Abstract
Background.
Tricuspid valve regurgitation (TR) is a common finding immediately after cardiac transplantation. However, there is a scarcity of data regarding its implication if left untreated on long-term outcomes and the role of early surgical repair.
Methods.
We retrospectively reviewed the Duke University Medical Center transplant database from January 2000 to June 2012 and identified 542 patients who underwent orthotropic heart transplantation. Patients were excluded if they underwent surgical repair for TR during the transplant or if the transplant was part of a multiorgan transplant or redo heart transplantation. TR was assessed intraoperatively after weaning from cardiopulmonary bypass. Independent variables were grade of TR and changes in TR grade during follow-up. TR grades were classified as insignificant (none or mild) versus significant (moderate or severe). Survival and need for posttransplant valve repair during follow-up were assessed.
Results.
Significant TR was detected in 114 patients (21%) after weaning from cardiopulmonary bypass, with no significant difference in preoperative recipient pulmonary vascular resistance. Significant TR was associated with increased maximum postoperative plasma creatinine (median [interquartile range], 2.2 [1.5 to 3.2] mg/dL vs 1.8 [1.4 to 2.6] mg/dL, p = 0.008), prolonged postoperative stay (median [interquartile range], 12 [9 to 21] days vs 10 [8 to 14] days; p < 0.001), and decreased adjusted survival. Significant TR regressed to insignificant in 91% of recipients by 1 year after transplant. Six recipients (1%) who had significant TR after cardiopulmonary bypass underwent delayed tricuspid valve repair for significant TR during follow-up.
Conclusions.
Significant TR is a common finding immediately after transplant and is associated with early morbidity and reduced adjusted survival. Most significant TR resolves by 1 year after transplant. Optimal algorithms for follow-up and treatment of significant TR after heart transplantation need to be defined.
Heart transplantation remains the gold standard therapy for refractory, end-stage heart failure [1]. Overall outcomes of heart transplantation have improved during the last decade, but a number of postoperative complications continue to limit further improvements in the therapy. Among those, tricuspid valve regurgitation (TR) has been implicated in decreased long-term survival after heart transplant, but some of these data are conflicting [1–3]. TR is a frequent finding after orthotropic heart transplantation (OHT), and it has been postulated that TR relates to right ventricular dysfunction and geometrical alteration of the tricuspid valve apparatus after transplantation [3–6]. The aim of this study was to assess the implications of TR after heart transplantation, identify factors associated with postoperative TR, and evaluate the effect of TR on long-term survival.
Material and Methods
Study Design and Patient Selection
After Institutional Review Board approval and waiver for the need of informed consent, all records of consecutive patients who underwent OHT at our institution between January 2000 and April 2012 were reviewed. Patient data were obtained from Duke Enterprise Data Unified Content Explorer, a Duke University enterprise data warehouse that provides investigators with clinical information collected as a by-product of patient care [7]. These data were supplemented and validated with manual record review as well as with data obtained from United Network for Organ Sharing site-specific reports and the Duke Database for Cardiovascular Disease. Survival data were matched and merged with our database and the Social Security Death Index and were supplemented with a record review.
The analysis excluded patients who underwent a concomitant cardiac surgical procedure during transplant (including tricuspid valve repair), pediatric heart transplants, redo heart transplant, or combined transplants. Also excluded were patients who underwent a biatrial technique for transplantation for any reason.
Assessment and Grading of TR
TR was assessed by board-certified echocardiographers with transesophageal echocardiography (TEE) intraoperatively and with transthoracic echocardiography postoperatively, according to published guidelines [8–10]. We analyzed patient data at the time of transplant (intraoperative TEE baseline TR), 30 days, 90 days, 6 months, and 1 year postoperatively. The degree of TR was defined as none (0), trace (1+), mild (2+), moderate (3+), and severe (4+). Two study groups were derived for further comparison: patients with evidence of none, trace, or mild TR (insignificant TR) and patients with moderate or severe TR (significant TR) as diagnosed by TEE after cessation of cardiopulmonary bypass.
Right atrial (RA) size was also assessed according to the most recent guidelines at the time of the study [11]. In brief, RA was defined as enlarged if the RA area exceeded 18 cm2, RA length exceeded 53 mm, or RA diameter exceeded 44 mm.
Outcome Measures
The primary end points of this study were survival at 30 days and at 1 year after transplantation. Secondary end points were changes in TR grade postoperatively, need for a posttransplant tricuspid valve intervention, kidney function and requirement for dialysis, length of postoperative hospitalization, and ventilator time.
Statistical Analysis
All data were coded, matched, merged, and analyzed by a statistical team from the biostatistics division at the Duke Clinical Research Institute. Percentages and proportions are reported for categorical variables. Continuous measures are reported with median and interquartile ranges (IQR) or mean with SD. The Pearson χ2 or exact test was used when appropriate to test for differences in categorical variables. Wilcoxon rank sum testing was used to assess differences in continuous variables. Kaplan-Meier analysis of survival with log-rank testing for statistical significance was conducted.
A logistic regression model was used to assess the relationship between TR and the short-term outcome variables. Cox proportional hazards regression models were used to assess time to event in modeling beyond 30 days with risk factor adjustment. To examine differences in the presence of TR over time, we fit a logistic regression model with time after baseline (30, 90, 180, and 366 days) as a fixed-effect class variable and a random intercept for each patient to account for repeated measures per patient. The Kenward-Roger method was used to estimate the denominator degrees of freedom for the test on association with time. Statistical analysis was performed using SAS 9.2 and 9.4 software (SAS Institute Inc, Cary, NC).
Results
From January 2000 to June 2012, 542 patients underwent cardiac transplantation at our institution with available postoperative imaging and follow-up (Fig 1). We excluded 8 patients who had a history of previous heart transplantation. Of the remaining patients, 114 (21%) had moderate or severe TR on intraoperative TEE and therefore were classified in the significant TR group, and 428 (79%) had no, trace, or mild TR at baseline and were classified in the insignificant TR group. During follow-up, 5 patients underwent tricuspid valve repair for persistent, significant TR. For the patients who underwent tricuspid procedures after transplant, these were all for severe tricuspid insufficiency. Some had severe TR immediately after the transplant that failed to improve. These typically had annular stretching or annular distortion. Of these 5 patients, 4 had significant TR at baseline, and 1 did not. None of these patients died within 30 days.
Fig 1.
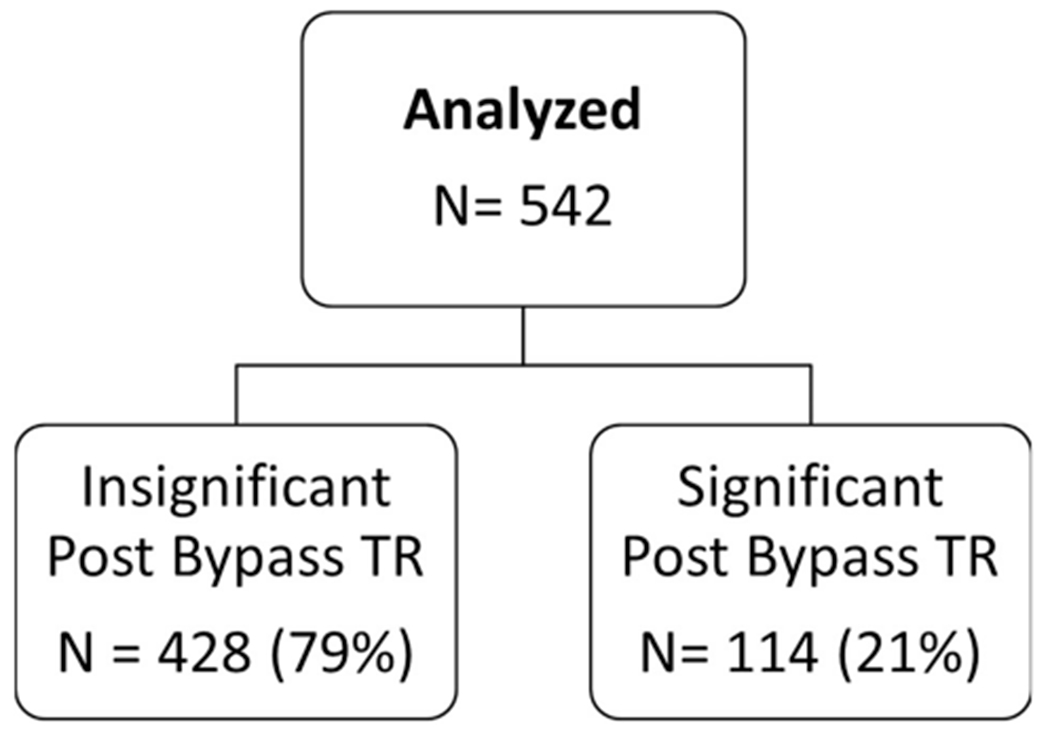
Study design and patient inclusion. (TR = tricuspid regurgitation.)
Differences in baseline demographics between the two study groups are reported in Table 1. There was a higher percentage of women in the significant posttransplant TR group (38% vs 22%, respectively; p < 0.001). Of note, no significant differences were noted between the two groups in preoperative blood urea nitrogen, creatinine, body mass index, age, pulmonary vascular resistance, and use of inotropes or mechanical support as a bridge to transplant. Donor characteristics were similar among the two study groups and are reported in Table 2. Female donor sex was more prevalent in recipients who had significant TR after transplant (47% vs 27%, respectively; p < 0.001). Graft ischemia time was not significantly different between the groups.
Table 1.
Preoperative Characteristics of the Recipients
| Variablesa | Total | No Significant TR | Significant TR | p Value |
|---|---|---|---|---|
| Patients | 542 (100) | 428 (79.0) | 114 (21.0) | |
| Age, years | 0.359 | |||
| Mean ± SD | 52.0 ± 12.4 | 53.0 ± 12.1 | 51.0 ± 13.4 | |
| Median (IQR) | 55 (45–61) | 55 (46–61) | 54 (45–62) | |
| Female sex | 137/542 (25.3) | 94/428 (22.0) | 43/114 (37.7) | 0.001 |
| Body mass index, kg/m2 | 0.611 | |||
| Mean ± SD | 26.9 ± 5.4 | 26.9 ± 5.4 | 26.7 ± 5.4 | |
| Median (IQR) | 26.4 (23.0–30.0) | 26.5 (23.2–30.1) | 26.1 (22.7–29.4) | |
| Creatinine, mg/dL | 0.632 | |||
| Mean ± SD | 1.34 ± 0.5 | 1.34 ± 0.6 | 1.35 ± 0.4 | |
| Median (IQR) | 1.30 (1.00–1.50) | 1.30 (1.00–1.50) | 1.30 (1.01.60) | |
| BUN, mg/dL | 0.557 | |||
| Mean ± SD | 23.5 ± 14 | 23.2 ± 13 | 24.7 ± 15 | |
| Median (IQR) | 20 (15–28) | 20 (14–28) | 21 (15–31) | |
| Albumin, g/dL | 0.551 | |||
| Mean ± SD | 3.28 ± 0.7 | 3.27 ± 0.7 | 3.33 ± 0.6 | |
| Median (IQR) | 3.3 (2.8–3.8) | 3.3 (2.7–3.8) | 3.4 (2.9–3.7) | |
| Diagnosis | 0.804 | |||
| Ischemic | 239/533 (44.8) | 188/422 (44.6) | 51/111 (46.0) | |
| Valvular | 9/533 (1.7) | 6/422 (1.4) | 3/111 (2.7) | |
| Idiopathic | 208/533 (39.1) | 166/422 (39.3) | 42/111 (37.8) | |
| Congenital | 16/533 (3.0) | 14/422 (3.3) | 2/111 (1.8) | |
| Other | 61/533 (11.4) | 48/422 (11.4) | 13/111 (11.7) | |
| Preoperative PVR, Woods unit | 0.236 | |||
| Mean ± SD | 2.7 ± 1.9 | 2.7 ± 1.8 | 2.8 ± 1.9 | |
| Median (IQR) | 2.4 (1.6–3.7) | 2.4 (1.5–3.6) | 2.6 (1.7–3.8) | |
| Inotrope(s) bridge | 262/538 (48.70) | 209/425 (49.18) | 53/113 (46.90) | 0.667 |
| Ventilator or MCS pretransplant | 281/538 (52.23) | 219/425 (51.53) | 62/113 (54.87) | 0.523 |
Categorical variables are shown as number (%) and continuous variables as indicated.
BUN = blood urea nitrogen; IQR = interquartile range; MCS = mechanical circulatory support; PVR = pulmonary vascular resistance; TR = tricuspid regurgitation.
Table 2.
Characteristics of the Donors
| Variablesa | Total | No Significant TR | Significant TR | p Value |
|---|---|---|---|---|
| Female | 168/538 (31.2) | 115/425 (27.1) | 53 (46.9) | <0.001 |
| Age, years | 0.492 | |||
| Mean ± SD | 36 ± 13 | 36 ± 13 | 37 ± 13 | |
| Median (IQR) | 36 (24–47) | 36 (24–47) | 38 (24–47) | |
| BMI, kg/m2 | 0.962 | |||
| Mean ± SD | 27.1 ± 6.6 | 27.0 ± 6.5 | 27.2 ± 6.8 | |
| Median (IQR) | 25.4 (22.5–30.1) | 25.4 (22.7–30.0) | 25.5 (22.0–30.6) | |
| Inotrope support | 209/378 (55.3) | 164/292 (56.2) | 45/86 (52.3) | 0.529 |
| LVEF | 0.015 | |||
| Mean ± SD | 0.602 ± 0.081 | 0.598 ± 0.082 | 0.617 ± 0.074 | |
| Median (IQR) | 0.60 (0.55–0.65) | 0.60 (0.55–0.65) | 0.60 (0.56–0.65) | |
| Diabetes mellitus | 34/532 (6.4) | 23/422 (5.5) | 11/110 (10.0) | 0.082 |
| History of | ||||
| Tobacco | 108/532 (33.8) | 149/421 (35.4) | 31/111 (27.9) | 0.139 |
| Cocaine | 109/530 (20.6) | 84/421 (20.0) | 25/109 (22.9) | 0.492 |
| Alcohol | 61/319 (19.1) | 49/241 (20.3) | 12/78 (15.4) | 0.334 |
| Donor-recipient BMI mismatch | 226/536 (42.2) | 173/423 (40.9) | 53/113 (46.9) | 0.251 |
| Ischemic time, minutes | 0.243 | |||
| Mean ± SD | 203.9 ± 54.9 | 204.9 ± 56.3 | 200.0 ± 49.2 | |
| Median (IQR) | 205 (169–238) | 205 (168–240) | 199 (175–221) |
Categorical variables are shown as number (%) and continuous variables as indicated.
BMI = body mass index; IQR = interquartile range; LVEF = left ventricular ejection fraction; TR = tricuspid regurgitation.
Operative and perioperative data are reported in Table 3. Days in hospital after transplant was longer for patients with intraoperative significant TR compared with those patients with intraoperative insignificant TR (median [IQR], 12 [9 to 12] days vs 10 [8 to 14] days; p < 0.001). Furthermore, the 30-day mortality rate was higher for patients with versus without significant TR (5.3% vs 0.7%, respectively; p = 0.004). Similarly, the 1-year mortality rate was also higher in patient with significant TR (19.3%) compared with those with insignificant TR (8.7%; p = 0.002). Of the 56 deaths at 1 year, examining the TR assessment that is closest to death, 42 (75%) did not have significant TR at that time, and 14 (25%) did have significant TR. When examining the last two TR assessments for these 56 patients, 36 (64%) were without significant TR, and 20 (36%) exhibited significant TR
Table 3.
Perioperative and Postoperative Outcomes
| Variablea | Total | No Significant TR | Significant TR | p Value |
|---|---|---|---|---|
| Length of stay post-Txp, days | <0.001 | |||
| Mean ± SD | 15 ± 15 | 14 ± 13 | 20 ± 20 | |
| Median (IQR) | 10 (8–15) | 10 (8–14) | 12 (9–21) | |
| Peak 7-day BUN, mg/dL | 0.008 | |||
| Mean ± SD | 51 ± 26 | 49 ± 24 | 58 ± 30 | |
| Median (IQR) | 45 (32–63) | 44 (32–60) | 50 (33–77) | |
| Peak 7-day creatinine, mg/dL | 0.004 | |||
| Mean ± SD | 2.1 ± 1.2 | 2.01 ± 1.1 | 2.42 ± 1.4 | |
| Median (IQR) | 2 (1–2) | 2 (1–2) | 2 (1–3) | |
| Peak hospital BUN, mg/dL | 0.003 | |||
| Mean ± SD | 56 ± 28 | 54 ± 26 | 64 ± 32 | |
| Median (IQR) | 48 (35–69) | 48 (35–65) | 56 (37–85) | |
| Peak hospital BUN, mg/dL | 0.008 | |||
| Mean ± SD | 2.27 ± 1.4 | 2.19 ± 1.3 | 2.57 ± 1.5 | |
| Median (IQR) | 1.9 (1.4–2.7) | 1.8 (1.4–2.6) | 2.2 (1.5–3.2) | |
| Post-Txp dialysis | 13 (2.4) | 6/428 (1.4) | 7/114 (6.1%) | 0.009 |
| Mortality | ||||
| 30 days | 9 (1.7) | 3/428 (0.7) | 6/114 (5.3) | 0.004 |
| 1 year (K-M method), % | 10.50 | 8.33 | 18.60 | <0.001 |
Categorical variables are shown as number (%) or as indicated and continuous variables as indicated.
BUN = blood urea nitrogen; IQR = interquartile range; K-M = Kaplan-Meier; TR = tricuspid regurgitation; Txp = transplant.
Finally, when assessing the 476 patients with both TR assessments and 1-year vital status known, and selecting the TR status closest to the last echo before the end of study, 28 of 476 patients (6%) had significant TR and 448 (94%) did not have significant TR. Of the 28 patients with significant TR, 14 (50%) died by year 1 and 14 (50%) were still alive. Among the 448 patients without significant TR, 42 (9%) had died and 406 (91%) were still alive.
Postoperative renal dysfunction was more common in the significant TR group, with a higher proportion of patients requiring dialysis (Table 3). Overall, the proportion of patients with significant TR decreased over time, with very few patients left with residual significant TR at 1-year after transplant (Fig 2).
Fig 2.
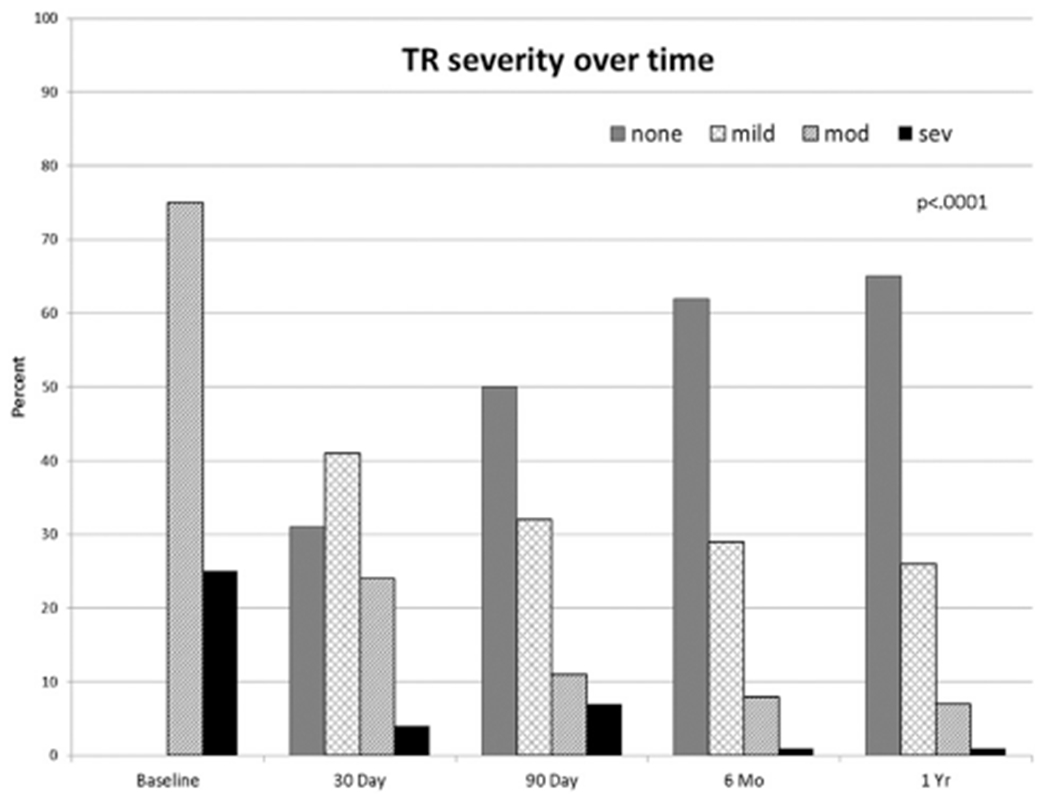
Proportion of patients with tricuspid regurgitation (TR) at different times after transplantation. (mod = moderate; sev = severe.)
Table 4 reports mitral regurgitation severity, RA size, and right ventricular function over time. Overall, there were small differences in right ventricular function and RA size between the groups that did not reach statistical significance (Fig 3 and Table 4). However, the significant TR group tended to have slightly more mitral regurgitation at 3 to 12 months after transplant, which was statistically significant.
Table 4.
Echocardiographic Variables
| Significant TR at Baseline |
||||
|---|---|---|---|---|
| Total |
No |
Yes |
||
| Variable | No. (%) | No. (%) | No. (%) | p Value |
| MR at 30 days | 0.374 | |||
| None | 164/418 (39.23) | 133/331 (40.18) | 31/87 (35.63) | |
| Trivial | 182/418 (43.54) | 146/331 (44.11) | 36/87 (41.38) | |
| Mild | 64/418 (15.31) | 47/331 (14.20) | 17/87 (19.54) | |
| Moderate | 8/418 (1.91) | 5/331 (1.51) | 3/87 (3.45) | |
| Severe | 0/418 (0.00) | 0/331 (0.00) | 0/87 (0.00) | |
| MR at 90 days | 0.017 | |||
| None | 169/455 (37.14) | 145/362 (40.06) | 24/93 (25.81) | |
| Trivial | 218/455 (47.91) | 164/362 (45.30) | 54/93 (58.06) | |
| Mild | 62/455 (13.63) | 50/362 (13.81) | 12/93 (12.90) | |
| Moderate | 6/455 (1.32) | 3/362 (0.83) | 3/93 (3.23) | |
| Severe | 0/455 (0.00) | 0/362 (0.00) | 0/93 (0.00) | |
| MR at 6 months | 0.018 | |||
| None | 177/430 (41.16) | 152/343 (44.31) | 25/87 (28.74) | |
| Trivial | 192/430 (44.65) | 149/343 (43.44) | 43/87 (49.43) | |
| Mild | 58/430 (13.49) | 40/343 (11.66) | 18/87 (20.69) | |
| Moderate | 3/430 (0.70) | 2/343 (0.58) | 1/87 (1.15) | |
| Severe | 0/430 (0.00) | 0/343 (0.00) | 0/87 (0.00) | |
| MR at 1 year | 0.001 | |||
| None | 185/440 (42.05) | 161/355 (45.35) | 24/85 (28.24) | |
| Trivial | 201/440 (45.68) | 157/355 (44.23) | 44/85 (51.76) | |
| Mild | 48/440 (10.91) | 35/355 (9.86) | 13/85 (15.29) | |
| Moderate | 4/440 (0.91) | 2/355 (0.56) | 2/85 (2.35) | |
| Severe | 2/440 (0.45) | 0/355 (0.00) | 2/85 (2.35) | |
| RA size at 30 days | 0.423 | |||
| Normal | 345/530 (65.09) | 272/419 (64.92) | 73/111 (65.77) | |
| Small | 0/530 (0.00) | 0/419 (0.00) | 0/111 (0.00) | |
| Mildly enlarged | 147/530 (27.74) | 120/419 (28.64) | 27/111 (24.32) | |
| Moderately enlarged | 35/530 (6.60) | 25/419 (5.97) | 10/111 (9.01) | |
| Severely enlarged | 3/530 (0.57) | 2/419 (0.48) | 1/111 (0.90) | |
| RA size at 90 days | 0.231 | |||
| Normal | 347/530 (65.47) | 282/422 (66.82) | 65/108 (60.19) | |
| Small | 1/530 (0.19) | 1/422 (0.24) | 0/108 (0.00) | |
| Mildly enlarged | 153/530 (28.87) | 114/422 (27.01) | 39/108 (36.11) | |
| Moderately enlarged | 26/530 (4.91) | 23/422 (5.45) | 3/108 (2.78) | |
| Severely enlarged | 3/530 (0.57) | 2/422 (0.47) | 1/108 (0.93) | |
| RA size at 6 months | 0.058 | |||
| Normal | 356/515 (69.13) | 274/411 (66.67) | 82/104 (78.85) | |
| Small | 0/515 (0.00) | 0/411 (0.00) | 0/104 (0.00) | |
| Mildly enlarged | 134/515 (26.02) | 113/411 (27.49) | 21/104 (20.19) | |
| Moderately enlarged | 22/515 (4.27) | 21/411 (5.11) | 1/104 (0.96) | |
| Severely enlarged | 3/515 (0.58) | 3/411 (0.73) | 0/104 (0.00) | |
| RA size 1 year | 0.451 | |||
| Normal | 372/499 (74.55) | 294/399 (73.68) | 78/100 (78.00) | |
| Small | 0/499 (0.00) | 0/399 (0.00) | 0/100 (0.00) | |
| Mildly enlarged | 108/499 (21.64) | 87/399 (21.80) | 21/100 (21.00) | |
| Moderately enlarged | 18/499 (3.61) | 17/399 (4.26) | 1/100 (1.00) | |
| Severely enlarged | 1/499 (0.20) | 1/399 (0.25) | 0/100 (0.00) | |
| RV function at 30 days | 0.058 | |||
| Normal | 165/194 (85.05) | 124/140 (88.57) | 41/54 (75.93) | |
| Mild dysfunction | 24/194 (12.37) | 14/140 (10.00) | 10/54 (18.52) | |
| Moderate dysfunction | 4/194 (2.06) | 2/140 (1.43) | 2/54 (3.70) | |
| Severe dysfunction | 0/194 (0.00) | 0/140 (0.00) | 0/54 (0.00) | |
| RV not well seen | 1/194 (0.52) | 0/140 (0.00) | 1/54 (1.85) | |
| RV function at 90 days | 0.316 | |||
| Normal | 176/208 (84.62) | 129/151 (85.43) | 47/57 (82.46) | |
| Mild dysfunction | 23/208 (11.06) | 17/151 (11.26) | 6/57 (10.53) | |
| Moderate dysfunction | 7/208 (3.37) | 3/151 (1.99) | 4/57 (7.02) | |
| Severe dysfunction | 2/208 (0.96) | 2/151 (1.32) | 0/57 (0.00) | |
| RV function at 6 months | 0.631 | |||
| Normal | 187/212 (88.21) | 136/157 (86.62) | 51/55 (92.73) | |
| Mild dysfunction | 17/212 (8.02) | 15/157 (9.55) | 2/55 (3.64) | |
| Moderate dysfunction | 5/212 (2.36) | 3/157 (1.91) | 2/55 (3.64) | |
| Severe dysfunction | 1/212 (0.47) | 1/157 (0.64) | 0/55 (0.00) | |
| Hypercontractile | 1/212 (0.47) | 1/157 (0.64) | 0/55 (0.00) | |
| RV not well seen | 1/212 (0.47) | 1/157 (0.64) | 0/55 (0.00) | |
| RV function at 1 year | 0.414 | |||
| Normal | 211/227 (92.95) | 158/168 (94.05) | 53/59 (89.83) | |
| Mild dysfunction | 11/227 (4.85) | 7/168 (4.17) | 4/59 (6.78) | |
| Moderate dysfunction | 5/227 (2.20) | 3/168 (1.79) | 2/59 (3.39) | |
| Severe dysfunction | 0/227 (0.00) | 0/168 (0.00) | 0/59 (0.00) | |
MR = mitral regurgitation; No. = number; RA = right atrium; RV = right ventricle; TR = tricuspid regurgitation.
Fig 3.
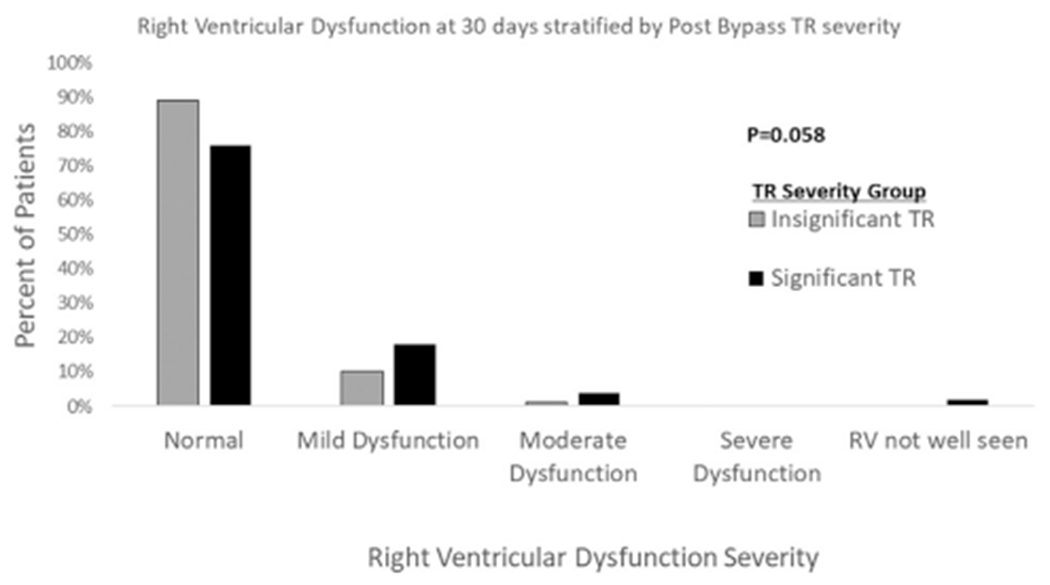
Right ventricular (RV) dysfunction at 30 days based on the immediate postoperative tricuspid regurgitation (TR) category.
Preoperative recipient and donor characteristics were examined in a multivariable Cox proportional hazards model using time-to-event methods for the likelihood of persistent moderate or severe TR during follow-up. Donor creatinine (hazard ratio, 1.175; 95% confidence interval [CI], 1.026 to 1.346) and donor age (hazard ratio, 1.022; 95% CI, 1.003 to 1.042) were identified as covariates associated with increased risk of persistent moderate or severe TR. Conversely, donor male sex (hazard ratio, 0.402; 95% CI, 0.246 to 0.657) was associated with a decreased risk of TR in our patient population. Logistic regression and a stepwise selection of candidate variables (baseline characteristics of recipients and donors) showed three variables were significant for estimating likelihood of moderate or severe TR at the baseline intraoperative assessment: (1) donor creatinine (odds ratio, 1.245; 95% CI, 1.073 to 1.443), (2) male recipient (odds ratio, 0.361; 95% CI, 0.223 to 0.584), and (3) sex mismatch (odds ratio, 2.503; 95% CI, 1.399 to 4.477).
A Kaplan-Meier analysis using risk adjustment from the United Network for Organ Sharing OHT risk adjust model, accounting for differences in recipient pulmonary vascular resistance, creatinine (both donor and recipient), ischemia time, donor age, body mass index, and sex mismatch, demonstrated a statistically significant difference in survival up to 10 years with a log-rank p = 0.0009 favoring patients without significant postoperative TR (Fig 4).
Fig 4.
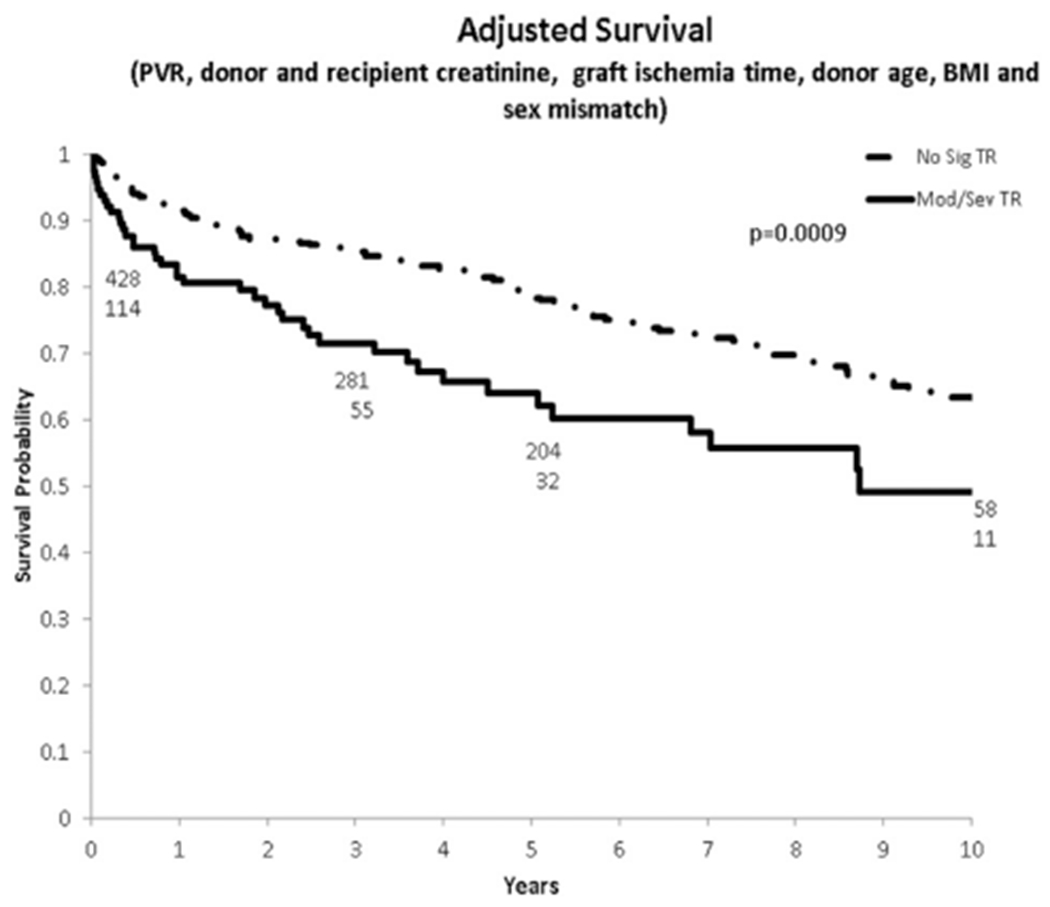
Adjusted Kaplan-Maier survival for patients with versus without significant TR after transplantation. (BMI = body mass index; PVR = pulmonary vascular resistance; No Sig TR = no significant tricuspid regurgitation; Mod/Sev TR = moderate/severe tricuspid regurgitation.)
Comment
There continues to be an ongoing debate regarding interventions on the tricuspid valve at the time of heart transplantation. This is mainly caused by a number of reports on the relationship between posttransplant TR and adverse outcomes [2, 6, 12–15]. Given these findings, some authors have suggested that routine donor tricuspid valve repair should be performed at the time of transplant [4, 16]. Others have introduced technical modifications to the inferior vena cava anastomosis to reduce the incidence of TR [17]. However, when data on the outcomes of patients who underwent donor tricuspid valve repair at the time of transplant were evaluated, no clear benefit was seen [18]. Of 330 patients, 52.4% underwent donor tricuspid valve repair, with no significant benefit or harm seen in regard to death, posttransplant tricuspid valve procedure, or dialysis [18].
A small randomized trial was conducted by Jeevanandam and colleagues [16] in 1997 in which 60 patients were randomized to bicaval heart transplantation (n = 30) or bicaval OHT with DeVega tricuspid valve annuloplasty on the donor heart before implantation (n = 30). They demonstrated improved immediate donor heart function (better right ventricular performance, lower perioperative mortality, and shorter reperfusion times) in the prophylactic tricuspid repair group. At 1 year, they noted less TR for the repair group but no difference in renal function between the groups. The study patients were monitored 5.7 to 6.7 years after transplant, and the tricuspid repair group had a survival advantage for cardiac-specific death [4]. Furthermore, the repair group had less long-term TR and better renal function. The group concluded that such repair should be performed as a routine adjunct to bicaval heart transplantation. The study, however, had a number of very important limitations, including being grossly underpowered, lack of blinding, and importantly, 1 surgeon performing the tricuspid repair was different from the surgeon performing the transplants without tricuspid repair. This could explain the overall shorter reperfusion, cardiopulmonary bypass, and ischemic times experienced with the repair group.
In this analysis, we report the largest single-center experience of patients who underwent heart transplantation with relatively prevalent posttransplant TR. In our series, 20% of recipients had clinically significant TR after weaning from cardiopulmonary bypass. Over time however, the TR improved in most of those patients, and at 1 year, the proportion of patients with severe TR was very low.
We currently have a poor ability to predict which patients with clinically significant TR will improve without the need for surgical intervention (given that most patients improve) and in which patients the TR will become clinically meaningful and lead to worse outcomes as reported in this study and demonstrated by others [4, 16]. Another clinical dilemma relates to early postoperative TR actually being a marker of overall graft dysfunction and not necessarily an isolated finding. In fact, worsening mitral regurgitation was also more likely to develop in patients in this study who had significant postoperative TR, and their survival was diminished long-term despite the TR improving within the first few months. Whether reversible TR after transplant is associated with similar survival to patients who did not have significant TR after transplant is unclear.
Female sex was a negative prognostic indicator for both the donor and the recipient for posttransplant TR in our study. This finding confirms a previous report of sex difference in TR presence and progression. A large-scale echocardiographic study of function in 946 TR patients identified female sex as an independent predictor for fast progression of TR over time [19].
This study had a number of important limitations. It is a single-center experience over a long period of time. The decision to intervene on the tricuspid valve was left to the discretion of the surgeon, which will add a number of important biases. Furthermore, significant TR could have developed in some patients that would have been missed if they did not present for follow-up. Finally, we did not have comprehensive myocardial biopsy data to relate new TR being caused by the biopsy.
Despite its limitations, this study provides the natural history of TR after heart transplantation at an experienced center that does not perform tricuspid surgical interventions at the time of the transplant.
In conclusion, significant TR early after transplant is a common finding and is associated with early morbidity and reduced unadjusted survival. Female donor and recipient sex appear to be associated with significant posttransplant TR. Most significant TR resolves by 1 year after transplant. Identifying patients with significant TR who will develop such complications remains difficult, and further clinical investigation is warranted.
Acknowledgments
The authors wish to thank the Duke Clinical Transplantation team for their help in caring for these patients. Muath Bishawi received funding from 1R38HL143612-01.
References
- 1.Yusen RD, Edwards LB, Kucheryavaya AY, et al. The registry of the International Society for Heart and Lung Transplantation: thirty-first adult lung and heart-lung transplant report—2014; focus theme: retransplantation. J Heart Lung Transplant 2014;33:1009–24. [DOI] [PubMed] [Google Scholar]
- 2.Anderson CA, Shernan SK, Leacche M, et al. Severity of intraoperative tricuspid regurgitation predicts poor late survival following cardiac transplantation. Ann Thorac Surg 2004;78:1635–42. [DOI] [PubMed] [Google Scholar]
- 3.Mastouri R, Batres Y, Lenet A, et al. Frequency, time course, and possible causes of right ventricular systolic dysfunction after cardiac transplantation: a single center experience. Echocardiography 2013;30:9–16. [DOI] [PubMed] [Google Scholar]
- 4.Jeevanandam V, Russell H, Mather P, Furukawa S, Anderson A, Raman J. Donor tricuspid annuloplasty during orthotopic heart transplantation: long-term results of a prospective controlled study. Ann Thorac Surg 2006;82:2089–95; discussion 2095. [DOI] [PubMed] [Google Scholar]
- 5.Kalra N, Copeland JG, Sorrell VL. Tricuspid regurgitation after orthotopic heart transplantation. Echocardiography 2010;27:1–4. [DOI] [PubMed] [Google Scholar]
- 6.Sahar G, Stamler A, Erez E, et al. Etiological factors influencing the development of atrioventricular valve incompetence after heart transplantation. Transplant Proc 1997;29:2675–6. [DOI] [PubMed] [Google Scholar]
- 7.Horvath MM, Winfield S, Evans S, Slopek S, Shang H, Ferranti J. The DEDUCE Guided Query tool: providing simplified access to clinical data for research and quality improvement. J Biomed Inform 2011;44:266–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mügge A, Daniel WG, Herrmann G, Simon R, Lichtlen PR. Quantification of tricuspid regurgitation by Doppler color flow mapping after cardiac transplantation. Am J Cardiol 1990;66:884–7. [DOI] [PubMed] [Google Scholar]
- 9.Rees AP, Milani RV, Lavie CJ, Smart FW, Ventura HO. Valvular regurgitation and right-sided cardiac pressures in heart transplant recipients by complete Doppler and color flow evaluation. Chest 1993;104:82–7. [DOI] [PubMed] [Google Scholar]
- 10.Suzuki Y, Kambara H, Kadota K, et al. Detection and evaluation of tricuspid regurgitation using a real-time, two-dimensional, color-coded, Doppler flow imaging system: comparison with contrast two-dimensional echocardiography and right ventriculography. Am J Cardiol 1986;57:811–5. [DOI] [PubMed] [Google Scholar]
- 11.Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 2010;23:685–713; quiz 786-8. [DOI] [PubMed] [Google Scholar]
- 12.Aziz M, Saad RA, Burgess MI, Campbell CS, Yonan NA. Clinical significance of tricuspid valve dysfunction after orthotopic heart transplantation. J Heart Lung Transplant 2002;21:1101–8. [DOI] [PubMed] [Google Scholar]
- 13.Chan MC, Giannetti N, Kato T, et al. Severe tricuspid regurgitation after heart transplantation. J Heart Lung Transplant 2001;20:709–17. [DOI] [PubMed] [Google Scholar]
- 14.Marelli D, Esmailian F, Wong SY, et al. Tricuspid valve regurgitation after heart transplantation. J Thorac Cardiovasc Surg 2009;137:1557–9. [DOI] [PubMed] [Google Scholar]
- 15.Sade LE, Özin B, Atar I, Demir Ö, Demirtas S, Müderrisoğlu H. Right ventricular function is a determinant of long-term survival after cardiac resynchronization therapy. J Am Soc Echocardiogr 2013;26:706–13. [DOI] [PubMed] [Google Scholar]
- 16.Jeevanandam V, Russell H, Mather P, et al. A one-year comparison of prophylactic donor tricuspid annuloplasty in heart transplantation. Ann Thorac Surg 2004;78:759–66; discussion 765–6. [DOI] [PubMed] [Google Scholar]
- 17.Marelli D, Silvestry SC, Zwas D, et al. Modified inferior vena caval anastomosis to reduce tricuspid valve regurgitation after heart transplantation. Tex Heart Inst J 2007;34:30–5. [PMC free article] [PubMed] [Google Scholar]
- 18.Greenberg J, Teman NR, Haft JW, et al. Association of donor tricuspid valve repair with outcomes after cardiac transplantation. Ann Thorac Surg 2018;105:542–7. [DOI] [PubMed] [Google Scholar]
- 19.Prihadi EA, van der Bijl P, Gursoy E, et al. Sex differences in progression of tricuspid regurgitation: results from a large-scale echocardiographic study. Circulation 2018;136: A16542. [Google Scholar]


