Abstract
The widespread of infections caused by methicillin-resistant Staphylococcus aureus (MRSA), has necessitated the search for alternative therapies; introduction of new agents being a suggestion. This study compares the in vitro and in vivo activities of zabofloxacin, a novel fluoroquinolone, with moxifloxacin, levofloxacin and ciprofloxacin against clinical isolates of MRSA from patients hospitalized in the Alexandria Main University hospital; a tertiary hospital in Alexandria, Egypt, where zabofloxacin has not been yet introduced. The strains tested showed the highest percentage of susceptibility to zabofloxacin (61.2%) among the tested fluoroquinolones with the most effective MIC50 and MIC90 (0.25 and 2 µg/ml, respectively). Time-kill curve analysis revealed a rapid bactericidal activity of zabofloxacin after 6 h of incubation with a quinolone-resistant isolate and complete killing when tested against a quinolone-sensitive isolate with inhibition of regrowth in both cases. PCR amplification and sequencing of QRDRs in selected strains revealed the following amino acid substitutions: Ser-84→Leu in GyrA, Ser-80→Phe in GrlA and Pro-451→Ser in GrlB. The in vivo studies demonstrated that zabofloxacin possessed the most potent protective effect against systemic infection in mice (ED50: 29.05 mg/kg) with lowest count in the dissected lungs (3.66 log10 CFU/ml). The histopathological examination of lung specimens of mice treated with zabofloxacin displayed least congestion, inflammation, oedema and necrosis with clear alveolar spaces and normal vessels. In conclusion, zabofloxacin was proved to possess high in vitro and in vivo efficacy encompassing its comparators and could be considered as a possible candidate for the treatment of infections caused by MRSA. To our knowledge, this is the first study evaluating the in vitro and in vivo activity of zabofloxacin against Egyptian MRSA clinical isolates.
Key words: MRSA, ED50, fluoroquinolones, quinolone resistance-determining regions, zabofloxacin
Introduction
Staphylococcus aureus has been considered as one of the pathogenic bacteria responsible for a wide variety of diseases. Such diseases range from slight skin infections to critical infections including septicemia (Tokajian 2014). After the introduction of methicillin in 1959, methicillin-resistant S. aureus (MRSA) has transpired as a significant hospital-associated pathogen (Tokajian 2014). The widespread of MRSA and its capability for escalating in hospitals and community settings represent a real threat that stands against infection control practices (Tokajian 2014). The epidemiology of MRSA differs noticeably worldwide even at regional levels (Borg et al. 2007). In comparison with other African, southern and eastern Mediterranean countries, Egypt showed the highest rates of MRSA occurrence among S. aureus clinical isolates (Abdel-Maksoud et al. 2016). According to the Antibiotic Resistance Surveillance and Control in the Mediterranean Region, more than 50% of the S. aureus isolates, obtained from blood cultures in Egypt, during a three-year study period (2003–2005), were recognized to be methicillin-resistant (Borg et al. 2007). Poor and problematic healthcare system in the majority of African countries, not excluding Egypt, inadequate financing, poor provision of properly trained healthcare professionals, poverty in infrastructure and occasional lack of adequate medications aggravate the problem, making the fight of these countries against this pathogen a real challenge (Falagas et al. 2013).
Fluoroquinolones have been recognized as promising and potent antibiotics which inhibit DNA gyrase and topoisomerase IV resulting in impeding the supercoiling of the DNA and causing bacterial death (Kocsis et al. 2016). The classic fluoroquinolones possess a considerable antimicrobial activity against Gram-negative bacteria, but their potency against Gram-positive bacteria has been controverted (Park et al. 2006). Therefore, research in the field of fluoroquinolones was oriented towards potentiating the antimicrobial efficacy against Gram-positive cocci, anaerobes and fluoroquinolone-resistant strains by finding substituents with better binding affinity to target enzymes (Kwon et al. 2006). Consequently, a number of new agents have been developed, among which was zabofloxacin (Kocsis et al. 2016). Zabofloxacin is a novel member in the family of fluoroquinolones (Park et al. 2006) with reported activity against methicillin-resistant coagulase-negative staphylococci, Streptococcus pyogenes, Streptococcus pneumoniae and Enterococcus faecalis. Its activity against these organisms is regarded to be superior to other fluoroquinolones as ciprofloxacin, moxifloxacin, and gemifloxacin (Park et al. 2006; Karpiuk and Tyski 2013). Zabofloxacin is slightly inferior in comparison with other fluoroquinolones against Enterobacteriaceae family. However, it is effective against the Gram-negative respiratory bacteria, such as Haemophilus influenzae and Moraxella catarrhalis (Karpiuk and Tyski 2013). Owing to the double mechanism of binding of zabofloxacin to the complex enzyme-DNA, the likelihood of the development of bacterial resistance is markedly diminished (Karpiuk and Tyski 2013).
The present study compared the in vitro activity of zabofloxacin with moxifloxacin, levofloxacin and ciprofloxacin against MRSA clinical isolates collected from hospitalized patients admitted to the Alexandria Main University hospital (AMUH); a tertiary hospital based in Alexandria, Egypt, where zabofloxacin has not been yet introduced. In addition, the in vivo protective activity of zabofloxacin and comparator fluoroquinolones against systemic infection caused by MRSA clinical isolate was evaluated using a mice model.
Experimental
Materials and Methods
Test organisms and bacterial identification. One hundred-sixteen clinical isolates of MRSA were collected from the routine laboratory of Alexandria Main University Hospital (AMUH) over a six-month period between January 2017 to July 2017. The clinical origins of these isolates were as follow: bronchoalveolar lavages (n = 66), blood (n = 19), urine (n = 16), pus (n = 11), and wound swabs (n = 4). As a quality control, a reference strain of methicillin-sensitive S. aureus (MSSA; ATCC 25923) and a strains of methicillin-resistant S. aureus (MRSA; ATCC 43300) strains were included in this study.
The specimens were cultured on Mannitol Salt Agar and identified by colony morphology, Gram stain, as well as, by biochemical characteristics, which were estimated by the commercial test system Dry Spot Staphytect Plus (Oxoid, UK). Methicillin resistance was confirmed by cefoxitin and oxacillin disc diffusion methods according to the Clinical and Laboratory Standards Institute guidelines (CLSI 2013).
Antibiotics. Ciprofloxacin, CIP, (Amryia Pharm. Ind., Egypt), levofloxacin, LEV, (Sanofi-Aventis, Germany) and moxifloxacin, MOX, (Alcon, Belgium) were purchased from pharmaceutical markets. Zabofloxacin powder, ZAB, was provided by the courtesy of Dongwha Pharm. Co., Ltd. (Seoul, Korea).
Laboratory animals. Five-week-old male Swiss albino mice (body weight, 17–21 g) were purchased from Theodor Bilharz Research Institute, Giza, Egypt. All mouse experiments were performed in accordance with the ethical guidelines of the Institutional Animal Care and Use Committee, Faculty of Pharmacy, Alexandria University (ACUC Project # ACUC18/28). Mice were housed in animal rooms maintained at 23 ± 2°C with 50 ± 20% relative humidity.
Antimicrobial susceptibility testing. Minimum inhibitory concentrations (MICs) were determined by the microtiter broth dilution method according to the guidelines of CLSI (2012). Microtiter plates containing 5.0 × 105 CFU/ml were incubated with serial dilutions of each antibiotic in Mueller-Hinton broth (Oxoid, UK) at 35°C for 18 h, and the lowest concentration of the antibiotic that prevented visible growth was considered as the MIC.
Time-kill analysis. The time-kill studies were performed following CLSI M26-A guidelines (1999). Two isolates, one resistant and one susceptible to the four tested fluoroquinolones, named S13 and S15 respectively, were selected for this analysis. The bacterial cultures in Muller-Hinton broth (Oxoid, UK) were diluted to reach an approximate count of 105 to 106 CFU/ml and preincu bated for 2 h. Then, ZAB, MOX, LEV and CIP were added to the cultures at concentrations of 0.5, 1, 2, and 4 MIC. The bacterial count was determined after 0, 3, 6 and 24 h of incubation with the antibiotics tested by culturing the bacteria on Muller-Hinton agar (Oxoid) at 37°C for 18 h following serial dilution. The anti bio tic was considered as bactericidal at a concentration, which decreased the control count by 3 log CFU/ml (99.9%) at specified time intervals. The procedure was performed in triplicates and a graph of the log CFU/ml was then plotted against time with calculation of standard deviation.
Quinolone resistance-determining region (QRDR) sequence analysis. PCR was used to amplify the QRDRs of the gyrA, parC and parE genes using extracted genomic DNA of the strains tested as a template and the previously described primer pairs and cycling conditions (Pan et al. 1996). To determine alterations in the DNA gyrase or topoisomerase IV, selected PCR products were purified using Zymo ResearchTM DNA Purification Kit and then sequenced using a system from LGC Co. Ltd. (Germany).
Determination of ED50. To determine and to compare the in vivo efficacy of ZAB to other fluoroquinolones in systemic infection murine model, four groups of four mice were challenged intraperitoneally with 0.2 ml of bacterial suspension adjusted with 5% gastric mucin (Oxoid, UK) in 0.9% NaCl solution at a dose 100 times higher than the minimal lethal dose. The challenge inoculum was sufficient to kill 100% of the untreated control mice, which died within 48 h after infection. An aliquot of 0.2 ml of each of ZAB, MOX, LEV and CIP was administrated twice orally to mice at 1 h and 4 h post infection. Four dose levels were used: 0.63, 2.5, 10 and 40 mg/kg of body weight for each fluoroquinolone. Mortality was recorded for seven days and the effective dose needed to protect 50% of the mice (ED50) was calculated by the method of Miller and Tainter with modifications (Randhawa 2009) from the survival rates on day 7 post infection.
Bacteriological and histopathological examinations. Twenty-five mice were allocated into five treatment groups: ZAB, MOX, LEV, CIP and normal saline (control). The doses of the antibiotics were adjusted to 10 mg/kg of body weight and injected intraperitoneally into mice twice daily beginning 24 h after infection. Mice were sacrificed by cervical dislocation on day 3 (12 h after the fourth administration). For bacteriological count, the lungs (n = 4 for each group) were dissected under aseptic conditions, suspended in 0.9% NaCl solution and homogenized. Serial dilutions were performed and bacterial count in homogenized lung was determined. Lung tissue for histological examination (n = 2 for each group) was fixed in 10% buffered formalin then stained with hematoxylin-eosin. Histopathological evaluation was done by an investigator who was blinded to the sample origin.
Results
In vitro activity. The microtiter broth dilution MICs were determined for 116 MRSA clinical isolates (Table I). The strains showed the highest percentage of susceptibility to zabofloxacin (61.2%) among the fluoro quinolones tested. Comparing the MIC50 and MIC90, ZAB showed the highest potency with values of 0.25 and 2 µg/ml, respectively. Moxifloxacin followed ZAB in activity (MIC50 0.5 µg/ml, MIC90 8 µg/ml), then LEV (MIC50 4 µg/ml, MIC90 16 µg/ml) and the least effective was CIP (MIC50 8 µg/ml, MIC90 64 µg/ml). The distribution of MICs values for the four fluoroquinolones are shown in Table II. The lowest MIC range among the tested fluoroquinolones was displayed by ZAB (0.03 – 4 µg/ml). A comparable range (0.06 – 16 µg/ml) was obtained by MOX while old generation fluoroquinolones, LEV and CIP, had a much higher range (0.125 – >64 µg/ml). In the presence of omeprazole, an efflux pump inhibitor (Vidaillac et al. 2007), the ZAB MIC values of all tested isolates remained unaffected (data not shown).
Table I.
In vitro activity of zabofloxacin, moxifloxacin, levofloxacin and ciprofloxacin against 116 MRSA clinical isolates.
| Antimicrobial agent | MIC (μg/ml) | % Susceptible | % Intermediate | % Resistant | ||
|---|---|---|---|---|---|---|
| MIC range | MIC50* | MIC90* | ||||
| Zabofloxacin | 0.03 – 4 | 0.25 | 2 | 61.2 | 0.9 | 37.9 |
| Moxifloxacin | 0.06 – 16 | 0.5 | 8 | 50.9 | 8.6 | 40.5 |
| Levofloxacin | 0.125 – 64 | 4 | 16 | 46.5 | 0.9 | 52.6 |
| Ciprofloxacin | 0.125 – > 64 | 8 | 64 | 42.3 | 1.7 | 56 |
MIC50 and MIC90, MICs (μg/ml) for 50% and 90% of isolates tested, respectively.
Table II.
MIC distribution of zabofloxacin, moxifloxacin, levofloxacin and ciprofloxacin determined by broth microdilution method for 116 MRSA isolates.
| Antimicrobial agent | MIC (μg/ml) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.03 | 0.06 | 0.125 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | > 64 | |
| Zabofloxacin | 10 | 17 | 25 | 12 | 7 | 1 | 32 | 12 | 0 | 0 | 0 | 0 | 0 |
| Moxifloxacin | 0 | 2 | 7 | 35 | 15 | 10 | 11 | 21 | 13 | 2 | 0 | 0 | 0 |
| Levofloxacin | 0 | 0 | 10 | 33 | 11 | 0 | 1 | 8 | 22 | 29 | 0 | 2 | 0 |
| Ciprofloxacin | 0 | 0 | 4 | 18 | 19 | 8 | 2 | 4 | 11 | 19 | 14 | 14 | 3a |
Bold figures indicate CLSI breakpoints applied for resistant isolates; zabofloxacin and moxifloxacin: 2 µg/ml, levofloxacin and ciprofloxacin: 4 μg/ml.
MIC for these isolates was greater than or equal to the indicated value.
The bactericidal activity of the fluoroquinolones tested against MRSA was compared by time-kill analysis. As illustrated in figure 1A–D, ZAB and MOX showed a comparable rapid bactericidal activity with a decrease in the viable count by ≥ 3 log10 CFU/ml after 6 h of contact when tested at the concentrations of 2 and 4 MIC against the fluoroquinolone-resistant clinical strain S13 (Fig. 1A and 1B). Against the fluoroquinolone-sensitive strain, S15, complete killing was achieved at 6 h when ZAB was used at a concentration of 2 MIC and at a concentration of 4 MIC when MOX was used (Fig. 2A and 2B). Regrowth of both isolates was completely inhibited by ZAB. Older classes fluoroquinolones, LEV and CIP, exhibited an inferior pattern of bactericidal activity (Fig. 2A–D).
Fig. 1.
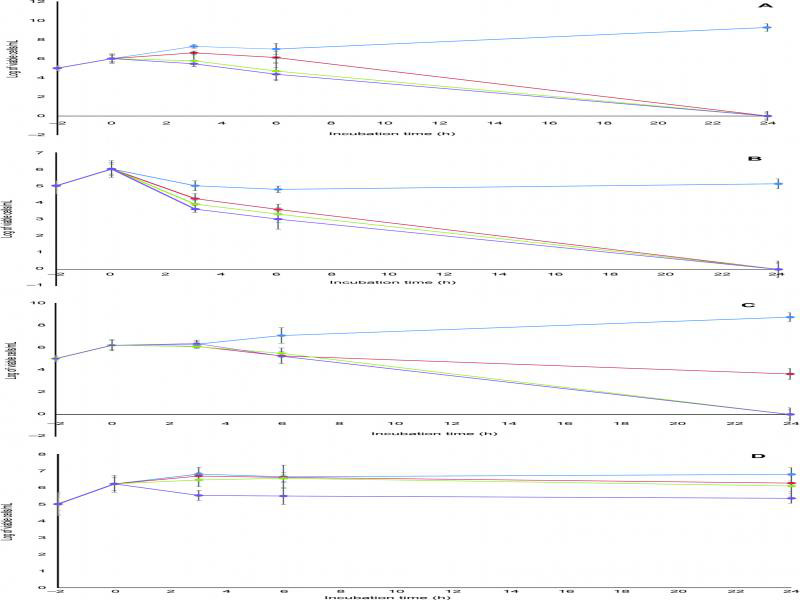
Bactericidal activities of (A) zabofloxacin, (B) moxifloxacin, (C) levofloxacin and (D) ciprofloxacin against fluoroquinolone-resistant MRSA clinical isolate S13. Blue line, 0.5 MIC; red line, 1 MIC; green line, 2 MIC; purple line, 4 MIC. Data points are geometric means with error bars being one standard deviation of replicate experiments (n = 3).
Fig. 2.
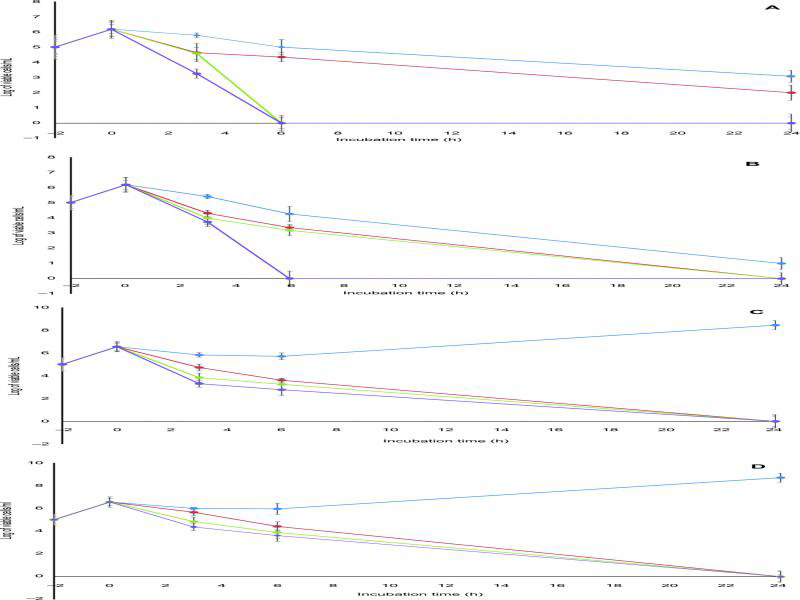
Bactericidal activities of (A) zabofloxacin, (B) moxifloxacin, (C) levofloxacin and (D) ciprofloxacin against fluoroquinolone-sensitive MRSA clinical isolate S15. Blue line, 0.5 MIC; red line, 1 MIC; green line, 2 MIC; purple line, 4 MIC. Data points are geometric means with error bars being one standard deviation of replicate experiments (n = 3).
Analysis of QRDRs in selected MRSA strains. PCR amplification of QRDR sequences of the gyrA, parC and parE genes were performed for fluoroquinolone-resistant isolates. Seven isolates showing different resistance patterns were selected for sequencing analysis to detect mutations in DNA gyrase and topoisomerase IV as shown in Table III. The detected mutations revealed the following amino acid substitutions: Ser-84→Leu in GyrA, Ser-80→Phe in GrlA and Pro-451→Ser in GrlB. Clinical isolates displaying resistance to ZAB had three mutations in the QRDRs of the gyrA, parC and parE genes, while isolates resistant to other fluoroquinolones, but not to ZAB, displayed a single or double point mutations.
Table III.
MICs of zabofloxacin, moxifloxacin, levofloxacin and ciprofloxacin for selected 7 clinical MRSA isolates with detected mutations in QRDRs.
| Strain No. | MIC (μg/ml) | Detected Mutation in QRDRs | |||||
|---|---|---|---|---|---|---|---|
| ZABa | MOX | LEV | CIP | gyrA | parC | parE | |
| S37 | 4 | 8 | 32 | 64 | S84Lb | S80Fc | P451Sd |
| S30 | 2 | 8 | 16 | 64 | S84L | S80F | P451S |
| S5 | 2 | 4 | 16 | 64 | S84L | S80F | P451S |
| S3 | 2 | 8 | 32 | 64 | S84L | S80F | P451S |
| S11 | 0.125 | 0.25 | 8 | 32 | S84L | None | P451S |
| S38 | 0.25 | 0.25 | 8 | 16 | None | S80F | None |
| S22 | 0.03 | 0.25 | 4 | 8 | None | S80F | None |
ZAB: zabofloxacin, MOX: moxifloxacin, LEV: levofloxacin and CIP: ciprofloxacin.
Serine → Leucine,
Serine → Phenylalanine,
Proline → Serine.
In vivo activity. The protective efficacy of ZAB against systemic infection in mice was compared with those of MOX, LEV and CIP (Table IV). Zabofloxacin exerted the most potent protective activity (MIC: 0.06 µg/ml; ED50: 29.05 mg/kg) followed by MOX (MIC: 0.06 µg/ml; ED50: 38.69 mg/kg), while LEV and CIP required a dose of more than 40 mg/kg to elicit a protective activity against the systemic infection (Table IV). These in vivo results were in accordance with the in vitro MICs values.
Table IV.
In vivo activities of zabofloxacin, moxifloxacin, levofloxacin and ciprofloxacin against mice infected with MRSA S19 clinical isolate.
| Microorganism inoculum (CFU/mouse) | Antimicrobial agenta | MIC (μg/ml) | Count in dissected lungs log10 (CFU/ml)b | ED50c (mg/kg) |
|---|---|---|---|---|
| Methicillin-resistant S. aureus clinical isolate S19 | Zabofloxacin | 0.06 | 3.66 | 29.05 |
| Moxifloxacin | 0.06 | 4.31 | 38.69 | |
| Levofloxacin | 8 | 4.01 | > 40.00 | |
| Ciprofloxacin | 16 | 4.24 | > 40.00 |
Each antimicrobial agent was administrated twice orally at 1 and 4 h post infection.
Count in control group receiving saline was log10 5.5 CFU/ml.
ED50: median effective dose needed to protect 50% of the mice.
The mice group treated with ZAB showed the lowest count in the dissected lungs (3.66 log10 CFU/ml), when compared to other tested fluoroquinolones or to the control receiving saline (Table IV). These results again agreed with the in vitro time-kill curve findings in which ZAB showed the highest bactericidal activity as compared to the fluoroquinolones under investigation (Fig. 1A–D and Fig. 2A–D).
The histopathological examination of lung tissue sections obtained from CIP-treated mice group showed obvious features of acute bronchopneumonia almost the same as those in the untreated control with marked congestion, oedema, inflammatory infiltration, necrosis with accumulation of necro-inflammatory exudate within the lumen of bronchioles (Fig. 3). The lungs of ZAB-treated mice group revealed very mild congestion and inflammation with scarce inflammatory cells in the interstitial spaces. The mice group treated with MOX and LEV displayed moderate interstitial inflammation, moderate congestion and oedema (Fig. 3). Figure 4 demonstrates a patent alveolus dissected from ZAB-treated mice group, showing clear alveolar spaces and normal vessels and bronchioles lined by a layer of pneumocytes. The degree of congestion, inflammation, oedema and necrosis was scored using a scoring system adapted from Dubin and Kolls (2007) and summarized in Table V. Mice groups infected with MRSA clinical isolate S19 and treated with ZAB at an adjusted dose of 20 mg/kg of body weight/day, displayed best scores with least detected congestion, inflammation, oedema and necrosis followed by MOX, then LEV and finally CIP, all administrated at same dose regimen.
Fig. 3.
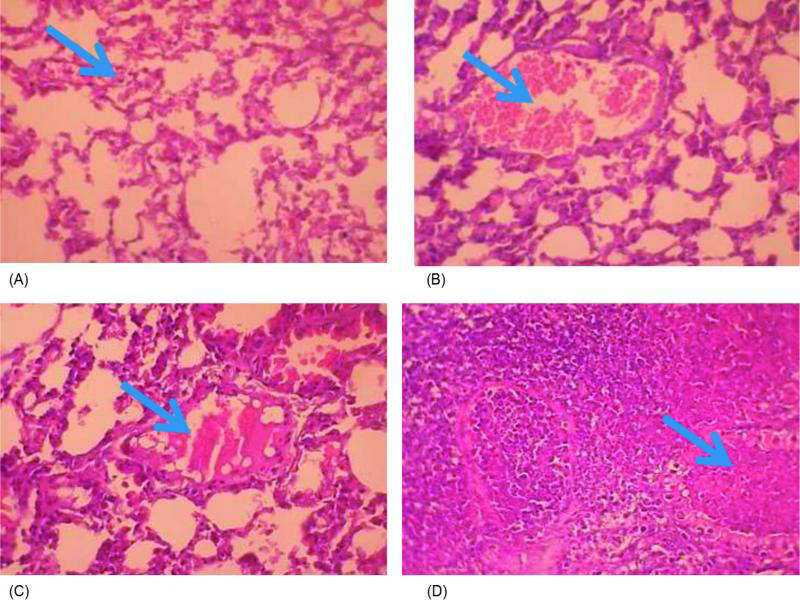
Lung tissue sections dissected from mice infected with MRSA clinical isolate and treated with (A) zabofloxacin showing very mild congestion (arrow) and inflammation with scarce inflammatory cells in the interstitial spaces; (B) moxifloxacin showing moderate congestion with ectatic vessels (arrow) and moderate interstitial inflammation; (C) levofloxacin showing moderate inflammation, congestion and oedema, with a dilated lymphatic vessel (arrow); and (D) ciprofloxacin showing bronchopneumonic changes with marked congestion, oedema, inflammatory infiltration and necrosis, with accumulation of necro-inflammatory exudate within the lumen of bronchioles (arrow) (H&E x400).
Fig. 4.
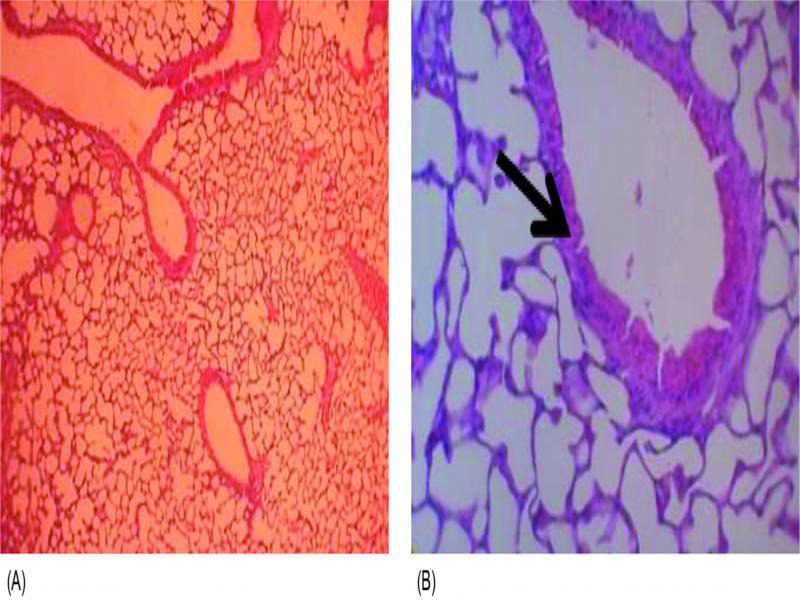
(A, × 100) cross section of lung tissue dissected from mice treated with zabofloxacin showing patent alveoli, (B, × 400) clear alveolar spaces and normal vessels, the bronchioles are lined by a layer of pneumocytes (arrow).
Table V.
Scoring of detected congestion, inflammation, oedema and necrosis in lung tissues of mice groups infected with MRSA clinical isolate S19 and treated with zabofloxacin, moxifloxacin, levofloxacin and ciprofloxacin at a dose of 20 mg/kg of body weight/day.
| Histological observationa | (Score 0–3) | |||
|---|---|---|---|---|
| ZABb | MOX | LEV | CIP | |
| Congestion | 0 (40%) | |||
| +1 (20%) | +1 (60%) | +2 (80%) | +3 (60%) | |
| +2 (40%) | +2 (40%) | +1 (20%) | +1 (40%) | |
| Inflammation | 0 (40%) | |||
| +1 (20%) | +1 (60%) | +2 (80%) | +3 (60%) | |
| +2 (40%) | +2 (40%) | +1 (20%) | +1 (40%) | |
| Edema | 0 (40%) | |||
| +1 (20%) | +1 (60%) | +2 (80%) | +3 (60%) | |
| +2 (40%) | +2 (40%) | +1 (20%) | +1 (40%) | |
| Necrosis | 0 (40%) | |||
| +1 (20%) | +1 (60%) | +2 (80%) | +3 (60%) | |
| +2 (40%) | +2 (40%) | +1 (20%) | +1 (40%) | |
Randomly selected sections blindly scored with a score applied to review of a whole lung section, scored at X10 magnification.
ZAB: Zabofloxacin, MOX: moxifloxacin, LEV: levofloxacin and CIP: ciprofloxacin.
Discussion
Zabofloxacin is a novel fluoroquinolone recently appro ved and launched in South Korea (Kocsis and Szabo 2016) with reported potent in vitro and in vivo activities against pathogens responsible for respiratory tract and uncomplicated skin infections such as S. pneumoniae, H. influenzae, M. catarrhalis and S. aureus (Kwon et al. 2006; Park et al. 2006). This fluoroquinolone targets DNA gyrase and topoisomerase IV, and due to its double mechanism of binding to the DNA-enzyme complex, mutations in both proteins are required for the development of high-level of resistance (Park et al. 2010). This agent has not been yet introduced to Egyptian healthcare facilities, including AMUH, a tertiary hospital with a heavy patient turnover and a significantly high prevalence rate (71%) of MRSA emerging as a nosocomial pathogen in its different departments (Sadaka et al. 2009). Therefore, the present study was designed to evaluate and to compare the in vitro and in vivo activity of ZAB against MRSA clinical isolates to older fluoroquinolones available on the Egyptian market.
The MIC results showed that ZAB was 4, 8 and 32-fold more active than MOX, LEV and CIP, respectively (Tables I and II). Among the 116 tested MRSA isolates, 61.2% showed susceptibility to ZAB, the highest rate obtained among comparators. The MIC50 and MIC90 of ZAB were 0.25 and 2 µg/ml, respectively. These values were lower than that reported by Kwon et al. (2006) and Park et al. (2006) for MRSA isolates; probably due to lack of exposure of Egyptian isolates to this new antibiotic. In addition, ZAB exhibited a rapid bactericidal activity with a decrease in the viable count by ≥ 3 log10 CFU/ml after 6 h of contact when tested against a fluoroquinolone-resistant isolate and complete killing when tested against a fluoroquinolone-sensitive strain with inhibition of regrowth in both cases (Fig. 1A–D and 2A–D). Similar rapid bactericidal activity was reported by Park et al. (2016), when ZAB was tested against clinical isolates of S. pneumoniae.
In S. aureus, topoisomerase IV and the A subunit of the DNA gyrase are the primary targets for quinolones (Sierra et al. 2002; Karpiuk and Tyski 2013; Fernandes and Martens 2017); consequently, the gyrA, parC and parE genes were screened for mutations in seven isolates showing different patterns of quinolone resistance. Isolates displaying resistance to ZAB (MIC: 2–4 µg/ml) were found to have their amino acids altered in positions: Ser84 in GyrA, Ser80 in ParC and Pro451 in ParE. Mutations at Ser84 in GyrA, Ser80 in ParC (Yun et al. 2005) and Pro451 in ParE (Hannachi-M’Zali et al. 2002) were reported to be associated with a high-level of quinolone resistance in S. aureus. A single point mutation or double point mutation were not able to elicit a considerable increase in MIC values of ZAB (Table III). Park et al. (2016) reported previously that multiple mutations are required to produce significant elevations in MIC values of ZAB against S. pneumoniae isolates. Dual targeting and high susceptibility are desirable features in ZAB underlying its importance as a possible candidate for the treatment of infections caused by MRSA. The association of efflux mechanism with elevated MICs values has been reported for CIP and MOX (Kaatz et al. 2002) while ZAB was shown to be a poor efflux substrate (Park et al. 2016), similar finding was obtained in the present study suggesting that ZAB is a poor substrate for NorA efflux pump.
The in vivo studies demonstrated that ZAB possessed the most potent protective effect against systemic infection caused by a MRSA strain isolated from bronchoalveolar lavage (Table IV), similar findings being reported by Kwon et al. (2006) and Park et al. (2006). The histopathological examination of lung specimens of mice infected with MRSA isolate and treated with ZAB displayed least congestion, inflammation, oedema and necrosis (Table V; Fig. 3); the examined alveoli displayed clear alveolar spaces and normal vessels (Fig. 4).
In conclusion, ZAB, a new fluoroquinolone not yet introduced to the Egyptian market, was proved to possess high in vitro and in vivo potency encompassing its comparators and might be regarded as an effective treatment option for infections caused by MRSA. The clinical usefulness of ZAB needs further establishment and confirmation in studies with larger number of isolates. To our knowledge, this is the first study evaluating the in vitro and in vivo activities of ZAB against Egyptian MRSA clinical isolates.
Acknowledgments
The authors acknowledge Dongwha Pharm. Co., Ltd. for kindly supplying zabofloxacin powder used in this study. No funding was provided.
Footnotes
Conflict of interest
The authors do not report any financial or personal connections with other persons or organizations, which might negatively affect the contents of this publication and/or claim authorship rights to this publication.
Literature
- Abdel-Maksoud M, El-Shokry M, Ismail G, Hafez S, El-Kholy A, Attia E, Talaat M.. Methicillin-Resistant Staphylococcus aureus recovered from healthcare- and community-associated infections in Egypt. Int J Bacteriol. 2016;2016:1–5. doi: 10.1155/2016/5751785 Medline [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg MA, de Kraker M, Scicluna E, van de Sande-Bruinsma N, Tiemersma E, Monen J, Grundmann H; ARMed Project Members and Collaborators.. Prevalence of methicillin-resistant Staphy lo coccus aureus (MRSA) in invasive isolates from southern and eastern Mediterranean countries. J Antimicrob Chemother. 2007; 60(6):1310–1315. doi: 10.1093/jac/dkm365 Medline [DOI] [PubMed] [Google Scholar]
- CLSI.. Methods for determining Bactericidal Activity of Antimicro bial Agents: approved guideline M26-A, 1999. Wayne (USA): Clinical and Laboratory Standards Institute. [Google Scholar]
- CLSI.. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically: approved standard-Ninth Edition M07-A9. 2012. Wayne (USA): Clinical and Laboratory Standards Institute. [Google Scholar]
- CLSI.. Performance standards for antimicrobial susceptibility testing. CLSI approved standard M100-S23. 2013. Wayne (USA): Clinical and Laboratory Standards Institute. [Google Scholar]
- Dubin PJ, Kolls JK.. IL-23 mediates inflammatory responses to mucoid Pseudomonas aeruginosa lung infection in mice. Am J Physiol Lung Cell Mol Physiol. 2007;292(2):L519–L528. doi: 10.1152/ajplung.00312.2006 Medline [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falagas ME, Karageorgopoulos DE, Leptidis J, Korbila IP.. MRSA in Africa: filling the global map of antimicrobial resistance. PLoS One. 2013;8(7):e68024. doi: 10.1371/journal.pone.0068024 Medline [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes P, Martens E.. Antibiotics in late clinical development. Biochem Pharmacol. 2017;133:152–163. doi: 10.1016/j.bcp.2016.09.025 Medline [DOI] [PubMed] [Google Scholar]
- Hannachi-M’Zali F, Ambler JE, Taylor CF, Hawkey PM.. Examination of single and multiple mutations involved in resistance to quinolones in Staphylococcus aureus by a combination of PCR and denaturing high-performance liquid chromatography (DHPLC). J Antimicrob Chemother. 2002;50(5):649–655. doi: 10.1093/jac/dkf243 Medline [DOI] [PubMed] [Google Scholar]
- Kaatz GW, Moudgal VV, Seo SM.. Identification and charac teriza tion of a novel efflux-related multidrug resistance phenotype in Staphylococcus aureus. J Antimicrob Chemother. 2002;50(6):833–838. doi: 10.1093/jac/dkf224 Medline [DOI] [PubMed] [Google Scholar]
- Karpiuk I, Tyski S.. Looking for the new preparations for antibacterial therapy III. New antimicrobial agents from the quinolones group in clinical trials. Przegl Epidemiol. 2013;67(3):455–460. Medline [PubMed] [Google Scholar]
- Kocsis B, Domokos J, Szabo D.. Chemical structure and pharmacokinetics of novel quinolone agents represented by avarofloxacin, delafloxacin, finafloxacin, zabofloxacin and nemonoxacin. Ann Clin Microbiol Antimicrob. 2016;15(1):34. doi: 10.1186/s12941-016-0150-4 Medline [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocsis B, Szabo D.. Zabofloxacin for chronic bronchitis. Drugs Today (Barc). 2016;52(9):495–500. doi: 10.1358/dot.2016.52.9.2530595 Medline [DOI] [PubMed] [Google Scholar]
- Kwon AR, Min YH, Ryu JM, Choi DR, Shim MJ, Choi EC.. In vitro and in vivo activities of DW-224a, a novel fluoroquinolone antibiotic agent. J Antimicrob Chemother. 2006;58(3):684–688. doi: 10.1093/jac/dkl304 Medline [DOI] [PubMed] [Google Scholar]
- Pan XS, Ambler J, Mehtar S, Fisher LM.. Involvement of topoisomerase IV and DNA gyrase as ciprofloxacin targets in Streptococcus pneumoniae. Antimicrob Agents Chemother. 1996;40(10):2321–2326. doi: 10.1128/AAC.40.10.2321 Medline [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HS, Jung SJ, Kwak JH, Choi DR, Choi EC.. DNA gyrase and topoisomerase IV are dual targets of zabofloxacin in Streptococcus pneumoniae. Int J Antimicrob Agents. 2010;36(1):97–98. doi: 10.1016/j.ijantimicag.2010.02.022 Medline [DOI] [PubMed] [Google Scholar]
- Park HS, Kim HJ, Seol MJ, Choi DR, Choi EC, Kwak JH.. In vitro and in vivo antibacterial activities of DW-224a, a new fluoronaphthyridone. Antimicrob Agents Chemother. 2006;50(6):2261–2264. doi: 10.1128/AAC.01407-05 Medline [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HS, Oh SH, Kim HS, Choi DR, Kwak JH.. Antimicrobial activity of zabofloxacin against clinically isolated Streptococcus pneumoniae. Molecules. 2016;21(11):1562. doi: 10.3390/molecules21111562 Medline [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randhawa MA. Calculation of LD50 values from the method of Miller and Tainter, 1944. J Ayub Med Coll Abbottabad. 2009;21(3):184–185. Medline [PubMed] [Google Scholar]
- Sadaka SM, El-Ghazzawy EF, Harfoush RA, Meheissen M.. Evaluation of different methods for the rapid diagnosis of methi cillin-resistance in Staphylococcus aureus. Afr J Microbiol Res. 2009;3:49–55. [Google Scholar]
- Sierra JM, Marco F, Ruiz J, Jiménez de Anta MT, Vila J.. Correlation between the activity of different fluoroquinolones and the presence of mechanisms of quinolone resistance in epidemiologically related and unrelated strains of methicillin-susceptible and -resistant Staphylococcus aureus. Clin Microbiol Infect. 2002;8(12):781–790. doi: 10.1046/j.1469-0691.2002.00400.x Medline [DOI] [PubMed] [Google Scholar]
- Tokajian S. New epidemiology of Staphylococcus aureus infections in the Middle East. Clin Microbiol Infect. 2014;20(7):624–628. doi: 10.1111/1469-0691.12691 Medline [DOI] [PubMed] [Google Scholar]
- Vidaillac C, Guillon J, Arpin C, Forfar-Bares I, Ba BB, Grellet J, Moreau S, Caignard DH, Jarry C, Quentin C.. Synthesis of omeprazole analogues and evaluation of these as potential inhibitors of the multidrug efflux pump NorA of Staphylococcus aureus. Antimicrob Agents Chemother. 2007;51(3):831–838. doi: 10.1128/AAC.01306-05 Medline [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun HJ, Min YH, Jo YW, Shim MJ, Choi EC.. Increased antibacterial activity of DW286, a novel fluoronaphthyridone antibiotic, against Staphylococcus aureus strains with defined mutations in DNA gyrase and topoisomerase IV. Int J Antimicrob Agents. 2005; 25(4):334–337. doi: 10.1016/j.ijantimicag.2004.11.013 Medline [DOI] [PubMed] [Google Scholar]


