Abstract
The aim of this study was to assess the periodontal status of cystic fibrosis (CF) adult patients and to evaluate whether there is a correlation between the bacterial population of the subgingival biofilm and the health status of the periodontal tissues in this group of adults. The study involved 22 cystic fibrosis adult patients. The periodontal condition was assessed using Plaque Index (PLI), Gingival Index (GI), and Probing Pocket Depth (PPD). The gingival sulcus samples were analyzed by the Real-Time PCR assay (RT-PCR). Majority of patients showed moderate or severe bacterial dental plaque accumulation, but none of them had clinical symptoms of periodontal diseases. RT-PCR showed the presence of periopathogens in 50% of patients. Red complex microorganisms were detected in 9.09%, orange complex in 27.27%, and green complex in 31.82% of the samples analyzed. In cystic fibrosis patients colonized by periopathogens, the periodontal markers were significantly higher in comparison to not colonized by periopathogens patients. Despite the widespread presence of bacterial dental deposits in the cystic fibrosis adult patients examined, none of them has clinical symptoms of periodontal disease; however, the presence of periodontal pathogens in subgingival biofilm may represent a possible risk factor of this disease in the future. An unsatisfactory level of oral hygiene in any patient with cystic fibrosis indicates a need to focus on standards of dental care for such patients.
Key words: cystic fibrosis, periodontal pathogens, periodontitis, Real-Time PCR
Introduction
The role of oral health, including the level of dental hygiene, accumulation of dental bacterial plaque, and periodontal diseases in the pathogenesis of respiratory infections is usually ignored in the medical care of cystic fibrosis (CF) patients. The oral cavity is a reservoir of many microorganisms; to date, more than 700 oral bacterial species have been identified. Over 400 of them form dental bacterial plaque biofilm and can be isolated from gingival sulcus or gingival pocket. The remaining 300 inhabit other areas, such as the tongue, oral mucosa, carious lesions, and teeth with endodontic infection (Haffajee et al. 2008; Dewhirst et al. 2010). Most oral bacteria are saprophytes of the oral cavity; the species composition of the dental plaque micro-biome is characterized by stability (homeostasis) that may break down, and, under favorable environmental conditions, the plaque microflora may express their virulence (Haffajee et al. 2008). The oral cavity, especially in individuals with poor oral hygiene or periodontal diseases, can also harbor various important pathogenic microorganisms, including respiratory pathogens (Wise and Williams 2013; Souto et al. 2014; Caldas et al. 2015; Vilela et al. 2015).
Periodontitis is a chronic infection of tooth-supporting tissues. The pathogenesis involves a complex of an immuno-inflammatory reaction of the host to a microbial community in the dental plaque. The severity of the periodontal disease results from the interaction between the dental plaque microbiota and the host’s defense mechanisms (Haffajee et al. 2008; Dewhirst et al. 2010). Subgingival plaque bacteria form a specific biofilm complex in which microorganisms become more effectively protected against host defense mechanisms and efficiently use the nutrients (Socransky et al. 1998). The concept of a bacterial complex, introduced by (Socransky et al. 1998), is based on the specific plaque hypothesis that assumes that a specific microbiota is associated with gingivitis or chronic or aggressive periodontitis, and that the pathogenicity of the plaque depends on the presence or increase in the number of specific micro-organisms. Based on the bacterial correlations all of the biofilm’s periopathogens are divided into four groups (complexes). The most pathogenic for periodontal tissues and considered as a marker of periodontitis is the red complex, which includes Porphyromonas gingivalis, Tannerella forsythia, and Treponema denticola. The orange complex, directly related to the red one, includes Fusobacterium nucleatum, Prevotella intermedia, Peptostreptococcus micros, and Campylobacter rectus. The yellow complex comprises Streptococcus mitis, Streptococcus oralis, Streptococcus sanguis, and the green complex includes Capnocytophaga gingivalis, Campylobacter concisus, Eikenella corrodens, and Aggregatibacter actinomycetemcomitans serotype a. Also, there are separate periopathogens such as A. actinomycetemcomitans serotype b, Selenomonas noxia, and Actinomyces naeslundii genospecies, which are not related to other groups. Particular importance in the etiopathogenesis of periodontitis is attributed to (Haffajee et al. 2008) that is considered the dominant etiological factor in the initiation and early stage of periodontal disease. Species of individual complexes are closely related. In most cases, species of a particular group appear together, or they are all absent together. This interaction and cooperation between the bacteria of dental plaque biofilm lead to a form of stabilization in which all species exist in harmony and equilibrium with their environment. This biofilm stabilization is named the climax community (Socransky et al. 1998; Haffajee et al. 2008).
The periopathogens are not only responsible for pathological changes in periodontal tissues, but may also be associated with several systemic conditions, including respiratory diseases (Gomes-Filho et al. 2010; Messika et al. 2018). Oral bacteria may disseminate into the lower respiratory tract, increasing the risk of pulmonary infections (Gomes-Filho et al. 2010). Thereby, to reduce the risk of possible autoinfection especially in patients at risk of such infection, there is a need to control periodontal condition regularly.
The aim of this study was to assess the periodontal status of cystic fibrosis patients and to evaluate whether there is a correlation between the bacterial population of the subgingival biofilm and the health status of the periodontal tissues in this group of adults.
Experimental
Material and Methods
Patients. The research was conducted in accordance with ethical principles, including the World Medical Association Declaration of Helsinki and was approved by an Ethical Committee of the Poznan University of Medical Sciences, Poland (No 427/16). The inclusion criteria were as follows: 1. diagnosis of cystic fibrosis confirmed by positive results of sweat test and a genetic test, 2. age above 18 years, 3. at least 10 teeth present in the mouth. Exclusion criteria included: 1. pregnancy or lactation, 2. diabetes or evidence of any other than CF systemic disease as a risk factor for periodontitis, 3. acute respiratory infections. After explaining the purpose and details of the study, written informed consent was obtained from all the subjects who were willing to participate. Twenty-two CF patients (14 women and 8 men) with an average age of 29.43 ± 6.78 years meetings all the above conditions were included to this study. All of them were patients of the Department of Pulmonology, Allergology and Respiratory Oncology of the Poznan University of Medical Sciences, Poland.
Periodontal examination. Assessment of periodontal condition was conducted following the World Health Organization criteria for epidemiological surveys (WHO 1998). Two professionals examined patients under artificial light using a dental mirror and a dental WHO 621 periodontal ballpoint probe. Prior to the clinical examination, examiners were calibrated and the inter-examiner agreement was determined by Cohen’s Kappa values of 0.85.
The clinical examination procedure included the evaluation of dental plaque accumulation, assessment of gingival bleeding and the measure the depth of gingival sulcus. For the presence and quantity of dental plaque recording, the Silness & Löe Plaque Index (PLI) was used (Silness and Löe 1964). The gingival status was assessed using the Löe & Silness Gingival Index (GI) (Löe and Silness 1967). The presence/absence of dental plaque and gingival bleeding were based on the examination of four surfaces (buccal, lingual/palatal, mesial, and distal) of six index teeth: upper first right molar (16), upper right central incisor (11), upper first left premolar (24), lower first left molar (36), lower left central incisor (31), and lower right premolar (44), as recommended by the indices. In the recording of dental plaque accumulation, all four surfaces of teeth were given the score from 0 to 3. The criteria for this index are: 0 – no plaque, 1 – plaque invisible but can be found with the periodontal probe at the gingival margin, 2 – moderate plaque was easily seen without probing, 3 – sample plaque easily seen. Then the scores form four surfaces were summed up and divided by four to give the plaque index for each tooth. The index for the patient was obtained by summing the indices for all six teeth and dividing by six. The PLI score: < 0.1 means no plaque, from 0.1 to 1.0 indicates a small quantity of plaque, from 1.1 to 2.0 a moderate amount and between 2.1 to 3.0 a considerable one.
The bleeding was assessed by probing gently along the wall of the soft tissue of the gingival sulcus. There were given scores from 0 to 3, were 0 means normal gingiva, 1 – mild inflammation – slight change in color and slight edema but no bleeding on probing, 2 – moderate inflammation – redness, edema, glazing, and bleeding on probing, and 3 – severe inflammation – marked redness and edema, ulceration with tendency to spontaneous bleeding. The sites were probed, waiting 10 s to verify the presence or absence of gingival bleeding. The mean index was calculated by dividing the sum of numbers from the scale by the total number of sites scored within the mouth. A score from 0.1 to 1.0 means mild inflammation, 1.1 – 2.0 moderate, and from 2.1 to 3.0 – severe inflammation.
Depth of the gingival sulcus (Probing Pocket Depth, PPD) on six dental sites: distofacial, facial, mesiofacial, distolingual, lingual, and mesiolingual of all teeth except third molar teeth, was examined using the periodontal probe 621. During the examination, the probe was introduced down to the bottom of the sulcus in parallel to the long dental axis with permanent contact with the tooth. The mean individual PPD index was calculated by dividing the sum of the PPD for each tooth by the total number of examined teeth.
Bacterial microflora evaluation. Gingival sulcus samples were collected for quantification of bacterial content and analysis for the presence of nine different microorganisms, using molecular biology tools and specific gene amplification by RT-PCR (Gołyńska et al. 2017). Microbiological analysis was performed to assess the presence and quantity of following periodontal pathogens: 1. A. actinomycetemcomitans, 2. P. gingivalis, T. forsythia, and T. denticola from the red complex, 3. F. nucleatum, P. intermedia, P. micros from the orange complex as well as Eubacterium nodatum from the complex associated with the orange one, 4. C. gingivalis from the green complex. We have used commercially RT-PCR kit (PET Test® plus).
Samples were collected from the gingival sulcus of four teeth – two posterior and two front teeth, two uppers and two lower, so that they were the same teeth (16, 11, 36, 31) in which the clinical indices (PLI, GI) were assessed. All samples were collected following the procedure recommended by the manufacturer. Before collecting the samples, the supragingival bacterial plaque was removed, and the examined area was dried and isolated with sterile swabs from the access of saliva. Using sterile tweezers, a sterile paper point included in the kit was introduced for 20 seconds. Then each of the four samples was loaded into one test tube, placed in a transportation set, and shipped to an MPI Pharma Laboratory.
Statistical analysis. Statistical analysis was done using Statistica, version 12 software (StatSoft Inc., Tulsa, USA). For statistical inference of the collected data, mean values with standard deviation and percentages were adopted. The normalcy of the variable distribution was tested using the Shapiro-Wilk test. Also, the Spearman’s rank correlation coefficient was used to determine the correlation between the received values of clinical indices (GI, PLI, PPD) and also between particular clinical index (GI, PLI or PPD) and count of bacteria in the subgingival biofilm. The level of significance was set at 0.05.
Results
Mild dental plaque accumulation (PLI = 0.1–1.0) was detected in 36% of cystic fibrosis patients, moderate (PLI = 1.1–2.0) in 45%, and severe (PLI = 2.1–3.0) in 18%. There was no patient with PLI < 0.1, but this condition is observed only directly after tooth brushing in patients with good oral hygiene. In the vast majority of patients (68%) the GI index ranged between 0.1 and 1.0 indicative of mild inflammation. In 27% of patients, no gingivitis was present (GI < 0.1), and a moderate inflammation (GI = 1.0–2.0) was found in 45%. No patient showed severe gingivitis (GI ≥ 2.1). There was no patient in whom periodontal pocket was recorded (PPD > 4 mm). In 45% of patients, the PPD ranged between 3 and 4 mm, in 18% between 0.5 and 2 mm, and 36% between 3 and 4 mm. There was a very high positive correlation between the following: GI and PLI, GI and PPD, PPD and PLI (p < 0.0001). Quantitative microbial analysis of gingival sulcus revealed that the mean total bacteria count in gingival sulcus in CF patients was 1.3 × 107 ± 3.8 × 107 CFU/ml. There was a very high positive correlation (p < 0.0001) between the number of bacteria and the depth of gingival sulcus (PPD index).
Periopathogens were detected in 50% of CF patients. Based on these results, the patients examined were divided into two groups: 1. CF patients with periopathogens, that is, for whom PCR tests showed the presence of periopathogens, and 2. CF patients without periopathogens, that is, in whom periopathogens were not found in the materials examined (Table I). There was no significant difference in the total bacteria count between the two groups of patients (p > 0.05), but in patients with periopathogens, the GI, PLI, and PPD were significantly higher in comparison to patients without periopathogens (p > 0.05).
Table I.
Clinical data of adult patients with cystic fibrosis that were colonised or not colonised by periopathogens.
| PLI | GI | PPD | TOTAL BACTERIA COUNT (CFU/ml) | |||||
|---|---|---|---|---|---|---|---|---|
| C | 1.76 ± 0.70 | p < 0.01 | 0.56 ± 0.36 | p < 0.05 | 1.33 ± 0.24 | p < 0.01 | 8.9 × 106 ± 1.5 × 107 | p > 0.05 |
| NC | 0.86 ± 0.28 | 0.21 ± 0.26 | 0.98 ± 0.12 | 1.8 × 107 ± 5.3 × 107 | ||||
| TOTAL | 1.31 ± 0.69 | 0.39 ± 0.36 | 1.16 ± 0.26 | 1.3 × 107 ± 3.8 × 107 | ||||
Data are presented as mean and standard deviation (SD)
CF - Cystic Fibrosis, PLI - Plaque Index by Silness & Loe, GI - Gingival Index by Loe & Silness, PPD - Probing Pocket Depth, C - patients colonised by periopathogens, NC - patients not colonised by periopathogens
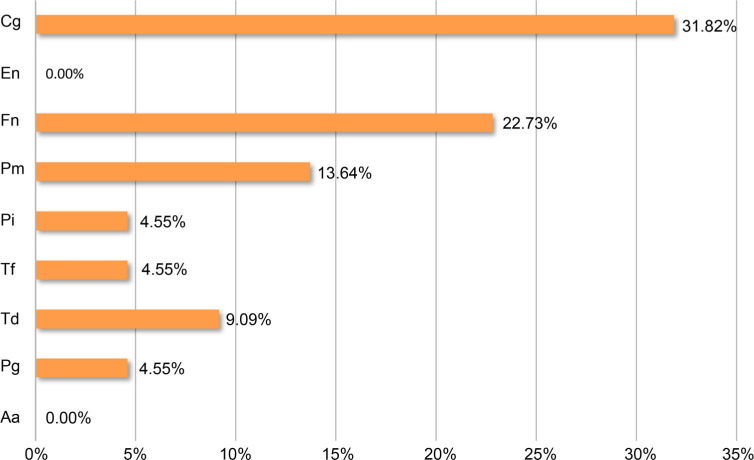
Seven out of nine tested periopathogens were detected in examined patients (Table II). A. actinomy-cetemcomitans and E. nodatum were not found in any of them. The average percentage of tested periopathogens within the total bacterial count was 2.42% and ranged from 0.01 to 21.56%. C. gingivalis from the green complex was the most frequently detected. Red complex microorganisms (P. gingivalis, T. forsythia, T. denticola) were present in 9.09% of samples, orange complex (F. nucleatum, P. intermedia, P. micros) in 22.73%, and green (C. gingivalis) in 31.82% (Fig. 1). Moreover, in 9.09% of patients a significantly increased number of periopathogens (C. gingivalis, F. nucleatum, P. intermedia, and P. micros) was detected.
Table II.
The number of bacteria isolated from gingival sulcus and the percentage of the species tested within the total bacterial count in samples.
| The periopathogens tested | Number of bacteria (CFU/ml) Mean ± SD | Percentage of the periopathogens tested within the total bacterial count (%) | |
|---|---|---|---|
| Aggregatibacter actinomycetemcomitans | 0.00 | 0.00 | |
| Red complex | Porphyromonas gingivalis | 2.0 × 10 ± 9.4 × 10 | 0.01 |
| Tannerella forsythia | 2.4 × 10 ± 1.1 × 102 | 0.01 | |
| Treponema denticola | 1.4 × 102 ± 6.3 × 102 | 0.01 | |
| Orange complex | Fusobacterium nucleatum | 7.3 × 102 ± 2.5 × 103 | 0.93 |
| Prevotella intermedia | 8.2 × 103 ± 3.9 × 104 | 0.01 | |
| Peptostreptococcus micros | 1.7 × 103 ± 8.2 × 103 | 1.50 | |
| Eubacterium nodatum | 0.00 | 0.00 | |
| Green complex | Capnocytophaga gingivalis | 6.8 × 104 ± 3.1 × 105 | 3.51 |
Fig. 1.
The percentage of the samples containing specific periopathogens.
Aa – Aggregatibacter actinomycetemcomitans, Pg – Porphyromonas gingivalis, Td – Treponema denticola, Tf – Tannerella forsythia, Pi – Prevotella intermedia, Pm – Peptostreptococcus micros, Fn – Fusobacterium nucleatum, En – Eubacterium nodatum, Cg – Capnocytophaga gingivalis. The mean value of these observations was 10.10% ± 10.85%.
Discussion
Among the many factors influencing the onset and progression of periodontal infection, periopathogens are of key importance, but their presence in the oral cavity is not always manifested. The clinical picture of periodontal disease depends on the balance of the interaction between microbial dental plaque biofilm and host immune response (Haffajee et al. 2008; Dewhirst et al. 2010). In the studied CF patients, there was a high positive correlation between the depth of the gingival sulcus (PPD) and the accumulation of dental plaque (PLI), also between the PPD and gingival bleeding (GI), and between PPD and total number of bacteria in gingival sulcus, confirming the pathomechanism of periodontitis and underscoring the importance of daily removal of dental bacterial plaque, by tooth brushing, as a preventive measure. The dental plaque accumulation index leads to conclusions not only about the current status, but also about the efficiency of hygienic procedures performed by the patient (daily mechano-chemical teeth brushing). However, it does not convey information about the host’s immune response to the bacterial infection or the severity of the inflammation. These factors can be analyzed by gingival indices describing the condition of the gums. The available literature regarding the periodontal status of cystic fibrosis patients shows that CF adult patients, compared to healthy individuals, have less gingival bleeding, with a similar bacterial plaque accumulation (Pawlaczyk-Kamieńska et al. 2018). In our study, despite the widespread presence of bacterial dental deposits in cystic fibrosis patients, none of them has clinical symptoms of periodontal disease. This can be explained by frequent and long-term antibiotic therapy, including via inhalation.
Microbiological analysis of bacterial subgingival plaque in CF patients was conducted by (Caldas et al. 2015). Their studies included 10 cystic fibrosis patients (five chronically colonized by Pseudomonas aeruginosa, and five not colonized) without clinical symptoms of gingivitis or periodontitis. The average age of the chronically colonized patients was 23.8 (17–34 years), and of the not colonized patients, it was age 16.6 (12–27 years). The qPCR PrioAnalyse assay was used for the analysis of bacterial plaque microbiological composition. This test identified nine periopathogens: A. actinomycetem-comitans, P. gingivalis, T. forsythia, and T. denticola from the red complex; F. nucleatum, P. intermedia, P. micros, and C. rectus from the orange complex; and E. corrodens from the green complex.
The analysis of samples revealed seven periopathogens in not colonized patients from the group, and five out of nine analyzed in the chronically colonized group. A. actinomycetemcomitans was not found in any patients. The most frequently detected was F. nucleatum (orange complex), and the second most frequent was E. corrodens (green complex). In our study, the PCR analysis showed seven out of nine the periopathogens tested. Similar to (Caldas et al. 2015), no A. actinomy-cetemcomitans were found. C. gingivalis (green complex) was the most common, followed by F. nucleatum (orange complex).
Reports published to date concerning the presence of oral periopathogens usually relate to generally healthy patients with varying degrees of periodontitis, diagnosed based on history, clinical, and radiological examination (Abiko et al. 2010; Kotslikov et al. 2015; Elamin et al. 2017). The cited studies exclude patients who had used antibiotics in the last three months or anti-inflammatory drugs in the last month. These results report a higher level of periopathogens in individuals with periodontitis compared to patients with healthy periodontium (Abiko et al. 2010). Furthermore, the authors found a positive correlation between the severity of periodontitis and the number of microorganisms, such as A. actinomycetemcomitans, P. gingivalis, P. intermedia, and T. forsythia (Abiko et al. 2010; Kotslikov et al. 2015; Elamin et al. 2017). Their numbers and the ratio to other microbes in the periodontal pockets determine the risk of onset and progression of periodontitis. A high titer of periopathogens does not guarantee disease development but informs of the risk (Abiko et al. 2010). The presented research included cystic fibrosis patients in whom the exclusion of systemic drugs application was not possible. Presumably, in those groups of patients, medications used may affect the microbiological oral condition, probably limiting the pathogenicity of the bacterial plaque biofilm. Unsatisfactory oral hygiene levels suggest that the periodontal risk cannot be ruled out.
Knowledge of the pathomechanisms of periodontitis and possible autoinfection by aspiration of oral microbiome should be taken into account in the medical care of CF patients. The dental biofilm cannot be eliminated, but it can be controlled by daily, regular chemo-mechanical oral hygiene practices performed by the patients. There is a necessity to improve the oral health condition in CF patients, and dentists should be a part of a multi-disciplinary cystic fibrosis team. There is also a need to implement effective strategies for care pathways directed toward maintaining oral health in this group of patients.
Conclusions
Despite the widespread presence of bacterial dental deposits in cystic fibrosis adult patients, none of them has clinical symptoms of periodontal disease, but the presence of periodontal pathogens in subgingival biofilm represents a possible risk factor of this disease in the future.
An unsatisfactory level of oral hygiene in any patient with cystic fibrosis indicates a need to focus on standards of dental care for such patients.
Acknowledgments
We are grateful to individuals with cystic fibrosis for contributing to this study.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Abbreviations used in this paper:
- CF
Cystic Fibrosis
- PCR
polymerase chain reaction
- PLI
Plaque Index by Silness & Löe
- GI
Gingival Index by Löe & Silness
- PPD
Probing Pocket Depth
- Aa
Aggregatibacter actinomycetemcomitans
- C
patients colonised by periopathogens
- NC
patients not colonised by periopathogens
Footnotes
Conflict of interest
The authors do not report any financial or personal connections with other persons or organizations, which might negatively affect the contents of this publication and/or claim authorship rights to this publication.
ORCID
Tamara Pawlaczyk-Kamieńska 0000-0003-3398-8812
Renata Śniatała 0000-0002-3123-7612
Halina Batura-Gabryel 0000-0003-4555-7905
Maria Borysewicz-Lewicka 0000-0001-6391-350X
Szczepan Cofta 0000-0002-8849-5487
Literature
- Abiko Y, Sato T, Mayanagi G, Takahashi N.. Profiling of subgingival plaque biofilm microflora from periodontally healthy subjects and from subjects with periodontitis using quantitative real-time PCR. J Periodontal Res. 2010;45(3):389–395. 10.1111/j.1600-0765.2009.01250.x [DOI] [PubMed] [Google Scholar]
- Dewhirst FE, Chen T, Izard J, Paster BJ, Tanner ACR, Yu WH, Lakshmanan A, Wade WG.. The human oral microbiome. J Bacteriol. 2010;192(19):5002–5017. 10.1128/JB.00542-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elamin A, Ali RW, Bakken V.. Putative periodontopathic bacteria and herpes viruses interactions in the subgingival plaque of patients with aggressive periodontitis and healthy controls. Clin Exp Dent Res. 2017;3(5):183–190. 10.1002/cre2.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gołyńska M, Polkowska I, Bartoszcze-Tomaszewska M, Sobczyńska-Rak A, Matuszewski Ł.. Molecular-level evaluation of selected periodontal pathogens from subgingival regions in canines and humans with periodontal disease. J Vet Sci. 2017;18(1):51–58. 10.4142/jvs.2017.18.1.51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes-Filho IS, Passos JS, Seixas da Cruz S.. Respiratory disease and the role of oral bacteria. J Oral Microbiol. 2010;2(1):5811 10.3402/jom.v2i0.5811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haffajee AD, Socransky SS, Patel MR, Song X.. Microbial complexes in supragingival plaque. Oral Microbiol Immunol. 2008;23(3): 196–205. 10.1111/j.1399-302X.2007.00411.x [DOI] [PubMed] [Google Scholar]
- Kotsilkov K, Popova C, Boyanova L, Setchanova L, Mitov I.. Comparison of culture method and real-time PCR for detection of putative periopathogenic bacteria in deep periodontal pockets. Biotechnol Biotechnol Equip. 2015;29(5):996–1002. 10.1080/13102818.2015.1058188 [DOI] [Google Scholar]
- Löe H, Silness J.. The gingival index, the plaque index and the retention index systems. J Periodontol. 1967;38(6):610–616. 10.1902/jop.1967.38.6.610 [DOI] [PubMed] [Google Scholar]
- Messika J, La Combe B, Ricard JD.. Oropharyngeal colonization: epidemiology, treatment and ventilator-associated pneumonia prevention. Ann Transl Med. 2018;6(20):426 10.21037/atm.2018.10.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlaczyk-Kamieńska T, Borysewicz-Lewicka M, Śniatała R, Batura-Gabryel H, Cofta Sz.. Dental and periodontal manifestations in patients with cystic fibrosis – a systematic review. J Cys Fibrosis. 2018. 10.1016/j.jcf.2018.11.007 [DOI] [PubMed] [Google Scholar]
- Rivas Caldas R, Le Gall F, Revert K, Rault G, Virmaux M, Gouriou S, Héry-Arnaud G, Barbier G, Boisramé S.. Pseudomonas aeruginosa and periodontal pathogens in the oral cavity and lungs of cystic fibrosis patients: a case control study. J Clin Microbiol. 2015;53(6):1898–1907. 10.1128/JCM.00368-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silness J, Löe H.. Correlation between oral hygiene and periodontal condition. Acta Odontol Scand. 1964;22(1):121–135. 10.3109/00016356408993968 [DOI] [PubMed] [Google Scholar]
- Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL Jr.. Microbial complexes in subgingival plaque. J Clin Periodontol. 1998; 25(2):134–144. 10.1111/j.1600-051X.1998.tb02419.x [DOI] [PubMed] [Google Scholar]
- Souto R, Silva-Boghossian CM, Colombo APV.. Prevalence of Pseudomonas aeruginosa and Acinetobacter spp. in subgingival biofilm and saliva of subjects with chronic periodontal infection. Braz J Microbiol. 2014;45(2):495–501. 10.1590/S1517-83822014000200017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilela MCN, Ferreira GZ, Santos PSS, Rezende NPM.. Oral care and nosocomial pneumonia: a systematic review. Einstein (Sao Paulo). 201;13(2):290–296. 10.1590/S1679-45082015RW2980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO Oral Health Surveys, Basic Methods 4th ed. Geneva (Switzerland): World Health Organization; 1998. [Google Scholar]
- Wise MP, Williams DW.. Oral care and pulmonary infection – the importance of plaque scoring. Crit Care. 2013;17(1):101 10.1186/cc11896 [DOI] [PMC free article] [PubMed] [Google Scholar]