Abstract
Gastric microbiota provides a biological barrier against the invasion of foreign pathogens from the oral cavity, playing a vital role in maintaining gastrointestinal health. Klebsiella spp. of oral origin causes various infections not only in gastrointestinal tract but also in other organs, with Klebsiella pneumoniae serotype K1 resulting in a liver abscess (KLA) through oral inoculation in mice. However, the relationship between gastric microbiota and the extra-gastrointestinal KLA infection is not clear. In our study, a 454 pyrosequencing analysis of the bacterial 16S rRNA gene shows that the composition of gastric mucosal microbiota in mice with or without KLA infection varies greatly after oral inoculation with K. pneumoniae serotype K1 isolate. Interestingly, only several bacteria taxa show a significant change in gastric mucosal microbiota of KLA mice, including the decreased abundance of Bacteroides, Alisptipes and increased abundance of Streptococcus. It is worth noting that the abundance of Klebsiella exhibits an obvious increase in KLA mice, which might be closely related to KLA infection. At the same time, the endogenous antibiotics, defensins, involved in the regulation of the bacterial microbiota also show an increase in stomach and intestine. All these findings indicate that liver abscess caused by K. pneumoniae oral inoculation has a close relationship with gastric microbiota, which might provide important information for future clinical treatment.
Key words: Klebsiella pneumoniae serotype K1, defensins, gastric mucosal microbiota, High-throughput pyrosequencing, liver abscess
Introduction
Klebsiella pneumoniae as one of the most important pathogens leads to many infections including nosocomial and community-acquired infections. K1 or K2 serotype K. pneumoniae-caused pyogenic liver abscess (KLA) is a highly invasive community-acquired infection and often develops serious complications (Siu et al. 2012). Previous studies show that liver abscess infection can be caused by many pathogens and that K. pneumoniae causes less than 10% liver infection (Lee et al. 1991; Zibari et al. 2000). Recently the rate of KLA infection has increased greatly even up to 70% in some regions (Kuo et al. 2013). And drug-resistant K. pneumoniae isolated from KLA infection has been reported to be increased in Beijing (Li et al. 2014).
It is well known that oral-gastrointestinal tract is one of the main pathways for the foreign pathogen, which causes human infection. Klebsiella spp. of oral origin causes various diseases not only in the gastrointestinal tract but also in other organs. Atarashi et al. (2017) hold that oral Klebsiella spp. colonizing the intestines causes inflammatory bowel disease (IBD). It has also been identified that oral inoculation of K. pneumoniae K1 or K2 serotype isolates causes liver abscess infections in mice (Tu et al. 2009; Chen et al. 2014).
Gastric microbiota provides a biological barrier against the invasion of foreign pathogens entering the oral pathway, playing a vital role in maintaining gastrointestinal health. (Bik et al. 2006). For example, Helicobacter pylori can successfully colonize stomach tissues via the oral pathway what results in infection. It has been identified that H. pylori infections can change the overall composition of stomach microbiota (Lofgren et al. 2011). Probiotics have been used for clinical treatment of gastritis caused by H. pylori (Sheu et al. 2002; Lesbros-Pantoflickova et al. 2007). But the relationship between the microbiota in gastric mucosal and extra-gastrointestinal liver abscess infection caused by K. pneumoniae through the oral pathway is not clear.
Experimental
Materials and Methods
Mouse model of KLA infection. In our previous study, K. pneumoniae K1 serotype clinical isolate Kp1002 was identified to cause KLA infection in C57BL/6 mice by oral inoculation (Chen et al. 2014). In the present study, six-week-old male C57BL/6 mice were orally inoculated with 106 CFU of Kp1002 using a 21-gauge feeding needle as previously described, with the phosphate buffered saline (PBS) given to the healthy control. All the mice were killed 48 h after oral inoculation. The serum, stomachs and cecum samples were retrieved for the following analysis. Mice with Kp1002 inoculation were divided into two groups: mice with KLA infection (KLA group) and mice without KLA infection (NKLA group) according to the criterion in our previous study (Chen et al. 2014). Five mice were randomly selected from each group and their samples were prepared for the following analysis. Mice in this study were raised in the Specific Pathogen Free (SPF) animal house. All the animal experiments in this study were conducted in accordance with the National Research Council Guide for Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the First Affiliated Hospital of Zhejiang Medical University (2013–012) and the Animal Welfare and Ethical Committee of Hebei University (2018026).
DNA extraction and high-throughput sequencing analysis. After mice sacrificed, the stomachs of the mice were isolated, with the corpus tissues divided and used in DNA extraction. DNA was extracted from 25 mg corpus tissue using a PureLink™ Genomic DNA Mini Kit (Invitrogen, USA) according to the manufacturer’s instructions, with addition of lysozyme at the concentration of 20 mg/ml (Sigma-Aldrich, USA) at the beginning of cell lysis. DNA concentrations were tested by NanoDrop ND-1000 spectrophotometer (Thermo Electron Corporation). DNA integrity and size were checked by agarose gel electrophoresis. The DNA was stored at –80°C for the 16S rRNA gene sequencing analysis. 16S rRNA gene was amplified by PCR with primers 27F (5’-AGAGTTTGATCCTGGCTCAG-3’) and 533R (5’-TTACCGCGGCTGCTGGCAC-3’) (Ling et al. 2015, Chen et al. 2018). The 16S rRNA gene amplification products were sequenced by the 454 Life Sciences genome sequencer FLX system (Roche, Switzerland). The metagenomic sequence data were deposited in the NCBI Sequence Read Archive under Accession number SRP111617. The sequencing data processing methods were described in our previous study (Ling et al. 2015, Chen et al. 2018).
Biosample collection and analysis of defensins and cytokines. After mice sacrificed, the stomachs and cecum of the mice were isolated. The contents of the stomachs or cecum were collected as previously described (Li et al. 2015). The contents were removed and washed off with an isopycnic 0.09% NaCl solution. The washout material was centrifuged at 10 000 rpm for 20 min at 4°C. Then, the supernatant was harvested for the defensins analysis using an ELISA test kit (Mlbio, Shanghai, China). Serum samples were separated and stored at –80°C for the analysis of the cytokines using an ELISA test kit (Mlbio, Shanghai, China).
Data processing and statistical analysis. Mothur (http://www.mothur.org) was used for diversity and taxonomy-based analyses at a 97% similarity level as previously described (Ling et al. 2015, Chen et al. 2018). The Mann-Whitney U test was performed with the SPSS 16.0 software, and the ELISA analysis data statistical analyses were performed using One-way ANOVA analysis as implemented in the SPSS 16.0 software.
Results
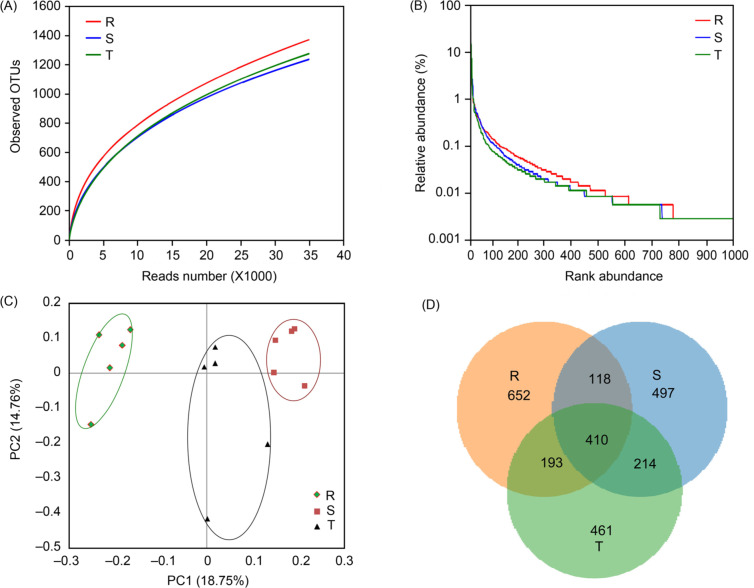
The overall structure of the gastric mucosal microbiota alteration after oral inoculation with K. pneumoniae K1 serotype isolate. Gastric mucus samples were obtained from KLA, NKLA, and Kp1002-untreated healthy control groups and the 16S rRNA gene was sequenced. Totally 153 397 high-quality reads were obtained after sequencing analysis, including 46 329 sequences from a healthy group, 53 863 sequences from NKLA group and 53 205 sequences from KLA group. These sequences had an average length of 477 bp (range from 400 bp to 521 bp). Operational taxonomic unit (OTU) was identified at the 97% similarity level. The coverage percentage was calculated by Good’s method (Ling et al. 2015, Chen et al. 2018) and the coverage percentage values of healthy, NKLA and KLA groups were 98.27%, 98.54%, and 98.40%, respectively. These values showed sufficient sequencing depth in this study. The richness estimators (ACE and Chao1 value) and diversity indices (Shannon and Simpson indices) displayed no obvious difference between healthy and Kp1002-treated groups (p > 0.05) (Fig. S1). Rarefaction analysis estimates showed that species richness differed between the healthy and Kp1002-treated groups (Fig. 1A). The rank-abundance curves generated from OTU analysis also revealed differences between the healthy and Kp1002-treated groups (Fig. 1B). Beta-diversity indices analysis was performed by the unweighted UniFrac method and principal coordinate analysis (PCoA). Unweighted PCoA revealed obvious differences among the three groups, indicating variations in microbial communities (Fig. 1C). At the same time, PCoA of weighted UniFrac analysis also showed the different trends among the three groups (Fig. S2). A Venn diagram showed different OTU data in three groups and that the overlapping OTU data. Totally 3890 OTUs were obtained from three groups. In addition, 624 common OTUs were shared by the KLA and NKLA groups, which was higher than that shared by the healthy group and any of the other groups (Fig. 1D). These results suggested that the overall structure of gastric mucosal microbiota was obviously altered in Kp1002-treated mice in comparison with that in healthy mice, whereas it did not show a great difference between KLA group and NKLA group.
Fig. 1.
Overall structure alteration in gastric microbiota.
The species richness (A) and Rank abundance curve (B) were based on OTUs analysis of the three groups. (C) PCoA of weighted UniFrac analysis plot of gastric microbiota of three groups. (D) Venn diagram showed the OTUs overlaps among the three groups. (R, healthy group; S, NKLA group; T, KLA group).
Major alterations in the composition of gastric mucosal microbiota in KLA mice. The sequences obtained in this study belonged to twelve bacterial phyla, with eleven of them found in healthy mice, including the most abundant phyla Bacteroidetes and Firmicutes. At the genus level, 93 genera were obtained. The healthy group consists of 46 genera with four abundant genera (> 1% of the total DNA sequences), including Lactobacillus, Bacteroides, Alistipes, and Clostridium sensu stricto. There were 47 genera in the NKLA group with six abundant genera, and 59 genera in the KLA group with six abundant genera.
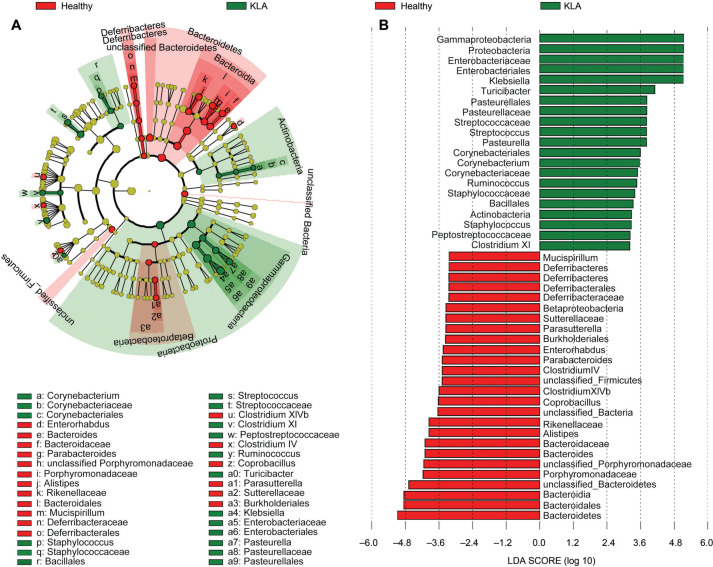
The linear discriminant analysis effect size (LEfSe) method was employed in this study to assess alterations in the composition of gastric mucosal microbiota in KLA mice. The gastric mucosal microbiota composition was significantly altered in KLA-infected mice when compared to that in healthy mice (Fig. 2). At the same time since the overall structure of gastric mucous microbiota showed differences between healthy mice and Kp1002-treated mice in Fig. 1, and for better understanding of the special changes in gastric microbiota from KLA infection mice, the significant differences in microbiota composition between NKLA and KLA mice were also explored. The specific changes of gastric mucosal microbiota in KLA mice were summarized in Fig. 3.
Fig. 2.
The differences in gastric microbiota composition between healthy and KLA groups by LEfSe analysis. Taxonomic cladogram (A) and Linear discriminative analysis (LDA) scores (B) exhibited the different enriched bacteria taxa between healthy group (Red) and KLA group (Green). All of the taxa showed in the figure with LDA threshold value > 2.
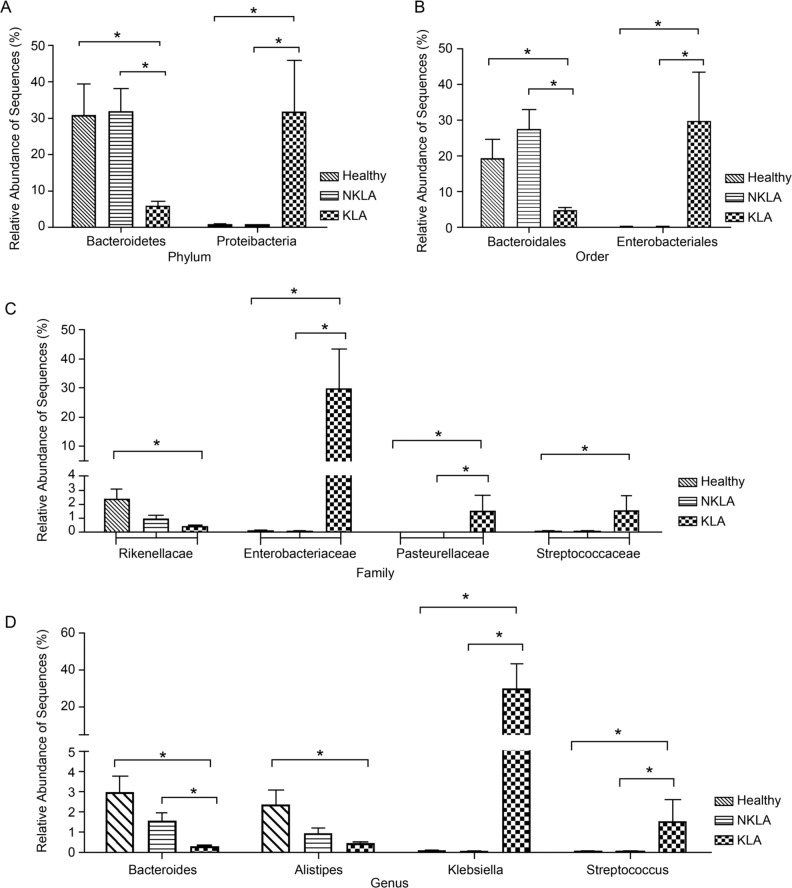
Fig. 3.
Comparing the differences at the relative abundance of bacterial sequences at the different levels including phylum, order, family and genus level among three groups. (Mann-Whitney U test, *: p < 0.05).
The KLA and healthy mice showed significant differences in the abundance of phylum Bacteroidetes, a marked decrease in the KLA group compared with that in the healthy group (p < 0.05). In addition, the abundance of the non-predominant phylum Proteobacteria in the KLA group significantly increased (p < 0.05) (Fig. 3A). At the order level analyses, the great changes were observed in KLA infection mice. Compared with the healthy mice, the KLA infection mice showed an obvious decrease in the abundance of Bacteroidales and a significant increase in Enterobacteriales (p < 0.05) (Fig. 3B). At the family level, the data showed that the KLA infection mice exhibited a great decrease in the abundance of Rikenellaceae and a marked increase in the abundance of Enterobacteriaceae, Pasteurellaceae, and Streptococcaceae compared to the healthy mice (p < 0.05) (Fig. 3C). Further detailed analyses at the genus level showed that a great decrease in the abundance of Bacteroides and Alistipes and an obvious increase in Klebsiella and Streptococcus in the KLA group compared to the healthy group (p < 0.05) (Fig. 3D). The differences between the KLA and NKLA groups displayed trends in gastric mucosal microbiota composition at the phylum, order, family and genus level similar to that between the healthy and KLA groups (Fig. 3).
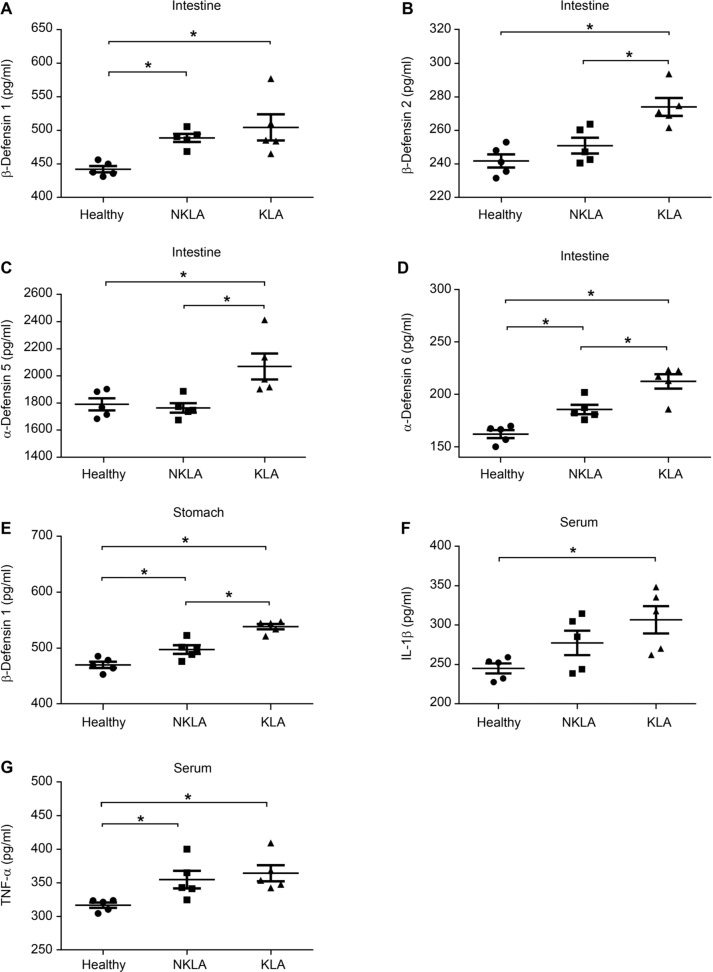
K. pneumoniae oral inoculation changes immune barrier. In our previous study, we found that cecal contents microbiota significant changes in KLA mice (Chen et al. 2018). Herein, we examined the immune barrier in the stomach and intestine. Fig. 4 shows alterations in defensins and cytokines in KLA mice. Compared with healthy mice, KLA infection mice showed significant increases in β-defensin 1, β-defensin 2, α-defensin 5, α-defensin 6 in the cecum, and β-defensin 1 in the stomach (p < 0.05). The levels of serum cytokines interleukin 1 beta (IL-1β) and tumor necrosis factor alpha (TNF-α) also significantly increased in the KLA mice compared to that of healthy mice (p < 0.05). On the other hand, compared with healthy mice, NKLA mice also showed a great increase in β-defensin 1 and α-defensin 6 in the cecum, and β-defensin 1 in the stomach when compared to that of healthy mice (p < 0.05).
Fig. 4.
Kp1002 oral inoculation changes gastric and intestinal defensins and serum inflammatory cytokines.
Both β-defensin 1 concentration in the intestine (A) and stomach (E) showed a great increase. The concentration of β-defensin 2 (B), α-defensin 5 (C) and α-defensin 6 (D) in intestine showed a significant increase. The levels of serum cytokines IL-1β (F) and TNF-α (G) also increased. (One-way ANOVA analysis, *: p < 0.05).
Discussion
Our previous study indicates that KLA infections are accompanied by changes in intestinal microbiota composition (Chen et al. 2018). The intestinal microbiota has been widely accepted to be an important biological barrier against foreign pathogens. The stomach has long been considered to be sterile due to the low pH of the gastric acid secreted by goblet cells. However, stomach actually consists of a microbial community, which provides a biological barrier against the invasion of foreign pathogens, especially pathogens of oral origin such as H. pylori, and maintains the gastrointestinal health (Bik et al. 2006; Andersson et al. 2008; Lofgren et al. 2011). To better understand the relationship between gastric microbiota and extra-gastrointestinal KLA infections, this study analyzes changes in gastric microbiota composition in KLA mice.
The present study observes great alterations in gastric mucosal microbiota composition in KLA mice. KLA mice show a significant reduction in Bacteroidetes in the gastric microbiota, with a great reduction in the abundance of Bacteroidetes in the gastric microbiota of H. pylori-infected mice (Lofgren et al. 2011). In human, positive H. pylori status is also associated with decreased abundance of Bacteroidetes (Maldonado-Contreras et al. 2011). Gastritis in male mice is accompanied by decreased Bacteroides species ASF519 colonization, indicating that inflammation-driven atrophy alters the gastric niche for the gastrointestinal commensal bacteria colonization (Lertpiriyapong et al. 2014). It seems that the decrease in the abundance of phylum Bacteroidetes or genus Bacteroides might be closely related to the gastrointestinal and extra-gastrointestinal infections caused by foreign pathogen through an oral pathway.
At the same time, the abundance of Klebsiella significantly increased in gastric mucosa of KLA infection mice. Sung et al. (2016) used 454-pyrosequencing to compare the microbiota composition between gastric fluid and gastric mucosa samples and found that using gastric mucosa samples was more effective in detecting meaningful bacteria, such as H. pylori, nitrosating or nitrate-reducing bacteria. In our study, the increase in the abundance of Klebsiella in gastric mucosa might be an effective bioindicator for KLA infection.
On the other hand, in the gastrointestinal tract, the constitutive expression of defensins imparts the regulation of the bacterial microbiota and immunomodulatory activity and is involved in the pathogenesis of various intestinal infection diseases (Wehkamp et al. 2005). α-defensin 5 has been reported to have microbicidal activity against various bacteria, including Escherichia coli and Staphylococcus aureus (Ouellette et al. 1994). The production of β-defensins 2, 3, and 4 is significantly higher in ulcerative colitis (Rahman et al. 2011). The present study also finds out that the defensin expression levels in the stomach or cecum are higher in KLA mice. Simultaneously, the elevated proinflammatory cytokines IL-1β and TNF-α could be induced by β-defensins (Ghosh et al. 2011). These findings show the changes in the gastrointestinal microbiota in response to K. pneumoniae, as well as an increase of the expression of defensins and cytokines.
It should not be ignored that Kp1002-treated mice without KLA infection also exhibited changes in the gastrointestinal microbiota and increased expression levels of defensins relative to that in healthy mice. We also find that after oral inoculation with the K. pneumoniae isolate, mouse infection rates continuously increase after 48 h (data not shown). These changes in gastrointestinal microbiota and defensins expression levels may be related to the infection process, thereby providing new insight into KLA treatment.
Supplementary materials are available on the journal’s website.
Acknowledgements
This study was supported by Natural Science Foundation of Hebei Province (No. H2015201076), Preferential funding for postdoctoral research projects of Hebei Province (No. B2017003032), and Graduate students’ innovative funding projects of Hebei University (No. hbu2018ss75).
We thank the support from the Affiliated Hospital of Hebei University Postdoctoral workstation and Hebei University Biology Postdoctoral research station. We also thank the support from Key Bioengineering Discipline of Hebei Province, Engineering Laboratory of Microbial Breeding and Preservation of Hebei Province.
Footnotes
Conflict of interest
The authors do not report any financial or personal connections with other persons or organizations, which might negatively affect the contents of this publication and/or claim authorship rights to this publication.
Literature
- Andersson AF, Lindberg M, Jakobsson H, Bäckhed F, Nyrén P, Engstrand L.. Comparative analysis of human gut microbiota by barcoded pyrosequencing. PLoS One. 2008. Jul 30;3(7):e2836. doi: 10.1371/journal.pone.0002836 Medline [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atarashi K, Suda W, Luo C, Kawaguchi T, Motoo I, Narushima S, Kiguchi Y, Yasuma K, Watanabe E, Tanoue T, et al.. Ectopic colonization of oral bacteria in the intestine drives TH1 cell induction and inflammation. Science. 2017. Oct 20;358(6361):359–365. doi: 10.1126/science.aan4526 Medline [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bik EM, Eckburg PB, Gill SR, Nelson KE, Purdom EA, Francois F, Perez-Perez G, Blaser MJ, Relman DA.. Molecular analysis of the bacterial microbiota in the human stomach. Proc Natl Acad Sci USA. 2006. Jan 17;103(3):732–737. doi: 10.1073/pnas.0506655103 Medline [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N, Ling ZX, Jin TT, Li M, Zhao S, Zheng LS, Xi X, Wang LL, Chen YY, Shen YL, et al.. Altered profiles of gut microbiota in Klebsiella pneumoniae-induced pyogenic liver abscess. Curr Microbiol. 2018. Jul;75(7):952–959. doi: 10.1007/s00284-018-1471-7 Medline [DOI] [PubMed] [Google Scholar]
- Chen N, Wang L, Xue J, Ma X, Zhao S, Rong R, Li H, Ding L, Zheng M, Chen Y, et al.. Different metabolic profiles of K1 serotype and non-serotype K1 and K2 Klebsiella pneumoniae isolates in oral infection mice model. Microb Pathog. 2014. Oct;75:41–48. doi: 10.1016/j.micpath.2014.08.006 Medline [DOI] [PubMed] [Google Scholar]
- Ghosh SK, Gupta S, Jiang B, Weinberg A.. Fusobacterium nucleatum and human beta-defensins modulate the release of antimicrobial chemokine CCL20/macrophage inflammatory protein 3α. Infect Immun. 2011. Nov;79(11):4578–4587. doi: 10.1128/IAI.05586-11 Medline [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo SH, Lee YT, Li CR, Tseng CJ, Chao WN, Wang PH, Wong RH, Chen CC, Chen SC, Lee MC.. Mortality in Emergency Department Sepsis score as a prognostic indicator in patients with pyogenic liver abscess. Am J Emerg Med. 2013. Jun;31(6):916–921. doi: 10.1016/j.ajem.2013.02.045 Medline [DOI] [PubMed] [Google Scholar]
- Lee KT, Sheen PC, Chen JS, Ker CG.. Pyogenic liver abscess: multivariate analysis of risk factors. World J Surg. 1991. May;15(3): 372–376, discussion 376–377. doi: 10.1007/BF01658732 Medline [DOI] [PubMed] [Google Scholar]
- Lertpiriyapong K, Whary MT, Muthupalani S, Lofgren JL, Gamazon ER, Feng Y, Ge Z, Wang TC, Fox JG.. Gastric colonisation with a restricted commensal microbiota replicates the promotion of neoplastic lesions by diverse intestinal microbiota in the Helicobacter pylori INS-GAS mouse model of gastric carcinogenesis. Gut. 2014. Jan;63(1):54–63. doi: 10.1136/gutjnl-2013-305178 Medline [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesbros-Pantoflickova D, Corthésy-Theulaz I, Blum AL.. Helicobacter pylori and probiotics. J Nutr. 2007. Mar 01;137(3) Suppl 2: 812S–818S. doi: 10.1093/jn/137.3.812S Medline [DOI] [PubMed] [Google Scholar]
- Li M, Liang P, Li Z, Wang Y, Zhang G, Gao H, Wen S, Tang L.. Fecal microbiota transplantation and bacterial consortium transplantation have comparable effects on the re-establishment of mucosal barrier function in mice with intestinal dysbiosis. Front Microbiol. 2015. Jul 07;6:692. doi: 10.3389/fmicb.2015.00692 Medline [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Sun G, Yu Y, Li N, Chen M, Jin R, Jiao Y, Wu H.. Increasing occurrence of antimicrobial-resistant hypervirulent (hypermucoviscous) Klebsiella pneumoniae isolates in China. Clin Infect Dis. 2014. Jan 15;58(2):225–232. doi: 10.1093/cid/cit675 Medline [DOI] [PubMed] [Google Scholar]
- Ling Z, Liu X, Cheng Y, Jiang X, Jiang H, Wang Y, Li L.. Decreased diversity of the oral microbiota of patients with hepatitis B virus-induced chronic liver disease: a pilot project. Sci Rep. 2015. Dec; 5(1):17098. doi: 10.1038/srep17098 Medline [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lofgren JL, Whary MT, Ge Z, Muthupalani S, Taylor NS, Mobley M, Potter A, Varro A, Eibach D, Suerbaum S, et al.. Lack of commensal flora in Helicobacter pylori-infected INS-GAS mice reduces gastritis and delays intraepithelial neoplasia. Gastroenterology. 2011. Jan;140(1):210–220.e4. doi: 10.1053/j.gastro.2010.09.048 Medline [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado-Contreras A, Goldfarb KC, Godoy-Vitorino F, Karaoz U, Contreras M, Blaser MJ, Brodie EL, Dominguez-Bello MG.. Structure of the human gastric bacterial community in relation to Helicobacter pylori status. ISME J. 2011. Apr;5(4):574–579. doi: 10.1038/ismej.2010.149 Medline [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouellette AJ, Hsieh MM, Nosek MT, Cano-Gauci DF, Huttner KM, Buick RN, Selsted ME.. Mouse Paneth cell defensins: primary structures and antibacterial activities of numerous cryptdin isoforms. Infect Immun. 1994. Nov;62(11):5040–5047. Medline [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman A, Fahlgren A, Sundstedt C, Hammarström S, Danielsson Å, Hammarström ML.. Chronic colitis induces expression of β-defensins in murine intestinal epithelial cells. Clin Exp Immunol. 2011. Jan;163(1):123–130. doi: 10.1111/j.1365-2249.2010.04282.x Medline [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheu BS, Wu JJ, Lo CY, Wu HW, Chen JH, Lin YS, Lin MD.. Impact of supplement with Lactobacillus- and Bifidobacterium-con taining yogurt on triple therapy for Helicobacter pylori eradication. Aliment Pharmacol Ther. 2002. Sep;16(9):1669–1675. doi: 10.1046/j.1365-2036.2002.01335.x Medline [DOI] [PubMed] [Google Scholar]
- Siu LK, Yeh KM, Lin JC, Fung CP, Chang FY.. Klebsiella pneumoniae liver abscess: a new invasive syndrome. Lancet Infect Dis. 2012. Nov;12(11):881–887. doi: 10.1016/S1473-3099(12)70205-0 Medline [DOI] [PubMed] [Google Scholar]
- Sung J, Kim N, Kim J, Jo HJ, Park JH, Nam RH, Seok YJ, Kim YR, Lee DH, Jung HC.. Comparison of gastric microbiota between gastric juice and mucosa by next generation sequencing method. J Cancer Prev. 2016. Mar 30;21(1):60–65. doi: 10.15430/JCP.2016.21.1.60 Medline [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu YC, Lu MC, Chiang MK, Huang SP, Peng HL, Chang HY, Jan MS, Lai YC.. Genetic requirements for Klebsiella pneumoniae-induced liver abscess in an oral infection model. Infect Immun. 2009. Jul 01;77(7):2657–2671. doi: 10.1128/IAI.01523-08 Medline [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehkamp J, Fellermann K, Herrlinger KR, Bevins CL, Stange EF.. Mechanisms of Disease: defensins in gastrointestinal diseases. Nat Clin Pract Gastroenterol Hepatol. 2005. Sep;2(9):406–415. doi: 10.1038/ncpgasthep0265 Medline [DOI] [PubMed] [Google Scholar]
- Zibari GB, Maguire S, Aultman DF, McMillan RW, McDonald JC.. Pyogenic liver abscess. Surg Infect (Larchmt). 2000. Apr;1(1):15–21. doi: 10.1089/109629600321254 Medline [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary materials are available on the journal’s website.