Abstract
Natural products such as essential oils (EOs) are secondary metabolites that can be obtained from either plant or animal sources or produced by microorganisms. Much attention has been given to exploring the use of secondary metabolites as natural antibacterial agents. This study investigates the antibacterial activity and mechanism of β-caryophyllene, a compound that can be found in various EOs, against Bacillus cereus. The minimum inhibitory concentration of β-caryophyllene against B. cereus was 2.5% (v/v), whereas killing kinetics of β-caryophyllene at minimum inhibitory concentration recorded complete bactericidal activity within 2 hours. Zeta-potential measurement in the cells treated with half the minimum inhibitory concentration of β-caryophyllene at 1.25% (v/v) showed an increase in the membrane permeability surface charge to –3.98 mV, compared to untreated cells (–5.46 mV). Intracellular contents leakage of UV-absorbing materials was detected in the cells treated with β-caryophyllene. Additionally, β-caryophyllene does not interfere with the efflux activity of B. cereus via the ethidium bromide influx/efflux activity. The results revealed that β-caryophyllene was able to alter membrane permeability and integrity of B. cereus, leading to membrane damage and intracellular content leakage, which eventually caused cell death.
Key words: antibacterial, β-caryophyllene, intracellular leakage, membrane damage, zeta potential
Introduction
Bacillus cereus is a Gram-positive, spore-forming, a rod-shaped bacterium present on almost all surfaces such as food, soil, and human skin (Glasset et al. 2018). Due to its universal distribution in the natural environment, this bacterium constitutes a part of the permanent microbiota in a variety of raw foods such as cereal grains and products, milk and dairy products, fruits, vegetables, spices, and instant meals and products (Berthold-Pluta et al. 2019). Bacillus species are widely known as spoilage organisms in the food industries. It has been estimated that Bacillus species have caused serious economic losses in the dairy industry up to 30% due to spoilage and lowered product quality (Majed et al. 2016). B. cereus is one of the most common agents of food poisoning outbreaks in which the symptoms can either be diarrheal or emetic.
In more severe cases outside of the gastrointestinal tract, B. cereus causes nosocomial systemic infections in immunocompromised patients including bacteremia, cerebral abscess, endocarditis meningitis, pneumonia, and septicemia (Sankararaman and Velayuthan 2013). To better manage the risks of food spoilage, which caused significant economic losses in the food industry, there is the need to search not only for new compounds with antimicrobial activity but more importantly of low toxicity. The compound can then be used in combination with conventional antibiotics as means to enhance the effectiveness of the antibiotics.
Currently, natural products such as essential oils (EOs) obtained from plant or animal sources as well as those produced by microorganisms are being studied extensively for their application as alternative antibacterial agents (Yap et al. 2017; Yang et al. 2018; Yang et al. 2019). EOs can be obtained from various plant parts such as flowers, seeds, buds, leaves, and bark. EOs are made up of a variety of constituents, including hemiterpenoids, monoterpenoids, sesquiterpenoids, diterpenoids, sesterpenoids, triterpenoids, tetrapenoids, and polyterpenoids. Each of these constituents has a different number of isoprene units, forming an aromatic ring structure. Depending on the characteristic of these isoprenes, the EO constituent can be either aromatic or non-aromatic compounds (Trombetta et al. 2005; Gallucci et al. 2009; Morsy 2017; Mahizan et al. 2019). Terpenoids are derived from terpenes with the modification through the addition or removal of functional groups (Mahizan et al. 2019). Terpenes can have different chemical functional groups including alcohol, aldehyde, phenol, ketone, ether, and hydrocarbon groups (Guimarães et al. 2019). These terpenes and terpenoids are secondary metabolites, which are often used as a functional food, food additives, medicines, nutritional supplements, and in the manufacture of cosmetics because they exhibit antibacterial, antioxidant, and anti-inflammatory properties (Zengin and Baysal 2014). Although it has been reported that the antibacterial activity of EOs can be attributed to the presence of terpenes and terpenoids (Nazzaro et al. 2013), and some non-terpenic compounds (Valdivieso-Ugarte et al. 2019), the mode of action of these compounds remain largely unknown. Commonly found terpenes and terpenoids in EOs include α-pinene, limonene, linalool, and β-caryophyllene (BCP) (Hodgson 2017).
BCP is a bicyclic sesquiterpene, with a cyclobutane ring, usually found in EOs of oregano, cinnamon, and black pepper (Gertsch et al. 2008). Studies have shown that BCP has great potential as an antimicrobial agent in the food industry, due to its low toxicity (Pieri et al. 2016). BCP has been known for its anticancer, antioxidant, and anti-inflammatory attributes (Dahham et al. 2015). Previously, studies conducted in the plant extracts containing BCP from Lantana sp., Lippia gracillis, Spiranthera odoratissima, Syzygium cumini, Thymus kotschyanus, and Vernonia remotiflorae V. brasiliana have shown their antimicrobial activity (Pieri et al. 2016). However, there has been paucity in information about the mechanisms of the reported antibacterial activity of BCP. Therefore, this study explores the use of BCP, as an antibacterial agent by evaluating the antibacterial activity and its mode of action against B. cereus.
Experimental
Materials and Methods
Chemicals. β-caryophyllene (BCP) (≥ 80% purity, food-grade), used in this study, was purchased from Sigma-Aldrich, USA. Mueller-Hinton broth (MHB) was purchased from Thermo Fisher Scientific, UK.
Bacterial strain and growth conditions. B. cereus ATCC 14579 was provided by UCSI University and cultured on Luria Bertani (LB) agar. A single colony was inoculated into MHB (pH 7.3 ± 0.1) and incubated overnight at 37°C, with continuous shaking at 200 rev min–1. All test conditions were carried out according to the guidelines described in the Clinical and Laboratory Standards Institute M100-S21 guidelines (CLSI 2016).
Resazurin microplate assay. This assay was performed to determine the minimum inhibitory concentration (MIC) of BCP against B. cereus via the broth microdilution method. This assay was carried out according to Yang et al. (2018) with slight modification. Tween 80 with the final concentration of 10% was incorporated into MHB to enhance the solubility of BCP. Resazurin at a final concentration of 0.03% was used to aid the visualization. Two-fold dilutions were performed in a 96-well plate. Each well consisted of 50 μl of BCP or antibiotic, 40 μl of a bacterial suspension at approximately 1 × 105 CFU ml–1, and 10 μl of 0.03% resazurin. The 96-well plate was incubated at 37°C at 200 rev min–1 for 20 hours. The MICs of BCP and antibiotics were determined qualitatively based on the observation of the color change of resazurin. The antibiotics served as positive control and this assay was carried out in triplicate.
Time-kill analysis. An inoculum of 1 × 105 CFU/ml was used in the time-kill analysis via the enumeration of viable colony-forming units. B. cereus was treated with BCP at a concentration equal to MIC (MIC value 2.5%, v/v) whereas the control was untreated. This analysis consisted of the untreated samples as (inoculum with MHB supplemented with 10% of Tween 80 to enhance BCP solubility) and the samples treated with BCP. Each treatment had a final volume of 20 ml. Samples were incubated at 37°C with continuous shaking at 200 rev min–1 for 24 hours. Viable counting was performed at 0, 30, 60, 120, 180, and 240 min and subsequently every 4 hours. The first viable counting time point was right after inoculation, and the assay was performed for 24 hours. Samples were serially diluted with 0.85% (w/v) sodium chloride, plated onto LB agar and incubated at 37°C for 16 hours. This analysis was performed in triplicate. In the subsequent assays, cells were treated with BCP at a concentration equal to half of the MIC (1.25%, v/v) to prevent the total killing of the bacteria.
Zeta potential measurement. The surface charge of BCP-treated and untreated (control) B. cereus was determined using a Zetasizer Nano ZS instrument (Malvern Instruments, Malvern, UK). Cells treatment time was based on time-kill analysis (2h) with the use of BCP at a concentration of 1.25% (v/v). The cells treated were washed with saline solution for at least three times and resuspended in the fresh saline solution for the zeta potential measurement. These experiments were carried out in triplicate according to Yang et al. (2018).
Measurement of UV-absorbing materials. Measurement was performed as described by Liu et al. (2016) with slight modification (2 hours treatment time). The untreated (control) and BCP-treated (1.25%, v/v) cell suspensions were incubated at 37°C with continuous shaking at 200 rev min–1 for 2 h. Cells were harvested by centrifugation (8000 g, 10 min, 4°C). The absorbance of the supernatants was taken at 260 nm and 280 nm to measure the content of nucleic acids and proteins, respectively, in the extracellular environment. These experiments were carried out in triplicate.
Ethidium bromide influx/efflux assay. This assay was performed as described by Viveiros et al. (2010) with slight modification, as detailed. The ethidium bromide (EtBr) accumulation (influx) and efflux were assessed by a Tecan microplate reader (Tecan Trading AG, Switzerland). During accumulation assay, B. cereus was grown in MHB with continuous shaking at 37°C until the optical density value of 0.6 was obtained. The cell pellet was washed, resuspended, and adjusted with 0.85% (w/v) sodium chloride to a final value of 0.3 at OD600 nm. EtBr was added to yield a final concentration of 1 mg/l. After the addition of EtBr, 1.25% (v/v) BCP was added. With the excitation wavelength of 530 nm and detection wavelength of 585 nm, the readings were taken at intervals of 5 min for 60 min. The supernatant was removed and resuspended in 0.85% (w/v) sodium chloride solution in the efflux assay. Then, 0.6% glucose was added with or without BCP to the cells. The fluorescent readings were measured every 5 min for 60 min. These experiments were performed in triplicate.
Statistical analysis. Statistical analysis was carried out using the Student’s t-test. A p < 0.05 was considered significant.
Results
Resazurin microplate assay. This assay was performed to determine the susceptibility of B. cereus to BCP. This method shows the color change from blue to pink-purple when the bacteria are growing. Based on the antimicrobial screening, BCP exhibited antibacterial activity against B. cereus and the MIC value was equal to 1.25% (v/v) (Supplementary Fig. S1).
Time-kill analysis. Time-kill analysis of BCP against B. cereus was performed to determine the killing kinetics of BCP and the optimum treatment time for the subsequent experiments. A complete killing of B. cereus treated with BCP at a concentration of 2.5% (v/v) was observed after 2 h whereas for the cells treated with half of the BCP MIC (1.25%, v/v) the suppression of cell growth was observed when compared to the untreated cells (control) (Fig. 1). Hence, a period of 2 h was used as the standard treatment time in subsequent assays.
Fig. 1.
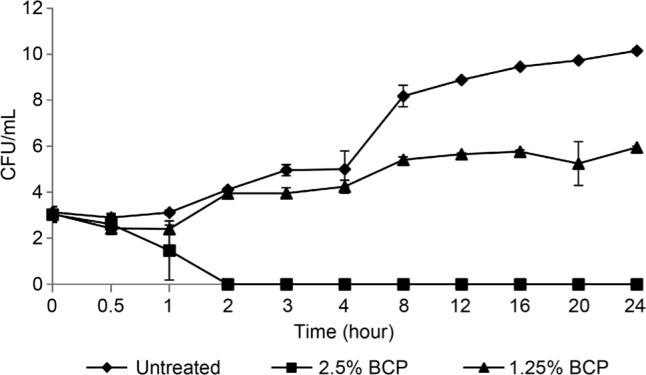
Time-kill analysis of B. cereus treated with BCP. At MIC 2.5% BCP (v/v), the bacteria were killed within 2 hours. Cells treated at half MIC showed a suppressed and slower growth rate than the untreated cells.
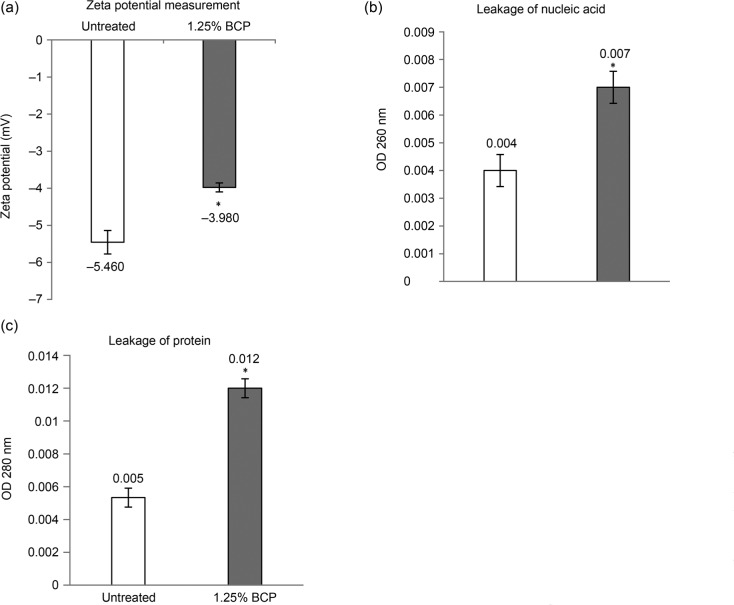
Zeta-potential measurement. Following the 2 h of treatment, based on time-kill analysis, the measurements were carried out to evaluate the surface charge of the bacterial cells by detecting the mobility of bacterial cells in the presence of an electrophoretic force. Untreated (control) B. cereus, under normal conditions, carried a negative value of –5.46 mV while cells treated with 1.25% (v/v) BCP for 2 h had a less negative value of –3.98 mV, compared to the untreated cells (Fig. 2A).
Fig. 2.
Assays performed to evaluate the effects of BCP on the membrane surface charge and integrity of B. cereus. (A) Zeta-potential value (mV) of untreated (–5.460 mV) and BCP-treated cells (–3.980 mV); (B) Nucleic acid content of untreated and 1.25% (v/v) BCP-treated B. cereus in the extracellular environment; and (C) Protein content of untreated and 1.25% (v/v) BCP-treated B. cereus in the extracellular environment. Results were presented in mean ± SD for triplicates and considered as significant when * p < 0.050.
Measurement of UV-absorbing materials. The recording of the absorbance values of the bacterial supernatants can indicate if there has been leakage of intracellular materials due to the non-selective pore formation (Liu et al. 2016). Based on the results obtained (Fig. 2B, 2C), the supernatants of BCP-treated cells at 260 nm (nucleic acids) and 280 nm (proteins) had an absorbance higher than untreated cells (control). The absorbance value for extracellular nucleic acids in the supernatants of BCP-treated cells at a concentration of 1.25% (v/v) was 0.007, a slightly higher than for the untreated cells, where the absorbance value was equal to 0.004. For proteins, the absorbance value in the supernatant of BCP-treated cells at a concentration of 1.25% (v/v) was 0.01167, whereas in untreated cells the absorbance value was 0.0053.
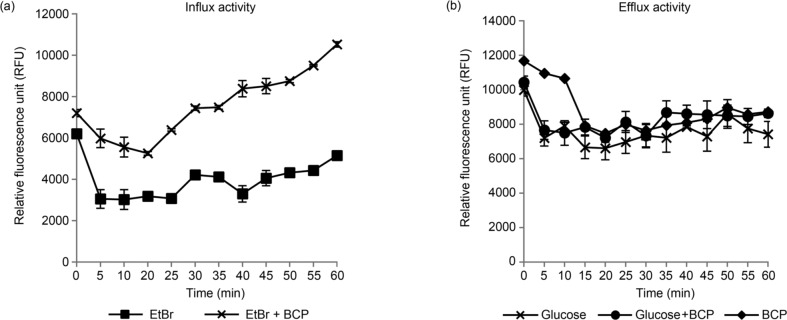
Ethidium bromide influx/efflux assay. To further determine the potential ability of BCP to interfere in an efflux, the efflux activity of B. cereus was analyzed using EtBr, a known efflux pump substrate. The cells were treated with EtBr and 1.25% (v/v) BCP to evaluate the accumulation of EtBr. Based on the fluorescence reading, the cells treated with BCP (10525 RFU) showed increased by two folds the EtBr accumulation level at the 60th min when compared to the untreated cells (5151 RFU) (Fig. 3A). During an efflux assay, glucose was added to act as an energy source to enhance active efflux and reenergize the cells (Machado et al. 2017). The cells treated with BCP showed only a slight reduction in fluorescence reading in the efflux assay, with or without glucose (Fig. 3B).
Fig. 3.
The influx and efflux of EtBr in B. cereus (A) EtBr influx assay with untreated and BCP-treated cells exposed to 1 mg/l of EtBr for 60 minutes and (B) EtBr efflux assay with the cells treated with glucose or BCP only and in a combination of glucose and BCP.
Discussion
Based on the resazurin microplate assay, the MIC value of BCP against B. cereus was 2.5% (v/v). The mode of antibacterial action of BCP was classified as bactericidal based on the time-kill analysis where the reduction of log number of the bacteria was observed. The compounds of antimicrobial activity are categorized as bactericidal when a reduction of ≥ 3 log10 in CFU/ml whereas reduction of < 3 log10 in CFU/ml is bacteriostatic relative to the initial inoculum concentration (Belley et al. 2008).
To date, the exact underlying antimicrobial mechanisms of BCP remained unknown. Nonetheless, owing to its nature of being one of the major constituents in many EOs, we postulated that BCP might involve altering of the permeability of the bacterial membrane. Previously, several studies have reported the involvement of EOs in the altering the bacterial membrane permeability and intracellular leakage (Trombetta et al. 2005; Yap et al. 2013; Yang et al. 2017; Yang et al. 2019). Hence, as a proof of concept, the measurement of zeta potential and UV-absorbing materials as well as the efflux pump assays were carried out on B. cereus treated with BCP to evaluate the mode of action of BCP.
The zeta potential value provides information about the membrane potential, which can be related to the metabolic state of bacteria whereby at higher growth rates, zeta potential values are more negative (Yang et al. 2018). The alteration in the zeta potential value, becoming less negative, together with the increased surface permeability eventually leads to decreased cell viability (Yang et al. 2017). Under normal physiological conditions, the net negative bacterial surface charge is balanced by positively charged counter ions in the surrounding media. Based on the result obtained, the zeta potential value of the treated B. cereus became less negative (Fig. 2A). This may indicate the interaction between BCP and bacterial surface that was governed by electrostatic interactions. Therefore, the zeta potential of the cell decreased, consequently destabilizing the cell surface that might lead to the membrane damage.
The absorbance value at 280 nm also showed an increase in the cells treated compared to the untreated cells. The absorbance values of UV-absorbing materials may indicate if there is an intracellular material leakage due to the non-selective pore formation. It was evident that intracellular nucleic acids and proteins were released into the extracellular environment following the exposure of bacterial cells to BCP, suggesting there was the non-selective pore formation inducing leakage of intracellular materials into the extracellular environment (Liu et al. 2016).
During the evaluation of the influx/efflux activity of B. cereus with ethidium bromide, the cells treated with BCP showed an increase in the EtBr accumulation level as compared to the untreated cells during their influx activities, while efflux activities showed a slight reduction of fluorescence readings for all the treatments. This indicated that BCP did not interfere with the efflux pumps. Although there was the influx of EtBr during the accumulation assay in the cells treated with BCP, the influx could be due to the membrane damage caused by BCP, rather than the inhibition of efflux pumps of the bacteria. It has been suggested that BCP altered the membrane permeability of B. cereus (by a change in surface charge of the cells), causing possible damage in the cell membrane. When the cell membrane integrity was lost, the essential role of cell membrane acting as a barrier to molecules was adversely affected, leading to an influx of EtBr into the cell.
The quantitative assessment of the altered membrane permeability and integrity via zeta potential and UV-absorbing materials measurement confirmed that BCP alters the membrane permeability and may cause non-selective pore formation on the cell membranes. The non-selective pore formation induced the leakage of intracellular contents into the extracellular environment, disrupting the cell membrane integrity. However, based on an efflux activity evaluation, BCP did not interfere with the efflux activity B. cereus.
Conclusion
In conclusion, the proposed mode of action of BCP is through altering the bacterial membrane permeability and causing non-selective pore formation. This induces the intracellular content leakage leading to damage and loss of the membrane integrity, and may eventually lead to cell death. BCP is a plant-derived natural product, which could act as an alternative antibacterial agent or adjuvant in combating antibiotic resistance. A new strategy such as combinatorial treatment using natural compound and antibiotic, disruptive metals or nanotechnologies has recently gained much attention in tackling antimicrobial resistance, especially in multi-resistant strains of bacteria (Kwiatkowski et al. 2017; Low et al. 2017; Moo et al. 2019). The results of this work could be extrapolated to a combinatorial treatment and also at the molecular level using high-throughput screening study before the application of BCP for treatment regimes.
Supplementary materials are available on the journal’s website.
Acknowledgments
Authors would like to thank all the members of Floral Biotechnology Laboratory; UCSI University, Malaysia for providing B. cereus. This work was funded by the Higher Colleges of Technology Interdisciplinary Research Grant 1319.
Footnotes
Conflict of interest
The authors do not report any financial or personal connections with other persons or organizations, which might negatively affect the contents of this publication and/or claim authorship rights to this publication.
Literature
- Belley A, Neesham-Grenon E, Arhin FF, McKay GA, Parr TR Jr, Moeck G.. Assessment by time-kill methodology of the synergistic effects of oritavancin in combination with other antimicrobial agents against Staphylococcus aureus. Antimicrob Agents Chemother. 2008. Oct 01;52(10):3820–3822. 10.1128/AAC.00361-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthold-Pluta A, Pluta A, Garbowska M, Stefańska I.. Prevalence and toxicity characterization of Bacillus cereus in food products from Poland. Foods. 2019. Jul 19;8(7):269 10.3390/foods8070269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLSI Performance standards for antimicrobial susceptibility testing. CLSI supplement M100S. Wayne (USA): Clinical and Laboratory Standards Institute; 2016. [Google Scholar]
- Dahham S, Tabana Y, Iqbal M, Ahamed M, Ezzat M, Majid A, Majid A.. The anticancer, antioxidant and antimicrobial properties of the sesquiterpene β-caryophyllene from the essential oil of Aquilaria crassna. Molecules. 2015. Jun 26;20(7):11808–11829. 10.3390/molecules200711808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gertsch J, Leonti M, Raduner S, Racz I, Chen JZ, Xie XQ, Altmann KH, Karsak M, Zimmer A.. Beta-caryophyllene is a dietary cannabinoid. Proc Natl Acad Sci USA. 2008. Jul 01;105(26): 9099–9104. 10.1073/pnas.0803601105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimarães AC, Meireles LM, Lemos MF, Guimarães MCC, Endringer DC, Fronza M, Scherer R.. Antibacterial activity of terpenes and terpenoids present in essential oils. Molecules. 2019. Jul 05;24(13):2471 10.3390/molecules24132471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson A. Terpenes in essential oils. VUV Analytics. [Internet]. 2017. [cited 2019 Feb 11]. Available from https://vuvanalytics.com/knowledge-base/better-living-through-flavor-chemistry-part-3-essential-oils-and-their-terpenes/
- Kwiatkowski P, Mnichowska-Polanowska M, Pruss A, Masiuk H, Dzięcioł M, Giedrys-Kalemba S, Sienkiewicz M.. The effect of fennel essential oil in combination with antibiotics on Staphylococcus aureus strains isolated from carriers. Burns. 2017. Nov;43(7): 1544–1551. 10.1016/j.burns.2017.04.014 [DOI] [PubMed] [Google Scholar]
- Liu G, Song Z, Yang X, Gao Y, Wang C, Sun B.. Antibacterial mechanism of bifidocin A, a novel broad-spectrum bacteriocin produced by Bifidobacterium animalis BB04. Food Control. 2016. Apr;62:309–316. 10.1016/j.foodcont.2015.10.033 [DOI] [Google Scholar]
- Low WL, Kenward K, Britland ST, Amin MCIM, Martin C.. Essential oils and metal ions as alternative antimicrobial agents: a focus on tea tree oil and silver. Int Wound J. 2017. Apr;14(2): 369–384. 10.1111/iwj.12611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado D, Fernandes L, Costa SS, Cannalire R, Manfroni G, Tabarrini O, Couto I, Sabatini S, Viveiros M.. Mode of action of the 2-phenylquinoline efflux inhibitor PQQ4R against Escherichia coli. PeerJ. 2017. Apr 26;5:e3168 10.7717/peerj.3168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahizan NA, Yang SK, Moo CL, Song AAL, Chong CM, Chong CW, Abushelaibi A, Lim SHE, Lai KS.. Terpene derivatives as a potential agent against antimicrobial resistance (AMR) pathogens. Molecules. 2019. Jul 19;24(14):2631 10.3390/molecules24142631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majed R, Faille C, Kallassy M, Gohar M.. Bacillus cereus biofilmssame, only different. Front Microbiol. 2016. Jul 07;7:1054 10.3389/fmicb.2016.01054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moo CL, Yang SK, Yusoff K, Ajat M, Thomas W, Abushelaibi A, Lim SHE, Lai KS.. Mechanisms of antimicrobial resistance (AMR) and alternative approaches to overcome AMR. Curr Drug Discov Technol. 2019. Mar 04;16 10.2174/1570163816666190304122219 [DOI] [PubMed] [Google Scholar]
- Morsy NFS. Chemical structure, quality indices and bioactivity of essential oil constituents In: El-Shemy HA, editor. Active ingredients from aromatic and medicinal plants. London (UK): InTech; 2017. p. 175–206. [Google Scholar]
- Nazzaro F, Fratianni F, De Martino L, Coppola R, De Feo V.. Effect of essential oils on pathogenic bacteria. Pharmaceuticals. 2013. Nov 25;6(12):1451–1474. 10.3390/ph6121451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieri FA, Souza MCC, Vermelho LLR, Vermelho MLR, Perciano PG, Vargas FS, Borges APB, da Veiga-Junior VF, Moreira MAS.. Use of β-caryophyllene to combat bacterial dental plaque formation in dogs. BMC Vet Res. 2016. Dec;12(1):216 10.1186/s12917-016-0842-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankararaman S, Velayuthan S.. Bacillus Cereus. Pediatr Rev. 2013. Apr 01;34(4):196–197. 10.1542/pir.34-4-196 [DOI] [PubMed] [Google Scholar]
- Trombetta D, Castelli F, Sarpietro MG, Venuti V, Cristani M, Daniele C, Saija A, Mazzanti G, Bisignano G.. Mechanisms of antibacterial action of three monoterpenes. Antimicrob Agents Chemother. 2005. Jun 01;49(6):2474–2478. 10.1128/AAC.49.6.2474-2478.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdivieso-Ugarte M, Gomez-Llorente C, Plaza-Díaz J, Gil Á.. Antimicrobial, antioxidant, and immunomodulatory properties of essential oils: A systematic review. Nutrients. 2019. Nov 15;11(11): 2786 10.3390/nu11112786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viveiros M, Martins M, Couto I.. Evaluation of efflux activity of bacteria by a semi-automated fluorometric system In: Gillespie S., McHugh T, editors. Antibiotic resistance protocols. Totowa (USA): Humana Press; 2010;642:159–172. [DOI] [PubMed] [Google Scholar]
- Yang SK, Yusoff K, Warren T, Akseer R, Alhosani MS, Abushelaibi A, Lim SHE, Lai KS.. Lavender essential oil induces oxidative stress which modifies the bacterial membrane permeability of carbapenemase producing Klebsiella pneumoniae. Sci Rep. 2020. Jan 21; 10:819 10.1038/s41598-019-55601-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SK, Yap PSX, Krishnan T, Yusoff K, Chan KG, Yap WS, Lai KS, Lim SHE.. Mode of action: synergistic interaction of peppermint (Mentha piperita L. Carl) essential oil and meropenem against plasmid-mediated resistant E. coli. Rec Nat Prod. 2018. Jun 30;12(6):582–594. 10.25135/rnp.59.17.12.078 [DOI] [Google Scholar]
- Yang SK, Yusoff K, Ajat M, Thomas W, Abushelaibi A, Akseer R, Lim SHE, Lai KS.. Disruption of KPC-producing Klebsiella pneumoniae membrane via induction of oxidative stress by cinnamon bark (Cinnamomum verum J. Presl) essential oil. PLoS One. 2019. Apr 2;14(4):e0214326 10.1371/journal.pone.0214326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap PSX, Lim SHE, Hu CP, Yiap BC.. Combination of essential oils and antibiotics reduce antibiotic resistance in plasmid-conferred multidrug resistant bacteria. Phytomedicine. 2013. Jun;20(8–9): 710–713. 10.1016/j.phymed.2013.02.013 [DOI] [PubMed] [Google Scholar]
- Yap PSX, Yang SK, Lai KS, Lim SHE.. Essential oils: The ultimate solution to antimicrobial resistance in Escherichia coli? In: Samie Amidou, editor. Escherichia coli – Recent advances on physiology, pathogenesis and biotechnological applications. London (UK): InTech; 2017. p. 299–313. [Google Scholar]
- Zengin H, Baysal A.. Antibacterial and antioxidant activity of essential oil terpenes against pathogenic and spoilage-forming bacteria and cell structure-activity relationships evaluated by SEM microscopy. Molecules. 2014. Nov 03;19(11):17773–17798. 10.3390/molecules191117773 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary materials are available on the journal’s website.