:122–8. 10.1177/107327481201900206 [DOI] [PubMed] [Google Scholar]
- 48.Wardak Z, Bland R, Ahn C, Xie XJ, Chason D, Morrill K, et al. A phase II clinical trial of SAbR followed by immediate vertebroplasty for spine metastases. Int J Radiat Oncol Biol Phys. (2019) 104:83–89. 10.1016/j.ijrobp.2019.01.072 [DOI] [PubMed] [Google Scholar]
- 49.Redmond KJ, Sciubba D, Khan M, Gui C, Lo SL, Gokaslan ZL, et al. A phase 2 study of post-operative stereotactic body radiation therapy (SBRT) for solid tumor spine metastases. Int J Radiat Oncol Biol Phys. (2020) 106:261–8. 10.1016/j.ijrobp.2019.10.011 [DOI] [PubMed] [Google Scholar]
- 50.Laufer I, Iorgulescu JB, Chapman T, Lis E, Shi W, Zhang Z, et al. Local disease control for spinal metastases following “separation surgery” and adjuvant hypofractionated or high-dose single-fraction stereotactic radiosurgery: outcome analysis in 186 patients. J Neurosurg Spine. (2013) 18:207–14. 10.3171/2012.11.SPINE12111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Giaj-Levra N, Niyazi M, Figlia V, Napoli G, Mazzola R, Nicosia L, et al. Feasibility and preliminary clinical results of linac-based Stereotactic Body Radiotherapy for spinal metastases using a dedicated contouring and planning system. Radiat Oncol. (2019) 14:184. 10.1186/s13014-019-1379-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Versteeg AL, Hes J, van der Velden JM, Eppinga W, Kasperts N, Verkooijen HM, et al. Sparing the surgical area with stereotactic body radiotherapy for combined treatment of spinal metastases: a treatment planning study. Acta Oncol. (2019) 58:251–6. 10.1080/0284186X.2018.1539240 [DOI] [PubMed] [Google Scholar]
- 53.Choi CH, Kim JH, Kim JI, Park JM. Comparison of treatment plan quality among MRI-based IMRT with a linac, MRI-based IMRT with tri-Co-60 sources, and VMAT for spine SABR. PLoS ONE. (2019) 14:e0220039. 10.1371/journal.pone.0220039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Glicksman RM, Tjong MC, Neves-Junior WFP, Spratt DE, Chua KLM, Mansouri A, et al. Stereotactic ablative radiotherapy for the management of spinal metastases: a review. JAMA Oncol. (2020) 6, 567–77. 10.1001/jamaoncol.2019.5351 [DOI] [PubMed] [Google Scholar]
- 55.Mossa-Basha M, Gerszten PC, Myrehaug S, Mayr NA, Yuh WT, Jabehdar Maralani P, et al. Spinal metastasis: diagnosis, management and follow-up. Br J Radiol. (2019) 92:20190211. 10.1259/bjr.20190211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thibault I, Chang EL, Sheehan J, Ahluwalia MS, Guckenberger M, et al. Response assessment after stereotactic body radiotherapy for spinal metastasis: a report from the SPIne response assessment in Neuro-Oncology (SPINO) group. Lancet Oncol. (2015) 16:e595–603. 10.1016/S1470-2045(15)00166-7 [DOI] [PubMed] [Google Scholar]