Abstract
Prebiotics inducing the growth or activity of beneficial intestinal bacteria – probiotics producing short-chain fatty acids (SCFA) have lately received wide recognition for their beneficial influence on host intestinal microbiota and metabolic health. Some non-starch polysaccharides (NSP) are defined as prebiotics and oats being one of richest sources of NSP in grains are considered as potentially having prebiotic effect. However, information on fermentation of specific NSP of oats is limited. Moreover, bacterial cross-feeding interactions in which fermentation of prebiotics is involved is poorly characterized. Here, we report the exploration of new candidates for the syntrophic bacterial interactions and fermentability of oat non-starch polysaccharides (NSP). The results obtained by differentiating composition, viscosity and concentration of oats NSP in fermentation medium showed that Bacillus licheniformis pre-digests oat NSP, degrades high viscosity of oat β-glucan and makes hemicellulose easier to access for other bacteria. Because of fermentation, B. licheniformis produces lactic and succinic acids, which further can be used by other bacteria for cross-feeding and SCFA production.
Key words: Oat NSP, prebiotics, B. licheniformis, B. ovatus, C. butyricum
Introduction
Oats are rich source of non-starch polysaccharides (NSP) (4.5 g/100 g); furthermore, 58% of the total NSP are soluble, which is higher than in all the other cereals (Miller and Fulcher 2011). The main component of soluble NSP of oats is (1/3), (1/4)-b-D-glucan (referred as β-glucan). Oats contains 3.2–6.8% β-glucan, which varies with cultivar and environmental effects (Holguin-Acun et al. 2011).
The largest tissue in all of the cereal grains is the endosperm, which may constitute up to 70% of the weight of the mature oat groat (Miller and Fulcher 2011). The fractionation characteristics of isolated endosperm cell wall suggested a layer model: a relatively thin outer layer, consisting of an insoluble polysaccharide skeleton (mostly cellulose, glucomannan, and arabinoxylan) and matrix polysaccharides (β-glucan and arabinoxylan), and also a large inner layer of soluble polysaccharides (mostly β-glucan and small amount of arabinoxylan (Miller and Fulcher 2011).
The cell walls of subaleurone layer of some oat varieties can be very thick. This is an important structural feature allowing production of bran fractions with high β-glucan content (Kaletunc and Breslauer 2003).
Some NSP are defined as prebiotics, which are non-viable food ingredients that can be fermented by specific enzymes derived from gut anaerobic bacteria (Roberfroid 2007). As a result, prebiotics induce the growth or activity of beneficial intestinal bacteria – probiotics, which produce short-chain fatty acids (SCFA). The SCFA are natural ligands for free fatty acid receptor 2 and 3 (FFAR 2/3), found in enteroendocrine and immune cells (Morrison and Preston 2016). Consequently, probiotics and prebiotics are widely used in human and animal nutrition because they beneficially influence the host intestinal microbiota and metabolic health.
Most probiotic strains belong to the genus Lactobacillus or Bifidobacterium, with only a few belonging to Enterococcus, Escherichia, or Streptococcus (Ulsemer et al. 2012).
The purpose of the current study was to characterize the syntrophic bacterial interaction and fermentability of both types of oat NSP: with β-glucan (BG25) or without β-glucan (BG0) by less studied bacterial consortia. New candidates for the probiotics: Bacillus licheniformis (McFarlin et al. 2017), Clostridium butyricum (Kanai et al. 2015) and Bacteroides ovatus (Martín et al. 2013) have been explored.
C. butyricum is a common human and animal strictly anaerobe, Gram-positive, gut commensal bacterium frequently found in the environment (Montoya et al. 2001). Some Clostridium strains can secrete α- or β-glucanase to degrade polysaccharides (Montoya et al. 2001; Nakajima et al. 2002). Recent studies in animal diabetic models showed positive effects of C. butyricum (Sun et al. 2016; Jia, et al. 2017), which could act as a beneficial probiotic for prevention and treatment of hyperglycaemia and associated metabolic dysfunction (Jia et al. 2017).
B. ovatus is also commonly found in mammalian intestine (Rajilić-Stojanović and de Vos 2014), the genus Bacteroides accounts for up to 40% of human colonic microbiota (Hong et al. 2008).
Most recent study discovered a naturally evolved cooperation between B. ovatus and B. vulgatus within the mammalian intestinal microbiota (Rakoff-Nahoum et al. 2016). A cross-feeding enzymes’ system in the gut symbiont B. ovatus digests polysaccharides at a cost to itself but at a benefit to another species, such as B. vulgatus (Rakoff-Nahoum et al. 2016). Furthermore, recent genetically engineered B. ovatus showed a significant prophylactic effect, limiting the development of intestinal inflammation both clinically and histopathologically (Hamady 2013).
In other studies in humans, the probiotic potential of B. licheniformis was identified, which was safe and effective for the treatment of diarrhea (Heo et al. 2014). Moreover, a short-term complex probiotic supplementation including B. licheniformis spores resulted in decreasing of dietary endotoxemia, the level of triglycerides, and potentially systemic inflammation (McFarlin et al. 2017). However, authors do not explain the nature of these results, where microbiota and SCFA as ligands for FFAR molecules might be involved.
Although it was reported that spores of B. licheniformis cause spoilage of milk, dairy products and bread (Pepe et al. 2003; Gopal et al. 2015), the latest study has shown that antimicrobial substances secreted by B. licheniformis may serve as useful templates for the development of food preservative agents of natural origin (Arbsuwan et al. 2017).
From the foregoing, it is clear that in the last decade interest in a new probiotic and prebiotic supplementation of human diets has increased due to its co-metabolic interactions between gut microbiota and the host. However, knowledge of the syntrophic fermentation of various oat fractions is still limited, and therefore the present study for the first time considered the fermentability of oat NSP and SCFA production by less studied bacterial consortia.
Experimental
Materials and Methods
Plant Material and Sample Preparation. Oat (Avena sativa) flakes were obtained from the “Rigas Dzirnavnieks” Ltd., Latvia. The flakes were milled and sieved through the 0.5 mm sieve. The milled flakes (50 g) were added to glass beaker filled with 500 ml of water (7°C) and mixed by an automatic mixer (Overhead Stirrer Multi Mixer MM-1000, Biosan, Latvia) for 30 min at 120 rpm. The solution was washed through a 160-μm sieve, then residue on sieve was added into beaker filled with 500 ml to repeat washing-sieving process, and to remove any water-soluble compounds (starch, proteins, low molecular weight β-glucan). The residue on sieve was weighted and dried for 4 hours at 60°C in an oven.
To prepare the sample free of β-glucan (BG0), the above-mentioned ready-made 50 g of substrate with a moisture of 7–9%, containing 25% (1,3;1,4)-β-D-glucan (BG25) was dissolved in 200 ml of water. Enzymatic hydrolysis of β-D-glucan to glucose and low molecular weight particles was performed using 0.1% β-glucanase with enzymatic activity ≥ 2 000 000 U/ml (Sunson Industry Group Co. Ltd., China). Hydrolysis was carried out for 90 minutes at 48–52°C, pH 5.3–5.5.
The resulting hydrolysate was cooled to a temperature of 20–25°C. The insoluble fraction of hydrolysate and supernatant containing β-D-glucan were separated by decantation process. The insoluble part of hydrolysate was centrifuged at 5000 rpm for 5 minutes (Thermo Scientific Heraeus X3). The resulting insoluble fraction of substrate was added to glass beaker filled with 500 ml of water (7°C) and washed through a 160 μm sieve, then washing-sieving process was repeated. Residue was dried at a temperature of 45–50°C until moisture of 7–9%. The resulting substrate further was used as BG0 sample.
Both oat substrates were analyzed for β-glucan content, which was determined by the specific enzymatic method (McCleary and Glennie-Holmes 1985) using a mixed-glucan linkage kit (Megazyme Int. Ireland Ltd. Wicklow, Irland).
Viscosity Measurement. Viscosity of the solutions containing 1% β-glucan and 0% β-glucan were measured before and after batch pasteurization process using a digital rotational viscometer equipped with Pt100 temperature sensor (Thermo Scientific HAAKE Rotational 7 Plus Viscometer). L1 spindle was used to measure dynamic of viscosity. 2.5 grams of BG25 and 2 grams of BG0 were separately mixed with 50 ml of water (20°C) at the room temperature (23°C). Solutions were placed into 100 ml 5 cm diameter glass beakers and gently mixed for 30 seconds, and then heated to 60°C.
Since the viscosity of the solution containing 1% β-glucan varied with its temperature (heating/cooling), different calibration geometries were chosen. After heating it up to 60°C and during its cooling to 40°C 30 rpm/range 200 mPas was chosen. Then, the viscosity reached upper viscosity limit of the selected calibration geometry, the calibration settings were changed to 12 rpm/500 mPas. After pasteurization, calibration parameters of 100 rpm/60 mPas were selected.
To measure the viscosity of solutions containing BG0 prior to the pasteurization process and, the viscosity of solutions containing BG0 and BG25 after the pasteurization process, calibration settings of 100 rpm/60 mPas were selected. All viscosity measurements were conducted in triplicates.
Growth Medium. Bacterial fermentation of oat NSP was conducted in a complex growth medium, which contained 10 g/l beef extract (BBLTM beef extract powder, Becton Dickinson and Co.), 10 g/l peptone (BactoTM peptone, Becton Dickinson and Co.), 5 g/l sodium chloride, 3 g/l sodium citrate, 3 g/l yeast extract (Powdered purified yeast autolysate for bacteriology, Bio-Rad, France), 0.25 g/l L-cysteine (sterile filtered and added after a process of batch pasteurization of the growth medium), all dissolved in distilled water. BG25 as carbon source was added into borosilicate jar with 50 ml growth medium to obtain 1% β-glucan concentration (2.5 g/50 ml). Such growth medium was prepared separately for C. butyricum and B. ovatus. The same procedure was carried out for the BG0 (2 g/50 ml).
To achieve the required level of sterility, a low temperature batch pasteurization method was used. Tightly sealed bottled samples were pasteurized in a water batch (fryer filled with water) at 80°C for 30 min and then stored at room temperature for repeated continuous process for 7 days.
To determine the level of sterility/contamination of growth medium after pasteurization, 0.1 ml of samples were inoculated on R2A medium (Becton & Dickinson). After 48 hours, bacterial colonies were identified using a commercial biochemical identification kit (BD Diagnostic Systems, Sparks, MD). An aliquot of 1 ml samples of media was transferred to Eppendorf centrifuge tubes and centrifuged at 5000 rpm for 4 minutes (Sigma, Germany). A 0.5 ml of supernatant was taken and diluted with 0.5 ml acetonitrile (ratio 1:1) and frozen at –18°C for further SCFA detection.
The initial pH of the growth medium and pH after 24, 72 and 96 hours of the incubation period of bacteria was measured using a pH meter (AD I 405).
Bacterial Strains and in vitro Fermentation. The bacterial strains used in this study were C. butyricum MSCL 1019 and B. ovatus MSCL 841, obtained from the Microbial Strain Collection of Latvia, where they were stored in liquid nitrogen. Before use, the strains were cultivated in Wilkins-Chalgren anaerobe agar (Oxoid, UK) under anaerobic conditions (GasPak Anaerobic Pouch, Becton & Dickinson) at 37°C for 48 hours. The harvested strains were adjusted for each growth medium to 4.8 × 105 CFU/ml for C. butyricum and 2.2 × 105 CFU/ml for B. ovatus. The growth media with bacterial strains were incubated in a thermostat at 37°C. In vitro fermentation was carried for 96 hours under anaerobic conditions. The SCFA concentration and pH were measured at 0, 24, 72, 96 hours. Aliquots of 1 mL samples were transferred to Eppendorf centrifuge tubes and centrifuged at 5000 rpm for 4 minutes (Sigma 1-14, Germany). A 0.5 ml of supernatant was taken and diluted with 0.5 ml acetonitrile (ratio 1:1). All samples were done at duplicates and were frozen at –18°C for further SCFA analysis.
SCFA Analysis by LC-TOF-MS. Concentrations of SCFA were determined by liquid chromatography time-of-flight mass spectrometry (LC-TOF-MS) in accordance with previously described analytical methods (Ferrer and Thurman 2003; Ibanez and Bauer 2014; Han et al. 2015). All solvents were of analytical grade. Formic acid was purchased from Sigma-Aldrich (St. Louis, USA). Deionized water (18.2 MΩ) was prepared by a Milli-Q water purification system from Millipore (Billerica, Massachusetts, USA). LC analyses were performed using Agilent 1290 Infinity series (Agilent Technologies, Germany) UHPLC equipped with an Agilent 1290 DAD photodiode array detector. Phenomenex (Torrance, CA) RezexTM ROA-Organic (Acid H+ (8%) (250 mm × 4.6 mm) column was employed for the separation. The column was thermostated at 55 ± 1°C. The injection volume was 2.0 μL.
The mobile phase was directly on-line degassed and its composition consisted of 0.5% (v/v) formic acid in water at a flow rate of 0.30 ml/min in isocratic mode. The high-resolution mass spectra (HRMS) were taken on an Agilent 6230 TOF LC/MS system (Agilent Technologies, Germany) with electrospray ionization (ESI). The source parameters were: negative ionization mode, drying gas flow 10.0 l/min and temperature 285°C, fragmentor ionisation 75 V. One full mass spectrum was acquired in a profile mode, with mass range from m/z 50 to 1100, 1 scan/s. The data of SCFA were obtained using the extraction of individual compound chromatogram at its individual m/z value. The internal reference masses of m/z 112.9856 and m/z 1033.9881 (G1969-85001 ESI-TOF Reference Mass Solution Kit, Agilent Technologies & Supelco) were used for all analyses of the samples. The experimental data were handled using the MassHunter version B07.00 software (Agilent Technologies).
Data Processing and Analysis. Identification of separated SCFA were based on the search for [M-H]-ions, using extracted ion mass chromatograms. The experimentally obtained mass spectra of SCFA approved the calculated values (lactic acid C3H6O3, [M-H]-Calculated – 89.0264, Found – 89.0244, Δ 0.0020; acetic acid C2H4O2, [M-H]-Calculated – 59.0150, Found – 59.0139, Δ 0.0011; succinic acid C4H6O4, [M-H]-Calculated – 117.0217, Found – 117.0193, Δ 0.0024; propionic acid C3H6O2, [M-H]-Calculated – 73.0303, Found – 73.0295, Δ 0.0008; butyric acid C4H8O2, [M-H]-Calculated – 87.0114, Found – 87.0452, Δ 0.0338). For quantification the calibration curves of acetic, propionic, succinic and lactic acids, the standard solutions were constructed by plotting the ratio of the average chromatographic peak area and mass concentration. According to the reflected data, the regression equation of the trend line was calculated. Standard solutions were injected in triplicate, and the corresponding peak areas were recorded. The relative standard deviation between all solutions was determined to be less than 1.5%. The calibration curves obtained showed linearity of the determination coefficient (R2 > 0.99) in the used concentration range (0.5–100 μg/ml). A coefficient of determination (R2) was calculated using Microsoft Excel 2013, p < 0.001. Analyses of SCFA measurements done at duplicates were averaged. To compare the means between viscosity measurements one-way analysis of variance (one-way ANOVA) was used, p value of less than 0.05 was considered significant. Data were expressed in μg/ml as means ± S.D.
Results and Discussion
The results of our study showed that the batch pasteurization of solutions with oat NSP did not inactivate the thermostable B. licheniformis (biotype validity 18185, confidence 0.9555). Since B. licheniformis is а source of β-1,3-1,4-glucanase (Planas 2000) its effect on β-glucan viscosity degradation and SCFA production by the bacteria alone and in consortium with C. butyricum and B. ovatus was further tested.
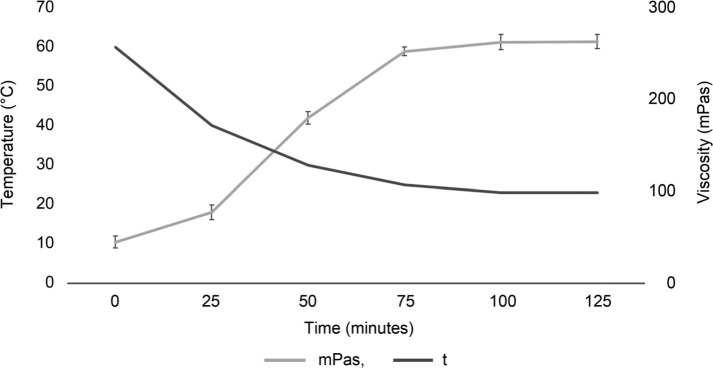
The viscosity of a solution containing 1% β-glucan before the pasteurization process, directly after mixing prepared substrate with water (20°C), was 14 ± 1 mPas (pH 6.9). After the solution was heated to 60°C and then cooled to room temperature, there was а tendency to increase the viscosity (Fig. 1).
Fig. 1.
Changes in the viscosity of a solution containing 1% oat β-glucan, after heating it up to 60°C and cooling to room temperature. Data represent mean ± SD of triplicates.
The final stable viscosity after cooling the solution to room temperature reached 260 mPas (Fig. 1). At the end of the pasteurization process, the viscosity of the solution containing BG25, degraded by B. licheniformis decreased to 4 ± 1 mPas and did not differ from the solution containing BG0, in which β-glucan was degraded using commercial enzymes (2 ± 1 mPas vs 4 ± 1 mPas, p = 0.18).
Oat β-glucan is able to form highly viscous solutions at low concentrations; however, the viscosity depends on the concentration and the molecular weight of β-glucan (Anttila et al. 2008).
Our results showed that fermentation of oat β-glucan by B. licheniformis resulted in degradation of β-glucan viscosity and, consequently, its molecular weight. As noted earlier (Sahasrabudhe et al. 2016), the enzymatic degradation of medium viscosity of oat β-glucan enhances its effect on immune receptors. The digested oat β-glucan activates type II transmembrane protein receptor Dectin-1 that binds β-1,3- and β-1,6-glucans more efficiently than non-digested β-glucan (Sahasrabudhe et al. 2016). In this way, pre-digestion of high molecular weight of oat β-glucan by B. licheniformis may have more visible host’s microbial-independent immune modulation effects in vivo through the pattern-recognition receptor Dectin-1 activation, what should be explored in future.
After pasteurizing of the growth medium containing B. licheniformis, lactic acid was detected in both BG25 (14.8 μg/ml) and BG0 (15.6 μg/ml), and also succinic acid at a concentration of 11.4 μg/ml in BG25 and 12.7 μg/ml in BG0, respectively. Propionic and butyric acids have not been detected in any of the growth media (BG25 and BG0), indicating that B. licheniformis does not have the potential to produce these SCFA. A very low concentration of acetic acid (1.8 μg/ml) produced by B. licheniformis was detected in BG0.
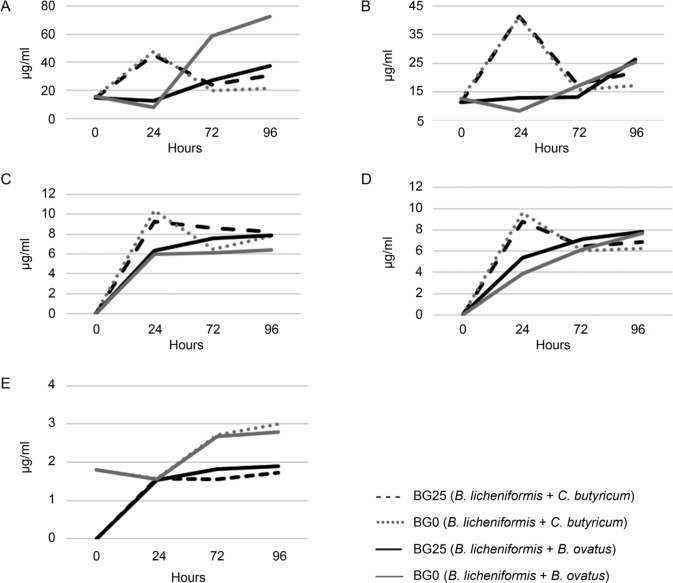
After B. ovatus was added to the growth medium containing B. licheniformis, the lactic acid concentration decreased in both BG25 and BG0 after 24 hours (Fig. 2A). Succinic acid decreased in BG0 after 24 hours period, but not in BG25 (Fig. 2B). These data show that B. ovatus within the 24 hours of fermentation consumed lactic and succinic acids produced by B. licheniformis (Fig. 2A, 2B).
Fig. 2.
Changes in concentration of lactic (A), succinic (B), butyric (C), propionic (D), and acetic (E) acids within 96 hours of syntrophic in vitro fermentation of BG0 and BG25 by B. licheniformis in the consortium with C. butyricum and B. ovatus.
Because B. licheniformis produces lactic and succinic acids, it could be involved in bacterial cross-feeding and butyrate synthesis through the butyril-CoA: acetate CoA-transferase pathway (Reichardt et al. 2014), as well as in the synthesis of propionate through either succinate synthesis pathway or acrylate pathway using lactate (Reichardt et al. 2014).
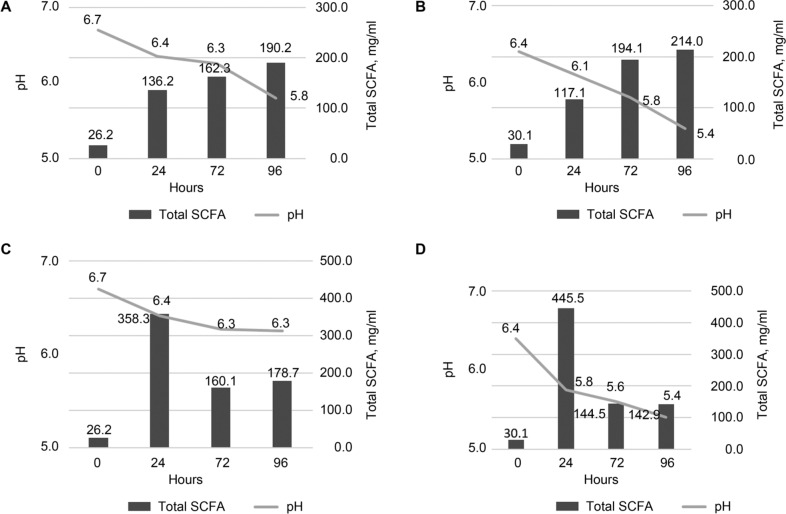
The concentration of lactic acid, produced by B. licheniformis in a consortium with B. ovatus in BG25 was lower than in BG0 (58.5 vs 27.1 μg/ml after 72 hours and 37.5 vs 72.6 μg/ml after 96 hours of fermentation for BG25 and BG0 (Fig. 2A). One of the hypotheses that explain this finding can be an assumption that degradation of β-glucan by commercial enzymes facilitates access to hemicellulose for bacteria. This hypothesis is also implied by the fact that the pH value in BG0 was lower than in BG25 (Fig. 3A, 3B).
Fig. 3.
The total acids concentrations and changes in pH within the 96 hours of syntrophic in vitro fermentation of BG25 (A, C) and BG0 (B, D) by B. ovatus (A, B) and C. butyricum (C, D) in consortium with B. licheniformis.
Since the β-xylosidases from B. ovatus have been found to be highly effective in digesting hemicellulose to xylooligosaccharides (Jordan et al. 2017), it is likely that oat hemicelluloses that were not digested by B. licheniformis, have been finally degraded by B. ovatus into xylooligosaccharides. These xylooligosaccharides can become substrates for B. licheniformis through the use of xylanase enzymes. As a result, further syntrophic fermentation of xylooligosaccharides led to the production of lactic and succinic acids by B. licheniformis.
The results of our study showed that fermentation of BG0 and BG25 during culture of the consortium consisted of B. licheniformis and B. ovatus or C. butyricum resulted in an increase of the concentration of propionic and butyric acids (Fig. 2C, 2D), while the production of butyrate and propionate by the same bacterium is not common. Only a few anaerobes, such as Roseburia inulinivorans and Coprococcus catus were previously mentioned having the ability to produce both (Reichardt et al. 2014).
The total acid concentration peak for B. licheniformis in consortium with C. butyricum was observed after 24 hours of fermentation in BG25 and BG0 (Fig. 3C, 3D). It was assumed that B. licheniformis degraded large β-glucan molecules and produced lactic and succinic acids, while the simple or/and complex sugars were degraded by C. butyricum, because of their previously mentioned enzymatic capacities (Montoya et al. 2001; Nakajima et al. 2002).
The acetic acid produced by B. licheniformis in consortium with C. butyricum or B. ovatus did not reach 3 μg/ml (Fig. 2E), which is the lowest concentration of all the acids analysed.
Because the current study did not consider antagonistic interactions between members of the bacterial consortium, a further study are needed to investigate if B. licheniformis competes for the substrate with other bacteria, and thereby reduces availability of the substrate to another bacterium (pathogenic or probiotic).
Conclusions
In conclusion, the results of this study show that B. licheniformis, a widespread food contaminant, acts as thermostable probiotic that ferments oat NSP. It pre-digests oat NSP, degrades the high viscosity of oat β-glucan and makes hemicellulose more accessible for other bacteria. As a result of fermentation, B. licheniformis produces lactic and succinic acids, which further can be used by other bacteria for cross-feeding and production of SCFA. Thus, B. licheniformis in syntrophic consortia with C. butyricum or B. ovatus produces SCFA, which were not produced when B. licheniformis was grown alone.
Footnotes
Disclosure of interest
The authors report that they have no conflicts of interest.
Literature
- Anttila H, Sontag-Strohm T, Salovaara H.. 2008. Viscosity of beta-glucan in oat products. AFSci. 13(1–2):80–87. [Google Scholar]
- Arbsuwan N, Payoungkiattikun W, Sirithorn P, Daduang S, Jangpromma N, Dhiravisit A, Tae Hahm Y, Kurt Neubert L, Klaynongsruang S.. 2017. Purification and characterization of macrolactins and amicoumacins from Bacillus licheniformis BFP011: a new source of food antimicrobial substances. CyTA – Journal of Food. 16(1):50–60. [Google Scholar]
- Ferrer I, Thurman EM.. 2003. Liquid chromatography time – of – flight/mass spectrometry (LC/TOF/MS) for the analysis of emerging contaminants. Trends Anal Chem. 22(10):750–756. [Google Scholar]
- Gopal N, Hill C, Ross PR, Beresford TP, Fenelon MA, Cotter PD.. 2015. The prevalence and control of Bacillus and related spore-forming bacteria in the dairy industry. Front Microbiol. 21(6):1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamady ZZR. 2013. Novel xylan-controlled delivery of therapeutic proteins to inflamed colon by the human anaerobic commensal bacterium. Ann R Coll Surg Engl. 95(4):235–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Lin K, Sequeira C, Borchers CH.. 2015. An isotope-labeled chemical derivatization method for the quantitation of short-chain fatty acids in human feces by liquid chromatography-tandem mass spectrometry. Anal Chim Acta. 7(854):86–94. [DOI] [PubMed] [Google Scholar]
- Heo J, Kim SK, Park KS, Jung HK, Kwon JG, Jang BI.. 2014. A double-blind, randomized, active drug comparative, parallelgroup, multi-center clinical study to evaluate the safety and efficacy of probiotics (Bacillus licheniformis, Zhengchangsheng® capsule) in patients with diarrhea. Intest Res. 12(3):236–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holguin-Acun AL, Ramos-Chavira N, Carvajal-Millan E, Santana-Rodriguez V, Rasco´n-Chu A, Niño-Medina G.. 2011. Non-starch polysaccharides in maize and oat In: Preedy VR, Watson RR, Patel VB, editors. Flour and breads and their fortification in health and disease prevention. 1st ed. San Diego, California (USA): Academic Press; p. 542. [Google Scholar]
- Hong PY, Wu JH, Liu WT.. 2008. Relative abundance of Bacteroides spp. in stools and wastewaters as determined by hierarchical oligonucleotide primer extension. Appl Environ Microbiol. 74(9):2882–2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibanez AB, Bauer S.. 2014. Analytical method for the determination of organic acids in dilute acid pretreated biomass hydrolysate by liquid chromatography-time-of-flight mass spectrometry. Biotechnol Biofuels. 7(1):145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia L, Li D, Feng N, Shamoon M, Sun Z, Ding L, Zhang H, Chen W, Sun J, Chen YQ.. 2017. Anti-diabetic effects of Clostridium butyricum CGMCC0313.1 through promoting the growth of gut butyrate-producing bacteria in type 2 diabetic mice. Sci Rep. 7(1):7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan DB, Stoller JR, Lee CC, Chan VJ, Wagschal K.. 2017. Biochemical characterization of a GH43 β-xylosidase from Bacteroides ovatus. Appl Biochem Biotechnol. 182(1): 250–260. [DOI] [PubMed] [Google Scholar]
- Kaletunc G, Breslauer K.. 2003. Characterization of cereals and flours. 1st ed. Boca Raton (USA): CRC Press. [Google Scholar]
- Kanai T, Mikami Y, Hayashi A.. 2015. A breakthrough in probiotics: Clostridium butyricum regulates gut homeostasis and anti-inflammatory response in inflammatory bowel disease. J Gastroenterol. 50(9):928–939. [DOI] [PubMed] [Google Scholar]
- Martín R, Miquel S, Ulmer J, Kechaou N, Langella P, Bermudez-Humaran LG.. 2013. Role of commensal and probiotic bacteria in human health: a focus on inflammatory bowel disease. Microb Cell Fact. 12:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCleary BV, Glennie-Holmes M.. 1985. Enzymic quantification of (1 → 3) (1 → 4)-β-D-glucan in barley and malt. JIB. 91(5): 285–295. [Google Scholar]
- McFarlin BK, Henning AL, Bowman EM, Gary MA, Carbajal KM.. 2017. Oral spore-based probiotic supplementation was associated with reduced incidence of post-prandial dietary endotoxin, triglycerides, and disease risk biomarkers. World J Gastrointest Pathophysiol. 8(3):117–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SS, Fulcher RG.. 2011. Microstructure and Chemistry of the Oat Kernel In: Webster FH, Wood PJ, editors. Oats: Chemistry and Technology. 2nd ed. St.Paul, Minnesota (U.S.A.): AACC International; p. 77–94. [Google Scholar]
- Montoya D, Arévalo C, Gonzales S, Aristizabal F, Schwarz WH.. 2001. New solvent-producing Clostridium sp. strains, hydrolyzing a wide range of polysaccharides, are closely related to Clostridium butyricum. J Ind Microbiol Biotech. 27(5):329–335. [DOI] [PubMed] [Google Scholar]
- Morrison DJ, Preston T.. 2016. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes. 7(3):189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima N, Ishihara K, Matsuura Y.. 2002. Dietary-fiber-degrading enzymes from a human intestinal Clostridium and their application to oligosaccharide production from nonstarchy polysaccharides using immobilized cells. Appl Microbiol Biotechnol. 59: 82–189. [DOI] [PubMed] [Google Scholar]
- Pepe O, Blaiotta G, Moshetti G, Greco T, Villani F.. 2003. Rope-producing strains of Bacillus spp. from wheat bread and strategy for their control by lactic acid bacteria. Appl Environ Microbiol. 69(4):2321–2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planas A. 2000. Bacterial 1,3-1,4-beta-glucanases: structure, function and protein engineering. Biochim Biophys Acta. 1543(2):361–382. [DOI] [PubMed] [Google Scholar]
- Rajilić-Stojanović M, de Vos W.. 2014. The first 1000 cultured species of the human gastrointestinal microbiota. FEMS Microbiol Rev. 38(5):996–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakoff-Nahoum S, Foster K, Comstock L.. 2016. The evolution of cooperation within the gut microbiota. Nature. 533(7602):255–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichardt N, Duncan SH, Young P, Belenguer A, McWilliam Leitch C, Scott KP, Flint HJ, Louis P.. 2014. Phylogenetic distribution of three pathways for propionate production within the human gut microbiota. ISME J. 8(6):1323–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberfroid M. 2007. Prebiotics: the concept revisited. J Nutr. 137(3 Suppl 2):830S–837S. [DOI] [PubMed] [Google Scholar]
- Sahasrabudhe NM, Tian L, van den Berg M, Bruggeman G, Bruininx E, Schols HA, Faas MM, de Vos P.. 2016. Endo-glucanase digestion of oat beta-glucan enhances Dectin-1 activation in human dendritic cells. J Funct Foods. 21:104–112. [Google Scholar]
- Sun J, Wang F, Ling Z, Yu X, Chen W, Li H, Jin J, Pang M, Zhang H, Yu J, Liu J.. 2016. Clostridium butyricum attenuates cerebral ischemia/reperfusion injury in diabetic mice via modulation of gut microbiota. Brain Res. 1642:180–188. [DOI] [PubMed] [Google Scholar]
- Ulsemer P, Toutounian K, Schmidt J, Karsten U, Goletz S.. 2012. Preliminary safety evaluation of a new Bacteroides xylanisolvens isolate. Appl Environ Microbiol. 78(2):528–535. [DOI] [PMC free article] [PubMed] [Google Scholar]