Abstract
In 2002, we discovered that mice carrying the hypomorphic Gata1low mutation that reduces expression of the transcription factor GATA1 in megakaryocytes (Gata1low mice) develop myelofibrosis, a phenotype that recapitulates the features of primary myelofibrosis (PMF), the most severe of the Philadelphia-negative myeloproliferative neoplasms (MPNs). At that time, this discovery had a great impact on the field because mutations driving the development of PMF had yet to be discovered. Later studies identified that PMF, as the others MPNs, is associated with mutations activating the thrombopoietin/JAK2 axis raising great hope that JAK inhibitors may be effective to treat the disease. Unfortunately, ruxolitinib, the JAK1/2 inhibitor approved by FDA and EMEA for PMF, ameliorates symptoms but does not improve the natural course of the disease, and the cure of PMF is still an unmet clinical need. Although GATA1 is not mutated in PMF, reduced GATA1 content in megakaryocytes as a consequence of ribosomal deficiency is a hallmark of myelofibrosis (both in humans and mouse models) and, in fact, a driving event in the disease. Conversely, mice carrying the hypomorphic Gata1low mutation express an activated TPO/JAK2 pathway and partially respond to JAK inhibitors in a fashion similar to PMF patients (reduction of spleen size but limited improvement of the natural history of the disease). These observations cross-validated Gata1low mice as a bona fide animal model for PMF and prompted the use of this model to identify abnormalities that might be targeted to cure the disease. We will summarize here data generated in Gata1low mice indicating that the TGF-β/P-selectin axis is abnormal in PMF and represents a novel target for its treatment.
Keywords: cancer supporting microenvironment, Gata1, hematopoietic stem cells, megakaryocytes, primary myelofibrosis, P-selectin, TGF-β
1 |. INTRODUCTION
Primary myelofibrosis (PMF) is the most severe of the Philadelphia-negative myeloproliferative neoplasms (MPNs), which include polycythemia vera (PV) and essential thrombocytopenia (ET).1,2 As all the other MPNs, PMF is a clonal disorder of the hematopoietic stem cells (HSC),3,4 which usually has sporadic occurrence, although cases of familiar PMF have been occasionally reported.1,2 PMF is manifested with thrombocytopenia and anemia associated with the presence of platelets larger than normal (megathrombocytes) and red blood cells with tear drop shape (tear drop poikilocytes) in the blood.1,2 The bone marrow of PMF patients presents distinctive microenvironment abnormalities with excessive deposition of extracellular matrix (fibrosis), increased proliferation of osteoblasts (osteosclerosis) and endothelial cells (neoangiogenesis). These abnormalities lead to hematopoietic failure in bone marrow, increased stem/progenitor cell trafficking, and development of hematopoiesis in extramedullary sites, mostly the spleen. The disease eventually evolves in leukemia.1,2 The complexity of the PMF phenotype makes unlikely that the disease is driven by the mutation of a single gene. Furthermore, the presence of thrombocytopenia and anemia, two traits regulated by the transcription factor GATA1,5 suggests that in some way the driver mutation(s) impair GATA1 functions. In spite of the great progresses made in recent years in our understanding of the genetic lesions driving PMF, the etiology of PMF is far to be completely understood and is the subject of numerous investigations. These investigations are prompted by the belief that, besides their therapeutic implications for PMF, studies on the etiology of MPN have the potential to share light also on the complex interactions between stem cell and microenvironmental abnormalities that support progression to the metastatic phase in other cancers and on mechanisms underlying development of the fibrosis preceding the fatal failure of other organs.
This review will summarize the role played by megakaryocyte abnormalities induced by hypomorphic GATA1 content on the etiology of PMF, with the hope that this information will be useful to understand the pathogenesis of other diseases as well.
2 |. THE MUTATION LANDSCAPE OF PMF DOES NOT INCLUDE Gata1
In 2005, four groups identified that MPNs are associated with the gain-of-function JAK2V617F mutation in the gene encoding the Janus Kinase-2 (JAK2, >95% of PV and 50–60% of ET and PMF).6–9 JAK2 is a tyrosine kinase that initiate the signaling from all the hematopoietic superfamily receptors, including the receptors for erythropoietin (EPO), thrombopoietin (TPO), and granulocyte-colony stimulating factor (G-SCF),10 and that controls the proliferation of normal hematopoietic cells of many lineages. It is not surprising, therefore, that its constitutive activation may drive hyperproliferation of several hematopoietic lineages inducing diseases associated with excessive red blood cell production, PV, or excessive production of megakaryocytes with (PMF) or without (ET) a block in platelet production. Since the discovery of JAK2V617F, the mutation landscape of MPNs has been refined with the identification of additional mutations in the JAK2/MPL axis in those patients who lack canonical JAK2 mutations.10,11 Furthermore, PMF patients may present gain-of-function mutations in additional genes involved in epigenetic and splicing regulation and in induction of apoptosis.10,11 Since these mutations are found across all the hematopoietic malignancies and their acquisition may either precede or follow that of the JAK2/MPL mutations, they are thought to drive the evolution of PMF to leukemia. In spite of the great progress made on the knowledge on the genetic basis of MPNs, the mechanism(s) that decides how the same JAK2/MPL mutation may induce hyperproliferation of either the erythroid (PV) or the megakaryocyte lineage (ET and PMF) is still far to be elucidated. Studies in vitro and in mouse models suggest that the erythroid or megakaryocyte phenotype is determined by the levels of activation of STAT5, the signaling element immediately downstream to JAK2V617F with high and low levels of STAT5 activation driving PV or ET, respectively.12–15 In addition, the mechanisms determining the block in maturation of the hyperproliferating megakaryocytes in PMF may be represented by a RSP14 ribosomopathy (reviewed in Reference 16).
3 |. PMF MEGAKARYOCYTES ARE HYPOMORPHIC FOR Gata1
The hypomorphic Gata1low mutation, which specifically deletes the first hypersensitive site (HS1) upstream to the gene, was developed in 1997 in the Stuart Orkin laboratory.17 At that time, the establishment of this mutation, and of the similar Gata10.5 mutation developed by the Iamamoto laboratory,15 represented a paradigm shift in our approach to study the genetic control of protein function. In fact, starting from these mutations, the focus of these studies switched from determining how the function of a protein was controlled by gene coding sequences on how it was instead controlled by noncoding sequences that regulate its levels of expression. Studies on Gata1low and Gata10.5 mice have identified the first lineage-specific regulatory sequences and assessed that erythroid and megakaryocyte differentiation is assured by an appropriate protein concentration window inside the cells.15,17,18 The mutation was originally established in the C57BL/6 background, where it induces a high (>90%) perinatal mortality due to severe anemia.17 In the CD1 background, however, the mutation is no longer perinatally lethal,19 and the mice have an apparently normal life span because, by recruiting within few weeks, the spleen as an extramedullary erythropoietic site expresses normal hematocrit levels from 1 to 18 months of age.19 The splenic erythropoiesis of Gata1low mice does not exhaust the response to “erythroid stress” since these mice are still capable of increasing hematocrit levels when treated with exogenous EPO and recover from hemolytic anemia induced by phenylhydrazine.19 It is thought that the splenic erythropoiesis occurring in Gata1low mice at steady state is sustained by a pathway different from that activated during canonical “stress erythropoiesis” because it does not involve the glucocorticoid/BMP pathway20 but rather hyperactivation of the TPO pathway.21,22 Hyperactivation of TPO signaling generates in the spleen of Gata1low mice a unique precursor cell that remains bipotent for the erythroid and megakaryocyte pathway until the very end of the maturation process.23,24 These precursor cells are similar to those observed in vitro when human CD34+ cells are cultured with a recombinant hyperactive TPO mimetic.25
Proof that almost all the red blood cells circulating in the blood of Gata1low mice are generated by the spleen is provided by the observation that splenectomy induces death of the mice by profound anemia within 2 weeks.26 In addition, in Gata1low mice almost all the hematopoietic stem cells are present in their spleen.27
The fact that the CD1 Gata1low mice do not die at birth allowed the identification that GATA1 also regulates terminal maturation of mast cells28 and of dendritic cells.29 In the latter cases, the mutation increases the cell proliferation rates but impairs their ability to present antigens in response to lipopolysaccharides, suggesting that Gata1low mice also have impaired ability to activate T cells.
The megakaryocyte abnormalities expressed by Gata1low mice include increased proliferation and delayed maturation and are extremely similar to those found in PMF (Figure 1).30–32 It is therefore not surprising that Gata1low mice develop myelofibrosis with robust levels of collagen fibers in the bone marrow with age.33,34
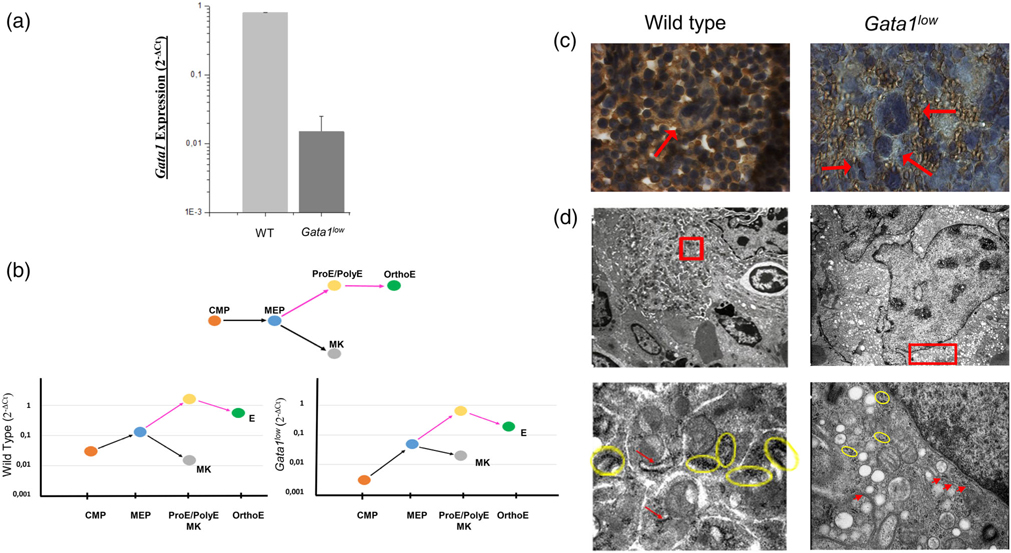
FIGURE 1.
Reduced frequency of ribosomes decreases the GATA1 content in the megakaryocytes from the spleen of Gata1low mice in spite of normal levels of Gata1 mRNA. (a) Quantitative RT-PCR determinations of the levels of Gata1 mRNA expressed by megakaryocytes prospectively isolated from the bone marrow of one-month old wild-type (WT) and Gata1low littermates showing that Gata1low megakaryocytes express reduced levels of Gata1 mRNA. (b) Quantitative RT-PCR determinations of the levels of Gata1 mRNA expressed by common myeloid progenitor cells (CMP, Lin− Sca1− Ly6c− FcεRIα− c-kitpos CD34low CD16/CD32+), bipotent megakaryocyte-erythroid progenitor cells (MEP, Lin− Sca1− Ly6c− FcεRIα− c-kitpos CD34low CD16/CD32low), early (proerythroblasts, ProE, and polychromatic erythroblasts, PolyE) and late (orthochromatic erythroblasts, OrthoE) erythroblasts and megakaryocytes (MK) prospectively isolated from the spleen of WT and Gata1low littermates, as indicated. Gata1low CMP, MEP and erythroid cells express levels of Gata1 mRNA onefold lower than that expressed by the corresponding WT cells while the levels of Gata1 mRNA in Gata1low megakaryocytes is within normal ranges. (c) Immunostaining with a Gata1 specific antibody of the spleen from WT and Gata1low littermates. The nuclei of WT megakaryocytes, but not those from Gata1low littermates, were readily stained by the antibody. Megakaryocytes are indicated by arrows. Magnification ×100. (d) Transmission electron microscopy of representative megakaryocytes, top panels, from WT and Gata1low littermates and details of their perinuclear area (bottom panels). The detail of the Gata1low megakaryocyte indicate the presence of a poorly developed rough endoplasmic reticulum with reduced numbers of polysomes (indicated by the yellow circles). Magnifications ×4,400 and ×30,000 in the top and bottom panels, respectively. Yellow Circles indicated polyribosomes and red arrows show poorly developed rough endoplasmic reticulum. Similar results were published in References 22 and 39
The similarity between the megakaryocyte abnormalities observed in Gata1low mice and PMF patients suggests that they are determined by the same mechanism(s). In fact, also megakaryocytes from PMF patients are hypomorphic for GATA1 because, in spite of normal levels of GATA1 mRNA,35 they express a RSP14 signature, which impairs the translation of GATA1 mRNA.36 The list of the RSP14-dependent genes included in the GeneSet database and how the expression of these genes is altered in Gata1low mice are included in Table S1. In addition, although at birth Gata1low megakaryocytes express, as expected, reduced levels of Gata1 mRNA (Figure 1a), in adult mice the mutation reduces the levels of Gata1 mRNA mostly in stem/progenitor cells, altering the GATA1/GATA2 switch that controls the proliferation of hematopoietic progenitor cells37 favoring their expansion38 but not in the megakaryocytes (Figure 1b). However, as in the case of PMF patients, the low GATA1 content of these cells (Figure 1c) is probably the result of a RSP14 ribosomopathy that reduces the numbers of ribosomes in these cells (Figure 1d) impairing the translation of Gata1 mRNA.22 Also in the case of Gata1low mice, the RSP14 signature is induced by a hyperactive MPL/JAK2 pathway (Table 1).22
TABLE 1.
Summary of the abnormalities expressed by primary myelofibrosis (PMF) patients that were first identified in Gata1low mice and that represent potential druggable targets to cure the disease
| Abnormality | Gata1low mice | PMF patients | |
|---|---|---|---|
| HSC | ↑CXCR4 | 39 | 39–42 |
| ↑Mobilization | 39 | 43 | |
| Megakaryocytes | ↑TGF-β | 27, 44 | 44–47 |
| ↑P-selectin | 27 | 48 | |
| ↑LOX2 | 34 | 34 | |
| ↑Neutrophil emperipolesis | 31 | 45 | |
| ↑Parapoptosis | 31 | 49 | |
| Neutrophils | ↑DNA trap | 50 | 51 |
| ↑Emperipolesis | 31 | 45 | |
| Bone marrow microenvironment | ↑TGF-β bioavailability | 44 | 44 |
| ↑Activated fibrocytes | 27 | 52 | |
| ↑Collagen fibers | 33, 35 | 1, 2 | |
| ↓Normal HSC | 27, 44 | 53, 54 | |
| T cells | ↓Activation | 50 | 55, 56 |
| Spleen microenvironment | ↑Malignant HSC | 27, 44 | 53, 54 |
| ↑Megakaryocytes | 33 | 1, 2 | |
| ↑Activated fibrocytes | 27, 44 | 44 | |
| ↑Abnormal endothelial cells | 33 | 57 | |
| ↑TGF-β bioavailability | 44 | 44 | |
| Plasma/serum | ↑TPO | 22 | - |
| ↑SDF-1 | 39 | 41, 58 | |
| ↑Bioactive TGF-β | 44 | 56 | |
| ↑Total TGF-β | 44 | 55, 56 | |
| ↑Antimitochondrial antibodies | - | 50 |
The discovery of the mutations that drive the development of MPNs prompted the development of mutation-specific mouse models (reviewed in Reference 59). These mutants rapidly develop a PV and/or ET phenotype, which eventually evolves in myelofibrosis, and are considered models for secondary myelofibrosis. The value of these mutants for PMF, however, is limited by the fact that their bone marrow contains high levels of reticulinic fibers (fibers with low levels of collagen polymerization detected at the early stage of the disease) but it is unclear if they ever develop the collagen fibers developed when the disease progress to myelofibrosis. These limitations and the similarities existing between the activation of the MPL/JAK2 pathway and the RSP14 ribosomapathy observed in PMF patients and Gata1low mice finally established Gata1low mutants as bona fide model for PMF.
3.1 |. Myelofibrosis is a disease of the microenvironment sustained by the abnormal megakaryocytes
In 2007, two papers by Walkey et al. established that mutations altering the microenvironment are necessary and sufficient to induce an MPN phenotype in mice, suggesting that the establishment of MPN does not require abnormal hematopoietic stem cells.60,61 More recently, two independent studies have refined this concept by demonstrating that expression of JAK2V617F in megakaryocytes, which are important regulators of the microenvironment,62 is necessary and sufficient to induce myelofibrosis in mice.63,64 Together, these observations suggest that PMF is driven by products produced by the abnormal megakaryocytes that alters the stem cell-supporting functions of the microenvironment and raise hope that the disease may be cured by targeting these products.
The hypothesis that megakaryocyte abnormalities drive the development of PMF is not new and has been supported by extensive morphological data. The presence of distinctive megakaryocyte abnormalities characterized by hyperproliferation with retarded maturation was the first cellular hallmark for PMF to be identified.32 In addition to be present in high number as immature cell clusters, an indication of hyperproliferation, the megakaryocytes present in the bone marrow of PMF patients contain high levels of the growth factor TGF-β44,45 and are embedded by high numbers of neutrophils entrapped in their cytoplasm by a process of pathological emperipolesis.31,65 It is important to mention that TGF-β is one of the growth factors responsible for the epithelial-mesenchymal transition, which induces the formation of activated fibrocytes suggested by Robert Weinberg to be responsible for fibrosis and disease progression in cancer.66 Furthermore, the bone marrow of PMF patients contains high numbers of naked nuclei with heavily condensed chromatin, which are probably the reminiscence of megakaryocytes that died by an immune-mediated process defined para-apoptosis.31,49 These morphological observations suggest that the microenvironment surrounding the para-apoptotic megakaryocytes is enriched for growth factors produced by these cells and activated by the neutrophil proteases.
Also the megakaryocytes from Gata1low mice remain immature and are subjected to death by para-apoptosis (Figure 2). In addition, they express high levels of TGF-β and P-selectin, the adhesion receptors that mediates their interaction with neutrophils (Figure 3). These observations lead to the pathobiological model for myelofibrosis summarized in Figure 4, which hypothesizes that the disease is sustained by progressive TGF-β accumulation in the microenvironment triggered by pathological P-selectin-dependent emperipolesis between megakaryocytes and neutrophils.
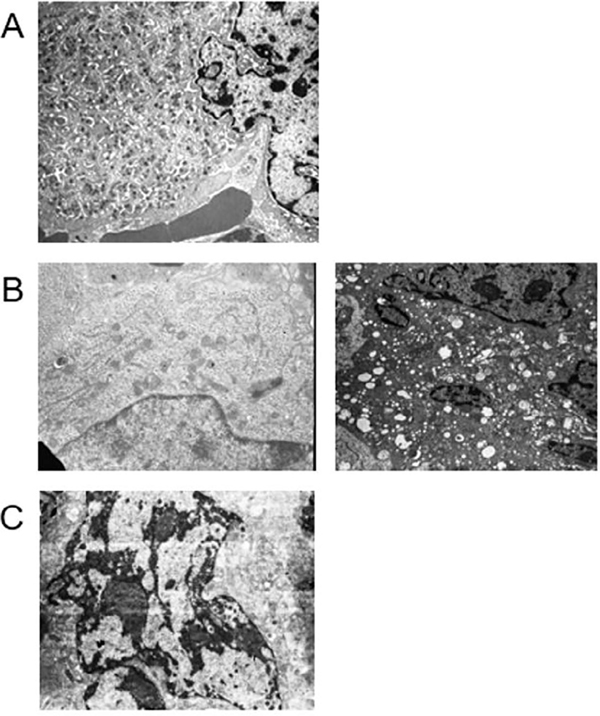
FIGURE 2.
Gata1low megakaryocytes remain immature and the nuclei of some of them present a tightly condensed chromatin characteristic of para-apoptosis, an immune-mediated form of cell death probably induced by pathological neutrophil emperipolesis. (a) Electron microscopy analyses of a mature wild-type megakaryocyte with properly developed demarcation membrane system and delineated platelet territories. (b) Electron microscopy analyses of one representative immature (left panel) and one representative para-apoptotic (right panel) Gata1low megakaryocyte. (c) Electron microscopy analyses of a representative mature megakaryocyte from a Gata1low mouse treated with the TGF-β inhibitor SB431542. Magnification ×4,400. Similar results were published in References 31 and 45
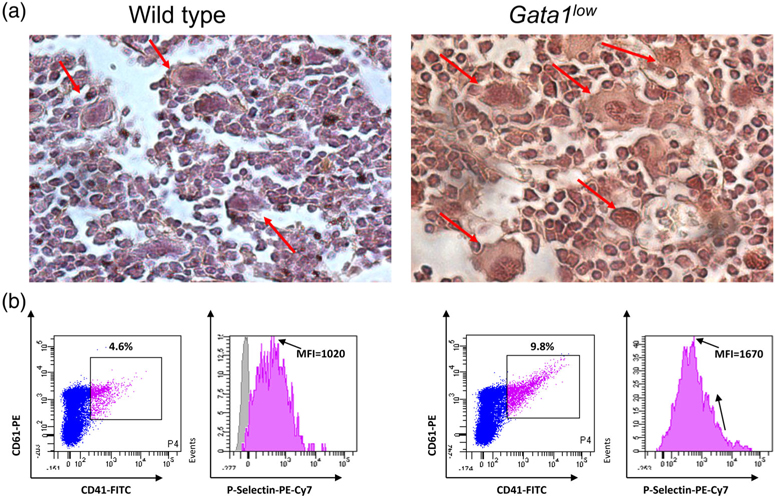
FIGURE 3.
Gata1low megakaryocytes express levels of TGF-β and of P-selectin greater than normal. (a) TGF-β-specific immunohistochemistry of bone marrow sections from representative wild-type and Gata1low littermates, as indicated. To be noted the intense immunostaining of the Gata1low megakaryocytes. Magnification ×40. Megakaryocytes are indicated by arrows. Similar results were published in Reference 44. (b) Flow cytometry analyses for the expression of P-selectin by megakaryocytes from wild-type and Gata1low littermates. Megakaryocytes were identified on the basis of CD41/CD61 markers, as indicated. Expression of high levels of P-selectin in Gata1low megakaryocytes was also detected by electron microscopy31
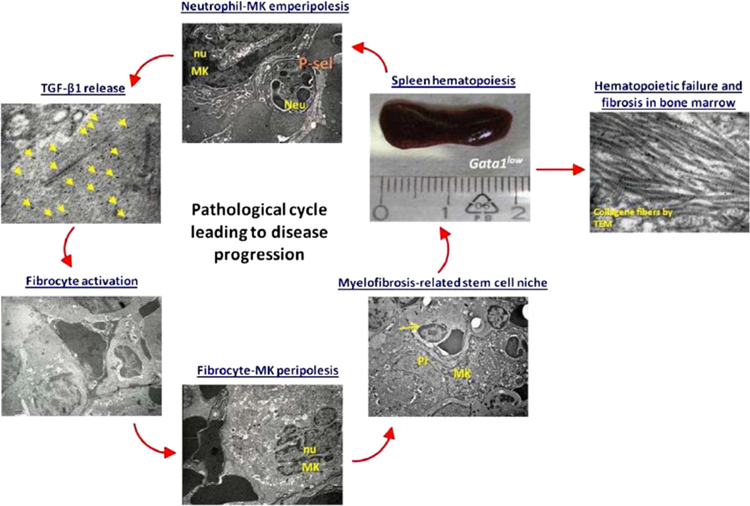
FIGURE 4.
An outline of the P-selectin/TGF-β circuit leading to disease progression in the Gata1low mouse model of myelofibrosis. We propose that in Gata1low mice, hematopoiesis in the spleen is sustained by a circuit between P-selectin and TGF-β and contributes to disease progression. This circuit is triggered by the abnormal expression of P-selectin on megakaryocytes (MK) that leads to neutrophilmegakaryocyte emperipolesis, increasing TGF-β content in the surrounding microenvironment and resulting in fibrocyte activation. Activated fibrocytes establish, possibly through P-selectin, peripolesis with MK forming “myelofibrosis-related stem cell niches” that sustain proliferation of the malignant stem cells in spleen generating more megakaryocytes and more neutrophils, establishing an amplification loop that contributes to disease progression. This loop may also determine hematopoietic failure and fibrosis in bone marrow. Neu, neutrophil; nu, nucleus; Pr, fibrocyte protrusion; yellow arrow, immature hematopoietic cell; yellow arrowheads, TGF-β-immuno, gold particles. The scale allows appreciation of the splenomegaly experienced by Gata1low mice
This model was tested by experiments indicating that chemical inhibition of TGF-β signaling44 or genetic ablation of P-selectin27 completely restores megakaryocyte maturation (Figure 2) and rescues the myelofibrotic phenotype of Gata1low mice. These observations support the therapeutic hypothesis that cure of myelofibrosis requires targeting the abnormalities of both the malignant stem cells and of their supporting microenvironment.67 This hypothesis is testable since both TGF-β and P-selectin are druggable by products developed for clinical use in other diseases. TGF-β may be inhibited either by galunisertib, a small molecule that inhibits AKL5, the first element of the TGF-β receptor 1 signaling68 or by the TGF-β receptor trap AVID200 developed by Forbious.69 P-selectin may instead be inhibited by crizanlizumab, an antibody developed by Novartis, which has been shown effective to prevent pain crisis in Sickle Cell Diseases.67 Phases 1–2 clinical trials with AVID200 (NCT03094169, open for accrual) and Crizanlizumab (NCT not available as yet) in combination with ruxolitinib are in progress.
The identification of biomarkers to evaluate disease progression or response to therapy is another important facet in the process to address the clinical need of a disease. The comparison of the expression signature of bone marrow from Gata1low mice and PMF patients identified common abnormalities in 12 genes of the noncanonical TGF-β signaling67,70 which include Jun, JUNB, FOS, FOSB, HIPK2, ID1, GADD45b, BMP4, BMP7, EVI1, STAT1, and IL8.50,71 Jun, JUNB, FOS, and FOSB are transcription factors involved in the formation of the complex AP-1 (activator protein 1), which regulate gene expression in myelomonocytic cells and recently suggested by the Weissman laboratory of be responsible to induce fibrosis in multiple organs, including in bone marrow.72 Altered expression of HIPK2 (homeodomain-interacting protein kinase 2) has been implicated in the development of kidney fibrosis.73 ID1 (DNA-binding protein inhibitor 1) and GADD45b (growth arrest and DNA-damage-inducible beta) are two genes implicated in the growth and progression of multiple cancers.74,75 BMP4 and BMP7 (bone morphogenetic proteins 4 and 7) are among the growth factors that regulate bone and cartilage formation and may be responsible for the osteosclerosis expressed by Gata1low mice76 while EVI1 (ecotropic virus integration site 1 protein homolog) was among the first transcription factors involved in tumor development in mice which has been implicated in drug resistance in human leukemia.77 On the other hand, the signal transducer and activator of transcription (STAT) 1 protein is another STAT family member downstream of JAK2 signaling and its activation has been implicated in development of myelofibrosis by the Skoda laboratory.78 Last but not least, IL-8 (Interleukin 8) is a cytokine produced by many cells known to be overexpressed in PMF, but not in ET the levels of which may represent an infaust prognostic criteria in PMF.79
These genes are included in arrays currently under development to be used as diagnostic or prognostic tool in PMF.
Besides the TGF-β/P-selectin circuit discussed above, the profile of Gata1low mice and PMF patients includes several additional abnormalities many of which are also druggable (Table 1). The preclinical studies which are in progress to validate their use as therapeutic targets for PMF are reviewed in Reference 16.
Last but not least, the expression signature of the bone marrow of Gata1low mice includes the transcription factor c-Jun which is expressed at altered levels also in the fibrosis state of other organs,72 raising hope that the same therapeutic strategy may be used to cure fibrosis in multiple organs. To test this hypothesis, we are currently investigating whether Gata1low mice develop fibrosis in organs other than bone marrow.
4 |. CONCLUSIONS
The interplay between hematopoietic stem cells and their supporting microenvironment in the regulation of hematopoiesis was originally identified thanks to mice carrying mutations at the steel and white locus. Steel mice carry mutations in the gene encoding stem cell factor, the stem cell specific growth factor and is expressed by the microenvironment. The white mice carry mutations in c-Kit, the gene encoding the receptor for stem cell factor expressed on the stem cells. The numerous studies on the effects exerted by mutations at these two single loci on adult hematopoiesis have greatly contributed to shape up our understanding of its regulation (reviewed in References 80–84). Studies on adult hematopoiesis in Gata1low mice have now clarified an important facet of the interplay between the stem cells and their niche. They have put in the picture megakaryocytes as the progeny of the hematopoietic stem cells responsible to modulate the supporting functions of their niche in response to physiological hematopoietic perturbations. In the case of Gata1low mice, however, chronic platelet deficiency activates a TPO/JAK2 axis, which sustains the maturation of abnormal megakaryocytes responsible for the activation of unique niches in the spleen that turn normal hematopoietic stem cells into malignant clones, inducing myelofibrosis. By putting megakaryocytes at the center of the myelofibrosis etiology, studies in Gata1low mice provide proof-of-principle in animal models that PMF may be prevented and reversed by targeting products (TGF-β, P-selectin, and possibly others) released by megakaryocytes to regulate the hematopoietic stem cell niches.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by grants from the National Cancer (P01-CA108671), Heart, Lung and Blood Institute (1R01-HL116329), and Associazione Italiana Ricerca Cancro (AIRC 17608).
Funding information
Associazione Italiana per la Ricerca sul Cancro, Grant/Award Number: 17608; Center for Strategic Scientific Initiatives, National Cancer Institute, Grant/Award Number: P01-CA108671; National Heart, Lung, and Blood Institute, Grant/Award Number: 1R01-HL116329
Abbreviations
- ALK5
Anaplastic Lymphome Kinase 5
- AP-1
Activator protein 1
- BMP4–7
Bone Morphogenetic Protein 4–7
- CD
Cluster of Differentiation
- CMP
Common Myeloid Progenitor
- CXCR4
C-X-C chemokine receptor type 4
- DNA
DeoxyriboNucleic Acid
- EMEA
European Medicines Evaluation Agency
- EPO
Erythropoietin
- ET
Essential Thrombocytopenia
- EVI1
Ecotropic Virus Integration site 1 protein homolog
- FcεRIα
High-affinity IgE receptor subunit α
- FDA
Food and Drug Administration
- FITC
Fluorescein isothiocyanate
- FOS
transforming gene of Fibroblast OsteoSarcoma Virus
- GADD45b
Growth Arrest and DNA Damage 45b
- GATA1–2
GATA-binding factor 1–2
- G-SCF
Granulocyte-Colony Stimulating Factor
- HIPK2
Homeodomain-Interacting Protein Kinase 2
- HS1
Hypersensitive Site 1
- HSC
Hematopoietic Stem Cells
- ID1
DNA-binding protein inhibitor
- IL
Interleukin
- JAK1–2
Just Another Kinase 1–2
- JUN, ju-nana
Japanese word for seventeen
- LIN
Lineage
- LOX2
Lipoxygenase-2
- Ly6c
Lymphocyte antigen 6 complex
- MEP
Megakaryocytes-Erythroid Progenitors
- MK
Megakaryocytes
- MPL
Myeloproliferative Leukemia Protein
- MPN
Myeloproliferative Neoplasm
- OrthoE
Orthichromatic Erythroblasts
- PE- Cy7
Phycoerythrin and Cyanine dye
- PMF
Primary Myelofibrosis
- PolyE
Polychromatic Erythroblasts
- ProE
ProErythroblast
- PV
Polycythemia Vera
- RSP14
Ribosome Protein 14
- RT-PCR
Reverse transcription polymerase chain reaction
- SCA1
Stem cells antigen-1
- SDF-1
Stromal cell-Derived Factor 1
- STAT1–5
Signal Transducer and Activator of Transcription 1–5
- TGF-β
Tumor/Trasforming Growth Factor β
- TPO
Thrombopoietin
- WT
Wild Type
Footnotes
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of this article.
REFERENCES
- 1.Tefferi A, Vainchenker W. Myeloproliferative neoplasms: Molecular pathophysiology, essential clinical understanding, and treatment strategies. J Clin Oncol. 2011;29:573–582. [DOI] [PubMed] [Google Scholar]
- 2.Barosi G, Hoffman R. Idiopathic myelofibrosis. Semin Hematol. 2005;42:248–258. [DOI] [PubMed] [Google Scholar]
- 3.Adamson JW, Fialkow PJ, Murphy S, Prchal JF, Steinmann L. Polycythemia vera: Stem-cell and probable clonal origin of the disease. N Engl J Med. 1976;295:913–916. [DOI] [PubMed] [Google Scholar]
- 4.Jamieson CHM, Gotlib J, Durocher JA, et al. The JAK2 V617F mutation occurs in hematopoietic stem cells in polycythemia vera and predisposes toward erythroid differentiation. Proc Natl Acad Sci USA. 2006;103:6224–6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crispino JD, Weiss MJ. Erythro-megakaryocytic transcription factors associated with hereditary anemia. Blood. 2004;123: 3080–3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.James C, Ugo V, Le Couédic J-P, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434:1144–1148. [DOI] [PubMed] [Google Scholar]
- 7.Kralovics R, Passamonti F, Buser AS, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352:1779–1790. [DOI] [PubMed] [Google Scholar]
- 8.Levine RL, Wadleigh M, Cools J, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7:387–397. [DOI] [PubMed] [Google Scholar]
- 9.Baxter EJ, Scott LM, Campbell PJ, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365:1054–1061. [DOI] [PubMed] [Google Scholar]
- 10.Vainchenker W, Constantinescu SN, Plo I. Recent advances in understanding myelofibrosis and essential thrombocythemia. F1000Res. 2016;5:700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guglielmelli P, Lasho TL, Rotunno G, et al. MIPSS70: Mutation-enhanced international prognostic score system for transplantation-age patients with primary myelofibrosis. J Clin Oncol. 2018;36:310–318. [DOI] [PubMed] [Google Scholar]
- 12.Teofili L, Martini M, Cenci T, et al. Different STAT-3 and STAT-5 phosphorylation discriminates among Ph-negative chronic myeloproliferative diseases and is independent of the V617F JAK-2 mutation. Blood. 2007;110:354–359. [DOI] [PubMed] [Google Scholar]
- 13.Olthof SG, Fatrai S, Drayer AL, Tyl MR, Vellenga E, Schuringa JJ. Downregulation of signal transducer and activator of transcription 5 (STAT5) in CD34 + cells promotes megakaryocytic development, whereas activation of STAT5 drives erythropoiesis. Stem Cells. 2008;26:1732–1742. [DOI] [PubMed] [Google Scholar]
- 14.Yan D, Hutchison RE, Mohi G. Critical requirement for Stat5 in a mouse model of polycythemia vera. Blood. 2012;119: 3539–3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shimizu R, Yamamoto M. GATA-related hematologic disorders. Exp Hematol. 2016;44:696–705. [DOI] [PubMed] [Google Scholar]
- 16.Eran Z, Zingariello M, Bochicchio M, Bardelli C, Migliaccio A. Novel strategies for the treatment of myelofibrosis driven by recent advances in understanding the role of the microenvironment in its etiogenesis. F1000Res. 2019;8:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mcdevitt MA, Fujiwara Y, Shivdasani RA, Orkin SH. An upstream, DNase I hypersensitive region of the hematopoietic-expressed transcription factor GATA-1 gene confers developmental specificity in transgenic mice. Proc Natl Acad Sci USA. 1997;94:7976–7981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shivdasani RA, Fujiwara Y, McDevitt MA, Orkin SH. A lineage-selective knockout establishes the critical role of transcription factor GATA-1 in megakaryocyte growth and platelet development. EMBO J. 1997;16:3965–3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vannucchi AM, Bianchi L, Cellai C, et al. Accentuated response to phenylhydrazine and erythropoietin in mice genetically impaired for their GATA-1 expression (GATA-1 low mice). Blood. 2001;97:3040–3050. [DOI] [PubMed] [Google Scholar]
- 20.Paulson RF, Shi L, Wu DC. Stress erythropoiesis: New signals and new stress progenitor cells. Curr Opin Hematol. 2011;18:139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vannucchi AM, Bianchi L, Paoletti F, et al. A pathobiologic pathway linking thrombopoietin, GATA-1, and TGF-β1 in the development of myelofibrosis. Blood. 2005;105:3493–3501. [DOI] [PubMed] [Google Scholar]
- 22.Zingariello M, Sancillo L, Martelli F, et al. The throm- bopoietin/MPL axis is activated in the Gata1low mouse model of myelofibrosis and is associated with a defective RPS14 signature. Blood Cancer J. 2017;7:e572–e572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vannucchi AM, Paoletti F, Linari S, et al. Identification and characterization of a bipotent (erythroid and megakaryocytic) cell precursor from the spleen of phenylhydrazine-treated mice. Blood. 2000;95:2559–2568. [PubMed] [Google Scholar]
- 24.Sanchez M, Weissman IL, Pallavicini M, et al. Differential amplification of murine bipotent megakaryocytic/erythroid progenitor and precursor cells during recovery from acute and chronic erythroid stress. Stem Cells. 2006;24:337–348. [DOI] [PubMed] [Google Scholar]
- 25.Belay E, Miller CP, Kortum AN, Torok-Storb B, Blau CA, Emery DW. A hyperactive Mpl-based cell growth switch drives macrophage-associated erythropoiesis through an erythroid-megakaryocytic precursor. Blood. 2015;125:1025–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Migliaccio AR, Martelli F, Verrucci M, et al. Gata1 expression driven by the alternative HS2 enhancer in the spleen rescues the hematopoietic failure induced by the hypomorphic Gata1low mutation. Blood. 2009;114:2107–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spangrude GJ, Lewandowski D, Martelli F, et al. P-selectin sustains extramedullary hematopoiesis in the Gata1low model of myelofibrosis. Stem Cells. 2016;34:67–82. [DOI] [PubMed] [Google Scholar]
- 28.Migliaccio AR, Rana RA, Sanchez M, et al. GATA-1 as a regulator of mast cell differentiation revealed by the phenotype of the GATA-1low mouse mutant. J Exp Med. 2003;197:281–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kozma GT, Martelli F, Verrucci M, et al. Dynamic regulation of Gata1 expression during the maturation of conventional dendritic cells. Exp Hematol. 2010;38:489–503.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vyas P, Ault K, Jackson CW, Orkin SH, Shivdasani RA. Consequences of GATA-1 deficiency in megakaryocytes and platelets. Blood. 1999;93:2867–2875. [PubMed] [Google Scholar]
- 31.Centurione L, Di Baldassarre A, Zingariello M, et al. Increased and pathologic emperipolesis of neutrophils within megakaryocytes associated with marrow fibrosis in GATA-1low mice. Blood. 2004;104:3573–3580. [DOI] [PubMed] [Google Scholar]
- 32.Zucker-Franklin D Ultrastructural studies of hematopoietic elements in relation to the myelofibrosis-osteosclerosis syndrome, megakaryocytes and p latelets (MMM or MOS). AdvBiosci. 1974;16:127–143. [Google Scholar]
- 33.Vannucchi AM, Bianchi L, Cellai C, et al. Development of myelofibrosis in mice genetically impaired for GATA-1 expression (GATA-1(low) mice). Blood. 2002;100:1123–1132. [DOI] [PubMed] [Google Scholar]
- 34.Eliades A, Papadantonakis N, Bhupatiraju A, et al. Control of megakaryocyte expansion and bone marrow fibrosis by lysyl oxidase. J Biol Chem. 2011;286:27630–27638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vannucchi AM, Pancrazzi A, Guglielmelli P, et al. Abnormalities of GATA-1 in megakaryocytes from patients with idiopathic myelofibrosis. Am J Pathol. 2005;167:849–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gilles L, Arslan AD, Marinaccio C, et al. Downregulation of GATA1 drives impaired hematopoiesis in primary myelofibrosis. J Clin Investig. 2017;127:1316–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bresnick EH, Lee HY, Fujiwara T, Johnson KD, Keles S. GATA switches as developmental drivers. J Biol Chem. 2010;285: 31087–31093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ghinassi B, Sanchez M, Martelli F, et al. The hypomorphic Gata1low mutation alters the proliferation/differentiation potential of the common megakaryocytic-erythroid progenitor. Blood. 2007;109:1460–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Migliaccio AR, Martelli F, Verrucci M, et al. Altered SDF-1/CXCR4 axis in patients with primary myelofibrosis and in the Gata1low mouse model of the disease. Exp Hematol. 2008;36:158–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abdelouahab H, Zhang Y, Wittner M, et al. CXCL12/CXCR4 pathway is activated by oncogenic JAK2 in a PI3K-dependent manner. Oncotarget. 2017;8:54082–54095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cho SY, Xu M, Roboz J, Lu M, Mascarenhas J, Hoffman R. The effect of CXCL12 processing on CD34+ cell migration in myeloproliferative neoplasms. Cancer Res. 2010;70:3402–3410. [DOI] [PubMed] [Google Scholar]
- 42.Bogani C, Ponziani V, Guglielmelli P, et al. Hypermethylation of CXCR4 promoter in CD34 + cells from patients with primary myelofibrosis. Stem Cells. 2008;26:1920–1930. [DOI] [PubMed] [Google Scholar]
- 43.Wang X, Zhang W, Ishii T, et al. Correction of the abnormal trafficking of primary myelofibrosis CD34+ cells by treatment with chromatin-modifying agents. Cancer Res. 2009;69:7612–7618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zingariello M, Martelli F, Ciaffoni F, et al. Characterization of the TGF-beta1 signaling abnormalities in the Gata1low mouse model of myelofibrosis. Blood. 2013;121:3345–3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schmitt A, Drouin A, Massé J-M, Guichard J, Shagraoui H, Cramer EM. Polymorphonuclear neutrophil and megakaryocyte mutual involvement in myelofibrosis pathogenesis. Leuk Lymphoma. 2002;43:719–724. [DOI] [PubMed] [Google Scholar]
- 46.Campanelli R, Rosti V, Villani L, et al. Evaluation of the bioactive and total transforming growth factor β1 levels in primary myelofibrosis. Cytokine. 2011;53:100–106. [DOI] [PubMed] [Google Scholar]
- 47.Ciurea SO, Merchant D, Mahmud N, et al. Pivotal contributions of megakaryocytes to the biology of idiopathic myelofibrosis. Blood. 2007;110:986–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alvarez-Larrán A, Arellano-Rodrigo E, Reverter JC, et al. Increased platelet, leukocyte, and coagulation activation in primary myelofibrosis. Ann Hematol. 2008;87:269–276. [DOI] [PubMed] [Google Scholar]
- 49.Thiele J, Lorenzen J, Manich B, Kvasnicka HM, Zirbes TK, Fischer R. Apoptosis (programmed cell death) in idiopathic (primary) osteo-/myelofibrosis: Naked nuclei in megakaryopoiesis reveal features of para-apoptosis. Acta Haematol. 1997;97: 137–143. [DOI] [PubMed] [Google Scholar]
- 50.Ciaffoni F, Cassella E, Varricchio L, Massa M, Barosi G, Migliaccio AR. Activation of non-canonical TGF-betal signaling indicates an autoimmune mechanism for bone marrow fibrosis in primary myelofibrosis. Blood Cells Mol Dis. 2015;54:234–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marin Oyarzún CP, Carestia A, Lev PR, et al. Neutrophil extracellular trap formation and circulating nucleosomes in patients with chronic myeloproliferative neoplasms. Sci Rep. 2016;6:38738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Verstovsek S, Manshouri T, Pilling D, et al. Role of neoplastic monocyte-derived fibrocytes in primary myelofibrosis. J Exp Med. 2016;213:1723–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang X, Prakash S, Lu M, et al. Spleens of myelofibrosis patients contain malignant hematopoietic stem cells. J Clin Invest. 2012;122:3888–3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.O’Malley DP, Kim YS, Perkins SL, et al. Morphologic and immunohistochemical evaluation of splenic hematopoietic proliferations in neoplastic and benign disorders. Mod Pathol. 2005;18:1550–1561. [DOI] [PubMed] [Google Scholar]
- 55.Barosi G An immune dysregulation in MPN. Curr Hematol Malig Rep. 2014;9:331–339. [DOI] [PubMed] [Google Scholar]
- 56.Massa M, Campanelli R, Fois G, et al. Reduced frequency of circulating CD4+CD25brightCD127lowFOXP3+ regulatory T cells in primary myelofibrosis. Blood. 2016;128:1660–1662. [DOI] [PubMed] [Google Scholar]
- 57.Qiu J, Salama ME, Hu CS, Li Y, Wang X, Hoffman R. The characteristics of vessel lining cells in normal spleens and their role in the pathobiology of myelofibrosis. Blood Adv. 2018;2:1130–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang X, Cho SY, Hu CS, et al. C-X-C motif chemokine 12 influences the development of extramedullary hematopoiesis in the spleens of myelofibrosis patients. Exp Hematol. 2015;43:100–9.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ling T, Crispino JD, Zingariello M, Martelli F, Migliaccio AR. GATA1 insufficiencies in primary myelofibrosis and other hematopoietic disorders: consequences for therapy. Expert Rev Hematol. 2018;11:169–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Walkley CR, Olsen GH, Dworkin S, et al. A microenvironment-induced myeloproliferative syndrome caused by RARy deficiency. Cell. 2008;129:1097–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Walkley CR, Shea JM, Sims NA, Purton LE, Stuart H. pRB extrinsically regulates hematopoietic stem cells via myeloid cell-bone marrow microenvironment interactions. Cell. 2007; 129:1081–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Malara A, Abbonante V, Zingariello M, Migliaccio A, Balduini A. Megakaryocyte contribution to bone marrow fibrosis: Many arrows in the quiver. Mediterr J Hematol Infect Dis. 2018;10:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhan H, Ma Y, Lin CHS, Kaushansky K. JAK2V617F-mutant megakaryocytes contribute to hematopoietic stem/progenitor cell expansion in a model of murine myeloproliferation. Leukemia. 2016;30:2332–2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jeremy Wen Q, Yang Q, Goldenson B, et al. Targeting megakaryocytic-induced fibrosis in myeloproliferative neoplasms by AURKA inhibition. Nat Med. 2015;21: 1473–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schmitt A, Jouault H, Guichard J, Wendling F, Drouin A, Cramer EM. Pathologic interaction between megakaryocytes and polymorphonuclear leukocytes in myelofibrosis. Blood. 2000;96:1342–1347. [PubMed] [Google Scholar]
- 66.Dongre A, Weinberg RA. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat Rev Mol Cell Biol. 2019;20:69–84. [DOI] [PubMed] [Google Scholar]
- 67.Ceglia I, Dueck AC, Masiello F, et al. Preclinical rationale for TGF-beta inhibition as a therapeutic target for the treatment of myelofibrosis. Exp Hematol. 2016;44:1138–1155.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gao X, Lee H-Y, da Rocha EL, et al. TGF-β inhibitors stimulate red blood cell production by enhancing self-renewal of BFU-E erythroid progenitors. Blood. 2016;128:2637–2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Varricchio L, Mascarenhas J, Migliaccio AR, et al. AVID200, a potent trap for TGF-$β$ ligands inhibits TGF-$β$1 signaling in human myelofibrosis. Blood. 2018;132:1791. [Google Scholar]
- 70.Akhmetshina A, Palumbo K, Dees C, et al. Activation of canonical Wnt signalling is required for TGF-$β$-mediated fibrosis. Nat Commun. 2012;3:735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ishikawa G, Fujiwara N, Hirschfield H, et al. Shared and tissue-specific expression signatures between bone marrow from primary myelofibrosis and that from essential thrombocythemia. Exp Hematol. 2019; 10.1016/j.exphem.2019.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wernig G, Chen S-Y, Cui L, et al. Unifying mechanism for different fibrotic diseases. Proc Natl Acad Sci USA. 2017;114: 4757–4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jin Y, Ratnam K, Chuang PY, et al. A systems approach identifies HIPK2 as a key regulator of kidney fibrosis. Nat Med. 2012;18:580–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ghosh AK, Quaggin SE, Vaughan DE. Molecular basis of organ fibrosis: Potential therapeutic approaches. Exp Biol Med. 2013; 238:461–481. [DOI] [PubMed] [Google Scholar]
- 75.Massagué J, Blain SW, Lo RS. TGFβ signaling in growth control, cancer, and heritable disorders. Cell. 2000;103: 295–309. [DOI] [PubMed] [Google Scholar]
- 76.Garimella R, Kacena MA, Tague SE, Wang J, Horowitz MC, Anderson HC. Expression of bone morphogenetic proteins and their receptors in the bone marrow megakaryocytes of GATA-1low mice: A possible role in osteosclerosis. J Histochem Cytochem. 2007;55:745–752. [DOI] [PubMed] [Google Scholar]
- 77.Niu Y, Yang X, Chen Y, et al. EVI1 induces autophagy to promote drug resistance via regulation of ATG7 expression in leukemia cells. Carcinogenesis. 2019. 10.1093/carcin/bgz167 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 78.Duek A, Lundberg P, Shimizu T, et al. Loss of Stat1 decreases megakaryopoiesis and favors erythropoiesis in a JAK2-V617F-driven mouse model of MPNs. Blood. 2014;123: 3943–3950. [DOI] [PubMed] [Google Scholar]
- 79.Tefferi A, Vaidya R, Caramazza D, Finke C, Lasho T, Pardanani A. Circulating Interleukin (IL)-8, IL-2R, IL-12, and IL-15 levels are independently prognostic in primary myelofibrosis: A comprehensive cytokine profiling study. J Clin Oncol. 2011;29:1356–1363. [DOI] [PubMed] [Google Scholar]
- 80.Fleischman RA. From white spots to stem cells: The role of the Kit receptor in mammalian development. Trends Genet. 1993; 9:285–290. [DOI] [PubMed] [Google Scholar]
- 81.Halaban R, Moellmann G. White mutants in mice shedding light on humans. J Invest Dermatol. 1993;100:S176–S185. [PubMed] [Google Scholar]
- 82.Besmer P, Manova K, Duttlinger R, et al. The kit-ligand (steel factor) and its receptor c-kit/W: Pleiotropic roles in gametogenesis and melanogenesis. Dev Suppl. 1993;1993:125–137. [PubMed] [Google Scholar]
- 83.Williams DE, de Vries P, Namen AE, Widmer MB, Lyman SD. The steel factor. Dev Biol. 1992;151:368–376. [DOI] [PubMed] [Google Scholar]
- 84.Broxmeyer HE, Maze R, Miyazawa K, et al. The kit receptor and its ligand, steel factor, as regulators of hemopoiesis. Cancer Cells. 1991;3:480–487. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.