Abstract
Microbiological contamination is a big challenge to the food industry, medicine, agriculture, and environmental protection. For this reason, scientists are constantly looking for alternative methods of decontamination, which ensure the effective elimination of unwanted biological agents. Cold plasma is a new technology, which due to its unique physical and chemical properties becomes a point of interest to a growing group of researchers. The previously conducted experiments confirm its effective action, e.g. in the disinfection of skin wounds, air, and sewage treatment, as well as in food preservation and decontamination. The reactive compounds present in the plasma: high-energy electrons, ionized atoms and molecules, and UV photons are the key factors that cause an effective reduction in the number of microorganisms. The mechanism and effectiveness of the cold plasma are complex and depend on the process parameters, environmental factors and the type and properties of the microorganisms that are to be killed. This review describes the current state of knowledge regarding the effectiveness of the cold plasma and characterizes its interaction with various groups of microorganisms based on the available literature data.
Key words: biofilm, cellular response, cold plasma, mycotoxin, viruses
Introduction
Cold plasma (CP) or nonthermal plasma (NTP) and, in particular, cold atmospheric plasma (CAP) is gaining increasing scientists’ interest, given the possibility of application thereof in medicine (Boudam and Moisan 2010; Isbary et al. 2010; Vandamme et al. 2010; Metelmann et al. 2018), food industry (Afshari and Hosseini 2014; Hojnik et al. 2017, Pignata et al. 2017), agriculture, and environmental protection (Bogaerts and Neyts 2018; Pawłat et al. 2018a; 2018b; Siddique et al. 2018). Many investigations have confirmed the positive effect of the plasma on the anticancer therapy (Kim et al. 2009; Vandamme et al. 2010), disinfection of skin wounds (Isbary et al. 2010; Lademann et al. 2011), surgical instruments and materials in contact with food (Boudam and Moisan 2010; Dasan et al. 2017b), purification of air, water, wastewater, and sewage, as well as preservation and decontamination of food (Gallagher et al. 2007; Korachi et al. 2009; Pawłat 2013; Chizoba Ekezie et al. 2017; Wolny-Koładka et al. 2017). Such a broad spectrum of the cold plasma applications is associated with its ability to inactivate biological factors as viruses (Terrier et al. 2009; Su et al. 2018), bacteria (Isbary et al. 2010; Samoń et al. 2014; Kartaschew et al. 2016), spores (Deng et al. 2006; Boudam and Moisan 2010), yeasts (Korachi et al. 2009; Metelmann et al. 2018), or fungi (Bayliss et al. 2012; Panngom et al. 2014; Siddique et al. 2018).
William Crookes discovered plasma in 1879 while the concept of the plasma was first used in the article by Irving Langmuir’s entitled “Oscillations in ionized gases” in 1928. Since then, plasma physics has become an important field of research. The plasma processing is used from the seventies when it was used to etch semiconductor materials. Then, in the 1980s, it was used in the computer industry, especially in the production of miniaturized circuits. In the last decade of the twentieth century, there has been a development of the plasma generation technology at an atmospheric pressure, which allowed to eliminate the expensive vacuum chambers. This has resulted in wider applications of cold plasma in medicine, environmental protection and food preservation (Misra et al. 2016).
Plasma, which is regarded as the fourth state of matter is a type of ionized gas containing many charged particles (OH–, H2O+, electrons), reactive compounds (reactive oxygen species – ROS, which include hydroxyl radical, superoxide anion hydrogen peroxide, and reactive nitrogen species – RNS, i.e., peroxynitrite), the molecules in the excited and basic states, and UV photons (UVB, UVC) (Brisset and Pawłat 2016; Bruggeman et al. 2016).
Plasma can be classified according to the generation conditions, i.e. atmospheric pressure (low-pressure plasma, high-pressure plasma), temperature (low-temperature plasma, high-temperature plasma), and the composition of plasma-generating gas (one-component plasma, multi-component plasma) (Dzimitrowicz et al. 2015; Bourke et al. 2017). The division of the plasma due to the temperature depends on the temperature of the electrons (Te). The high-temperature plasma is characterized by Te = 106 ~ 108 K, whereas for the low-temperature plasma the Te value is in the range from 104 to 105 K (Fridman et al. 2005). In addition, the low-temperature plasma is divided into thermal and non-thermal plasma due to the thermodynamic equilibrium. Inactivation of biological factors uses a non-thermal plasma that is characterized by a thermodynamic imbalance. Therefore, the electrons have a higher temperature than the temperature of the neutral particles (Tn), and the temperature of the gas (Tg), and thus, the temperature of the process increases slightly (Laskowska et al. 2016; Liao et al. 2017).
Non-equilibrium plasma (with temperatures of electrons substantially exceeding the temperatures of the other gas components) can be generated with the use of various electric discharges, e.g. corona, microwave, gliding arc, and dielectric barrier discharges as plasma sources. As a result of these discharges, the energy from the electric field is collected by electrons as a result of their collision, and only part of the energy is transferred to neutral molecules which results in the formation of the Te ≥ Tn state, characterized by non-thermal plasma. The type of the plasma source has directed the effect on the composition and the number of components generated by the plasma and determines the technological application of the plasma. The temperature of the plasma is extremely important in the processing of the materials that are sensitive to high temperatures. The optimization of the power supply system, an appropriate geometry of the discharge system, and the type of gas are essential when active agents generated by cold plasma (e.g. reactive compounds), whose temperature does not exceed several tens of degrees Celsius, have to be standardized. For the applications in agriculture, food industry, and medicine, the dielectric barrier discharges (DBD) and plasma jets are used most frequently for the generation of cold plasma due their simple structure and ease of modification (Pawłat et al. 2016; Bourke et al. 2017).
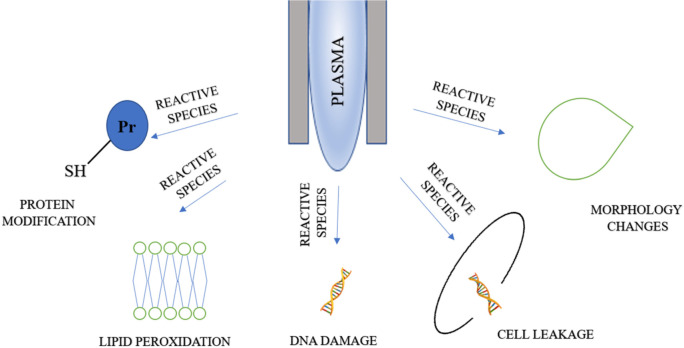
The mechanism involved in the cold plasma sterilization process has not yet been elucidated. The numerous attempts made by scientists confirm the inactivation of virus particles and microbial cells by the plasma, but the relationships between them have not been explored in details (Liao et al. 2017). The reactive compounds, high-energy electrons, ionized atoms and particles, and UV radiation are involved in the process of biological inactivation (Bourke et al. 2017). When in contact with biological material, the compounds derived from oxygen or nitrogen, i.e., O, O2, O3, OH·, NO, and NO2 are characterized by high reactivity. Their effects include oxidation of membrane lipids and proteins, which results in the disturbances of the proper membrane function and, finally, the disruption of the cell membrane (Afshari and Hosseini 2014). Membrane integrity is highly influenced by electrostatic forces. The charged particles generated by the plasma accumulate on the outer side of the membrane, thereby leading to its disintegration (Liao et al. 2017). The discontinuity of the cell surface structures can also be an effect of the electroporation process. This phenomenon involves an increase in the number of the existing cell micropores and the emergence of new ones induced by the pulsed electric field (Wiktor et al. 2013). The degradation of DNA caused by UV radiation could also be involved in the inactivation of microorganisms. Photons present in the plasma can alter the structure of the nucleic acids, leading to the formation of nitrogen base dimers and impairment of DNA replication capacity (Beggs 2002; Liao et al. 2017). The contribution of each of the mechanisms and their effectiveness in biological inactivation vary and depend primarily on the parameters of the plasma generation process, environmental factors, and the type and properties of the microorganisms (Fig. 1) (Bayliss et al. 2012).
Fig. 1.
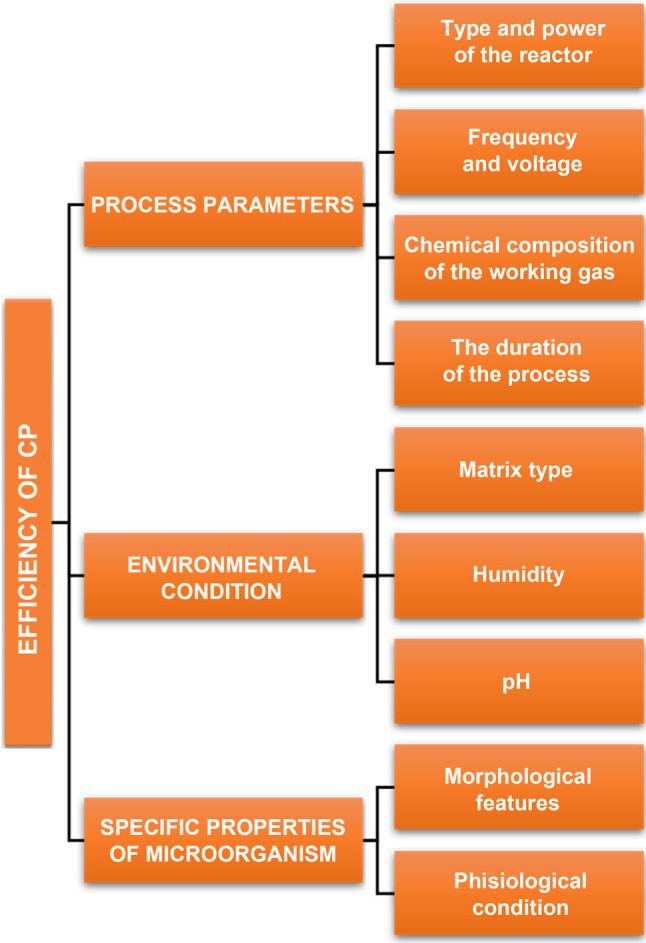
Factors influencing the effectiveness of decontamination using cold plasma. Based on Liao et al. 2017.
The aim of this review is to present the current knowledge on the antimicrobial activity of the plasma and to discuss the molecular mechanism of interactions between cold atmospheric plasma and various groups of microorganisms.
Effect of cold plasma on viruses
The cold plasma technology becomes a promising solution for inactivation of pathogenic viruses that cause infections in humans, animals, and plants (Bourke et al. 2017). The specific mechanisms of CP inactivation of viruses have not been elucidated yet. The studies carried out so far demonstrated that exposure to cold plasma can lead to the modification and/or degradation of proteins, nucleic acids, and lipids of viral envelopes (Pradeep and Chulkyoon 2016). The researches on the impact of the plasma on bacteriophages (a λ phage model system) suggested that the damage of capsid proteins is directly involved in the inactivation of viruses (Yasuda et al. 2010).
Cold plasma has been demonstrated to inactivate animal viruses. Terrier et al. (2009) investigated three types of viruses with the considerable clinical importance, which cause respiratory infections, e.g. respiratory syncytial virus (RSV), human parainfluenza virus type 3 (HPIV-3), and influenza A virus subtype H5N2. The plasma generated with air as the working gas contributed to a significant decrease in the titer of all viruses tested. Researchers indirectly associated this finding with the presence of ozone in the generated plasma that induces the protein damage and lipid peroxidation. The effect of the plasma on adenoviruses was analyzed by Zimmermann et al. (2011), who used 4.7-kV micro-discharges for the generation of the plasma. These viruses have double-stranded DNA (dsDNA) and exhibit low sensitivity to physical and chemical factors. They are causative agents of ophthalmological, respiratory, and gastrointestinal diseases. Using a green fluorescent protein (GFP) and a firefly luciferase, the researchers demonstrated that both viral infectivity and replication were inhibited. The activity of reactive nitrogen species (RNS) and their intermediates was proposed as a mechanism of the plasma effect on the DNA structure and immunogenicity of the virus. Some investigations were focused on the susceptibility of noroviruses (NoV), which cause gastrointestinal infections and pose a major problem in the food industry. Ahlfeld et al. (2015) studied the effect of CP on the inactivation of NoV from fecal samples. They treated the samples with a plasma stream and evaluated the effectiveness of inactivation at a variable time of the process. They achieved 1.23 log and 1.69 log reduction in the viral titer after 10 and 15 minutes, respectively (Bourke et al. 2017). The recent research also suggests that plasma-activated solutions (PASs) can contribute to effective decontamination. The effectiveness of inactivation of the Newcastle disease virus (ND) by the plasma-activated solutions (H2O, 0.9% NaCl, 0.3% H2O2) was assessed. The scanning electron microscope’s (SEM) images revealed morphological changes in virus particles, and the reduction of their titer and RNA degradation have also been shown (Su et al. 2018).
Effect of cold plasma on microbial cells
Microorganisms are a key target in the investigation of the plasma efficiency, as susceptibility to the sterilization process may vary between microorganisms, even within species and strains (Fig. 2). It largely depends on the structure of cellular envelopes and the microbial growth phase (Liao et al. 2017).
Fig. 2.
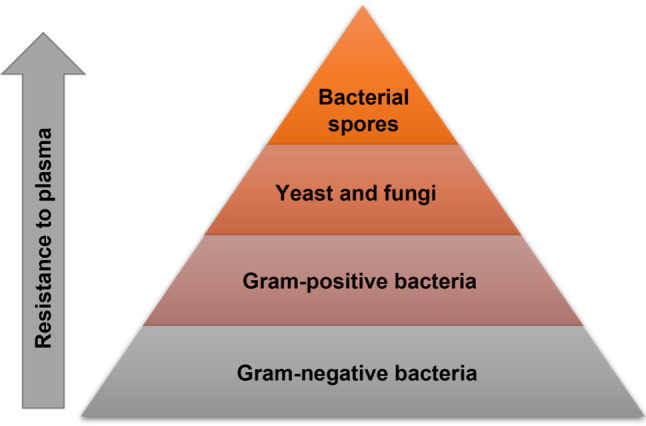
Pyramid of the sensitivity of microorganisms to plasma*. Based on Klämpfl T.G. et al. 2012; and Liao et al. 2017.
* Sensitivity of individual groups of microorganisms can vary depending on the conditions of the process.
Bacteria
The exact mechanism of inactivation of bacterial cells by cold plasma is still unknown, although the issue has been extensively studied. Permeabilisation of the cell membrane or cell wall leading to leakage of intracellular components, i.e. potassium, nucleic acids, and proteins, is regarded as one of the mechanisms of inactivation of this group of microorganisms. Furthermore, inactivation of bacteria occurs via the oxidative damage to intracellular proteins and DNA related to the effect of the plasma compounds (Mai-Prochnow et al. 2014). The overall mechanisms of microbial inactivation with plasma reactive species are presented in Fig. 3. The impact of the plasma stream on the external bacterial structures was investigated by Laroussi et al., who use the plasma generated with dielectric barrier discharge (DBD) against Escherichia coli and Bacillus subtilis cells. For Gram-negative E. coli with a cell wall composed of an outer membrane and thin peptidoglycan (murein), there was substantial damage to the membrane resulting in the cytoplasm leakage. The authors suggest that the visible changes resulted from the electrostatic rupture of the outer membrane. In turn, the microscopic image of Gram-positive B. subtilis cells with a thick cell wall did not show the significant morphological modifications and the decontamination was most probably due to interactions of reactive compounds with the intracellular components. The differences in the effect of the plasma on this microbial group are related to a different structure of cell walls in these microorganisms (Laroussi et al. 2003). A comprehensive analysis of chemical modifications induced by CAP in the structure of Gram-positive (B. subtilis) and Gram-negative (E. coli) bacteria was performed by Kartaschew et al. (2016) with use of a Fourier Transform Infrared Spectroscopy (FTIR) technique. The spectral images revealed that the plasma caused an increase in the number of symmetric stretching vibrations that reveal the formation of carboxylic groups (COO–). The investigators also noted the spectra in the absorbance range of 1720 cm–1 that indicate the formation of carbonyl groups (C = O) related to the presence of aldehydes, ketones, or acids. The resulting signals were associated with the destruction of the cell wall and cellular membrane by ROS. Another phenomenon induced by the activity of the plasma is the change of the cell membrane potential. This leads to disturbances in the function of the protonomotoric force and, consequently, abnormal ATP synthesis and the impairment of cell metabolism and division (Brun et al. 2018).
Fig. 3.
Mechanisms of microbial inactivation with plasma reactive species. Based on Bourke et al. 2017.
The changes in the membrane integrity may have a direct effect on DNA, mainly through the discontinuation of interactions with membrane proteins and formation of pores in the cell membrane by which nucleic acids can be released from the cell (Coutinho et al. 2018). Plasma-induced release of DNA is possible after the prior DNA fragmentation, and the effectiveness of this process depends on the conformation of the nucleic acid. In comparison with linear DNA, its supercoiled circular form is regarded as the resistant form of the nucleic acids (Moreau et al. 2008; Alkawareek et al. 2014). In general, it is believed that the plasma effect on DNA is a result of the activity of both reactive compounds and UV photons (Coutinho et al. 2018). In the cell, the DNA-protein crosslinks (DPCs) can be formed, which lead to the formation of hard-to-repair damage. Reactive oxygen and nitrogen species produced by the plasma oxidize proteins by the generation of hydroperoxide groups in their structure, which can form strong intramolecular crosslinks with the nucleic acids. In the experiment conducted by Guo et al. (2018), E. coli cells after treatment with the plasma stream with an air and 1% He were subjected to two versions of a Comet assay (with and without proteinase K) to assess the formation of DPC in DNA of the analyzed bacterium. In the fluorescent DNA image of the cells treated with the plasma, the researchers observed short “tails” indicating the damaged fragments without proteinase treatment, and much longer tails after the treatment with proteinase. This suggested the presence of DPC crosslinks, which were destroyed by the enzyme digesting the proteins. Then, the proteins were by the CsCl density gradient ultracentrifugation, treated with a nuclease, and their analysis revealed a higher concentration and diversity of the proteins than in the samples that were not treated with the plasma. This may be another piece of evidence supporting the hypothesis that the plasma-induced damage to nucleic acids is a result of the protein-DNA covalent bonding. An important issue is the CP effect on the genes encoding antibiotic resistance. The resistance of methicillin-resistant Staphylococcus aureus (MRSA) is associated with the mecA gene, which encodes protein PBP2a characterized by low affinity to β-lactam antibiotics. The most recent investigations of the CP applications for inactivation of S. aureus MRSA demonstrated that the cumulative energy delivered to the surface of the plasma-treated sample was 0.12 kJ/cm2 and 0.35 kJ/cm2, respectively, when air was used as the working gas. Five-log reduction in the number of S. aureus MRSA was observed even at the lower energy values, whereas higher values (0.35 kJ/cm2) were required for more effective degradation of the resistance gene. The isolated mecA gene exhibited higher sensitivity to the plasma activity compared to the intracellular gene. This is closely related to the protective effect of cell constituents, which are the first to interact with the reactive plasma components (Liao et al. 2018). The investigations also highlighted the changes in the membrane potential and integrity induced by the various intensities of the plasma activity. After application of the plasma treatment with a cumulative energy level of 0.12 kJ/cm2, the authors observed that a membrane potential coefficient was close to the depolarization value (Liao et al. 2018).
The use of cold plasma leads to the degradation of cellular proteins. Large proteins (50–90 kDa) are destroyed as the first. It was revealed by analysis of the samples where the concentration of the proteins with a molecular mass below 25 kDa increased after the plasma treatment (Hosseinzadeh Colagar et al. 2013). The probable mechanism of the degradation consists of the destruction of hydrogen, sulfide, and peptide bonds by reactive compounds present in the plasma. This results in the changes of the primary, secondary, and tertiary protein structure that lead to a decline in the enzymatic activity of the cell (Mai-Prochnow et al. 2014). Recently, researchers have investigated the effect of CP on proteins in thermophilic bacteria that pose a serious problem in the food industry due to their high resistance to the classical decontamination methods, i.e. high temperature or chemical denaturing agents. Attri et al. (2018) treated a highly stable MTH 1880 protein from the thermophilic bacterium Methanobacterium thermoautotrophicum with CP generated by DBD and analyzed it with the circular dichroism (CD), fluorescence, and nuclear magnetic resonance (NMR) spectroscopy techniques. Additionally, molecular dynamics (MD) simulation was carried out for both the native and plasma-treated protein. The investigators achieved a partial destabilization of MTH protein only after a long time of exposure to CP (20 min), which resulted in a decrease of 5°C in its melting point and an increase in its susceptibility to thermal denaturation. To elucidate the mechanism of the plasma effect on cellular proteins, the proteomic profile of Salmonella enteritidis was determined and as the result, 1096 proteins were identified with 249 of them present only in the plasma-treated samples and nine only in control samples. Under the impact of CP, the proteins that were overexpressed came mainly from the carbohydrate and nucleotide metabolism pathways and were associated with RNA transcription. It indicates an increased energy metabolism in cells as a defense response of bacteria (Ritter et al. 2018). The plasma-induced changes in bacterial metabolism were reported by Laroussi et al. (2003), who demonstrated the reduced utilization of L-lactic acid and increased consumption of D-sorbitol (Moreau et al. 2008). Interestingly, the effect of plasma on microorganisms does not always lead to cell death but can also reduce their metabolism, therefore, the cells do not undergo division and become viable but not culturable (VBNC). Dolezalova et al. (2015) treated a suspension of E. coli cells with the plasma and measured bacterial viability with a conventional culture method and by assessment of the fluorescent images of the cells stained with the LIVE/DEAD kits. The result of the former method suggested a 7.0-log reduction of the number of cells, whereas a reduction of only 1.0 log was indicated with the latter technique. These findings suggest that the plasma may induce the VBNC state of the cells.
Spores. Through evolutionary adaptation, bacterial spores have acquired the possibility to survive in adverse environmental conditions. They are characterized by resistance to disinfectants, chemical sterilants, drying processes, and thermal inactivation. This poses a serious threat to food and pharmaceutical industries as well as medicine, where spores are a permanent source of contamination (Liao et al. 2017). The major cause of such high resistance of spores is their structure, which differs substantially from that of vegetative forms of bacteria. Spores are composed of impermeable outer layers creating a specific barrier for the external factors. Additionally, they are characterized by low water content, which accounts for approximately 15% of the entire cell (Olesiak and Stępniak 2012). The interior of the spore contains a rigid structure, i.e. an inner membrane permeable only to small molecules (< 200 Daltons). When the membrane is damaged, dipicolinic acid (DPA) is released and endospore germination is not induced. DPA ensures considerably higher (up to 50-fold) resistance to UV radiation in spores (Olesiak and Stępniak 2012). In the very core of the spore, the DNA protection function against chemical and physical agents is fulfilled by small acid-soluble SASP proteins, which are closely related to the nucleic acids (Kądzielska et al. 2012). The precise mechanism of the plasma effect on spores has not been clarified to date. Some researchers suggest that spores are inactivated mainly via interactions between the reactive plasma compounds and the external spore structures (Hong et al. 2009). The morphological changes in B. subtillis endospores induced by various CF-related factors were presented by Deng et al. (2006) on scanning electron microscopy (SEM) images. The changes in the spore size, leakage of the cytoplasm content, and final membrane disruption were observed. These results suggest that oxidation by reactive oxygen species is the main factor contributing to the reduction of the number of viable B. subtilis cells, whereas the electric field, UV photons, and charged particles play a minor role in this process. Other investigators demonstrated that inactivation of endospores was primarily caused by damage to their outer layers resulting in leakage of DPA and hydration of the core (Tseng et al. 2012). The importance of the external structures for resistance to cold plasma was analyzed by Raguse et al. (2016), who investigated wild-type B. subtilis spores and mutants deficient in some surface structures. Additionally, the investigators examined the effect of the gas applied on the effectiveness of sterilization. Their results indicated a substantial impact of surface structures and the type of gas on the resistance of spores. A mixture of oxygen and argon was the most effective in inactivation of bacterial spores, as it caused significant damage to the external layers. In contrast, the mutants deficient in surface structures exhibited the highest sensitivity to plasma. The recent studies conducted by Connor et al. (2017) also have emphasized the impact of environmental conditions on plasma efficiency. The studies on the resistance of Clostridium difficile spores to plasma were conducted in three different environments (the spores suspended in water and dry spores with or without 0.03% BSA). The greatest reduction in the spore number in the shortest time was observed in a dry environment. In contrast, organic matter and moist environment extended the time of the spore inactivation by plasma (Klampf et al. 2014).
Another aspect that should be explored more deeply is the mechanism of CP effect on sporal DNA. There is a contradictory data on the effect of UV photons generated in the sterilization process on nucleic acids. The results of investigations conducted by Tseng et al. (2012) did not show DNA degradation in B. subtilis spores after a 20-min exposure to the plasma. This finding can be supported by the conclusion reported by Fiebrandt et al. (2016), who suggested that cell layers absorb UV radiation, thereby protecting DNA from damage. This mechanism is plausible given the structure of spores. Surface structures of spores are characterized by a high content of proteins with amino acid side chains forming endogenous chromophores. These compounds can be the main target of photooxidation, thereby protecting the cell interior from harmful radiation.
Some investigations have indicated that the plasma inactivates spores by the impact on key metabolic proteins. In their study, Dobrynin et al. (2010) suggested that reactive oxygen species penetrating the cell interior could cause oxidation of proteins involved in germination or inactivate germination receptors located in the inner membrane of spores. In turn, Wang et al. (2011) compared the kinetics of germination of the cold plasma-treated B. subtilis spores and untreated spores. They conducted the experiments in an environment enriched with nutrient germinants (L-valine) and non-nutrient germinants (dodecylamine, Ca2+DPA). Their results indicated the potential inactivation effects of the plasma on germination receptors but the germination induced by L-valine was inhibited.
Bacterial biofilm. Many microorganisms live in the environment as biofilms rather than free-living organisms. Biofilm was defined as the cells adhering to a solid surface and surrounded by an extracellular matrix produced by them (Czapka et al. 2018). Such populations exhibit higher resistance to adverse external factors (antibiotics, temperature, and pH); therefore, they pose a serious challenge in both medicine and food industry (Maciejewska et al. 2016). There are numerous reports demonstrating the sensitivity of biofilms to CF; however, the time required for full inactivation thereof is longer than for planktonic cells (Mai-Prochnow et al. 2014; Flynn et al. 2015). This was confirmed by Jahid et al. (2014), who compared the effect of CP on planktonic Aeromonas hydrophila cells and bacterial biofilm on the surface of lettuce. The experiment showed that a 15-s plasma treatment was sufficient to reduce the number of planktonic populations by > 5 logs. In contrast, the cell population on the lettuce surface was substantially reduced after a 5-min process of the plasma treatment (Jahid et al. 2014; Bourke et al. 2017). The extracellular matrix constitutes approx. 90% of biofilm and its presence largely determines the effectiveness of sterilization processes (Czyzewska-Dors et al. 2018). The matrix composition varies and depends on species of microorganisms forming the biofilm. The basic components of the extracellular matrix are polysaccharides, lipids, proteins, and nucleic acids. These compounds constitute a protective barrier against antibiotics or temperature as well as photons, reactive compounds, and charged particles in the plasma. Since these agents have to penetrate the protective layer, the biofilm inactivation time is prolonged (Mai-Prochnow et al. 2014). The results of investigations conducted by Ermolaeva et al. (2011) demonstrated the differences in the survival of bacteria in different biofilm layers, suggesting that the effectiveness of CP depends on the biofilm thickness. Microscopic evaluation of the viability of P. aeruginosa biofilm showed a greater number of bacterial cells in its deeper layers. This probably explains the proportional decline of the sterilization effectiveness with the increasing biofilm thickness. Another determinant of the sterilization effectiveness is the species of biofilm-forming microorganisms. This issue was investigated by Mai-Prochnow et al. (2016) who compared the effects of CP on bacterial biofilm formed by Gram-positive B. subtillis and Gram-negative P. aeruginosa. Almost complete reduction of the bacteria in Gram-negative biofilm was observed in contrast to Gram-positive biofilm where a 10-min plasma treatment resulted in < 1 log reduction. The presence of reactive oxygen compounds contributing to the cell wall disintegration in the emission spectra suggests that the cell wall thickness may be correlated with the duration of the cold-plasma inactivation. In conclusion, the sterilants present in the plasma inactivate biofilms through damage to the extracellular matrix, cell wall, cellular membrane, and internal cell structures. They can also induce the VBNC state in the cells (Ziuzina et al. 2015; Bourke et al. 2017).
Bacterial cell response to cold-plasma treatment. Elucidation of mechanisms of cell response to the cold plasma treatment is an important issue and requires further exploration. Hitherto, the changes in gene transcription and expression induced by exposure of cells to plasma have been already analyzed. The available scientific publications demonstrated a potential increase in the expression of SOS regulon, oxidation-related genes, and the genes encoding DNA repair processes. In turn, the expression of housekeeping genes was reduced (Sharma et al. 2009; Roth et al. 2010). Sharma et al. investigated the effect of plasma on genomic DNA in E. coli and performed a microarray analysis of the samples after 2-min plasma exposure. The increased expression of the gene involved in superoxide radical scavenging (katG) and the recA gene responsible for DNA recombination and repair was noted (Sharma et al. 2009). Similar results were reported by Roth et al. (2010) who analyzed the samples of highly radiation-resistant bacteria (Deinococcus radiodurans) and found an increase in the expression of the genes involved in DNA repair, oxidative stress responses, and cell wall synthesis. The process of the nucleic acids repair may result in the emergence of mutations that will determine increased resistance to CP. UV radiation, which induces the formation of nitrogen base dimers in nucleic acids is the main mutagenic factor. On the other hand, ROS and RNS present in the plasma exert a destructive effect on cellular components; thus, contribute to bacterial death and minimize the conservation of mutation effects (Boxhammer et al. 2013).
Yeast
Yeasts, which are a valuable source of many enzymes, are widely used in food biotechnology and microbiology in both fermentation and food-enrichment processes (Krzyczkowska et al. 2008). With their low pathogenicity as well as a unique structure and properties, some yeast species, e.g. Saccharomyces cerevisiae or Schizosaccharomyces pombe have become an inseparable element of molecular biology, serving as a model for an eukaryotic organism. Furthermore, a simple and cost-efficient culture of yeasts has contributed to the increasing interest in these organisms as objects for the elucidation of the effect of CP on cells (Wawrzycka 2011). The experiments were performed by Nishime and coworkers (2017) on the effectiveness of the plasma generated by DBD discharges with helium addition against various microorganisms. The researchers studied Enterococcus faecalis (Gram-positive) and P. aeruginosa (Gram-negative) bacteria as well as Candida albicans yeasts. They reported an inactivating effect of the plasma on all of the microorganisms investigated. Nevertheless, in comparison with the bacterial cells, C. albicans exhibited higher resistance, which can be explained mainly by the differences in their cell structure. In turn, the investigations conducted by Colonna et al. (2017) were focused on the effect of the plasma generated with dry air and a gas mixture (65% O2, 30% CO2, 5% N2) on S. cerevisiae cells at a different density of cellular suspension and duration of the exposure. The results confirmed a correlation between the effectiveness of sterilization and the process parameters. For the samples with higher cellular density, a longer timer of exposure of the suspension to the plasma was required for a complete cell degradation. Moreover, the reduction effect monitored at various time points indicated higher efficiency of a plasma stream with a gas mixture. The effect of environmental conditions on the effectiveness of inactivation was examined by Ryu et al. (2013), who observed a decrease of the number of S. cerevisiae cells suspended in different media (water, saline, and YPD). The most serious CP-induced damage to membrane lipids and genomic DNA was observed in yeast cells suspended in water. This was associated with the highest content of hydroxyl radicals generated in the water medium during the process.
Polcic et al. (2018), who used genetic mutants of yeasts to identify the role of oxidative stress and apoptosis in the decontamination process made an attempt at the elucidation of the mechanisms of CP effect on S. cerevisiae. The results of the experiments indicated higher susceptibility of strains with superoxide dismutase deficiency than those deprived of the key components of the apoptotic pathway, as their sensitivity to the plasma activity did not change. This proves that reactive oxygen species are one of the most important factors involved in the inactivation of yeast cells, and the apoptosis process itself does not play a key role in this case. An important issue requiring comprehensive investigations is the impact of CP on intracellular proteins. The treatment of S. cerevisiae with argon plasma was found to lead to protein ubiquitination and formation of the insoluble protein aggregates in the yeast cytoplasm. These researchers also underlined the potential of the CP-induced generation of endoplasmic reticulum (ER) stress, which is characteristic of eukaryotic cells. This was confirmed by an increase in the activity of the endoplasmic reticulum transmembrane protein Ire1p induced by accumulation of unfolded proteins in ER (Itooka et al. 2018). As demonstrated in many research reports, the cold-plasma technique can be used for inactivation of enzymes (Li et al. 2011; Surowsky et al. 2013; Tolouie et al 2017; Tolouie et al. 2018). The authors suggest that the ability of plasma to inhibit enzymes is associated with loss of the protein secondary structure. It is an effect of interactions with reactive compounds generated in the gas used in the process (Misra et al. 2016). Colonna et al. (2017) investigated the impact of CP on enzymes produced by yeasts in a study on the plasma generated with dry air. Their analyses were focused on S. cerevisiae yeast invertase, which lost its activity at a level of > 96% after 75 s of the treatment.
Fungi
Plant diseases caused by fungal pathogens pose a serious crisis in agriculture, as they cause huge economic losses worldwide. As demonstrated in the recent research reports, the cold-plasma technology can become an alternative plant protection method either by inactivating fungal cells or improving the resistance of infected hosts (Dasan et al. 2017a; Siddique et al. 2018). The investigations that confirmed the susceptibility of this microbial group to the CP activity have been conducted by many researchers (Suhem et al. 2013; Sohbatzadeh et al. 2016; Nikmaram et al. 2018). Some of them suggested that the inactivation of fungal cells using CP mainly involves the production of reactive compounds which seems to have their destructive effect on the cell wall and inner membrane of fungi (Ye et al. 2012; Lu et al. 2014; Dasan et al. 2016; Dasan et al. 2017a). In their study, Ye et al. (2012) assessed the effect of the plasma (working gas: air) generated by corona discharges on the cells of Penicillium expansum, i.e. one of the most important pathogens causing spoilage of the stored fruit. SEM images revealed disruption of the external structures of P. expansum, which resulted in cytoplasmic leakage. In turn, an analysis of transmission electron microscopy (TEM) images indicated the plasma-induced alterations in the cell, i.e. an increased volume of the protoplasm, stretching of vacuoles, and disintegration of the membrane, which contributed to cell lysis.
Besides their impact on external structures and intracellular organelles, the reactive compounds present in the plasma and the generated UV radiation cause damage to nucleic acids and oxidation of proteins and lipids. Lu et al. (2014) have investigated the effect of CP on Cladosporium fulvum by determination of the concentration of malondialdehyde (MDA), i.e. the basic product of lipid peroxidation. The findings reported confirmed the hypothesis that the activity of sterilizing agents in fungal cells triggers the peroxidation process. The similar observations were reported by another research team investigating the effect of CP generated at atmospheric pressure on Aspergillus flavus cells. The investigators determined the activity of thiobarbituric acid (TBA) in the samples, which showed a linkage between the membrane damage and lipid oxidation process (Simoncicova et al. 2018).
The other mechanism that may reduce the number of fungal cells is apoptosis or necrosis, as suggested by Panngom et al. (2014) to identify the mechanism of inactivation of Fusarium oxysporum cells, the authors stained the cells with Annexin V and propidium iodide and treated them with argon plasma for 1, 5, and 10 min. The numbers of viable, necrotic, and apoptotic cells were analyzed with flow cytometry. The majority of the cells were propidium iodide-stained after 5- and 10-min of the treatment with CP. This indicates that the necrosis process was the main mechanism of inactivation of the fungal cells.
An important aspect that requires further research is the increase in resistance to fungal pathogens in the plasma-treated plants. The experiments conducted by Filatova et al. (2016) demonstrated a substantially reduced incidence of diseases caused by Fusarium spp. and Ustilago maydis in wheat, lupine, and maize after exposure of the seeds to CP. The stimulating and fungicidal effect of the plasma resulted in an increase in the yield of the spring wheat, maize, and lupine seeds by 4–6%, 1.5–2%, and 20–40%, respectively, in comparison with the control. After additional assays that should be carried out in the future, CP may become an alternative to chemical plant protection agents, which may effectively minimize the negative impact of fungicides on the environment (Siddique et al. 2018).
Cold-plasma inactivation of mycotoxins. The UN Food and Agriculture data indicate that 25% of world crops are contaminated with mycotoxins produced by fungi during plant growth or crop storage. Aspergillus, Fusarium, and Penicillium molds are the major producers of toxic secondary metabolites, including aflatoxin, fumonisin, zearalenone, ochratoxin, and deoxynivalenol, which are most toxic to mammals. The elimination of these compounds from food products is problematic due to their high thermostability; for instance, the temperature of aflatoxin degradation ranges from 237 to 306°C (Pankaj et al. 2017). The latest physicochemical methods that can potentially be used for elimination of mycotoxins include the cold-plasma sterilization (Ouf et al. 2015; Hojnik et al. 2017; Pankaj et al. 2017; Shi et al. 2017). There is a possibility of the complete degradation and reduction of aflatoxin B1 (AFB1), deoxy nivalenol (DON), and nivalenol (NIV) cytotoxicity with the use of argon plasma generated by microwave discharges (Hojnik et al. 2017). The reduction of the toxicity of these compounds may result from structural changes induced by the sterilization process. Wang et al. (2015) applied low-temperature radio-frequency plasma and reported 88.3% reduction in AFB1 concentration after a 10-minute process. The analysis of degradation products revealed five different compounds characterized by loss of the double bond between C8 and C9 in the furan ring. Besides its effect on the standard mycotoxin solutions, the plasma has been found to exert a reductive effect on the compounds contained in food products (Ouf et al. 2015; Siciliano et al. 2016; Shi et al. 2017). Aflatoxins present in hazelnuts were decontaminated using CP (1000 W, 12 min), which resulted in an over 70% decline in the AFB1 concentration (Siciliano et al. 2016). In turn, the concentration of this maize contaminant was reduced by 62% and 82% after the 1- and 10-min plasma treatments, respectively (Shi et al. 2017). However, the atmospheric plasma treatment of contaminated nuts did not allow complete removal of the mycotoxin. The best results were obtained with the highest power (1150 W) and the longest operating time (12 min), which enabled the reduction of AFB1 approx. by 70%. Given the high efficiency of degradation of standard mycotoxin solutions and mycotoxins contained in food, the cold plasma method is becoming a promising solution that may replace conventional techniques in the future. In addition to the high efficiency of compound degradation, the relatively low cost of the process, as well as environmental safety, also favor the use of CP (Hojnik et al. 2017).
Summary
The cold plasma technology is becoming a promising solution with the potential to replace conventional techniques of decontamination of food products, medical materials, and air in the future. This technique has many advantages, e.g. high efficiency in reducing the viral particles load and the number of microorganisms, formation of non-toxic by-products, and a relatively low cost of the process (Liao et al. 2016). Although many studies focused on this issue, the precise molecular mechanism of the plasma effect on cells of different microbial groups has not been clarified yet. The available reports on the possibility of induction of VBNC state in some bacteria raise doubts about the safety of this sterilization method (Dolezalova and Lukes 2015). In addition, this technology has some disadvantages, i.e. a small working surface and poor permeability. In food products, this technique may cause increased lipid oxidation, increased acidity of the product, reduced color intensity and decrease in firmness of fruits (Misra et al. 2016; Chizoba Ekezie et al. 2017). Therefore, it is necessary to conduct further research that will allow for the optimization of process parameters, explain the doubts concerning the biting mechanisms of plasma operation and promote the application of cold plasma in the industry with no negative effects on human health or the environment.
Footnotes
Conflict of interest
The authors do not report any financial or personal connections with other persons or organizations, which might negatively affect the contents of this publication and/or claim authorship rights to this publication.
ORCID
Iwona Niedźwiedź 0000-0003-1832-9528 Adam Waśko 0000-0001-9329-1043 Magdalena Polak-Berecka 0000-0003-3832-8610
Literature
- Afshari R, Hosseini H.. Non-thermal plasma as a new food preservation method, its present and future prospect. J Paramed Sci. 2014;5:116–120. [Google Scholar]
- Ahlfeld B, Li Y, Boulaaba A, Binder A, Schotte U, Zimmermann JL, Morfill G, Klein G.. Inactivation of a foodborne norovirus outbreak strain with nonthermal atmospheric pressure plasma. MBio. 2015. Feb 27;6(1):e02300–e02314. doi: 10.1128/mBio.02300-14 Medline [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkawareek MY, Alshraiedeh NH, Higginbotham S, Flynn PB, Algwari QT, Gorman SP, Graham WG, Gilmore BF.. Plasmid DNA damage following exposure to atmospheric pressure nonthermal plasma: kinetics and influence of oxygen admixture. Plasma Med. 2014;4(1-4):211–219. doi: 10.1615/PlasmaMed.2015011977 [DOI] [Google Scholar]
- Attri P, Han J, Choi S, Choi EH, Bogaerts A, Lee W.. CAP modifies the structure of a model protein from thermophilic bacteria: mechanisms of CAP-mediated inactivation. Sci Rep. 2018. Dec; 8(1):10218. doi: 10.1038/s41598-018-28600-w Medline [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayliss DL, Walsh JL, Iza F, Shama G, Holah J, Kong MG.. Complex responses of microorganisms as a community to a flowing atmospheric plasma. Plasma Process Polym. 2012. Jun;9(6):597–611. doi: 10.1002/ppap.201100104 [DOI] [Google Scholar]
- Beggs CB.. A quantitative method for evaluating the photoreactivation of ultraviolet damaged microorganisms. Photochem Photobiol Sci. 2002. Jun 7;1(6):431–437. doi: 10.1039/b202801h Medline [DOI] [PubMed] [Google Scholar]
- Bogaerts A, Neyts EC.. Plasma technology: an emerging technology for energy storage. ACS Energy Lett. 2018. Apr 13;3(4):1013–1027. doi: 10.1021/acsenergylett.8b00184 [DOI] [Google Scholar]
- Boudam MK, Moisan M.. Synergy effect of heat and UV photons on bacterial-spore inactivation in an N2 –O2 plasma-afterglow sterilizer. J Phys D Appl Phys. 2010. Jul 28;43(29):295202. doi: 10.1088/0022-3727/43/29/295202 [DOI] [Google Scholar]
- Bourke P, Ziuzina D, Han L, Cullen PJ, Gilmore BF.. Microbiological interactions with cold plasma. J Appl Microbiol. 2017. Aug;123(2):308–324. doi: 10.1111/jam.13429 Medline [DOI] [PubMed] [Google Scholar]
- Boxhammer V, Li YF, Köritzer J, Shimizu T, Maisch T, Thomas HM, Schlegel J, Morfill GE, Zimmermann JL.. Investigation of the muta genic potential of cold atmospheric plasma at bactericidal dosages. Mutat Res-Gen Tox En. 2013;753(1):23–28. [DOI] [PubMed] [Google Scholar]
- Brisset JL, Pawłat J.. Chemical effects of air plasma species on aqueous solutes in direct and delayed exposure modes: discharge, post-discharge and plasma activated water. Plasma Chem Plasma Process. 2016. Mar;36(2):355–381. doi: 10.1007/s11090-015-9653-6 [DOI] [Google Scholar]
- Bruggeman PJ, Kushner MJ, Locke BR, Gardeniers JGE, Graham WG, Graves DB, Hofman-Caris RCHM, Maric D, Reid JP, Ceriani E, et al.. Plasma–liquid interactions: a review and roadmap. Plasma Sources Sci Technol. 2016. Sep 30;25(5):053002. doi: 10.1088/0963-0252/25/5/053002 [DOI] [Google Scholar]
- Brun P, Bernabè G, Marchiori C, Scarpa M, Zuin M, Cavazzana R, Zaniol B, Martines E.. Antibacterial efficacy and mechanisms of action of low power atmospheric pressure cold plasma: membrane permeability, biofilm penetration and antimicrobial sensitization. J Appl Microbiol. 2018. Aug;125(2):398–408. doi: 10.1111/jam.13780 Medline [DOI] [PubMed] [Google Scholar]
- Chizoba Ekezie F-G, Sun DW, Cheng JH.. A review on recent advances in cold plasma technology for the food industry: current applications and future trends. Trends Food Sci Technol. 2017. Nov; 69:46–58. doi: 10.1016/j.tifs.2017.08.007 [DOI] [Google Scholar]
- Colonna W, Wan Z, Pankaj SK, Keener KM.. High-voltage atmospheric cold plasma treatment of yeast for spoilage prevention. Plasma Med. 2017;7(2):97–107. doi: 10.1615/PlasmaMed.2017019201 [DOI] [Google Scholar]
- Connor M, Flynn PB, Fairley DJ, Marks N, Manesiotis P, Graham WG, Gilmore BF, McGrath JW.. Evolutionary clade affects resistance of Clostridium difficile spores to Cold Atmospheric Plasma. Sci Rep. 2017. Dec;7(1):41814. doi: 10.1038/srep41814 Medline [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutinho NM, Silveira MR, Rocha RS, Moraes J, Ferreira MVS, Pimentel TC, Freitas MQ, Silva MC, Raices RSL, Ranadheera CS, et al.. Cold plasma processing of milk and dairy products. Trends Food Sci Technol. 2018. Apr;74:56–68. doi: 10.1016/j.tifs.2018.02.008 [DOI] [Google Scholar]
- Czapka T, Maliszewska I, Olesiak-Bańska J.. Influence of atmospheric pressure non-thermal plasma on inactivation of biofilm cells. Plasma Chem Plasma Process. 2018. Nov;38(6):1181–1197. doi: 10.1007/s11090-018-9925-z [DOI] [Google Scholar]
- Czyzewska-Dors E, Dors A, Pomorska-Mól M.. Właściwości biofilmu bakteryjnego warunkujące oporność na antybiotyki oraz metody jego zwalczania. Zycie Wet. 2018;93(11):765–771. [Google Scholar]
- Dasan BG, Boyaci IH, Mutlu M.. Nonthermal plasma treatment of Aspergillus spp. spores on hazelnuts in an atmospheric pressure fluidized bed plasma system: impact of process parameters and surveillance of the residual viability of spores. J Food Eng. 2017a. Mar;196:139–149. doi: 10.1016/j.jfoodeng.2016.09.028 [DOI] [Google Scholar]
- Dasan BG, Mutlu M, Boyaci IH.. Decontamination of Aspergillus flavus and Aspergillus parasiticus spores on hazelnuts via atmospheric pressure fluidized bed plasma reactor. Int J Food Microbiol. 2016. Jan;216:50–59. doi: 10.1016/j.ijfoodmicro.2015.09.006 Medline [DOI] [PubMed] [Google Scholar]
- Dasan BG, Onal-Ulusoy B, Pawłat J, Diatczyk J, Sen Y, Mutlu M.. A new and simple approach for decontamination of food contact surfaces with gliding arc discharge atmospheric non-thermal plasma. Food Bioprocess Technol. 2017b. Apr;10(4):650–661. doi: 10.1007/s11947-016-1847-2 [DOI] [Google Scholar]
- Deng X, Shi J, Kong MG.. Physical mechanisms of inactivation of Bacillus subtilis spores using cold atmospheric plasmas. IEEE Trans Plasma Sci. 2006. Aug;34(4):1310–1316. doi: 10.1109/TPS.2006.877739 [DOI] [Google Scholar]
- Dobrynin D, Fridman G, Mukhin YV, Wynosky-Dolfi MA, Rieger J, Rest RF, Gutsol AF, Fridman A.. Cold plasma inactivation of Bacillus cereus and Bacillus anthracis (anthrax) spores. IEEE Trans Plasma Sci. 2010. Aug;38(8):1878–1884. doi: 10.1109/TPS.2010.2041938 [DOI] [Google Scholar]
- Dolezalova E, Lukes P.. Membrane damage and active but nonculturable state in liquid cultures of Escherichia coli treated with an atmospheric pressure plasma jet. Bioelectrochemistry. 2015. Jun;103:7–14. doi: 10.1016/j.bioelechem.2014.08.018 Medline [DOI] [PubMed] [Google Scholar]
- Dzimitrowicz A, Jamróz P, Nowak P.. Sterylizacja za pomocą niskotemperaturowej plazmy, generowanej w warunkach ciśnienia atmosferycznego. Postepy Mikrobiol. 2015;54(2):195–200. [Google Scholar]
- Ermolaeva SA, Varfolomeev AF, Chernukha MY, Yurov DS, Vasiliev MM, Kaminskaya AA, Moisenovich MM, Romanova JM, Murashev AN, Selezneva II, et al.. Bactericidal effects of nonthermal argon plasma in vitro, in biofilms and in the animal model of infected wounds. J Med Microbiol. 2011. Jan 01;60(1):75–83. doi: 10.1099/jmm.0.020263-0 Medline [DOI] [PubMed] [Google Scholar]
- Fiebrandt M, Lackmann JW, Raguse M, Moeller R, Awakowicz P, Stapelmann K.. VUV absorption spectroscopy of bacterial spores and DNA components. Plasma Phys Contr Fusion. 2017. Jan 01; 59(1):014010. doi: 10.1088/0741-3335/59/1/014010 [DOI] [Google Scholar]
- Filatova I, Azharonok V, Lyushkevich V, Zhukovsky A, Mildažienė V, Pauzaite G, Zukiene RAM.. 2016. The effect of presowing plasma seeds treatment on germination, plants resistance to pathogens and crop capacity. Paper presented at: 1st International Workshop on Plasma Agriculture; Camden, NJ. [Google Scholar]
- Flynn PB, Higginbotham S, Alshraiedeh NH, Gorman SP, Graham WG, Gilmore BF.. Bactericidal efficacy of atmospheric pressure non-thermal plasma (APNTP) against the ESKAPE pathogens. Int J Antimicrob Agents. 2015. Jul;46(1):101–107. doi: 10.1016/j.ijantimicag.2015.02.026 Medline [DOI] [PubMed] [Google Scholar]
- Fridman A, Chirokov A, Gutsol A.. Non-thermal atmospheric pres sure discharges. J Phys D Appl Phys. 2005. Jan 21;38(2):R1–R24. doi: 10.1088/0022-3727/38/2/R01 [DOI] [Google Scholar]
- Gallagher MJ, Vaze N, Gangoli S, Vasilets VN, Gutsol AF, Milovanova TN, Anandan S, Murasko DM, Fridman AA.. Rapid inactivation of airborne bacteria using atmospheric pressure dielectric barrier grating discharge. IEEE Trans Plasma Sci. 2007. Oct; 35(5):1501–1510. doi: 10.1109/TPS.2007.905209 [DOI] [Google Scholar]
- Guo L, Zhao Y, Liu D, Liu Z, Chen C, Xu R, Tian M, Wang X, Chen H, Kong MG.. Cold atmospheric-pressure plasma induces DNA – protein crosslinks through protein oxidation. Free Radic Res. 2018. Jul 03;52(7):783–798. doi: 10.1080/10715762.2018.1471476 Medline [DOI] [PubMed] [Google Scholar]
- Hojnik N, Cvelbar U, Tavčar-Kalcher G, Walsh J, Križaj I.. Cold atmospheric pressure plasma versus “classic” decontamination. Toxins (Basel). 2017. Apr 28;9(5):151. doi: 10.3390/toxins9050151 Medline [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong YF, Kang JG, Lee HY, Uhm HS, Moon E, Park YH.. Sterilization effect of atmospheric plasma on Escherichia coli and Bacillus subtilis endospores. Lett Appl Microbiol. 2009. Jan;48(1):33–37. doi: 10.1111/j.1472-765X.2008.02480.x Medline [DOI] [PubMed] [Google Scholar]
- Hosseinzadeh Colagar A, Memariani H, Sohbatzadeh F, Valinataj Omran A.. Nonthermal atmospheric argon plasma jet effects on Escherichia coli biomacromolecules. Appl Biochem Biotechnol. 2013. Dec;171(7):1617–1629. doi: 10.1007/s12010-013-0430-9 Medline [DOI] [PubMed] [Google Scholar]
- Isbary G, Morfill G, Schmidt HU, Georgi M, Ramrath K, Heinlin J, Karrer S, Landthaler M, Shimizu T, Steffes B, et al.. A first prospective randomized controlled trial to decrease bacterial load using cold atmospheric argon plasma on chronic wounds in patients. Br J Dermatol. 2010. May;163(1):78–82. doi: 10.1111/j.1365-2133.2010.09744.x Medline [DOI] [PubMed] [Google Scholar]
- Itooka K, Takahashi K, Kimata Y, Izawa S.. Cold atmospheric pressure plasma causes protein denaturation and endoplasmic reticulum stress in Saccharomyces cerevisiae. Appl Microbiol Biotechnol. 2018. Mar;102(5):2279–2288. doi: 10.1007/s00253-018-8758-2 Medline [DOI] [PubMed] [Google Scholar]
- Jahid IK, Han N, Ha SD.. Inactivation kinetics of cold oxygen plasma depend on incubation conditions of Aeromonas hydrophila biofilm on lettuce. Food Res Int. 2014. Jan;55:181–189. doi: 10.1016/j.foodres.2013.11.005 [DOI] [Google Scholar]
- Kądzielska J, Obuch-Woszczatyński P, Pituch H, Młynarczyk G.. Clostridium perfringens jako czynnik etiologiczny biegunki poantybiotykowej. Postepy Mikrobiol. 2012;51(1):17–25. [Google Scholar]
- Kartaschew K, Baldus S, Mischo M, Bründermann E, Awakowicz P, Havenith M.. Cold atmospheric-pressure plasma and bacteria: understanding the mode of action using vibrational microspectroscopy. J Phys D Appl Phys. 2016. Sep 21;49(37):374003. doi: 10.1088/0022-3727/49/37/374003 [DOI] [Google Scholar]
- Kim GC, Kim GJ, Park SR, Jeon SM, Seo HJ, Iza F, Lee JK.. Air plasma coupled with antibody-conjugated nanoparticles: a new weapon against cancer. J Phys D Appl Phys. 2009. Feb 07;42(3):032005. doi: 10.1088/0022-3727/42/3/032005 [DOI] [Google Scholar]
- Klämpfl TG, Isbary G, Shimizu T, Li YF, Zimmermann JL, Stolz W, Schlegel J, Morfill GE, Schmidt HU.. Cold atmospheric air plasma sterilization against spores and other microorganisms of clinical interest. Appl Environ Microbiol. 2012. Aug 01;78(15):5077–5082. doi: 10.1128/AEM.00583-12 Medline [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klämpfl TG, Shimizu T, Koch S, Balden M, Gemein S, Li YF, Mitra A, Zimmermann JL, Gebel J, Morfill GE, et al.. Decontamination of nosocomial bacteria including Clostridium difficile spores on dry inanimate surface by cold atmospheric plasma. Plasma Process Polym. 2014. Oct;11(10):974–984. doi: 10.1002/ppap.201400080 [DOI] [Google Scholar]
- Korachi M, Turan Z, Şentürk K, Şahin F, Aslan N.. An investigation into the biocidal effect of high voltage AC/DC atmospheric corona discharges on bacteria, yeasts, fungi and algae. J Electrost. 2009. Jul;67(4):678–685. doi: 10.1016/j.elstat.2009.03.002 [DOI] [Google Scholar]
- Krzyczkowska J, Stolarzewicz I, Bellok D, Bellok M, Białecka-Florjańczyk E.. Wpływ modyfikacji pożywki na biokatalityczne właściwości drożdży. Żywn Nauka Technol Jakość. 2008;15(5):299–306. [Google Scholar]
- Lademann O, Kramer A, Richter H, Patzelt A, Meinke MC, Roewert-Huber J, Czaika V, Weltmann K-D, Hartmann B, Koch S.. Antisepsis of the follicular reservoir by treatment with tissue-tolerable plasma (TTP). Laser Phys Lett. 2011. Apr;8(4):313–317. doi: 10.1002/lapl.201010123 [DOI] [Google Scholar]
- Laroussi M, Mendis DA, Rosenberg M.. Plasma interaction with microbes. New J Phys. 2003;5(1):41.1–41.10. [Google Scholar]
- Laskowska M, Bogusławska-Wąs E, Kowal P, Hołub M, Dąbrow-ski W.. Skuteczność wykorzystania niskotemperaturowej plazmy w mikrobiologii i medycynie. Postepy Mikrobiol. 2016;55(2):172–181. [Google Scholar]
- Li HP, Wang LY, Li G, Jin LH, Le PS, Zhao HX, Xing XH, Bao CY.. Manipulation of lipase activity by the helium radio-frequency, atmospheric-pressure glow discharge plasma jet. Plasma Process Polym. 2011. Mar 22;8(3):224–229. doi: 10.1002/ppap.201000035 [DOI] [Google Scholar]
- Liao X, Cullen PJ, Liu D, Muhammad AI, Chen S, Ye X, Wang J, Ding T.. Combating Staphylococcus aureus and its methicillin resistance gene (mecA) with cold plasma. Sci Total Environ. 2018. Dec;645:1287–1295. doi: 10.1016/j.scitotenv.2018.07.190 Medline [DOI] [PubMed] [Google Scholar]
- Liao X, Liu D, Xiang Q, Ahn J, Chen S, Ye X, Ding T.. Inactivation mechanisms of non-thermal plasma on microbes: A review. Food Control. 2017. May;75:83–91. doi: 10.1016/j.foodcont.2016.12.021 [DOI] [Google Scholar]
- Lu Q, Liu D, Song Y, Zhou R, Niu J.. Inactivation of the tomato pathogen Cladosporium fulvum by an atmospheric-pressure cold plasma jet. Plasma Process Polym. 2014. Nov;11(11):1028–1036. doi: 10.1002/ppap.201400070 [DOI] [Google Scholar]
- Maciejewska M, Bauer M, Dawgul M.. Nowoczesne metody zwalczania biofilmu bakteryjnego. Postepy Mikrobiol. 2016;55(1):3–11. [Google Scholar]
- Mai-Prochnow A, Clauson M, Hong J, Murphy AB.. Gram positive and Gram negative bacteria differ in their sensitivity to cold plasma. Sci Rep. 2016. Dec;6(1):38610. doi: 10.1038/srep38610 Medline [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai-Prochnow A, Murphy AB, McLean KM, Kong MG, Ostrikov KK.. Atmospheric pressure plasmas: infection control and bacterial responses. Int J Antimicrob Agents. 2014. Jun;43(6):508–517. doi: 10.1016/j.ijantimicag.2014.01.025 Medline [DOI] [PubMed] [Google Scholar]
- Metelmann HR, Seebauer C, Miller V, Fridman A, Bauer G, Graves DB, Pouvesle J-M, Rutkowski R, Schuster M, Bekeschus S, et al.. Clinical experience with cold plasma in the treatment of locally advanced head and neck cancer. Clin Plasma Med. 2018. Mar;9:6–13. doi: 10.1016/j.cpme.2017.09.001 [DOI] [Google Scholar]
- Misra NN, Pankaj SK, Segat A, Ishikawa K.. Cold plasma interactions with enzymes in foods and model systems. Trends Food Sci Technol. 2016. Sep;55:39–47. doi: 10.1016/j.tifs.2016.07.001 [DOI] [Google Scholar]
- Misra NN, Schlüter O, Cullen PJ.. Plasma in food and agriculture. Amsterdam (Netherlands): Academic Press; 2016. p. 1–16. [Google Scholar]
- Moreau M, Orange N, Feuilloley MGJ.. Non-thermal plasma technologies: new tools for bio-decontamination. Biotechnol Adv. 2008. Nov;26(6):610–617. doi: 10.1016/j.biotechadv.2008.08.001 Medline [DOI] [PubMed] [Google Scholar]
- Nikmaram H, Rezaei Kanavi M, Ghoranneviss M, Balagholi S, Ahmadieh H, Roshandel D, Amini M.. Cold atmospheric pressure plasma jet for the treatment of Aspergillus keratitis. Clin Plasma Med. 2018. Mar;9:14–18. doi: 10.1016/j.cpme.2017.12.075 [DOI] [Google Scholar]
- Nishime TMC, Borges AC, Koga-Ito CY, Machida M, Hein LRO, Kostov KG.. Non-thermal atmospheric pressure plasma jet applied to inactivation of different microorganisms. Surf Coat Tech. 2017. Feb;312:19–24. doi: 10.1016/j.surfcoat.2016.07.076 [DOI] [Google Scholar]
- Olesiak P, Stępniak L.. Skuteczność wybranych związków dezynfekcyjnych wobec przetrwalników Bacillus. Inżynieria i Ochrona Środowiska. 2012;15:41–50. [Google Scholar]
- Ouf SA, Basher AH, Mohamed AAH.. Inhibitory effect of double atmospheric pressure argon cold plasma on spores and mycotoxin production of Aspergillus niger contaminating date palm fruits. J Sci Food Agric. 2015. Dec;95(15):3204–3210. doi: 10.1002/jsfa.7060 Medline [DOI] [PubMed] [Google Scholar]
- Pankaj SK, Shi H, Keener KM.. A review of novel physical and chemical decontamination technologies for aflatoxin in food. Trends Food Sci Technol. 2018. Jan;71:73–83. doi: 10.1016/j.tifs.2017.11.007 [DOI] [Google Scholar]
- Panngom K, Lee SH, Park DH, Sim GB, Kim YH, Uhm HS, Park G, Choi EH.. Non-thermal plasma treatment diminishes fungal viability and up-regulates resistance genes in a plant host. PLoS One. 2014. Jun 9;9(6):e99300. doi: 10.1371/journal.pone.0099300 Medline [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawłat J, Kwiatkowski M, Terebun P, Murakami T.. RF-powered atmospheric-pressure plasma jet in surface treatment of high-impact polystyrene. IEEE Trans Plasma Sci. 2016. Mar;44(3): 314–320. doi: 10.1109/TPS.2015.2436061 [DOI] [Google Scholar]
- Pawłat J, Starek A, Sujak A, Kwiatkowski M, Terebun P, Budzeń M.. Effects of atmospheric pressure plasma generated in GlidArc reactor on Lavatera thuringiaca L. seeds’ germination. Plasma Process Polym. 2018a. Feb;15(2):1700064. doi: 10.1002/ppap.201700064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawłat J, Starek A, Sujak A, Terebun P, Kwiatkowski M, Budzeń M, Andrejko D.. Effects of atmospheric pressure plasma jet operating with DBD on Lavatera thuringiaca L. seeds’ germination. PLoS One. 2018b. Apr 9;13(4):e0194349. doi: 10.1371/journal.pone.0194349 Medline [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawłat J.. Atmospheric pressure plasma jet for decontamination purposes. Eur Phys J Appl Phys. 2013;61:1–11. [Google Scholar]
- Pignata C, D’Angelo D, Fea E, Gilli G.. A review on microbiological decontamination of fresh produce with nonthermal plasma. J Appl Microbiol. 2017. Jun;122(6):1438–1455. doi: 10.1111/jam.13412 Medline [DOI] [PubMed] [Google Scholar]
- Polčic P, Pakosová L, Chovančíková P, Machala Z.. Reactive cold plasma particles generate oxidative stress in yeast but do not trigger apoptosis. Can J Microbiol. 2018. Jun;64(6):367–375. doi: 10.1139/cjm-2017-0753 Medline [DOI] [PubMed] [Google Scholar]
- Pradeep P, Chulkyoon M.. Non-thermal plasmas (NTPs) for inactivation of viruses in abiotic environment. Res J Biotechnol. 2016;11:91–96. [Google Scholar]
- Raguse M, Fiebrandt M, Denis B, Stapelmann K, Eichenberger P, Driks A, Eaton P, Awakowicz P, Moeller R.. Understanding of the importance of the spore coat structure and pigmentation in the Bacillus subtilis spore resistance to low-pressure plasma sterilization. J Phys D Appl Phys. 2016. Jul 20;49(28):285401. doi: 10.1088/0022-3727/49/28/285401 [DOI] [Google Scholar]
- Ritter AC, Santi L, Vannini L, Beys-da-Silva WO, Gozzi G, Yates J 3rd, Ragni L, Brandelli A.. Comparative proteomic analysis of foodborne Salmonella Enteritidis SE86 subjected to cold plasma treatment. Food Microbiol. 2018. Dec;76:310–318. doi: 10.1016/j.fm.2018.06.012 Medline [DOI] [PubMed] [Google Scholar]
- Roth S, Feichtinger J, Hertel C.. Response of Deinococcus radiodurans to low-pressure low-temperature plasma sterilization processes. J Appl Microbiol. 2010. Jun;109(5):1521–1530. doi: 10.1111/j.1365-2672.2010.04771.x Medline [DOI] [PubMed] [Google Scholar]
- Ryu YH, Kim YH, Lee JY, Shim GB, Uhm HS, Park G, Choi EH.. Effects of background fluid on the efficiency of inactivating yeast with non-thermal atmospheric pressure plasma. PLoS One. 2013. Jun 14;8(6):e66231. doi: 10.1371/journal.pone.0066231 Medline [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samoń R, Czapiński J, Grządziel J, Płonka M, Pawłat J, Diatczyk J.. Ocena działania bakteriobójczego niskotemperaturowej plazmy nie równowagowej generowanej w reaktorze RF. Eur J Med Technol. 2014;2:17–26. [Google Scholar]
- Sharma A, Collins G, Pruden A.. Differential gene expression in Escherichia coli following exposure to nonthermal atmospheric pressure plasma. J Appl Microbiol. 2009. Nov;107(5):1440–1449. doi: 10.1111/j.1365-2672.2009.04323.x Medline [DOI] [PubMed] [Google Scholar]
- Shi H, Ileleji K, Stroshine RL, Keener K, Jensen JL.. Reduction of aflatoxin in corn by high voltage atmospheric cold plasma. Food Bioprocess Technol. 2017. Jun;10(6):1042–1052. doi: 10.1007/s11947-017-1873-8 [DOI] [Google Scholar]
- Siciliano I, Spadaro D, Prelle A, Vallauri D, Cavallero M, Garibaldi A, Gullino M.. Use of cold atmospheric plasma to detoxify hazelnuts from aflatoxins. Toxins (Basel). 2016. Apr 26;8(5):125. doi: 10.3390/toxins8050125 Medline [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddique SS, Hardy GESJ, Bayliss KL.. Cold plasma: a potential new method to manage postharvest diseases caused by fungal plant pathogens. Plant Pathol. 2018. Jun;67(5):1011–1021. doi: 10.1111/ppa.12825 [DOI] [Google Scholar]
- Šimončicová J, Kaliňáková B, Kováčik D, Medvecká V, Lakatoš B, Kryštofová S, Hoppanová L, Palušková V, Hudecová D, Ďurina P, et al.. Cold plasma treatment triggers antioxidative defense system and induces changes in hyphal surface and subcellular structures of Aspergillus flavus. Appl Microbiol Biotechnol. 2018. Aug;102(15): 6647–6658. doi: 10.1007/s00253-018-9118-y Medline [DOI] [PubMed] [Google Scholar]
- Sohbatzadeh F, Mirzanejhad S, Shokri H, Nikpour M.. Inactivation of Aspergillus flavus spores in a sealed package by cold plasma streamers. J Theor Appl Phys. 2016. Jun;10(2):99–106. doi: 10.1007/s40094-016-0206-z [DOI] [Google Scholar]
- Su X, Tian Y, Zhou H, Li Y, Zhang Z, Jiang B, Yang B, Zhang J, Fang J.. Inactivation efficacy of non-thermal plasma activated solutions against Newcastle disease virus. Appl Environ Microbiol. 2018. Feb 23;84(9):e02836-17. doi: 10.1128/AEM.02836-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suhem K, Matan N, Nisoa M, Matan N.. Inhibition of Aspergillus flavus on agar media and brown rice cereal bars using cold atmospheric plasma treatment. Int J Food Microbiol. 2013. Feb;161(2): 107–111. doi: 10.1016/j.ijfoodmicro.2012.12.002 Medline [DOI] [PubMed] [Google Scholar]
- Surowsky B, Fischer A, Schlueter O, Knorr D.. Cold plasma effects on enzyme activity in a model food system. Innov Food Sci Emerg Technol. 2013. Jul;19:146–152. doi: 10.1016/j.ifset.2013.04.002 [DOI] [Google Scholar]
- Terrier O, Essere B, Yver M, Barthélémy M, Bouscambert-Duchamp M, Kurtz P, VanMechelen D, Morfin F, Billaud G, Ferraris O, et al.. Cold oxygen plasma technology efficiency against different airborne respiratory viruses. J Clin Virol. 2009. Jun;45(2): 119–124. doi: 10.1016/j.jcv.2009.03.017 Medline [DOI] [PubMed] [Google Scholar]
- Tolouie H, Mohammadifar MA, Ghomi H, Hashemi M.. Cold atmospheric plasma manipulation of proteins in food systems. Crit Rev Food Sci Nutr. 2018;58(15):2583–2597. doi: 10.1080/10408398.2017.1335689 Medline [DOI] [PubMed] [Google Scholar]
- Tolouie H, Mohammadifar MA, Ghomi H, Yaghoubi AS, Hashemi M.. The impact of atmospheric cold plasma treatment on inactivation of lipase and lipoxygenase of wheat germs. Innov Food Sci Emerg Technol. 2018. Jun;47:346–352. doi: 10.1016/j.ifset.2018.03.002 [DOI] [Google Scholar]
- Tseng S, Abramzon N, Jackson JO, Lin WJ.. Gas discharge plasmas are effective in inactivating Bacillus and Clostridium spores. Appl Microbiol Biotechnol. 2012. Mar;93(6):2563–2570. doi: 10.1007/s00253-011-3661-0 Medline [DOI] [PubMed] [Google Scholar]
- Vandamme M, Robert E, Pesnel S, Barbosa E, Dozias S, Sobilo J, Lerondel S, Le Pape A, Pouvesle JM.. Antitumor effect of plasma treatment on U87 glioma xenografts: preliminary results. Plasma Pro cess Polym. 2010. Mar 22;7(3-4):264–273. doi: 10.1002/ppap.200900080 [DOI] [Google Scholar]
- Wang G, Zhang P, Setlow P, Li Y.. Kinetics of germination of wet-heat-treated individual spores of Bacillus species, monitored by Raman spectroscopy and differential interference contrast microscopy. Appl Environ Microbiol. 2011. May 15;77(10):3368–3379. doi: 10.1128/AEM.00046-11 Medline [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SQ, Huang GQ, Li YP, Xiao JX, Zhang Y, Jiang WL.. Degradation of aflatoxin B1 by low-temperature radio frequency plasma and degradation product elucidation. Eur Food Res Technol. 2015. Jul;241(1):103–113. doi: 10.1007/s00217-015-2439-5 [DOI] [Google Scholar]
- Wawrzycka D.. Drożdże jako model w badaniach chorób neurodege neracyjnych. Postepy Hig Med Dosw. 2011. Jun 2;65:328–337. doi: 10.5604/17322693.945767 [DOI] [PubMed] [Google Scholar]
- Wiktor A, Śledź M, Nowacka M, Witrowa-Rajchert D.. Możliwości zastosowania niskotemperaturowej plazmy w technologii żywności. Żywn Nauka Technol Jakość. 2013. 5:5–14. [Google Scholar]
- Wolny-Koładka K, Pawłat J, Terebun P, Kwiatkowski M, Diatczyk J.. Ocena możliwości zastosowania plazmy niskotemperaturowej w celu higienizacji zmieszanych odpadów komunalnych służących do produkcji paliwa alternatywnego. Przegl Elektrotechn. 2017. Nov 5;1(11):211–215. doi: 10.15199/48.2017.11.43 [DOI] [Google Scholar]
- Yasuda H, Miura T, Kurita H, Takashima K, Mizuno A.. Biological evaluation of DNA damage in bacteriophages inactivated by atmospheric pressure cold plasma. Plasma Process Polym. 2010. Mar 22;7(3-4):301–308. doi: 10.1002/ppap.200900088 [DOI] [Google Scholar]
- Ye S, Song X, Liang JL, Zheng S, Lin Y.. Disinfection of airborne spores of Penicillium expansum in cold storage using continuous direct current corona discharge. Biosyst Eng. 2012. Oct;113(2): 112–119. doi: 10.1016/j.biosystemseng.2012.06.013 [DOI] [Google Scholar]
- Zimmermann JL, Dumler K, Shimizu T, Morfill GE, Wolf A, Boxhammer V, Schlegel J, Gansbacher B, Anton M.. Effects of cold atmospheric plasmas on adenoviruses in solution. J Phys D Appl Phys. 2011. Dec 21;44(50):505201. doi: 10.1088/0022-3727/44/50/505201 [DOI] [Google Scholar]
- Ziuzina D, Boehm D, Patil S, Cullen PJ, Bourke P.. Cold plasma inactivation of bacterial biofilms and reduction of quorum sensing regulated virulence factors. PLoS One. 2015. Sep 21;10(9):e0138209. doi: 10.1371/journal.pone.0138209 Medline [DOI] [PMC free article] [PubMed] [Google Scholar]