Abstract
Antimicrobial peptides (AMPs) are cationic small peptide chains that have good antimicrobial activity against a variety of bacteria, fungi, and viruses. AMP-17 is a recombinant insect AMP obtained by a prokaryotic expression system. However, the full antifungal activity, physicochemical characteristics, and cytotoxicity of AMP-17 were previously unknown. AMP-17 was shown to have good antifungal activity against five pathogenic fungi, with minimum inhibitory concentrations (MIC) of 9.375–18.75 μg/ml, and minimum fungicidal concentrations (MFC) of 18.75–37.5 μg/ml. Notably, the antifungal activity of AMP-17 against Cryptococcus neoformans was superior to that of other Candida spp. In addition, the hemolytic rate of AMP-17 was only 1.47%, even at the high concentration of 16× MIC. AMP-17 was insensitive to temperature and high salt ion concentration, with temperatures of 98°C and –80°C, and NaCl and MgCl2 concentrations of 50–200 mmol/l, having no significant effect on antifungal activity. However, AMP-17 was sensitive to proteases, trypsin, pepsin, and proteinase K. The elucidation of antifungal activity, physicochemical properties and cytotoxicity of AMP-17 provided an experimental basis for its safety evaluation and application, as well as indicated that AMP-17 might be a promising drug.
Key words: antimicrobial peptides, AMP-17, antifungal activity, stability, hemolytic activity
Introduction
In recent years, the number of patients presenting with fungal infections has increased, and the emergence of pathogenic fungi has seriously affected human health and safety. Of the several common pathogenic fungi that occur clinically, Candida spp., dominated by Candida albicans, and non-Candida spp., dominated by Cryptococcus neoformans, are the most common (Wang et al. 2012; Jensen 2016; Lovero et al. 2016). Candidiasis is mainly an acute, subacute or chronic infection caused by C. albicans. It is the most common fungal disease, often invading the skin and mucous membranes, and can also cause visceral or systemic infections. The clinical symptoms are intricate and inconsistent. However, cryptococcal infections are usually seen in patients with low immune function, especially in the case of acquired immunodeficiency syndrome (AIDS) and pulmonary tuberculosis (PTB) (Chopra et al. 2015). Non-candida disease is mainly caused by the invasion of C. neoformans spores into the respiratory tract, which subsequently causes hematogenous dissemination, leading to central nervous system infection (Lortholary et al. 2004; Burnik et al. 2007). Patients with cryptococcal lung infection often develop non-specific symptoms such as weight loss, fever, cough, and general weakness. Moreover, clinical manifestations vary from asymptomatic infection to severe pneumonia and respiratory failure (Brizendine et al. 2011; Debenedectis et al. 2013). At present, azoles, polyenes, echinocyclines, and fluorocytosines are the most commonly used drugs for the treatment of fungal infections; however, all have certain toxicity and side effects, and drug-resistant strains are constantly emerging. (Bellmann and Smuszkiewicz 2017; Spitzer et al. 2017). Therefore, the development of new antifungal drugs is urgently needed for clinical anti-infection treatment.
Antimicrobial peptides (AMPs), also known as host defense peptides, are biologically active molecules produced by various organisms as important components of their innate immune response (Yan et al. 2018). AMPs not only have strong antimicrobial efficacy but also have activity against viruses, parasites and tumor cells (Ren et al. 2012; Patnaik et al. 2013; Tindwa et al. 2013). In addition to strong antimicrobial efficacy, AMPs have low hemolytic and cytotoxic activities (Edwards et al. 2016). For example, (Quintana et al. 2014) found that the AMP subtilosin has antiviral and viricidal effects against herpes simplex virus type 2 (HSV-2). (Kovalchuk et al. 2007) showed that a complex of natural cytokines and AMPs (CCAP or Superlymph) inhibits virus proliferation in vitro. Therefore, due to their small molecular weight, good thermal stability and broad antimicrobial spectrum, AMPs are expected to replace antibiotics as new and highly effective antibacterial drugs (Presicce et al. 2009; Seydlová et al. 2017).
AMP-17 is encoded by a specific highly expressed gene extracted from the Musca domestica transcriptome database constructed after 12 h of microbial infection. The AMP-17 gene has no functional annotation in GeneBank and is of unknown function. To further assess the antimicrobial activity of the protein encoded by this gene, the prediction tools of the Antimicrobial Peptide (APD) and Collection of Anti-Microbial Peptides (CAMP) databases were used (Wang et al. 2004; Thomas et al. 2010; Wang et al. 2016). The two databases used four different algorithms, support vector machines (SVM), random forest (RF), artificial neural networks (ANN), and discriminant analysis (DA) to predict the protein structure, and the scores obtained were 1, 0.944, 1 and 1, respectively. All the results determined that the protein was an AMP. The antifungal function of recombinant AMP-17 protein has been reported by (Guo et al. 2017) who showed its strong activity against C. albicans, indicating it had good development potential.
In this study, the in vitro antifungal activity of AMP-17 protein was assessed against C. albicans, Candida krusei, Candida tropicalis, Candida parapsilosis, and C. neoformans. To further understand the physical and chemical properties of AMP-17 protein, the salt, acid-base, and thermal stabilities were also assayed. In addition, cytotoxicity and hemolytic activity of AMP-17 protein were determined, as a prelude to more extensive safety evaluation.
Experimental
Materials and Methods
Materials. AMP-17 protein was obtained from the Guoguo research team. It was produced in a prokaryotic expression system and purified by a nickel ion metal chelator affinity chromatography (Guo et al. 2017). Fluconazole (FLC), Sabouraud dextrose agar (SDA) and Sabouraud dextrose broth (SDB) were purchased from Solarbio (Beijing, China). Human red blood cells were donated by the Affiliated Hospital of Guizhou Medical University, Guiyang, China.
Microbial strains. C. albicans ATCC 10231 was routinely preserved by the Key Laboratory of Modern Pathogenic Biology, Guizhou Medical University, Guiyang, China). C. krusei IFM56881, C. tropicalis IFM57016, C. parapsilosis ATCC 22019, and C. neoformans IFM51426 were obtained from the Department of Microbiology, Guizhou Medical University, Guiyang, China. They were stored in 30% glycerol at –80°C.
MIC determination. A single actively growing microbial colony was inoculated into 5 ml sterile SDB medium and incubated overnight at 37°C. The turbidity of the fungal solution was adjusted to 1–5× 106 colony-forming units (CFU)/ml using a blood cell counting plate. The fungal suspension was then diluted with SDB to 0.5–2.5 × 103 CFU/ml. An aliquot of 100 μl of the final suspension was added into each well of a sterile 96-well plate containing 100 μl of medium containing antimicrobial agents at double-diluted concentrations. Phosphate-buffered saline (PBS) was used as a negative control and fluconazole as a positive control. The plate was assessed for MIC values of AMP-17 after 24 h or 48 h of incubation at 37°C. The MIC value was determined to be the minimum concentration at which microscopic growth could not be observed by the naked eye, as recommended by the Clinical Laboratory and Standards Institute CLSI (2008) methods (Cantón et al. 2008; Fothergill 2012). The experiment was repeated three times, three biological replicates at a time.
MFC determination. Based on the MIC value, the criteria for fungal MFC values were slightly adjusted. Briefly, samples from each well of a 96-well plate, prepared as described above, were used to determine MFC. Ten μl samples were plated on SDA, with CFUs being counted after incubation at 37°C for 24 h (C. albicans, C. krusei, C. tropicalis, C. parapsilosis) or 48 h (C. neoformans). The experiment was repeated three times, three biological replicates at a time.
Time-kill curves. Overnight cultures of fungi were diluted in sterile SDB medium and adjusted to 1–5×106 CFU/ml. An aliquot of 100 μl of this fungal suspension was added to a 96-well plate containing 900 μl of a specific concentration of AMP-17 and then incubated at 37°C. The optical density of the culture was recorded every two hours at a wavelength of 562 nm. With different sampling time (h) as the abscissa, the A562 value of each plate well was plotted on the ordinate, generating a time-kill curve. PBS and fluconazole were used as negative and positive controls, respectively. The experiment was repeated three times, three biological replicates at a time.
Determination of salt ion strength stability. An overnight culture of C. albicans was diluted in sterile SDB medium and adjusted to 1.0 – 2.5 × 103 CFU/ml. One hundred μl of this fungal suspension was added to a 96-well plate, where each well contained 100 μl of AMP-17, at 1–6 × MIC, and NaCl or MgCl2 at final concentrations of 0, 50, 100, and 200 mmol/l. The MIC value of AMP-17 against C. albicans, under the influence of different concentrations of NaCl or MgCl2, was determined as recommended by the CLSI (2008) methods. The experiment was repeated three times, three biological replicates at a time.
Determination of heat stability and freeze-thaw resistance. An overnight culture of C. albicans was diluted in sterile SDB and adjusted to 1.0 – 2.5 × 103 FU/ml. One hundred μl AMP-17, at 1×MIC, was maintained at 98°C in a water bath for 5, 20, 30, 60, 90, and 120 minutes (heat-resistant group). One hundred μl AMP-17, at 1×MIC was also repeatedly frozen at −80°C, 1, 2, 4, 6, 8, and 10 times (freeze-thawed group). One hundred μl aliquots of the fungal suspension were added to a 96-well plate containing the heat-resistant group, the freeze-thawed group, and a negative control group, and incubated at 37°C for 24 hours. Ten μl samples from the heat-resistant group plate were then directly inoculated onto SDA, and CFU counts were taken after incubation at 37°C for 24 h. Samples taken from the freeze-thaw group and the negative control group were diluted 1:10 000, and then 100 μl was plated on SDA. CFU counts were taken after incubation at 37°C for 24 h. ddH20 was used as a negative control. The experiment was repeated three times, three biological replicates at a time.
Stability to treatment with trypsin, pepsin and proteinase K. An overnight culture of C. albicans was diluted in sterile SDB and adjusted to 1.0 –2.5×103 CFU/ml Fifty μl 0.1 mg/ml proteases (the pH values of trypsin, pepsin, and protease K solutions were: 7, 5, and 7, respectively) and 50 μl 37.5 μg/ml AMP-17 (2 × MIC) were mixed in a 96-well plate and placed in a 37°C water bath for 2 min, 20 min, 40 min, 60 min, 80 min, 100 min, and 120 min. One hundred μl of the fungal suspension was then added to the 96-well plate, followed by incubation at 37°C for 18 h. Subsequently, 10 μl of the fungal suspension was diluted 1:10 000, and 50 μl of this was incubated on SDA for 24 hours, followed by CFU counting. PBS was used as a negative control. The experiment was repeated three times, three biological replicates at a time.
Determination of hemolytic activity. Hemolytic activity of AMP-17 was evaluated using the method of (Souza et al. 2013). Briefly, freshly isolated and washed human erythrocyte suspensions were diluted with 0.01 M PBS, pH 7.4, and then centrifuged at 125 ×g and 4°C for 10 min. This process was repeated three times. The final pellets were resuspended in PBS at a concentration of 4% (v/v). One hundred μl of different concentrations of AMP-17 (2.344–300 μg/ml) was added to a 96-well plate containing 100 μl 4% human red blood cell suspension, and this was incubated at 37°C for 1 h and then centrifuged (225 × g, 4°C, 10 min). The absorbance of the supernatants at 450 nm was then measured using an ultra-microplate spectrophotometer (Model: US Biotekepoch 2). PBS (0.01 M) was used as a negative control and 0.1% Triton X-100 was a positive control. The experiment was repeated three times, three biological replicates at a time. Percentage hemolysis was determined by the formula:
Statistical analysis. Statistical differences were analyzed using the GraphPad Prism 6 software. The comparison between groups was carried out by using a Student’s t-test. The p-value < 0.05 was statistically significant. All data for the experiment were expressed as mean ± SD.
Results
Antifungal activity of AMP-17. In a previous study, AMP-17 was shown to have strong antifungal activity against C. albicans ATCC76615, with a MIC of 2 μg/ml (Guo et al. 2017). To further validate its antifungal effect against several common clinical pathogens, actively growing fungal cells of C. albicans, C. tropicalis, C. krusei, C. parapsilosis, and C. neoformans) were treated with purified AMP-17 protein. Notably, 100 μg/ml AMP-17 could inhibit or kill C. albicans, C. tropicalis, C. krusei, C. parapsilosis,and C. neoformans in SDB medium. Compared to the PBS negative control, AMP-17 showed antifungal activity against each fungus from 2 to 24 h of culture. When fungi were cultured for 24 h, the fungicidal effect of AMP-17 was slightly different between species. The fungicidal curves of C. albicans and C. tropicalis increased slightly, but were lower than the PBS control group, while the fungicidal curves of C. krusei, C. parapsilosis, and C. neoformans were linear. Among them, AMP-17 had the strongest antifungal effect on C. neoformans. The efficiency with which 100 μg/ml AMP-17 inhibited the growth of several common clinical pathogens was comparable to that of the clinical fungicide fluconazole at 100 μg/ml, and the fungistatic efficiency of AMP-17 was slightly stronger than that of fluconazole (Fig. 1).
Fig. 1.
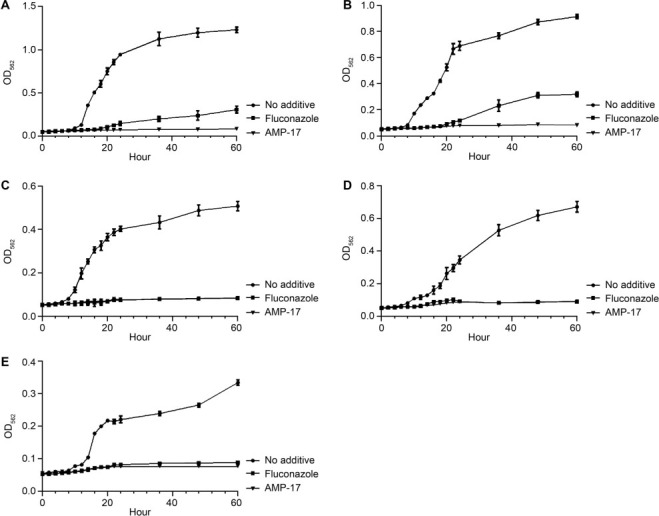
Time- kill curves of the AMP-17 against the fungal species tested. The growth of five fungi was monitored in the presence of 100 μg/ml AMP-17. Growth of C. albicans (A), C. tropicalis (B), C. krusei (C), C. parapsilosis (D), and C. neoformans with AMP-17 were monitored by reading the optical density (OD562) of the cultures. 100-μg/ml fluconazole was used as a positive control for fungi. Sabouraud’s dextrose broth (0 μg/ml AMP-17) was used as a negative control. The OD562 values of the remaining viable cells were monitored in 96-well plates at different times.
To better evaluate the antifungal activity of AMP-17, the MICs and MFCs for several common clinical pathogens were determined. MIC and MFC values of AMP-17 against C. albicans, C. tropicalis, C. krusei, and C. parapsilosis were 18.75 μg/ml and 37.5 μg/ml, respectively. For C. neoformans, MIC and MFC values of AMP-17 were 9.375 μg/ml and 18.75 μg/ml, respectively (Table I–II). These values demonstrated that AMP-17 exhibited significant antifungal activity against several common clinical pathogenic yeasts.
Table I.
MIC values of AMP-17 against the fungi species tested.
| Peptide | MIC (μg/ml) | ||||
|---|---|---|---|---|---|
|
C. albicans ATCC10231 |
C. krusei IFM56881 |
C. tropicalis IFM57016 |
C. parapsilosis ATCC22019 |
C. neoformans IFM51426 |
|
| AMP-17 | 18.75 | 18.75 | 18.75 | 18.75 | 9.375 |
Table II.
MFC values of AMP-17 against the fungi species tested.
| Peptide | MIC (μg/ml) | ||||
|---|---|---|---|---|---|
|
C. albicans ATCC10231 |
C. krusei IFM56881 |
C. tropicalis IFM57016 |
C. parapsilosis ATCC22019 |
C. neoformans IFM51426 |
|
| AMP-17 | 37.5 | 37.5 | 37.5 | 37.5 | 18.75 |
Physicochemical properties of AMP-17. Physical and chemical factors such as ionic strength, temperature, protease, and pH were some of the most important factors that might affect the AMP activity. In the presence of NaCl and MgCl2 at a concentration from 0 to 200 mmol, the MIC value of C. albicans increased with an increase in the metal ion concentration, indicating a decrease in antifungal activity for AMP-17. The effect of NaCl on the antifungal activity of AMP-17 was stronger than that of MgCl2 (Fig. 2). By contrast, AMP-17 did not show any loss of activity when exposed to temperatures up to 98°C for 120 min and was enhanced. AMP-17 also still had a strong antifungal activity after 10 cycles of freezing and thawing at –80°C. Therefore, the antifungal activity associated with AMP-17 protein was both heat tolerant and freeze-thaw resistant (Tables III). However, the antifungal activity of AMP-17 was still significant after treatment with trypsin, pepsin, and proteinase K for 20 min (p < 0.05). The antifungal activity of AMP-17 was significantly decreased after treatment with trypsin, pepsin and proteinase K for 40 min (p < 0.05). This phenomenon revealed that the anti-C. albicans activity of AMP-17 decreased significantly after long treatment with trypsin, pepsin and protease K, indicating that AMP-17 was sensitive to trypsin, pepsin and protease K (Fig. 3).
Fig. 2.
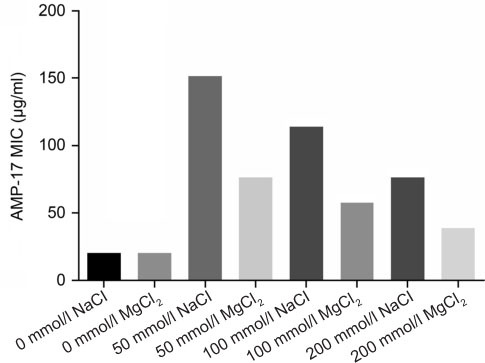
Influences of various NaCl or MgCl2 concentrations on the antifungal activity of AMP-17. The MIC value of AMP-17 against C. albicans was determined under the influence of different concentrations of NaCl or MgCl2 according to the conditions recommended by CLSI (2008).
Table III.
Influences of temperature and the number of freeze-thaw times on the antifungal activity of AMP-17.
| Temperature (98°C) (min) |
Colony count ( ± SD) |
Temperature (−80°C) (Times) |
Colony count ( ± SD) |
|---|---|---|---|
| 5 | 69.50 ± 4.95** | 1 | (240.50 ± 3.54**) × 102 |
| 20 | 6.00 ± 2.83** | 2 | (273.50±3.54**) × 102 |
| 30 | 4.50 ± 2.12** | 4 | (342.00 ± 8.49**) × 102 |
| 60 | 6.50 ± 0.71** | 6 | (357.00 ± 1.41**) × 102 |
| 90 | 9.50 ± 3.54** | 8 | (351.50 ± 6.36**) × 102 |
| 120 | 3.50 ± 0.71** | 10 | (370.50 ± 3.54**) × 102 |
| Negative control (ddH2O) | 565900.00 ± 17112.00 | Negative control (ddH2O) | (5507.00 ± 147.08) × 102 |
Note: Compared with ddH2O as a control, ** p < 0.01
Fig. 3.
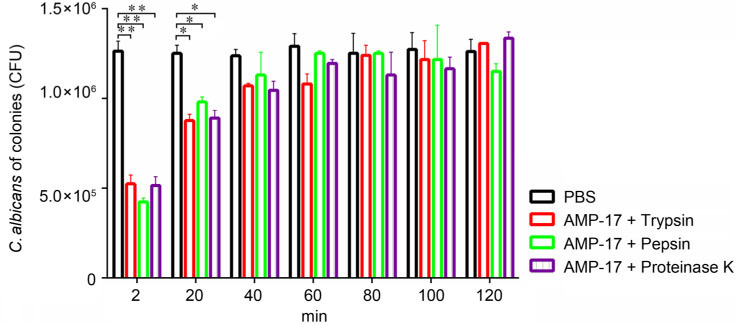
Influences of trypsin/pepsin/proteinase K on AMP-17 when used against C. albicans. Fifty μl of the proteases (at a concentration of 1 mg/ml) and 50 μl AMP-17 (at a concentration of 37.5 μg/ml) were mixed in a 96-well plate and placed in a 37°C water bath for 2, 20, 40, 60, 80, 100, and 120 min. Then, it was subjected to CFU enumeration after being treated with 100 μl of the fungal suspension for 18 hours. PBS was used as a negative control. Compared with PBS control, *P < 0.05; **P < 0.01.
Hemolytic assay of AMP-17. The toxicity of the new AMP, AMP-17 of M. domestica, was evaluated with human red blood cells. The human red blood cells were incubated with AMP-17 at 37°C for 1 h, and the level of hemolysis was determined. Notably, the hemolysis rate of AMP-17 at 16× MIC was only 1.47%, which was far below that of the prescribed drug standard (5%). As AMP-17 was not hemolytic to human red blood cells, it may lead to the conclusion that it has great drug development and application potential (Fig. 4).
Fig. 4.
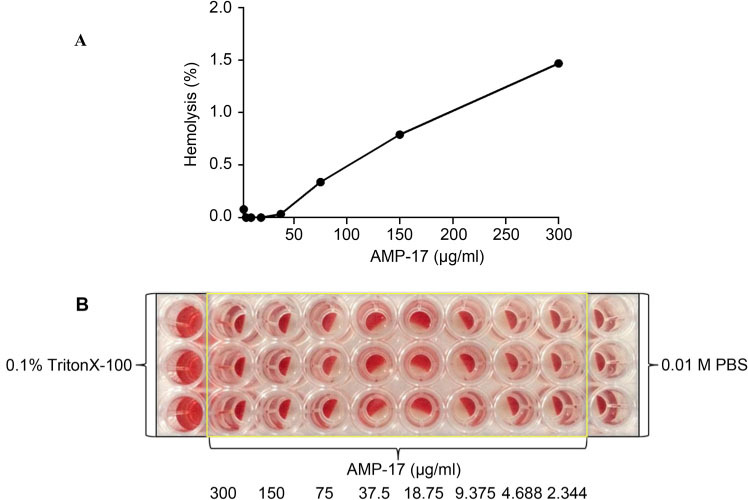
Hemolytic activities of AMP-17 in vitro. The diluted human red blood cells (100 μl) were incubated with AMP-17 at 37°C for 1 h and spun at 225×g for 10 minutes. The supernatants (100 μl) were transferred to a 96-well plate for measurement of the absorbance of the supernatants at 450 nm by the ultra-microplate spectrophotometer. 0.1% Triton-X 100 and 0.01M PBS were used as a positive control and a negative control, respectively. Fig. 4A is based on Fig. 4B calculated by the hemolysis rate formula. Fig. 4B was the original experimental diagram.
Discussion
With the increase in the incidence of pathogenic fungal infections, antifungal drugs are increasingly being used in clinical practice; however, drug-resistant strains are also gradually increasing, posing a serious health problem (Loeffler and Stevens 2003). Therefore, the development of new natural antifungal drugs has become a hot topic of current research, with research on AMP functions from M. domestica being carried out in-depth. In addition to traditional AMPs, more new classes of high-efficiency molecules have been discovered. For example, (Liu et al. 2016) isolated the anti-fungal peptide termicin from the salivary glands and blood cells of the termite Pseudacanthotermes spiniger. (Iijima et al. 1993) isolated an antifungal peptide (AFP) from the hemolymph of Sarcophaga peregrina larvae, which inhibits the growth of C. albicans, causing fungal cytoplasmic leakage and fungal death.
(Guo et al. 2017) identified AMP-17 protein as a new AMP, which has a strong antifungal activity against C. albicans. In the current study, these findings were advanced by evaluating antifungal spectrum, time-kill curves, physicochemical properties, and cytotoxicity of AMP-17. In vitro antifungal test results showed that AMP-17 protein had strong antifungal activity against several common clinical pathogenic fungi, with MICs and MFCs ranging from 9.375 μg/ml to 18.75 μg/ml and from 18.75 μg/ml to 37.5 μg/ml, respectively. Among them, AMP-17 had better antifungal activity against C. neoformans than other Candida species. These results indicated that different organisms had different biological effects, suggesting that the antifungal activity of AMP-17 was selective. This might be related to the surface structure of the fungal cell, the type, and content of the membrane proteins or differences in physiological metabolism. Klotz et al. ( 1985) observed the effects of environmental factors on cell surface hydrophobicity (CSH), and found that it changed rapidly on the surface of C. albicans cells after changing the culture environment, while a change for C. glabrata was not obvious. In addition, the results of a time-sterilization dynamic curve showed that the fungus began to grow after 6 h of incubation in the PBS group, and this increased in an upward trend. The positive control group (FLC) and the experimental group (AMP-17) were almost inhibited by several common pathogenic yeasts within 0–60 h, which showed the phenomenon of fungistasis after sterilization; however, the effect had some differences. The antifungal effect of AMP-17 on C. albicans and C. tropicalis was almost completely inhibited within 0–60 h, while the antifungal effect of FLC on both was gradually reduced after 20 h. The antifungal effects of AMP-17 and FLC on C. krusei, C. parapsilosis and Cryptococcus neoformans were almost completely inhibited within 0–60 h. Therefore, this phenomenon suggested that in addition to the membrane-bound fungicidal mode of conventional cationic AMPs, AMP-17 might also bind target sites in different antifungal mechanisms in the cytoplasm, though this needs further verification.
Although AMP-17 has good antifungal activity against several common clinical pathogenic fungi, its physicochemical properties were not known, which prompted us to carry out the experiments in the current study. It was found that AMP-17 protein had good thermal stability, antifreeze and salt stability; however, its antifungal activity was easily destroyed by protease. Under the action of proteases (trypsin, pepsin and proteinase K), the antifungal activity of AMP-17 protein decreased with time of treatment, to the point when it was lost. (Tang et al. 2015) also found that an AMP was weakened by protease action. It was suggested that under certain temperature conditions, a variety of proteases could hydrolyze the carboxyl-terminal peptide bond of AMP-17 protein, destroying the spatial structure of the protein and leading to loss of antifungal activity; however, the specific mechanisms need further verification. In this study, it was also found that on exposure to a high-temperature environment (98°C) for 5 to 120 minutes, the antifungal activity of AMP-17 did not decrease, but increased. This was in contrast to (Zhang et al. 2017). The reason for the increase in activity of AMP-17 protein against C. albicans might be that the spatial structure of the AMP-17 protein changes after high-temperature treatment; however, the specific reasons need further verification.
Cytotoxicity, as measured by human red blood cell hemolysis, is an important factor in new drug development. Hemolysis concentration-50 (HC50), as one of the most commonly used indicators of cytotoxicity, provides strong technical support in the development of new drugs (Konai et al. 2014). At present, although AMPs are expected to be the best substitute for antibiotics, most AMPs still show cell hemolysis, which limits their use as drugs. For example, (Chang et al. 2017) found that the AMP TP4 residue A12I/A15I had a higher hemolytic activity, which may be due to the increased hydrophobicity in the main helix caused by the A12I/A15I mutation, which reduces bacterial outer membrane target protein, OprI, binding, and bactericidal activity, but increases hemolytic activity. The phenomenon is similar to that of (Chen et al. 2005), who also found that the hemolysis of AMP was related to the content of hydrophobic residues. As the hydrophobicity increased, the helicity and self-assembly ability of the AMP also increased. As a new type of potential AMP, the cell hemolysis characteristics of AMP-17 could be key in drug design. Surprisingly, hemolysis by AMP-17 protein was much lower than the prescribed drug hemolysis standard (5%), making it a promising antifungal agent.
In conclusion, AMP-17 protein has extensive antifungal activity against several common pathogens. Besides, it has strong stability and low hemolytic activity. These characteristics make AMP-17 protein an attractive molecule for development and application in medicine.
Acknowledgments
We are grateful to the School of Basic Medicine of Guizhou Medical University for providing scientific research equipment. We are also grateful for the fungal strains provided by Professor Kang Yingqian from the Department of Microbiology.
Footnotes
Conflict of interest
The authors do not report any financial or personal connections with other persons or organizations, which might negatively affect the contents of this publication and/or claim authorship rights to this publication.
Funding
This study was funded by the National Natural Science Foundation of China [grant numbers: 81760647, 81560337] and Guiyang Science and Technology Bureau project [construction contract [2017] 5–30].
Literature
- Bellmann R, Smuszkiewicz P.. Pharmacokinetics of antifungal drugs: practical implications for optimized treatment of patients. Infection. 2017;45(6):737–779. 10.1007/s15010-017-1042-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brizendine K, Baddley J, Pappas P.. Pulmonary Cryptococcosis. Semin Respir Crit Care Med. 2011;32(06):727–734. 10.1055/s-0031-1295720 [DOI] [PubMed] [Google Scholar]
- Burnik C, Altintaş ND, Özkaya G, Serter T, Selçuk ZT, Firat P, Arikan S, Cuenca-Estrella M, Topeli A. Acute respiratory distress syndrome due to Cryptococcus albidus pneumonia: case report and review of the literature. Med Mycol. 2007;45(5):469–473. 10.1080/13693780701386015 [DOI] [PubMed] [Google Scholar]
- Cantón E, Pemán J, Espinel-Ingroff A, Martín-Mazuelos E, Carrillo-Muñoz A, Martínez JP. Comparison of disc diffusion assay with the CLSI reference method (M27-A2) for testing in vitro posaconazole activity against common and uncommon yeasts. J Antimicrob Chemother. 2008;61(1):135–138. 10.1093/jac/dkm442 [DOI] [PubMed] [Google Scholar]
- Chang TW, Wei SY, Wang SH, Wei HM, Wang YJ, Wang CF, Chen C, Liao YD. Hydrophobic residues are critical for the helix-forming, hemolytic and bactericidal activities of amphipathic anti-microbial peptide TP4. PLoS One. 2017;12(10):e0186442 10.1371/journal.pone.0186442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Mant CT, Farmer SW, Hancock REW, Vasil ML, Hodges RS.. Rational design of alpha-helical antimicrobial peptides with enhanced activities and specificity/therapeutic index. J Biol Chem. 2005;280(13):12316–12329. 10.1074/jbc.M413406200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra S, Capoor MR, Mallik R, Gupta S, Ray A, Khanna G, Suri JC, Bhattacharya D, Raghavan S. Pulmonary Cryptococcosis in HIV- sero-negative patients: case series from India. Mycoses. 2015;58(5):288–293. 10.1111/myc.12313 [DOI] [PubMed] [Google Scholar]
- Debenedectis CM, McCulloh RJ, Healey TT.. Pulmonary Cryptococcosis. R I Med J (2013). 2013;96(5):53–54. [PubMed] [Google Scholar]
- Edwards IA, Elliott AG, Kavanagh AM, Zuegg J, Blaskovich MAT, Cooper MA.. Contribution of amphipathicity and hydrophobicity to the antimicrobial activity and cytotoxicity of β-hairpin peptides. ACS Infect Dis. 2016;2(6):442–450. 10.1021/acsinfecdis.6b00045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fothergill AW.. Antifungal susceptibility testing: Clinical Laboratory and Standards Institute (CLSI) methods In: Hall GS, editor. Interactions of yeasts, moulds, and antifungal agents. New York (USA): Humana Press; 2012. [Google Scholar]
- Guo G, Tao R, Li Y, Ma H, Xiu J, Fu P, Wu J. Identification and characterization of a novel antimicrobial protein from the housefly Musca domestica. Biochem Biophys Res Commun. 2017;490(3):746–752. 10.1016/j.bbrc.2017.06.112 [DOI] [PubMed] [Google Scholar]
- Iijima R, Kurata S, Natori S. Purification, characterization, and cDNA cloning of an antifungal protein from the hemolymph of Sarcophaga peregrina (flesh fly) larvae. J Biol Chem. 1993;268(16): 12055–12061. [PubMed] [Google Scholar]
- Jensen RH.. Resistance in human pathogenic yeasts and filamentous fungi: prevalence, underlying molecular mechanisms and link to the use of antifungals in humans and the environment. Dan Med J. 2016;63(10):B5288. [PubMed] [Google Scholar]
- Klotz SA, Drutz DJ, Zajic JE.. Factors governing adherence of Candida species to plastic surfaces. Infect Immun. 1985;50(1):97–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konai MM, Ghosh C, Yarlagadda V, Samaddar S, Haldar J. Membrane active phenylalanine conjugated lipophilic norspermidine derivatives with selective antibacterial activity. J Med Chem. 2014; 57(22):9409–9423. 10.1021/jm5013566 [DOI] [PubMed] [Google Scholar]
- Kovalchuk LV, Gankovskaya LV, Gankovskaya OA, Lavrov VF.. Herpes simplex virus: treatment with antimicrobial peptides. Adv Exp Med Biol. 2007;601:369–376. 10.1007/978-0-387-72005-0_39 [DOI] [PubMed] [Google Scholar]
- Liu Z, Yuan K, Zhang R, Ren X, Liu X, Zhao S, Wang D. Cloning and purification of the first termicin-like peptide from the cockroach Eupolyphaga sinensis. J Venom Anim Toxins Incl Trop Dis. 2016;22(1):5 10.1186/s40409-016-0058-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeffler J, Stevens DA.. Antifungal drug resistance. Clin Infect Dis. 2003;36 Supplement_1:S31–S41. 10.1086/344658 [DOI] [PubMed] [Google Scholar]
- Lortholary O, Nunez H, Brauner M, Dromer F. Pulmonary Cryptococcosis. Semin Respir Crit Care Med. 2004;25(02):145–157. 10.1055/s-2004-824899 [DOI] [PubMed] [Google Scholar]
- Lovero G, De Giglio O, Montagna O, Diella G, Divenuto F, Lopuzzo M, Rutigliano S, Laforgia N, Caggiano G, Montagna MT.. Epidemiology of candidemia in neonatal intensive care units: a persistent public health problem. Ann Ig. 2016;28(4):282–287. [DOI] [PubMed] [Google Scholar]
- Patnaik B, Kang S, Seo G, Lee H, Patnaik H, Jo Y, Tindwa H, Lee Y, Lee B, Kim N, et al. Molecular cloning, sequence characterization and expression analysis of a CD63 homologue from the coleopteran beetle, Tenebrio molitor. Int J Mol Sci. 2013;14(10):20744–20767. 10.3390/ijms141020744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presicce P, Giannelli S, Taddeo A, Villa ML, Della Bella S.. Human defensins activate monocyte-derived dendritic cells, promote the production of proinflammatory cytokines, and up-regulate the surface expression of CD91. J Leukoc Biol. 2009;86(4):941–948. 10.1189/jlb.0708412 [DOI] [PubMed] [Google Scholar]
- Quintana VM, Torres NI, Wachsman MB, Sinko PJ, Castilla V, Chikindas M. Antiherpes simplex virus type 2 activity of the anti-microbial peptide subtilosin. J Appl Microbiol. 2014;117(5):1253–1259. 10.1111/jam.12618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren SX, Cheng ASL, To KF, Tong JHM, Li MS, Shen J, Wong CCM, Zhang L, Chan RLY, Wang XJ, et al.. Host immune defense peptide LL-37 activates caspase-independent apoptosis and suppresses colon cancer. Cancer Res. 2012;72(24):6512–6523. 10.1158/0008-5472.CAN-12-2359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seydlová G, Pohl R, Zborníková E, Ehn M, Šimák O, Panova N, Kolář M, Bogdanová K, Večeřová R, Fišer R, et al.. Lipophosphonoxins II: design, synthesis and properties of novel broad spectrum antibacterial agents. J Med Chem. 2017;60(14):6098–6118. 10.1021/acs.jmedchem.7b00355 [DOI] [PubMed] [Google Scholar]
- Souza ALA, Díaz-Dellavalle P, Cabrera A, Larrañaga P, Dalla-Rizza M, De-Simone SG.. Antimicrobial activity of pleurocidin is retained in Plc-2, a C-terminal 12-amino acid fragment. Peptides. 2013;45(7):78–84. 10.1016/j.peptides.2013.03.030 [DOI] [PubMed] [Google Scholar]
- Spitzer M, Robbins N, Wright GD.. Combinatorial strategies for combating invasive fungal infections. Virulence. 2017;8(2):169–185. 10.1080/21505594.2016.1196300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W, Yuan H, Zhang H, Wang L, Qian H, Qi X. An antimicrobial peptide screened from casein hydrolyzate by Saccharomyces cerevisiae cell membrane affinity method. Food Control. 2015;50(3):413–422. 10.1016/j.foodcont.2014.09.030 [DOI] [Google Scholar]
- Thomas S, Karnik S, Barai RS, Jayaraman VK, Idicula-Thomas S.. CAMP: a useful resource for research on antimicrobial peptides. Nucleic Acids Res. 2010;38(Database issue) suppl_1:D774–D780. 10.1093/nar/gkp1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tindwa H, Patnaik B, Kim D, Mun S, Jo Y, Lee B, Lee Y, Kim N, Han Y. Cloning, characterization and effect of TmPGRP-LE gene silencing on survival of Tenebrio molitor against Listeria monocytogenes infection. Int J Mol Sci. 2013;14(11):22462–22482. 10.3390/ijms141122462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Li X, Wang Z. APD3: the antimicrobial peptide database as a tool for research and education. Nucleic Acids Res. 2016;44 D1:D1087–D1093. 10.1093/nar/gkv1278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Xiao M, Chen SCA, Kong F, Sun ZY, Liao K, Lu J, Shao HF, Yan Y, Fan H, et al.. In vitro susceptibilities of yeast species to fluconazole and voriconazole as determined by the 2010 National China Hospital Invasive Fungal Surveillance Net (CHIF-NET) study. J Clin Microbiol. 2012;50(12):3952–3959. 10.1128/JCM.01130-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Wang G. APD: the antimicrobial peptide database. Nucleic Acids Res. 2004;32(90001):D590–D592. 10.1093/nar/gkh025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan H, Yun J, Ai D, Zhang W, Bai J, Guo J. Two novel cationic antifungal peptides isolated from Bacillus pumilus HN-10 and their inhibitory activity against Trichothecium roseum. World J Microbiol Biotechnol. 2018;34(2):21 10.1007/s11274-017-2392-5 [DOI] [PubMed] [Google Scholar]
- Zhang S, Huang J, Hu R, Guo G, Shang X, Wu J. Characterization of a new multifunctional beta-glucosidase from Musca domestica. Biotechnol Lett. 2017;39(8):1219–1227. 10.1007/s10529-017-2351-0 [DOI] [PubMed] [Google Scholar]


