Abstract
The root of Paris polyphylla var. yunnanensis, a famous and endangered traditional Chinese herb, has a significant medicinal value. The aim of this study was to analyze the composition and functional characteristics of bacterial endophytes in roots, stems, and leaves of P. polyphylla var. yunnanensis. The 16S rRNA gene sequencing and functional prediction of bacterial endophytes in roots, stems, and leaves of P. polyphylla var. yunnanensis were conducted. The Chao and Shannon indices of the bacteria in roots were significantly higher than those in stems and leaves. The dominant endophyte phyla were Cyanobacteria, Proteobacteria, Bacteroidetes, Firmicutes, and Actinobacteria. The main genera detected in roots were unclassified Cyanobacteria, Rhizobium, Flavobacterium, and Sphingobium; the main genera in stems were norank_c__Cyanobacteria, Bacillus, and Pseudomonas; the main genera in leaves were norank_c__Cyanobacteria and Rhizobium. The microbiota in roots was particularly enriched in functional categories “extracellular structures” and “cytoskeleton” compared with stems and leaves (p < 0.05). Our study reveals the structural and functional characteristics of the endophytic bacteria in roots, stems, and leaves of P. polyphylla var. yunnanensis, which aids in the scientific understanding of this plant.
Key words: Paris polyphylla var. yunnanensis, roots, stems, leaves, endophytic microbiota
Introduction
Paris polyphylla var. yunnanensis, a famous Chinese herb, is cultivated in the southwestern regions of China and was derived from the dry root of P. polyphylla var. Smith, a plant of the lily family (Song et al. 2015). P. polyphylla var. yunnanensis has the medical functions of cooling, detoxification, detumescence, pain relief, and tranquilizing shock (Song et al. 2017). It is widely used to treat furuncles, carbuncles, sore throat, snakebite, pain, convulsion, and other health symptoms. In addition, it is an effective component of a medical preparation such as “Yunnan Baiyao” (Song et al. 2015).
In recent years, many studies have been carried out on P. polyphylla var. yunnanensis. It is considered that the main medicinally effective components are steroidal saponins (Liang et al. 2019). Because of its medicinal value, there is an increasing demand in China for this plant. However, its growth cycle is relatively long, generally, it takes at least five years, and the annual growth of the root is too small to satisfy demand. So, P. polyphylla var. yunnanensis medicinal resources are close to depletion (Qi et al. 2013; Song et al. 2015). At present, the cultivation methods of P. polyphylla var. yunnanensis are mainly root block propagation and tissue culture, but tissue culture seedlings are easily infected by pathogenic bacteria, resulting in low yield (Duan et al. 2011).
To make effective use of above-ground stems and leaves of P. polyphylla var. yunnanensis, and to overcome the limited supply of root tissue, many studies have focused on the stems and leaves of this plant (Qin et al. 2018; Wang et al. 2018). Indeed, some studies have found that stems and leaves of P. polyphylla var. yunnanensis have some similar functions to the roots (Li et al. 2015). It remains of great significance for the planting and medicinal use of P. polyphylla var. yunnanensis to determine microbial characteristics of the stem, leaf and root.
Endophytic microbes are abundant in plants and play important roles in resisting the invasion of harmful foreign bacteria and promoting plant growth and metabolism (Huang et al. 2009; Zhao et al. 2009). However, little is known about the biodiversity within P. polyphylla var yunnanensis. This study aimed to characterize the composition and structure of the endophytic microbiota in P. polyphylla var. yunnanensis by the 16S rDNA gene sequencing. The data were analyzed in terms of roots, stems, and leaves. We also performed functional prediction according to biological characteristics.
Experimental
Materials and Methods
Experimental samples. P. polyphylla var. yunnanensis is mainly produced in Yunnan Province, southwest China. In this study, the 4-year-old cultivated plants were taken as the samples, but because the growth years of the wild plant could not be identified, we selected the wild plants with a similar weight of rhizome as the wild samples. There were both wild and artificially cultivated samples in each sample site, and the collection time was August. Finally, we screened two plants from each of Yulong County (Longitude: 100.05172; Latitude: 27.18273), Gengma County (Longitude: 100.11838674545288; Latitude: 23.886563634489097), and Tengchong City (Longitude: 98.49106; Latitude: 25.02081), all in Yunnan Province (total of six fresh plants). Half the six plants were wild type and half of them were cultivated. The plant surfaces were cleaned with sterile distilled water, disinfected with 70% ethanol, and then with sterile distilled water again. All six plants were separately collected as roots, stems, and leaves, and then were marked as R1 to R6, S1 to S2, L1 to L6.
DNA extraction and bacterial diversity analysis. DNA was extracted from roots, stems and leaves using a TruSeq™ DNA Sample Prep Kit according to the manufacturer’s protocols (Shanghai Majorbio Bio-pharm Technology Co., Ltd.). The final DNA concentration and purification were determined by NanoDrop 2000 UV-vis spectrophotometer (Thermo Scientific, Wilmington, USA), and DNA quality was checked by 1% agarose gel electrophoresis. The V3-V4 hypervariable regions of the bacteria the16S rRNA gene were amplified with primers 338F (5’-ACTCCTACGGGAGGCAGCAG-3’) and 806R (5’-GGACTACHVGGGTWTCTAAT-3’) by thermocycler PCR system (GeneAmp 9700, ABI, USA) (Cregger et al. 2018). The PCR reactions were conducted using the following program: 3 min of denaturation at 95°C, 27 cycles of 30 s at 95°C, 30 s for annealing at 55°C, and 45s for elongation at 72°C, and a final extension at 72°C for 10 min. Purified amplicons were pooled in equimolar and paired-end sequenced (2 × 300) on an Illumina MiSeq platform (Illumina, San Diego, USA) according to the standard protocols by Majorbio Bio-Pharm Technology Co. Ltd. (Shanghai, China).
Data analysis. 16S rDNA data analysis was mainly performed in the QIIME package and R software. The resulting sequences were used for the analysis of operational taxonomic units (OTUs), with a sequence similarity threshold of 97%. Then the Ribosomal Database Program classifier Bayesian algorithm was used to perform taxonomic analysis using representative sequences of OTUs (Ye et al. 2017). To calculate alpha diversity, we calculated the Chao1 and Shannon indices (Ye et al. 2017). In order to better understand the differences of microbiota characteristics among the three groups, we analyzed the composition of different sample communities and used PCA (Principal Component Analysis) to analyze the beta diversity, reflecting the difference and distance between the samples (Ye et al. 2017). Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt) is a software package that predicts the sequencing results of 16S amplifiers. According to the information of COG (Clusters of Orthologous Groups) database, the description information and function information of each COG can be parsed from eggNOG database, thus the functional abundance spectrum can be obtained. PICRUSt was performed for functional analysis using specialized software PICRUSt (Chen et al. 2017). The data was represented by the mean ± SD. There were six repeated samples in each group. Comparison between two groups was conducted by t-test; p < 0.05 was considered to be statistically significant.
Results
OTU abundances in roots, stems, and leaves. The average number of sequences cross all 18 samples was 61,955, the average number of nucleotides was 26,691,907 bp, and the average read length was 431 bp (Supplementary file 1). We found 1,258 OTUs in a group R (root samples), 1,119 OTUs in a group S (stems), and 910 OTUs in a group L (leaves). There were 902 OTUs shared by the groups S and R, 754 OTUs shared by the groups R and L, and 812 OTUs shared by the groups S and L; 670 OTUs were found in all three groups (Fig. 1).
Fig. 1.
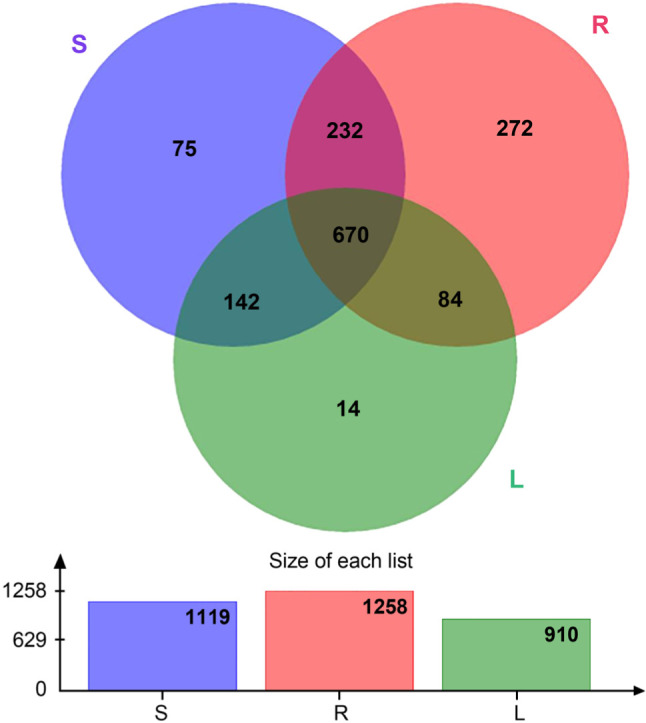
OTU relative abundances of roots, stems, and leaves (n = 6). S represents stems, R represents root, and L represents leaf.
Diversity. The microbial diversities of different groups were statistically analyzed by the t-test (Table I). According to the Chao diversity index, diversity in a group R was significantly higher than that in groups L and S (p < 0.05) (Fig. 2). In addition, the Shannon diversity index of a group R was significantly higher than that of groups L and S, and that of a group S was higher than that of group L (p < 0.05) (Fig. 3). At last, through PCA analysis, we found that there were significant differences in the composition of endophytic bacteria among three different groups, indicating that the endophytic bacterial communities in stems and leaves were more similar than those in roots (R = 0.2951, p = 0.005) (Fig. 4).
Table I.
Microbial diversity indices in roots, stems, and leaves (mean ± SD) (n = 6).
| Index | Mean ± SD (R) | Mean ± SD (S) | Mean ± SD (L) | p-value (R-S) | p-value (L-S) | p-value (L-R) |
|---|---|---|---|---|---|---|
| Shannon | 2.66 ± 0.72 | 1.55 ± 0.58 | 0.72 ± 0.28 | 1.51E-02 | 1.07E-02 | 1.10E-04 |
| Chao1 | 614.36 ± 44.24 | 467.46 ± 111.12 | 386.30 ± 66.16 | 1.32E-02 | 1.55E-01 | 3.63E-05 |
p < 0.05 was considered to be statistically significant
Fig. 2.
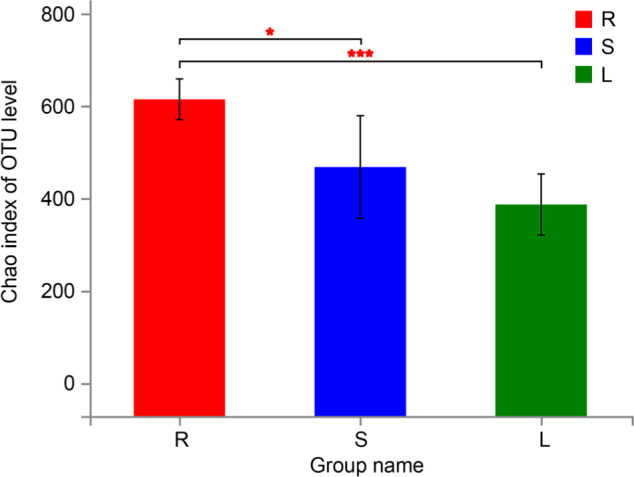
Chao index of OTU level of roots, stems, and leaves (mean ± SD) (n = 6). S represents stems, R represents root, and L represents leaf. (0.01 < p ≤ 0.05 marked as *, 0.001 < p ≤ 0.01 marked as **, p ≤ 0.001 marked as ***).
Fig. 3.
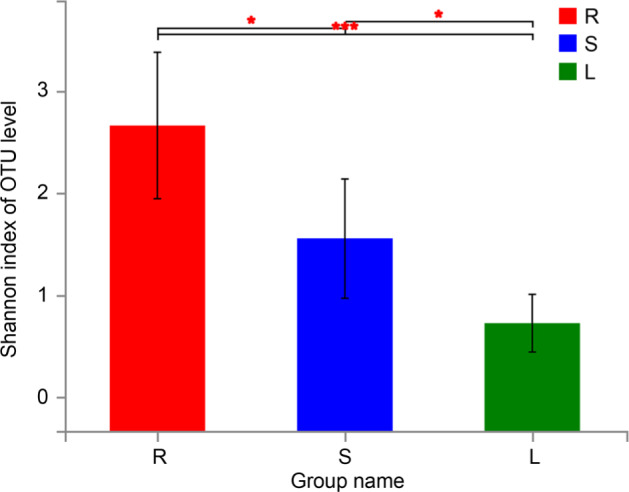
Shannon index of OTU level of roots, stems, and leaves (mean ± SD) (n = 6). S represents stems, R represents root, and L represents leaf. (0.01 < p ≤ 0.05 marked as *, 0.001 < p ≤ 0.01 marked as **, p ≤ 0.001 marked as ***).
Fig. 4.
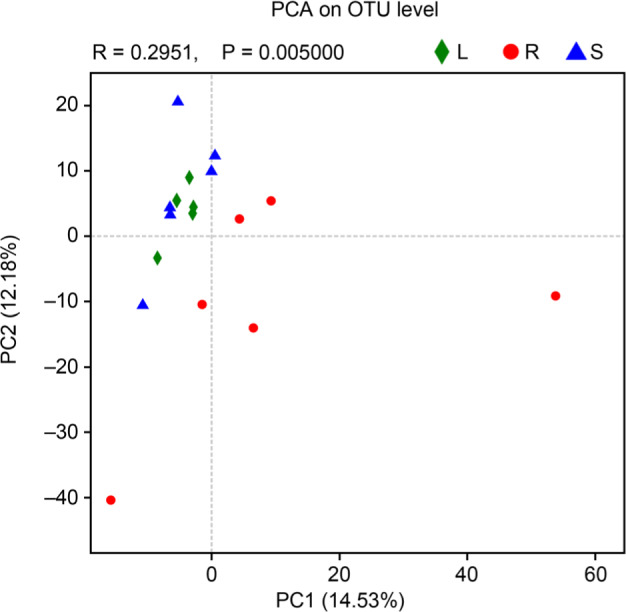
PCA of roots, stems, and leaves (n = 6). S represents stems, R represents root, and L represents leaf.
Relative microbial abundances. The dominant bacterial phyla detected in roots, stems, and leaves of P. polyphylla var. yunnanensis were Cyanobacteria, Proteobacteria, Bacteroidetes, Firmicutes, and Actinobacteria (Fig. 5). In the roots, the main phyla were Cyanobacteria (53.47% of all detected bacteria), Proteobacteria (30.89%), Bacteroidetes (6.54%), Firmicutes (3.88%), and Actinobacteria (4.35%). The main phyla in stems were Cyanobacteria (73.45%), Proteobacteria (12.41%), Firmicutes (8.28%), Actinobacteria (4.20%), and Bacteroidetes (1.39%). The main phyla in leaves were Cyanobacteria (90.37%), Proteobacteria (6.79%), Firmicutes (0.88%), Actinobacteria (1.07%), and Bacteroidetes (0.78%). The differences between Cyanobacteria, Proteobacteria, Firmicutes, and Bacteroidetes were statistically significant (p < 0.05 or 0.01, Supplementary file 2).
Fig. 5.
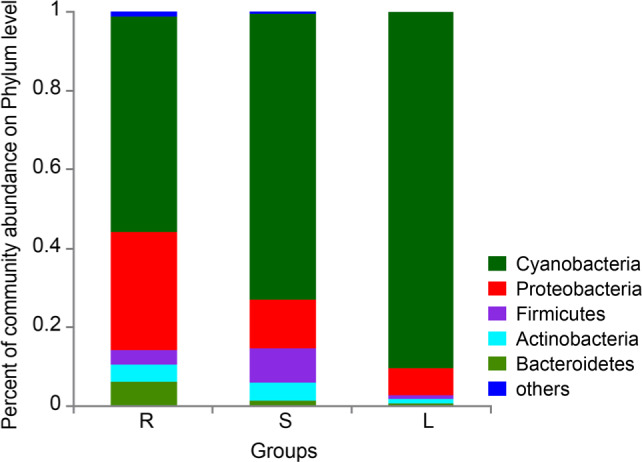
Percent of community abundance on the phylum level of roots, stems, and leaves (n = 6). S represents stems, R represents root, and L represents leaf.
We identified 13 genera present in any tissue in a proportion of more than 1% (Fig. 6). The main genera observed in roots were norank_c__Cyanobacteria (53.47%), Rhizobium (4.04%), Flavobacterium (3.14%), norank_f__Mitochondria (2.81%), and Sphingobium (2.27%). The main genera in stems were norank_c__Cyanobacteria (73.45%), Bacillus (5.12%), norank_f__Mitochondria (2.75%), and Pseudomonas (3.81%). The main genera observed in leaves were norank_c__Cyanobacteria (90.37%), norank_f__Mitochondria (1.29%), and Rhizobium (0.66%). The differences of norank_c__Cyanobacteria, Bacillus and Sphingobium were statistically significant (p < 0.05 or 0.01, Supplementary file 3).
Fig. 6.
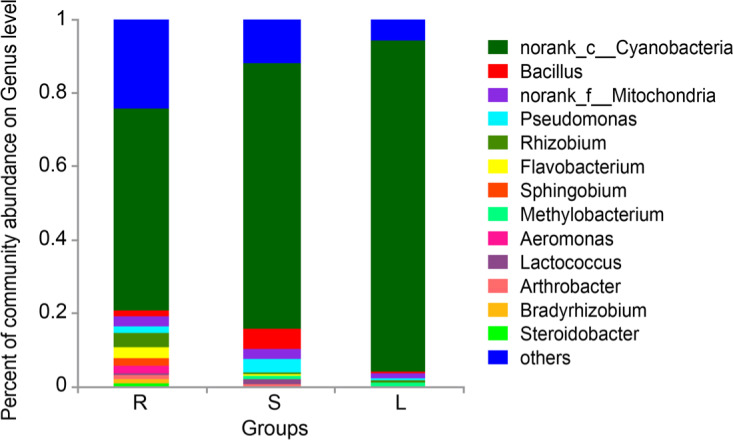
Percent of community abundance on Genus level of roots, stems, and leaves (n = 6). S represents stems, R represents root, and L represents leaf.
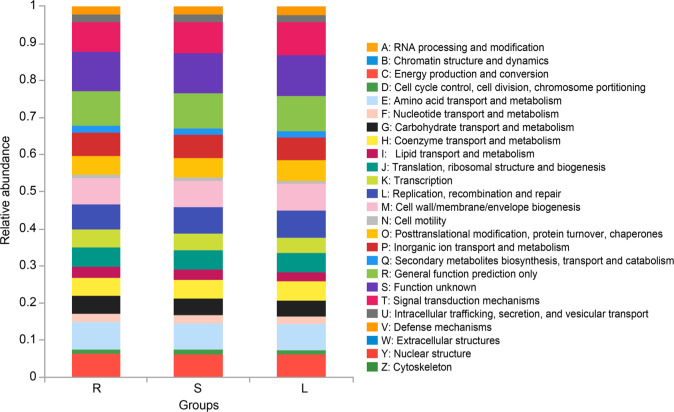
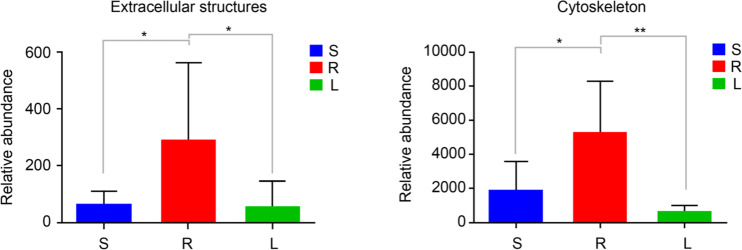
Functional characteristics of bacteria. According to the results of function prediction based on 16S rDNA analysis, we found that the microbiota in roots, stems and leaves had relatively similar functions in 25 COG functions, and were mainly enriched in the signal transduction mechanisms; energy production and conversion; replication, recombination, and repair; cell wall/membrane/envelope biogenesis; amino acid transport and metabolism; etc. (Fig. 7). This result indicated diverse metabolic functions of the microbiota in P. polyphylla var. yunnanensis. The group R was significantly more enriched than the groups S and L in functional categories extracellular structures and cytoskeleton (p < 0.05 or 0.01) (Fig. 8).
Fig. 7.
Functional relative abundance of roots, stems, and leaves (n = 6). S represents stems, R represents root, and L represents leaf.
Fig. 8.
Significantly relative abundance of roots, stems, and leaves (mean ± SD) (n = 6). A: enriched in extracellular structures. B: enriched in the cytoskeleton. S represents stems, R represents root, and L represents leaf. (0.01 < p ≤ 0.05 marked as *, 0.001 < p ≤ 0.01 marked as **).
Discussion
The 16S rDNA sequencing, facilitated by the rapid development of sequencing technology, now plays an important role in the study of bacterial community diversity and composition (Chen et al. 2017). Endophytic microbiota has a positive effect on promoting plant host metabolism and preventing infection by external bacteria (Tan et al. 2017; Ren et al. 2019). Data can be used to predict microbial enrichment functions in microbiota (Xu et al. 2018; Zegarra-Ruiz et al. 2019). Here, our study used the 16S rDNA sequencing technology to study the composition and structure of the endophytic microbiota in P. polyphylla var. yunnanensis, then predicted microbial functions, and compared the bacteria found in root, stem, and leaf.
The results of OTU abundances in roots, stems, and leaves indicated that there were the same OTUs in these three sample groups, and their number was greater in P. polyphylla var. yunnanensis roots than in leaves and stems. This preliminarily revealed the difference between roots, leaves, and stems regarding the OTUs. The results of Chao and Shannon diversity index in roots were significantly higher than that in leaves and steams indicated that the richness of endophytes was greater in P. polyphylla var. yunnanensis roots than in leaves and stems. In addition, the PCA analysis revealed that the endophytic bacterial communities in stems and leaves were more similar than those in roots and this observation also illustrated the idea from another perspective. Overall, our data showed that the number and richness of endophytes were greater in P. polyphylla var. yunnanensis roots than in leaves and stems. Some studies have underlined that bacteria (Pseudomonas, Fusarium, etc.) could produce a variety of metabolites, which might be involved in promoting the production of medicinal ingredients in plants (Wu et al. 2015; Chen et al. 2018). Therefore, we speculate that it may be one of the reasons why the roots of P. polyphylla var. yunnanensis are typically used as the medicinal parts, rather than the stems and leaves. Meanwhile, the number and abundance of endophytic microbes may be highest in the roots of P. polyphylla var. yunnanensis. Because the roots are closer to the soil than the stems and leaves and are directly supplied from the soil, which is a sink of bacteria including endophytes.
Furthermore, the dominant phyla in each of P. polyphylla var. yunnanensis roots, stems, and leaves were Cyanobacteria, Proteobacteria, Bacteroidetes, Firmicutes, and Actinobacteria, but the relative proportions of these groups differed between the plant tissues. At the relative abundance of the phylum level, these microbial composition structures of roots, stems, and leaves were different. Previous studies have shown that Cyanobacteria and Proteobacteria play an important role in medicinal plants, actively maintain the stability of the endophytic microbiota, and participate in host metabolism (Li et al. 2019; Yu et al. 2019). Furthermore, the relative abundance of Proteobacteria was highest in roots, while the value of the ratio of Proteobacteria/Cyanobacteria was highest in roots. Therefore, the differences in bacterial composition between the plant tissues may be related to their higher medicinal value.
Our study showed there were many differences in the main bacterial genera found in the roots, stems, and leaves. norank_c_Cyanobacteria were dominant in the roots, stems, and leaves. Rhizobium, Flavobacterium, and Sphingobium were more common in the roots than in the other tissues. Previous studies have shown that Sphingobium might promote metabolism and energy conversion (Lucas et al. 2018; Li et al. 2019). Rhizobium was thought to participate in the production of steroidal saponins, a medicinal compound rich in roots of P. polyphylla var. yunnanensis, and it could even fix nitrogen (Figueiredo et al. 2008; Qin et al. 2016). However, the structure and composition of the endophytic microbiota may indicate that the leaves and stems have, to some extent, the same medicinal properties as the roots, which may be the reason of the leaves and stems are less effective than the roots. It is a common phenomenon that different tissues of medical plants replace the originally used medicinal tissues (Liu and Si 2018). Of course, this work is a pioneer one in the analysis of the biodiversity of this plant; however, it is necessary to perform other studies that link the biodiversity of endophytes to the medicinal contents of the different plant tissues.
Previous studies have suggested that endophytic microbiota produce a variety of secondary metabolites and physiological activities (Wani et al. 2015; Liotti et al. 2018). Functional prediction indicated diverse metabolic functions of the microbiota in the roots, stems, and leaves of P. polyphylla var. yunnanensis. The functional classifications “extracellular structures” and “cytoskeleton” were significantly enriched in roots compared with stems and leaves. Moreover, studies have shown that the soil environment has a significant regulatory effect on plant growth (Tan et al. 2017; Urbina et al. 2018). The roots are directly affected by the soil environment, which is also related to the functions of root endophytes.
The study shows the biodiversity within P. polyphylla var. yunnanensis and characterizes the composition and structure of the endophytic microbiota in roots, stems, and leaves of P. polyphylla var. yunnanensis by the 16S rRNA gene sequencing. Although a lot of conjectures have been formed, whether the difference of the medicinal value of different tissues is related to the composition and function of endophytic bacteria still needs further verification.
Conclusions
In conclusion, our study reveals the structural and potential functional characteristics of the endophytic bacteria in roots, stems, and leaves of P. polyphylla var. yunnanensis, which aids scientific understanding of this plant. However, there are still much more researches to be carried out in the future.
Acknowledgements
The present work was supported by the National Natural Science Foundation of China (Nos: 31600018, 21620248), and Yunnan Basic Applied Research Project (No: 2016FD049).
Footnotes
Conflicts of interest
The authors do not report any financial or personal connections with other persons or organizations, which might negatively affect the contents of this publication and/or claim authorship rights to this publication.
Authors’ Contributions
Tian-hao Liu and Shou-zheng Tian participated in its design, and Tian-hao Liu, Wen-cong Tao, Yang Liu searched databases and helped to draft the manuscript. Xiao-mei Zhang, Yang Liu, and Tian-hao Liu carried out the statistical analysis of data.
Literature
- Chen C, Song X, Wei W, Zhong H, Dai J, Lan Z, Li F, Yu X, Feng Q, Wang Z, et al. The microbiota continuum along the female reproductive tract and its relation to uterine-related diseases. Nat Commun. 2017. Dec;8(1):875 10.1038/s41467-017-00901-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MY, Shao L, Zhang W, Wang CZ, Zhou HH, Huang WH, Yuan CS.. Metabolic analysis of Panax notoginseng saponins with gut microbiota-mediated biotransformation by HPLC-DAD-Q-TOF-MS/MS. J Pharm Biomed Anal. 2018. Feb;150:199–207. 10.1016/j.jpba.2017.12.011 [DOI] [PubMed] [Google Scholar]
- Cregger MA, Veach AM, Yang ZK, Crouch MJ, Vilgalys R, Tuskan GA, Schadt CW.. The Populus holobiont: dissecting the effects of plant niches and genotype on the microbiome. Microbiome. 2018. Dec;6(1):31 10.1186/s40168-018-0413-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan Y, He Z, Liang S, Wang Y, Xiong J, Zhan F, Zeng R.. [Study on correlations between total saponins content in rhizome or mycorrhizal infection rate of Pairs polyphylla var. yunnanensis and soil factors]. Zhongguo Zhong Yao Za Zhi. 2011. Nov;36(22): 3091–3095. [PubMed] [Google Scholar]
- Figueiredo MVB, Martinez CR, Burity HA, Chanway CP.. Plant growth-promoting rhizobacteria for improving nodulation and nitrogen fixation in the common bean (Phaseolus vulgaris L.). World J Microb Biot. 2008;24:1187–1193. [Google Scholar]
- Huang Y, Zhao J, Zhou L, Wang M, Wang J, Li X, Chen Q.. Antimicrobial compounds from the endophytic fungus Fusarium sp. Ppf4 isolated from the medicinal plant Paris polyphylla var. yunnanensis. Nat Prod Commun. 2009. Nov;4(11):1455–1458. 10.1177/1934578X0900401102 [DOI] [PubMed] [Google Scholar]
- Li G, Wang YF, Tang L, Yang CY, Li RY, Ma XJ.. [Phenotypic trait variation, principal component, correlation and path analysis of Paris polyphylla var. yunnanensis]. Zhong Yao Cai. 2015. Jul;38(7): 1339–1342. [PubMed] [Google Scholar]
- Li Q, Guo R, Li Y, Hartman WH, Li S, Zhang Z, Tringe SG, Wang H.. Insight into the bacterial endophytic communities of peach cultivars related to crown gall disease resistance. Appl Environ Microbiol. 2019. Mar 01;85(9):e02931-18. 10.1128/AEM.02931-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang MY, Wang YZ, Qiao X, Lu YW, Chen MH, Li P, Wen XD, Yang J.. Structural characterisation and discrimination of the aerial parts of Paris polyphylla var. yunnanensis and Paris polyphylla var. chinensis by UHPLC-QTOF-MS coupled with multivariate data analysis. Phytochem Anal. 2019. Jul;30(4):437–446. 10.1002/pca.2826 [DOI] [PubMed] [Google Scholar]
- Liotti RG, da Silva Figueiredo MI, da Silva GF, de Mendonça EAF, Soares MA.. Diversity of cultivable bacterial endophytes in Paullinia cupana and their potential for plant growth promotion and phytopathogen control. Microbiol Res. 2018. Mar;207:8–18. 10.1016/j.micres.2017.10.011 [DOI] [PubMed] [Google Scholar]
- Liu JJ, Si JP.. [Herbal textual research on Chinese medicine “Huangjing” (Polygonati Rhizoma) and some enlightenments]. Zhongguo Zhong Yao Za Zhi. 2018. Feb;43(3):631–636. [DOI] [PubMed] [Google Scholar]
- Lucas M, Balbín-Suárez A, Smalla K, Vetterlein D.. Root growth, function and rhizosphere microbiome analyses show local rather than systemic effects in apple plant response to replant disease soil. PLoS One. 2018. Oct 8;13(10):e0204922 10.1371/journal.pone.0204922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi J, Zheng N, Zhang B, Sun P, Hu S, Xu W, Ma Q, Zhao T, Zhou L, Qin M, et al. Mining genes involved in the stratification of Paris polyphylla seeds using high-throughput embryo Transcriptome sequencing. BMC Genomics. 2013;14(1):358 10.1186/1471-2164-14-358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin XJ, Ni W, Chen CX, Liu HY.. Untiring researches for alternative resources of Rhizoma paridis. Nat Prod Bioprospect. 2018. Aug;8(4):265–278. 10.1007/s13659-018-0179-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin XJ, Yu MY, Ni W, Yan H, Chen CX, Cheng YC, He L, Liu HY.. Steroidal saponins from stems and leaves of Paris polyphylla var. yunnanensis. Phytochemistry. 2016. Jan;121:20–29. 10.1016/j.phytochem.2015.10.008 [DOI] [PubMed] [Google Scholar]
- Ren F, Dong W, Yan DH.. Endophytic bacterial communities of Jingbai Pear trees in north China analyzed with Illumina sequencing of 16S rDNA. Arch Microbiol. 2019. Mar;201(2):199–208. 10.1007/s00203-018-1597-9 [DOI] [PubMed] [Google Scholar]
- Song Y, Li MF, Xu J, Zhao Z, Chen NZ.. Polymorphic microsatellite markers in the traditional Chinese medicinal plant Paris polyphylla var. yunnanensis. Genet Mol Res. 2015;14(3):9939–9942. 10.4238/2015.August.19.29 [DOI] [PubMed] [Google Scholar]
- Song Y, Xu J, Chen N, Li M.. The complete chloroplast genome of traditional Chinese medical plants Paris polyphylla var. yunnanensis. Mitochondrial DNA A DNA Mapp Seq Anal. 2017. Mar 04; 28(2):159–160. 10.3109/19401736.2015.1115489 [DOI] [PubMed] [Google Scholar]
- Tan Y, Cui Y, Li H, Kuang A, Li X, Wei Y, Ji X.. Diversity and composition of rhizospheric soil and root endogenous bacteria in Panax notoginseng during continuous cropping practices. J Basic Microbiol. 2017. Apr;57(4):337–344. 10.1002/jobm.201600464 [DOI] [PubMed] [Google Scholar]
- Urbina H, Breed MF, Zhao W, Lakshmi Gurrala K, Andersson SGE, Ågren J, Baldauf S, Rosling A.. Specificity in Arabidopsis thaliana recruitment of root fungal communities from soil and rhizosphere. Fungal Biol. 2018. Apr;122(4):231–240. 10.1016/j.funbio.2017.12.013 [DOI] [PubMed] [Google Scholar]
- Wang YH, Shi M, Niu HM, Yang J, Xia MY, Luo JF, Chen YJ, Zhou YP, Li H.. Substituting one Paris for another? In vitro cytotoxic and in vivo antitumor activities of Paris forrestii, a substitute of Paris polyphylla var. yunnanensis. J Ethnopharmacol. 2018. May; 218:45–50. 10.1016/j.jep.2018.02.022 [DOI] [PubMed] [Google Scholar]
- Wani ZA, Ashraf N, Mohiuddin T, Riyaz-Ul-Hassan S.. Plant-endophyte symbiosis, an ecological perspective. Appl Microbiol Biotechnol. 2015. Apr;99(7):2955–2965. 10.1007/s00253-015-6487-3 [DOI] [PubMed] [Google Scholar]
- Wu Z, Hao Z, Zeng Y, Guo L, Huang L, Chen B.. Molecular characterization of microbial communities in the rhizosphere soils and roots of diseased and healthy Panax notoginseng. Antonie van Leeuwenhoek. 2015. Nov;108(5):1059–1074. 10.1007/s10482-015-0560-x [DOI] [PubMed] [Google Scholar]
- Xu J, Zhang Y, Zhang P, Trivedi P, Riera N, Wang Y, Liu X, Fan G, Tang J, Coletta-Filho HD, et al. The structure and function of the global citrus rhizosphere microbiome. Nat Commun. 2018. Dec; 9(1):4894 10.1038/s41467-018-07343-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J, Joseph SD, Ji M, Nielsen S, Mitchell DRG, Donne S, Horvat J, Wang J, Munroe P, Thomas T.. Chemolithotrophic processes in the bacterial communities on the surface of mineral-enriched biochars. ISME J. 2017. May;11(5):1087–1101. 10.1038/ismej.2016.187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Guo H, Xie J, Luo J, Li Y, Liu L, Ou S, Zhang G, Peng X.. The alternate consumption of quercetin and alliin in the traditional Asian diet reshaped microbiota and altered gene expression of colonic epithelial cells in rats. J Food Sci. 2019. Mar;84(3):678–686. 10.1111/1750-3841.14473 [DOI] [PubMed] [Google Scholar]
- Zegarra-Ruiz DF, El Beidaq A, Iñiguez AJ, Lubrano Di Ricco M, Manfredo Vieira S, Ruff WE, Mubiru D, Fine RL, Sterpka J, Greiling TM, et al. A diet-sensitive commensal Lactobacillus strain mediates TLR7-dependent systemic autoimmunity. Cell Host Microbe. 2019. Jan;25(1):113–127.e6. 10.1016/j.chom.2018.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Shan T, Huang Y, Liu X, Gao X, Wang M, Jiang W, Zhou L.. Chemical composition and in vitro antimicrobial activity of the volatile oils from Gliomastix murorum and Pichia guilliermondii, two endophytic fungi in Paris polyphylla var. yunnanensis. Nat Prod Commun. 2009. Nov;4(11):1491–1496. 10.1177/1934578X0900401111 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.