Abstract
Proteus spp. is an etiological factor of urinary tract and bloodstream infections. The aim of this study was the retrospective analysis of susceptibility of Proteus spp. strains isolated from bloodstream infections (BSIs) as well as similarity evaluation of the strains isolated from different clinical samples. Proteus spp. strains were isolated in 2009–2017 from hospital patients. Identification was based on the colony’s morphology and biochemical or MALDI-TOF MS analyzes. The antibiotic susceptibility test was done using the diffusion method. Biofilm formation was evaluated with microplate method using TTC. Bacteremia caused by Proteus spp. was found in 97 patients, mainly secondary to urinary tract infection. Most of the strains were susceptible to piperacillin with tazobactam (95.9%) and amikacin (86.7%). Elderly patients have a higher risk of mortality after BSIs caused by Proteus spp. A detailed analysis was made for randomly chosen 26 strains isolated from 11 patients with Proteus mirabilis bacteremia. Using PFGE, we found that 10 (90.9%) isolates, collected from different clinical specimens of the same patient, were genetically identical.
Key words: antimicrobial susceptibility; bacteremia; biofilm, Proteus spp
Introduction
Proteus spp. is widely spread in the environment. It may occur in water, soil, manure or sewage, and it is also a component of human and animal gastrointestinal tract microbiome (O’Hara et al. 2000; Drzewiecka 2016). In the current taxonomy of Proteus genus, eight species are included: Proteus mirabilis, Proteus vulgaris, Proteus penneri, Proteus hauseri, Proteus cibarius, Proteus columbae, Proteus incostans, and Proteus terrae. P. mirabilis is the most common species of this genus isolated from human infections (Różalski et al. 2007; Armbruster and Mobley 2012).
In addition to be a leading cause of urinary tract infections (UTI) and chronic wound infections (Endimiani et al. 2005), P. mirabilis can also cause bloodstream infections. P. mirabilis is found in 1–3% of all BSIs (Tumbarello et al. 2012). Other Proteus species are rare in BSIs and occur at rates of less than 0.1 per 100 000 (Laupland et al. 2014).
The most common source of bacteria in BSIs caused by P. mirabilis is the urinary tract (UT) (Watanakunakorn and Perni 1994). According to Watanakunakorn and Perni (1994) and Kim et al. (2003), UT is a source of 47.6–52.8% BSIs caused by P. mirabilis (Watanakunakorn and Perni 1994; Kim et al. 2003).
Among infectious diseases, BSIs are a major cause of mortality worldwide (Laupland and Church 2014). Endimiani et al. (2005) found that mortality rate attributable to P. mirabilis BSIs is higher in BSIs cases caused by strains with extended spectrum beta-lactamases (ESBL). The presence of beta-lactamases may seriously limit BSIs treatment options. Proteus spp. is inherently resistant to polymyxins that are used, beside carbapenems, in empiric treatments of BSIs caused by Gram-negative bacilli. P. mirabilis might produce likewise, the AmpC-type cephalosporinases and carbapenemases (KPC) (Wang et al. 2014; Di Pilato et al. 2016).
The aims of this study were: a) retrospective susceptibility analysis of Proteus spp. strains isolated from BSIs, b) the similarity evaluation of 26 P. mirabilis strains isolated from clinical samples collected from randomly chosen 11 hospital patients.
Experimental
Materials and Methods
Identification and susceptibility testing of clinical Proteus spp. strains. Proteus spp. strains were isolated from 2009 to 2017 from patients of the Antoni Jurasz University Hospital No 1 in Bydgoszcz, Poland. Basic information, i.e., age, sex of patients, and the samples collected were determined using laboratory data. Details, i.e.: short-term (14 days) mortality after bacteremia, length of stay, period from admission to the hospital to the onset of Proteus spp. bacteremia, presence of urinal or central venal catheters, the previous surgery, and additional diseases were determined by using electronic administrative data. We did not find any detailed information about 15 patients hospitalized in 2009–2010; they were excluded from epidemiological analyzes.
The strains were identified at the Department of Microbiology: a) between 2009 and 2011 the identification was based on colony’s morphology and the results of biochemical reactions (API 32E, BioMériuex), b) between 2011 and 2014 – on biochemical reaction results included in the VITEK GN cards (BioMériuex), and c) since 2014 – by using MALDI-TOF MS technique (Microflex, Bruker).
Susceptibility of Proteus spp. strains was examined: in 2009 and 2010 by using the disk diffusion method and interpreted in accordance with the Clinical and Laboratory Standard Institute (CLSI 2009) recommendation; since 2011 – using VITEK AST cards (BioMériuex) and interpreted according to the European Committee on Antimicrobial Susceptibility Testing (EUCAST 2016). The meropenem susceptibility was evaluated since 2016.
Extended-spectrum beta-lactamases (ESBLs) were examined according to the CLSI recommendation by using the double disk synergic test (DDST).
Primary bacteremia was defined as the bacteremia for which no source of infection was documented. Bacteremia was defined as secondary when laboratory examination showed infection by the same microorganism at a distant site in the same time or up to three days earlier.
Until the investigation, the selected strains were stored in BHI with 20.0% glycerol at 70°C.
Genetic similarity and phenotypic characterization of P. mirabilis strains. A detailed analysis was performed for 26 strains isolated from 11 (A-K) randomly chosen patients with P. mirabilis bacteremia. Strains were isolated from three different samples from four patients, and from two different samples – from seven patients. Two patients had urethral catheters, and one – peripheral venous catheter.
Susceptibility patterns. The susceptibility assays of P. mirabilis strains were done using the diffusion method according to CLSI recommendation, including the following antibiotics in discs (Emapol): trimethoprim TRM (5 μg), norfloxacin NOR (10 μg), netilmicin NET (30 μg), tobramicin TOB (10 μg), ticarcilin TIC (75 μg), amikacin AMK (30 μg), imipenem IMP (10 μg), ciprofloxacin CIP (5 μg), cephoperazone CEP (75 μg), piperacillin PIP (100 μg), ampicillin AM (10 μg), ticarcillin with clavulanic acid TIM (85 μg), cefuroxime CXM (30 μg), ampicillin with sulbactam UNA (20 μg), gentamicin GEN (10 μg). All results were interpreted according to EUCAST (2016).
Biofilm formation. Biofilm formation by 26 P. mirabilis strains was evaluated based on the method described previously by Kwiecińska-Piróg et al. (2013a). The method is based on the measurement of absorbance of formazan (dissolution product of 2,3,5-triphenyl-tetrazolium chloride, TTC; Avantor; λ = 470 nm) (BioTek; Synergy HT Multi-detection; KC4 program). The assay was done in three independent repetitions. Two reference strains from the American Type Culture Collection (ATCC): Staphylococcus aureus ATCC 6538 (strong biofilm former) and Escherichia coli ATCC 35218 (weak biofilm former) were used as the positive and negative control, respectively.
The A value was calculated based on the average (x) and threefold standard deviation (SD) of blank absorbance. The strains which had an absorbance above the A value were classified as being able to biofilm formation. All strains with an absorbance in the range between A and 2 × A were classified as weak biofilm formers (W), in the range between 2 × A and 4 × A – as moderate biofilm formers (M), and above 4 × A – as strong biofilm formers (S).
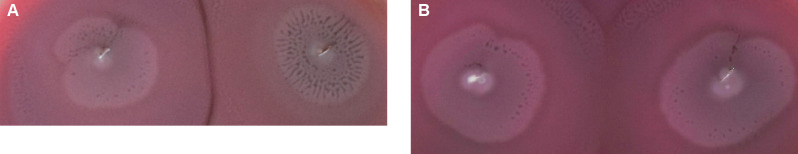
Dienes phenomenon testing. To examine the Dienes phenomenon of P. mirabilis strains, single colonies, previously cultured on MacConkey Agar, were inoculated as points on blood agar plates (Oxoid). The strains isolated from the same patient were inoculated on one plate. Plates were incubated at 37°C. After 24 hours, demarcation lines between strains were observed. When strains were non-identical, colonies were separated by the demarcation line (0.5–2 mm wide) (Fig. 1A). When strains were identical – there was any demarcation line (Sabbuda et al. 2003) (Fig. 1B).
Fig. 1.
The Dienes phenomenon: A) non-identical strains, B) identical strains.
Genetic similarity of P. mirabilis strains. Genetic similarity of Proteus spp. strains was evaluated by pulsed field gel electrophoresis (PFGE) using SfiI (Fermentas) restriction enzyme cleavage, as previously described by Sabbuba et al. (2003) with own modifications, described by Kwiecińska-Piróg et al. (2013b). Comparative analysis was performed using the Quantitative One software according to Dice.
Statistical analysis. Statistical analysis was performed using Statistica 12.5 PL (StatSoft) software. The confidence interval for all tests was α = 0.05. To compare variables, the chi2 test with Yates’ correction was used, if necessary.
Results
Proteus spp. blood stream infections. In the examined time period (2009–2017), BSIs were found in 3956 cases in 2829 patients. BSIs caused by Proteus spp. were confirmed in 98 cases (2.5%) in 97 patients (3.4%). In one female patient, secondary urinary tract bacteremia was recognized two times with a six-months interval between symptoms (Table II).
Table II.
The strains isolated from patients with P. mirabilis (n = 93) and P. vulgaris (n = 5) bacteremia.
| Year | spp. bacteremia Protteus | Secondary Proteus spp. bacteremia | Isolation of Proteus spp. not related directly to bacteremia | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Source of secondary bacteremia (strains isolated at the time/up to three days before isolation from blood sample) | Before bacteremia (more than three days) | After bacteremia | ||||||||||||||
| Urinary tract | Respiratory tract | Wounds | Blood catheter | Peritoneum | Respiratory tract | Wounds | Urinary tract | Blood catheter | Peritoneum | Respiratory tract | Wounds | Urinary tract | Blood catheter | Peritoneum | |||
| 2009 | 12 | 8 (66.7%) | 7 | 1 | 0 | 0 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 1 | 1 | 0 | 0 |
| 2010 | 12 | 8 (66.7%) | 4 | 0 | 2 | 2 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 4 | 1 | 2 | 0 |
| 2011 | 7 | 4 (57.1%) | 1 | 0 | 2 | 0 | 1 | 0 | 2 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 |
| 2012 | 4 | 4 (100.0%) | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| 2013 | 14 | 8 (57.1%) | 2 | 2 | 2 | 2 | 0 | 3 | 2 | 1 | 0 | 0 | 0 | 2 | 0 | 1 | 1 |
| 2014 | 14 | 9 (64.3%) | 5 | 0 | 3 | 1 | 0 | 0 | 2 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 |
| 2015 | 9 | 4 (44.4%) | 1 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 |
| 2016 | 15 | 8 (53.3%) | 5 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 |
| 2017 | 11 | 5 (45.5%) | 1 | 2 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 2 | 2 | 1 | 1 | 0 |
| Total | 98 | 58 (59.2%) | 27 | 8 | 14 | 7 | 2 | 4 | 9 | 7 | 1 | 1 | 5 | 11 | 5 | 5 | 2 |
Proteus spp. bacteremia was caused by P. vulgaris in five (5.2%) patients and by P. mirabilis in other patients (94.8%). In P. vulgaris bacteremia cases, four strains were isolated only from blood samples. One P. vulgaris bacteremia was related with a previous isolation of the same species strain from a chronic wound.
Median age for all patients with Proteus spp. bacteremia was 71 years. We found that 14-day mortality ratio of Proteus spp. bacteremia was 28.9%. None of P. vulgaris strain was related with short-term mortality. We observed that elderly patients have a higher risk of mortality (Table I).
Table I.
Demographic and clinical parameters of patients with Proteus spp. BSIs (n = 83).
| Variable | Death in 14 days (n = 24) | Survival (n = 59) | p-value* | ||
|---|---|---|---|---|---|
| Age (years), median (min-max) | 77.5 (55–94) | 67.0 (12–88) | 0.0025** | ||
| Sex | Men, No. (%) | 13 (54.2) | 33 (55.9) | 0.9229 | |
| Women, No. (%) | 11 (45.8) | 26 (44.1) | |||
| Length of stay in hospital (days), median (min-max) | 19.5 (1–139) | 38 (5–406) | 0.0142** | ||
| Length of stay in hospital before Proteus spp. BSI (days), median (min-max) | 9.5 (0–138) | 7.5 (0–380) | 1.0000** | ||
| Primary bacteremia, No. (%) | 11 (45.8) | 23 (39.0) | 0.7420 | ||
| Secondary bacteremia, No. (%) | 13 (54.2) | 36 (61.0) | |||
| Adequate empiric antibiotic therapy, No. (%) | 16 (66.7) | 43 (72.9) | 0.7648 | ||
| More than one strain in blood culture, No. (%) | 9 (37.5) | 20 (34.0) | 0.9537 | ||
| ESBL Proteus spp., No. (%) | 4 (16.7) | 11 (18.6) | 0.9185 | ||
| Predisposing factors | Urinal catheter, No. (%) | 18 (75.0) | 44 (67.8) | 0.8117 | |
| Central Venus Catheter, No. (%) | 15 (62.5) | 28 (47.5) | 0.3168 | ||
| Diabetes, No. (%) | 7 (29.2) | 10 (16.9) | 0.3419 | ||
| Hospitalization during past half year, No. (%) | 13 (54.2) | 25 (42.4) | 0.4625 | ||
| Cancer, No. (%) | 3 (12.5) | 4 (6.8) | 0.6784 | ||
| Surgery during past half year, No. (%) | 9 (37.5) | 33 (55.9) | 0.2003 | ||
| Cardio-vascular diseases, No. (%) | 15 (62.5) | 23 (39.0) | 0.0879 | ||
| Chronic wounds, No. (%) | 7 (29.2) | 13 (22.0) | 0.6849 | ||
Chi2 test,
U Mann-Whitney test
During the period analyzed, the most commonly used antibiotic for empiric therapy of BSIs was imipenem. Amongst 16 patients treated with imipenem, three (20.0%) of them died within 14 days. All P. mirabilis strains isolated from blood samples of these patients were susceptible to imipenem. Piperacillin with tazobactam was used in 15 cases of BSIs, and ceftriaxone – in 13 cases. The 14-days mortality rate for piperacillin with tazobactam was 26.7%, and for ceftriaxone – 44.0%.
Primary bacteremia was reported in 40 (40.8%) cases. The source of P. mirabilis secondary bacteremia was confirmed in 58 (59.2%) cases. In patients with secondary bacteremia, the main source of infection was the urinary tract (27; 46.6%), wounds of skin or soft tissues (14; 24.1%). In nine (9.2%) cases, Proteus spp. bacteremia occurred three days prior to isolation of the same bacterial species from wound swab, and in seven (7.1%) – from urine samples (Table II). One or more weeks after P. mirabilis bacteremia, strains of the same species were isolated from wound swabs (11; 11.2%), central venous catheter samples (5; 5.1%) and urine collected by a catheter (5; 5.1%).
All Proteus spp. strains isolated in 2016 and 2017 (n = 26) were susceptible to meropenem. Most strains were susceptible to piperacillin with tazobactam (95.9%), ceftazidime (87.8%) and amikacin (86.7%) (Table III). More than half of the strains examined (51.0%) were resistant to co-trimoxazole. Resistance to ciprofloxacin was reported in 30 (30.6%) strains. All 40 strains isolated from primary bacteremia cases were susceptible to piperacillin with tazobactam. No significant differences in the antimicrobial susceptibility of strains isolated from primary and secondary bacteremia were found (Table III).
Table III.
Antibacterial agents (Aas) susceptibility of Proteus spp. strains (n = 98) isolated from bacteremia.
| Aas | Primary bacteremia Aas susceptibility | p* | Secondary bacteremia Aas susceptibility | Total Aas susceptibility | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S | I | R | S | I | R | S | I | R | |||||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | ||
| AMC | 9 | 22.5 | 24 | 60.0 | 7 | 17.5 | 0.4588 | 16 | 27.6 | 28 | 48.3 | 14 | 24.1 | 25 | 25.5 | 52 | 53.1 | 21 | 21.4 |
| TZP | 40 | 100.0 | 0 | 0.0 | 0 | 0.0 | 0.1316 | 54 | 93.1 | 0 | 0.0 | 4 | 6.9 | 94 | 95.9 | 0 | 0.0 | 4 | 4.1 |
| CMX | 28 | 70.0 | 0 | 0.0 | 12 | 30.0 | 0.6037 | 45 | 77.6 | 0 | 0.0 | 13 | 22.4 | 73 | 74.5 | 0 | 0.0 | 25 | 25.5 |
| CTX | 31 | 77.5 | 0 | 0.0 | 9 | 22.5 | 0.3393 | 46 | 79.3 | 0 | 0.0 | 12 | 20.7 | 77 | 78.6 | 0 | 0.0 | 21 | 21.4 |
| CAZ | 35 | 87.5 | 2 | 5.0 | 3 | 7.5 | 0.1697 | 51 | 87.9 | 0 | 0.0 | 7 | 12.1 | 86 | 87.8 | 2 | 2.0 | 10 | 10.2 |
| FEP | 32 | 80.0 | 2 | 5.0 | 6 | 15.0 | 0.4254 | 48 | 82.8 | 4 | 6.9 | 6 | 10.3 | 80 | 81.6 | 6 | 6.1 | 12 | 12.2 |
| IMP | 30 | 75.0 | 8 | 20.0 | 2 | 5.0 | 0.6062 | 49 | 84.5 | 8 | 13.8 | 1 | 1.7 | 79 | 80.6 | 16 | 16.3 | 3 | 3.1 |
| GEN | 29 | 72.5 | 1 | 2.5 | 10 | 25.0 | 0.3662 | 46 | 79.3 | 1 | 1.7 | 11 | 19.0 | 75 | 76.5 | 2 | 2.0 | 21 | 21.4 |
| AMK | 33 | 82.5 | 3 | 7.5 | 4 | 10.0 | 0.6311 | 52 | 89.7 | 4 | 6.9 | 2 | 3.4 | 85 | 86.7 | 7 | 7.1 | 6 | 6.1 |
| CIP | 24 | 60.0 | 1 | 2.5 | 15 | 37.5 | 0.4336 | 38 | 65.5 | 5 | 8.6 | 15 | 25.9 | 62 | 63.3 | 6 | 6.1 | 30 | 30.6 |
| SXT | 20 | 50.0 | 0 | 0.0 | 20 | 50.0 | 0.3165 | 28 | 48.3 | 0 | 0.0 | 30 | 51.7 | 48 | 49.0 | 0 | 0.0 | 50 | 51.0 |
chi2 test – differences in the susceptibility of Proteus spp. strains isolated from primary and secondary bacteremia S – susceptible, I – intermediate, R – resistant, AMC – amoxicillin + clavulanic acid, TZP – piperacillin with tazobactam, CXM – cefuroxime, CTX – cefotaxime, CAZ – ceftazidime, FEP – cefepime, IMP – imipenem, GEN – gentamicin, AMK – amikacin, CIP – ciprofloxacin, SXT – co-trimoxazole
ESBLs were found in 18 (18.3%) out of 98 Proteus spp. strains. Among all ESBL positive strains, 6 (33.3%) were isolated in 2016, and 5 (27.8%) in 2017. None of the P. vulgaris strains produced ESBLs.
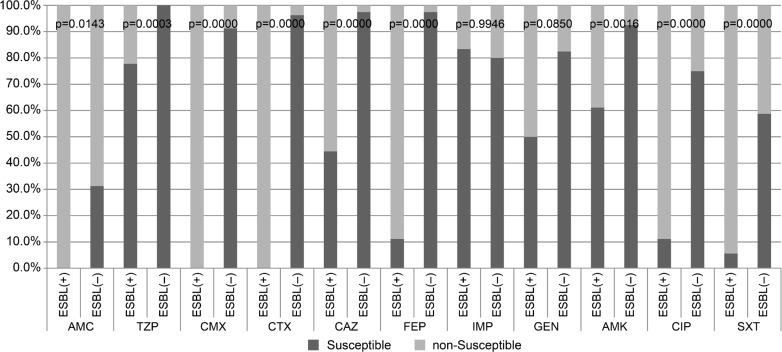
We compared the antimicrobial drugs susceptibility of ESBLs-positive (ESBLs(+)) and ESBLs-negative (ESBLs(–)) strains using the chi2 test (Fig. 2). Among the ESBLs(–) strains, all (n = 80) were susceptible to piperacillin with tazobactam. The ESBLs(+) strains were more resistant to all penicillins tested with beta-lactamases inhibitors and cephalosporins. Among the ESBLs(+) strains, more strains were non-susceptible to ciprofloxacin and co-trimoxazole than among ESBLs(–) bacteria (Fig. 2).
Fig. 2.
Antibiotic susceptibility of ESBLs(+) and ESBLs(–) Proteus spp. strains (n = 98) isolated from bacteremia; p-value of chi2 testing between susceptible and non-susceptible (resistant and intermediate) strains; abbreviations of antibiotic names (see Table III).
Susceptibility patterns. The susceptibility patterns of isolates from different specimens of the same patient were similar, except of the isolate B3 that was genetically different from B1 and B2 and was more resistant to antibiotics than other two isolates collected from the same patient. Isolate J2, collected from urine four days before the bacteremia, were resistant to trimethoprim while J1 were susceptible to this antibiotic (Table IV).
Table IV.
Genetic similarity of P. mirabilis isolates (n = 26).
ESBLs were detected in five (19.2%) P. mirabilis isolates from two patients (D and G), treated in the Intensive Care Unit (G) and the Cardiology Department (D). Isolates D1, D2, G1, G2 and G3 had the same DNA profiles.
Biofilm formation. The A value was equal to 0.0654, based on the formazan absorbance of blank sample. The mean values (± SD) of absorbance recorded for biofilms formed by the references strains, were as follows: for E. coli ATCC 35218 – 0.1031 ± 0.01, and for S. aureus ATCC 6538 – 0.6931 ± 0.03. The results obtained for clinical P. mirabilis strains were shown in Table IV.
Weak biofilms were formed by 17 (65.4%) isolates, moderate biofilms by four (15.4%), and strong by five (19.2%) isolates. Most of the strains (8; 72.7%) isolated from blood samples formed weak biofilms. Two of three isolates collected from chronic wounds prior to bacteremia were able to form stronger biofilms than strains collected from blood samples. Two isolates from urine samples of catheterized patients formed moderate and strong biofilms, while isolates cultured from blood sample – weak biofilms. The isolates from venal catheters formed weak biofilms, similarly to isolates from blood samples.
Dienes phenomenon results. We found demarcation lines between strains J1 and J2, B1 and B3, and B2 and B3 (Table IV). Those strains were not genetically identical. Strain J1, from urine collected by a catheter, formed a stronger biofilm than J2 cultured from the blood sample. The B3 strain, cultured from the bedsore swab sample, formed a strong biofilm, in contrast to B1 and B2 isolates that formed a weak biofilm.
Genetic similarity of P. mirabilis isolates. Using PFGE we found that isolates collected from different clinical specimens were identical in 10 of 11 patients (Table IV). In five patients (K, G, H, E, and J), the same strains were found in other localizations before the bacteremia was confirmed.
Strains J1 and J2, isolated from patient J, were genetically different and formed biofilms with various capacity, but they presented similar susceptibility patterns. The J2 strain was isolated from urine sample four days before J1 was cultured from blood sample.
Two strains, B1 and B2, were genetically identical as it was shown by their macrorestriction profiles. They formed weak biofilms and had the same susceptibility toward antibiotics. Strain B3, isolated from chronic wound swab nine days after confirmation of P. mirabilis bacteremia, was similar to B1 and B2, but it formed a strong biofilm and was resistant to higher number of the antibiotics tested than B1 and B2 isolates.
Discussion
The P. mirabilis is isolated from 1% to 3% of all BSIs (Laupland et al. 2007; Sohn et al. 2011). Our study confirms these data. We also observed Proteus spp. BSIs in 2.5% of all BSIs investigated. Among Enterobacterales, P. mirabilis is the fourth Gram-negative bacteria species after E. coli, Klebsiella pneumoniae, and Enterobacter spp. isolated from hospital-acquired BSIs (Sohn et al. 2011). BSIs are well-known cause of high mortality. We found that mortality rate of P. mirabilis BSIs was 28.9%. It is similar value to that obtained by Endimiani et al. (2005). They found that mortality rate attributable to P. mirabilis BSIs was 33.0%.
Laupland et al. (2014) showed that most cases of P. mirabilis bacteremia (18; 72.0%) have no documented sources and were recognized as primary bacteremia. In another seven (28.0%) cases, the urinary tract was confirmed to be the source of bacteremia. The opposite results were reported by Sohn et al. (2011). They detected primary bacteremia caused by Proteus spp. in 32.4% patients. The source of bacteria in secondary bacteremia was found mostly (35.1%) in the urinary tract and intra-abdominal cavity (21.6%) (Ahn et al. 2017). Infections of the urinary tract were admitted as the main source of BSIs also in the study by Tumbarello et al. (2012). They showed this phenomenon in BSIs caused by 19 (52.8%) multidrug resistant (MDR) and 28 (4.4%) non-MDR P. mirabilis strains.
Our data confirms the findings of Sohn et al. (2011). In most cases (58; 59.2%), the bacteremia caused by P. mirabilis was secondary due to the presence of these bacteria in other body sites, particularly in the urinary tract (27; 46.6%). In 14 (24.1%) patients, we found P. mirabilis in wound swabs. Wound infected by P. mirabilis was confirmed as the source of BSIs in the patient of the Traumatic Care Unit in India (Endimiani et al. 2005).
Proteus spp. is inherently susceptible to co-trimoxazole (trimethoprim-sulfamethoxazole). In the present study we found that more than half (51.0%) of the Proteus spp. strains, isolated from patients with bacteremia, were resistant to co-trimoxazole. High resistance to cotrimoxazole was reported also in another paper (Wang et al. 2014). Among 158 examined strains isolated from blood samples during longitudinal nationwide study from the Taiwan Surveillance of Antimicrobial Resistance (TSAR) program, the resistance of P. mirabilis to co-trimoxazole was found in 65.2% strains. Similar percentage (69.2%) of co-trimoxazole resistance was reported also for ESBLs-producing P. mirabilis strains isolated from blood samples (Lebeaux et al. 2014). In this study, we found that almost all ESBLs(+) strains (17; 94.4%) were non-susceptible to co-trimoxazole. Lower percentages of resistant strains were found in the study by Tumbarello et al. (2012): 33.3% among non-MDR, and 30.6% among MDR P. mirabilis strains involved in BSIs development.
ESBLs were found by Sohn et al. (2010) in nine (24.3%) out of 37 P. mirabilis strains causing bacteremia from 2000 to 2009. In our study, we detected ESBLs in 18.4% P. mirabilis isolates causing bacteremia during the period evaluated. The highest number of ESBL-positive strains was found in 2016 (6; 33.3%). Tumbarello et al. (2012) noted that ESBLs were confirmed in 36 (36.4%) out of 99 P. mirabilis strains causing BSIs. MDRs were recorded for 36 (36.6%) strains tested.
Tumbarello et al. (2012) diagnosed P. mirabilis BSIs in 103 adult patients. The growth of the strains was inhibited by meropenem, amoxicillin with clavulanic acid, piperacillin with tazobactam, and amikacin. Ahn et al. (2017) confirmed that most P. mirabilis isolates from BSIs were susceptible to piperacillin with tazobactam (98.3%) and amikacin (93.5%). In the study by Wang et al. (2014), 58.9–87.3% of P. mirabilis strains isolated from blood samples were susceptible to gentamicin and amikacin, respectively. This is in agreement with the present results. Proteus spp. strains examined in this study were mostly susceptible to piperacillin with tazobactam (95.3%) and amikacin (86.7%).
Proteus spp. strains were generally susceptible to ciprofloxacin (67.1–73.4% of the strains) (Tumbarello et al. 2012; Wang et al. 2014). Tumbarello et al. (2012) found that susceptibility to ciprofloxacin is rare among MDR strains and was showed only by three (8.3%) out of 36 strains. Sohn et al. (2010) claim that a correlation between ciprofloxacin resistance and ESBLs production in P. mirabilis bacteremia may occur. This is in agreement with our results. We found significant differences in the susceptibility of ESBLs(+) and ESBLs(–) Proteus spp. strains to ciprofloxacin.
Sabbuba et al. (2003) investigated the genetic similarity of P. mirabilis strains isolated from hospitalized patients. They confirmed identical DNA profiles of the isolates from blood and urine of the same patient. In the present study, we report that strains isolated from blood samples of 10 out of 11 patients were identical with isolates cultured from other clinical specimens. The same strains were isolated from various specimens also a few weeks after the bacteremia, that it is because of the survival of Proteus spp. strains despite the antibiotic treatment. The decrease in the effectiveness of antibiotic treatment may be related to the biofilm forming ability.
In most cases we observed that strains from clinical samples collected from infections related to biomaterials were able to form stronger biofilms than identical strains from other samples collected from these patients. The presence of biomaterials in body sites probably promotes biofilm formation, which is in agreement with the results obtained by Lebeaux et al. (2014) and Di Domenico et al. (2017). We also reported that isolates collected from chronic wounds prior to bacteremia were able to form stronger biofilms than isolates cultured from blood samples.
Conclusions
In most cases bacteremia caused by Proteus spp. was secondary to urinary tract infection.
Most of the strains isolated from Proteus spp. BSIs were susceptible to piperacillin with tazobactam, third generation of cephalosporins, and amikacin.
Elderly patients have a higher risk of mortality in consequence of Proteus spp. BSI.
Most strains isolated from blood samples were identical to isolates cultured from other clinical specimens of the same patient.
Acknowledgments
This research was financially supported by the Nicolaus Copernicus University with funds from the research potential maintenance of the Department of Microbiology, DS-UPB no.933.
Footnotes
Conflict of interest
Author does not report any financial or personal connections with other persons or organizations, which might negatively affect the contents of this publication and/or claim authorship rights to this publication.
Literature
- Ahn JY, Ann HW, Jeon Y, Ahn MY, Oh DH, Kim YC, Kim EJ, Song JE, Jung IY, Kim MH, et al. . 2017. The impact of production of extended-spectrum β-lactamases on the 28-day mortality rate of patients with Proteus mirabilis bacteremia in Korea. BMC Infect Dis. 17:327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armbruster CE, Mobley HLY. 2012. Merging mythology and morphology: the multifaceted lifestyle of Proteus mirabilis. Nat Rev Microbiol. 10(11):743–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLSI 2009. Performance Standards for Antimicrobial Disk Susceptibility Tests 11th ed. CLSI standard M02. Wayne (USA): Clinical and Laboratory Standards Institute. [Google Scholar]
- Di Domenico EG, Farulla I, Prignano G, Gallo MT, Vespaziani M, Cavallo I, Sperduti I, Pontone M, Bordignon V, Cilli L, et al. . 2017. Biofilm is a major virulence determinant in bacterial colonization of chronic skin ulcers independently from the multidrug resistant phenotype. Internat J Mol Sci, 18(5):1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pilato V, Chiarelli A, Boinett CJ, Riccobono E, Harris SR, D’Andrea M, Thomson NR, Rossolini GM, Giani T. 2016. Complete genome sequence of the first KPC-type carbapenemase-positive Proteus mirabilis strain from a bloodstream infection. Genome Announc. 4(3):e00607–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drzewiecka D. 2016. Significance and roles of Proteus spp. bacteria in natural environments. Microb Ecol. 72(4):741–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endimiani A, Luzzaro F, Brigante G, Perilli M, Lombardi G, Amicosante G, Rossolini GM, Toniolo A. 2005. Proteus mirabilis bloodstream infections: risk factors and treatment outcome related to the expression of extended-spectrum beta-lactamases. Antimicrob Agents Chemother. 49:2598–2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EUCAST 2016. Breakpoint tables for interpretation of MICs and zone diameters, version 8.0 [Internet] London (UK): The European Committee on Antimicrobial Susceptibility Testing; [cited 2018 June 27]. Available from http://www.eucast.org/clinical_breakpoints/ [Google Scholar]
- Kim BN, Kim NJ, Kim MN, Kim YS, Woo JH, Ryu J. 2003. Bacteremia due to tribe Proteeae: a review of 132 cases during a decade (1991–2000). Scand J Infect Dis. 35(2):98–103. [DOI] [PubMed] [Google Scholar]
- Kwiecińska-Piróg J, Bogiel T, Gospodarek E. 2013a. Effects of ceftazidime and ciprofloxacin on biofilm formation in Proteus mirabilis rods. J Antibiot. 66(10):593–597. [DOI] [PubMed] [Google Scholar]
- Kwiecińska-Piróg J, Skowron K, Zniszczoł K, Gospodarek E. 2013b. The assessment of Proteus mirabilis susceptibility to ceftazidime and ciprofloxacin and the impact of these antibiotics at subinhibitory concentrations on Proteus mirabilis biofilms. Biomed Res Int. 2013:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laupland KB, Church DL. 2014. Populations-based epidemiology and microbiology of community-onset bloodstream infections. Clin Microbiol Rev. 27:647–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laupland KB, Parkins MD, Ross T, Pitout JDD. 2007. Population-based laboratory surveillance for tribe Proteeae isolates in a large Canadian health region. Clin Microbiol Infect. 13(7):683–688. [DOI] [PubMed] [Google Scholar]
- Lebeaux D, Ghigo JM, Beloin C. 2014. Biofilm-related infections: bridging the gap between clinical management and fundamental aspects of recalcitrance toward antibiotics. Microbiol Mol Biol Rev. 78(3):510–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hara CM, Brenner FW, Miller JM. 2000. Classification, identification and clinical significance of Proteus, Providencia, and Morganella. Clin Microbiol Rev. 13(4):534–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Różalski A, Kwil I, Torzewska A, Baranowska M, Stączek P. 2007. Proteus bacilli: features and virulence factors. Post Hig Med Dosw. 61:204–219. [PubMed] [Google Scholar]
- Sabbuba NA, Mahenthiralingam E, Stickler DJ. 2003. Molecular epidemiology of Proteus mirabilis infections of the catheterized urinary tract. J Clin Microbiol. 41(11):4961–4965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn KM, Kang ChI, Joo EJ, Ha YE, Chung DR, Peck KR, Lee NY, Song JH. 2011. Epidemiology of ciprofloxacin resistance and its relationship to extended-spectrum beta-lactamase production in Proteus mirabilis bacteriemia. Korean J Intern Med. 26:89–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumbarello M, Trecarichi EM, Fiori B, Losito AR, D’Inzeo T, Campana L, Ruggeri A, Di Meco E, Liberto E, Fadda G, et al. . 2012. Multidrug-Resistant Proteus mirabilis bloodstream infections: risk factors and outcomes. Antimicrob Agents Chemother. 56(6):3224–3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JT, Chen PC, Chang SC, Shiau YR, Wang HY, Lai JF, Huang IW, Tan MC, Lauderdale TL. 2014. TSAR Hospitals. Antimicrobial susceptibilities of Proteus mirabilis: a longitudinal nationwide study from the Taiwan surveillance of antimicrobial resistance (TSAR) program. BMC Infec Dis. 14(1):486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanakunakorn C, Perni SC. 1994. Proteus mirabilis bacteremia: a review of 176 cases during 1980–1992. Scand J Infect Dis. 26:361–367. [DOI] [PubMed] [Google Scholar]