Abstract
The proteomes of outer membrane vesicles (OMVs) secreted by C. jejuni 81–176 strain, which was exposed to oxygen or antibiotic stress (polymyxin B), were characterized. We also assessed the OMVs production and their content in two mutated strains – ∆dsbI and ∆htrA. OMVs production was significantly increased under the polymyxin B stress and remained unaltered in all other variants. Interestingly, the qualitative load of OMVs was constant regardless of the stress conditions or genetic background. However, certain proteins exhibited notable quantitative changes, ranging from 4-fold decrease to 10-fold increase. Up- or downregulated proteins (e.g. major outer membrane protein porA, iron ABC transporter, serine protease- htrA, 60 kDa chaperonin-groL, enolase) represented various cell compartments (cytoplasm, periplasm, and membrane) and exhibited various functions; nevertheless, one common group was noted that consisted of components of flagellar apparatus, i.e., FlaA/B, FlgC/E, which were mostly upregulated. Some of these proteins are the putative substrates of DsbI protein. Further investigation of the regulation of C. jejuni OMVs composition and their role in virulence will allow a better understanding of the infectious process of C. jejuni.
Key words: Campylobacter jejuni, outer membrane vesicles (OMVs), proteomics
Introduction
The outer membrane (OM) of Gram-negative bacteria can naturally bulge out, entraps periplasmic content and pinches off to form spheres of 50–250 nm in diameter called outer membrane vesicles (OMVs). OMVs contain mostly outer membrane-associated and periplasmic proteins, but also cytoplasm-located molecules, quorum-signaling molecules, DNA and RNA and, thus, provide bacteria with means to interact adaptively with their environment, host and other microorganisms (Schwechheimer and Kuehn 2015). Alterations in size, number, and content of OMVs are frequently regarded as bacterial responses to changes in the environment (such as pH, temperature, ionic strength, and antibiotic pressure), including endogenous stress (Mcbroom et al. 2007, Schwechheimer and Kuehn 2015). This notion is supported by a number of observations. A treatment with polymyxin B (a cyclic cationic antimicrobial peptide that alters the membrane permeability) or with ciprofloxacin (an antibiotic that damages DNA and activates the SOS system) increased OMVs formation by Pseudomonas aeruginosa PA14 strain (MacDonald and Kuehn 2013). The hyper-vesiculating yieM mutant (ΔyieM) of Escherichia coli grew faster in the presence of polymyxin B than the nonmutated strain (Manning and Kuehn 2013). P. aeruginosa cells could respond with increased OMVs production to hydrogen peroxide treatment and temperature change (MacDonald and Kuehn 2013). Overexpression of a periplasmic fusion protein to imitate misfolded OM protein in E. coli resulted in hypervesiculation and tenfold increase of its concentration in OMVs; moreover, increased OMV production accompanied mutations in the σE heat shock response in E. coli (Mcbroom et al. 2007). Furthermore, the genes participating in cellular stress response (degS, depP, rseA) are engaged in the vesicle formation (Mcbroom et al. 2006). Mutations of degP, encoding periplasmic serine protease-chaperone responsible for the degradation of misfolded proteins in E. coli, as well as mutations of mucD, its homolog in P. aeruginosa, increase OMVs production (MacDonald and Kuehn 2013). Thus, bacteria may use OMVs to selectively dispose of misfolded proteins, particularly large aggregates, which are resistant to proteolytic cleavage and are too big to be discarded through OM pores (Mcbroom et al. 2007). OMVs may, therefore, help bacteria to survive under harsh conditions and establish a lasting resistance.
In this study, we investigated whether the production of C. jejuni OMVs and their protein composition correlates to environmental stress conditions. We also examined the influence of mutations in the htrA and dsbI genes on the level of OMVs production. The C. jejuni htrA gene is the E. coli degP gene homolog, while the dsbI gene is a disulfide oxidoreductase – a component of the Dsb system in C. jejuni, involved in the introduction of disulfide bonds into periplasmic proteins for securing their proper fold and function.
Experimental
Materials and Methods
Bacterial strains, media, and culture conditions. Bacterial strains and plasmids used are listed in Table I. C. jejuni strains were grown on Blood Agar No. 2 (BA, Oxoid) plates supplemented with 5% horse blood, Campylobacter Selective Supplement (Blaser-Wang) (Oxoid), and tetracycline (10 μg/ml) at 37°C or 42°C for 16–24 h under microaerobic conditions in a Mart anaerobic jar (flushed with 6% O2, 10% CO2, 85% N2 gas mixture using an Anoxomat Mart II system).
Table I.
Strains used in this study.
| Strain or plasmid | Genotype / resistance / description | Reference |
|---|---|---|
| Campylobacter jejuni | ||
| 81–176 (ATCC BAA-, 2151) | pVir, TcR (Wild type, human isolate) | |
| 81–176 htrA− | pVir, htrA::aphA3, TcR, KmR | This study |
| 81–176 dsbI− | pVir, cfafolvcaf TcR, CmR | (Raczko et al. 2005) |
If necessary, plates were also supplemented with chloramphenicol (20 μg/ml) and/or kanamycin (25 μg/ml). Campylobacter strains used for isolation of OMVs were grown in Mueller-Hinton broth for ~16 hours with shaking under microaerobic conditions at 37°C.
Stress was induced by either addition of polymyxin B to a final concentration of 5.5 µg/ml (~15 × higher than in Campylobacter Selective Supplement, minding an intrinsic resistance of C. jejuni to this compound (Iovine 2013)) or by incubation in the atmosphere of 15% O2 and 6% CO2. Higher oxygen atmosphere was generated by CO2 Gen sachets (Thermo Scientific Oxoid Microbiology Products) in the 2.5-litre gas jar. Cultures in optimal and stress conditions were conducted parallel. Overnight culture of C. jejuni was diluted 1:100. The same number of bacterial cells was used as inoculum for fresh media and grown in optimal and stress conditions in 37°C with shaking. After ~16 h optical density of each culture was measured, and bacteria were plated on BA medium to assess the number of bacteria cells (CFU/ml). The bacterial concentration ranged between 8 × 106 – 1 × 107 CFU/ml in the presence of polymyxin B while in 15% O2 atmosphere was between 1.5 × 107 – 5.5 × 107 CFU/ml. In optimal growth conditions, the concentration of bacteria was in the range from 1 × 108 to 5 × 108 CFU/ml.
Construction of C. jejuni mutants. The C. jejuni htrA mutant was obtained following the approach of Brøndsted et al. (2005). Mutagenesis was performed with a gene replacement method. A suicide mutagenesis plasmid was constructed in 2-step PCR. First, the upstream and downstream fragments of 5’ and 3’ end of the htrA gene were amplified with use of C. jejuni 81–176 chromosomal DNA as a template. For the fragment containing 3’ end of the htrA gene (421 bp), primers UW564 (5’-CGAGCTCAAATGCAGTGCTTTCTTATC-3’) and UW565 (5’-CTC-GAGCTGCAGTCTAGACAACATCTCCTTC-CATTAAATC-3’) were used, while primers UW566 (5’-CCGGTACCCAAATCGCTTTGTACGCCTTTAG-3’) and UW567 5’-TCTAGACTGCAGCTCGAG-TTTCTCGTGGTGGTGGAAATAAC-3’) were used for amplification of the fragment containing 5’ end of the gene (428 bp). In the sequences of the primers above, the restriction enzyme sites are underlined, and bases complementary to the htrA gene are indicated in bold.
In the second round of PCR, the amplified htrA fragments were joined, creating PCR fragments containing a deletion of 186 bp in htrA and introducing a XhoI-PstI-XbaI restriction sites instead. The final PCR product was cloned into the C. jejuni nonreplicable pBluescript II SK cloning vector with the use of SacI-KpnI enzymes, resulting in plasmid pUWM1321. Finally, the cat gene encoding for chloramphenicol resistance in C. jejuni cells (obtained from pRY109) was cloned into the PstI site of pUWM1321, resulting in the final mutagenesis plasmid denoted pUWM1329. The cat gene in this construct is transcribed in the same direction as the htrA gene.
C. jejuni 81–176 was electroporated with pUWM1329, and several chloramphenicol-resistant colonies were isolated. The disruption of the htrA gene as a result of double cross-over recombination and lack of wild-type htrA allele in the genome of the strain obtained was verified by PCR.
C. jejuni dsbI mutant was obtained in our lab and described earlier (Raczko et al. 2005).
OMV purification and quantification. OMVs were isolated from C. jejuni strain 81–176 by the method we used before (Godlewska et al. 2016) and described by Elmi et al. (2012) and Chutkan et al. (2013). Briefly, C. jejuni 81–176 strain was grown in the Mueller-Hinton broth under microaerobic conditions at 37°C. An overnight culture was diluted 1:100 into 330 ml of fresh growth media and grown 16–18 h to a mid-log phase. The cells were pelleted twice using centrifugation at 6000 × g for 2 × 20 min at 4°C. Supernatants were filtered through a 0.22 µm filter device to remove the remaining cells. The filtrate was ultracentrifuged in a Beckman L7-55 Ultracentrifuge at 150 000 × g for 3 h at 4°C using a 50.2 Ti rotor. OMVs preparations were plated on BA plates and incubated under microaerobic conditions to confirm the absence of viable bacteria.
OMVs production was quantified by determining protein concentration (BCA assay) and normalized to CFU at the time of harvest. This method allows indirect estimate the concentration of OMV based on total protein content but does not provide additional information about the size or quantity of OMV. Relative OMVs production levels were determined by comparison to the wild-type cell results in the control samples. Means of three independent experiments with standard deviations (error bars) were calculated.
Sample preparation for MS analysis. Membrane proteins were extracted by suspending OMVs in 1.5% sodium deoxycholate and 0.1% SDS in 25 mM ammonium bicarbonate, followed by sonication for 10 minutes (50% cycle). Protein concentration was estimated with Direct Detect (Milipore) and equalized to 40 µg per sample. Proteins were subsequently reduced with tris(2-carboxyethyl)phosphine for 30 min at 60°C, alkylated with methyl methanethiosulfonate and digested with 0.5 µg of trypsin at 37°C overnight. The reaction was quenched with trifluoroacetic acid (TFA).
LC-MS-MS/MS analysis. The resulting peptide mixtures were analyzed by LC-MS-MS/MS (liquid chromatography coupled to tandem mass spectrometry) using a Nano-Acquity (Waters) LC system and an Orbitrap Velos mass spectrometer (Thermo Electron Corp). Peptides were applied to RP-18 precolumn (nanoACQUITY Symmetry® C18) using water containing 0.1% TFA as a mobile phase and then transferred to nano-HPLC RP-18 column (nanoACQUITY BEH C18) using an acetonitrile gradient 5–35% in 180 minutes in the presence of 0.05% formic acid with the flow rate of 250 ml/min. Column outlet was directly coupled to the ion source of the spectrometer working in the regime of data dependent MS to MS/MS switch. To ensure reproducibility, sample preparations and MS analysis were repeated four times.
Database searching. The data acquired were processed by a Mascot Distiller followed by Mascot Search (Matrix Science, on-site license) against the UniProt database restricted to Campylobacter jejuni 81–176 and NCTC11168 sequences. The search parameters for precursor and product ions mass tolerance were 20 ppm and 0.1 Da, respectively, enzyme specificity: trypsin, missed cleavage sites allowed: 1, fixed modification of cysteine methylation and variable modification of methionine oxidation. To estimate the false-positive discovery rate (FDR), the decoy search option was enabled. The label-free quantitation was performed as described previously (Bakun et al. 2009) using in-house MScan software to select the proteins with an FDR < 1% and identified by at least two peptides.
LC-MS data were converted by an in-house MsConvert tool into 2D heat maps recognized by MSparky – an in-house modification of the Sparky NMR (http://www.cgl.ucsf.edu/home/sparky) – and the qualitative information from MScan was correlated with quantitative data from MSparky on the basis of m/z, retention time and isotopic profile fitting, generating a list of identified peptides with signal intensity values, subsequently subjected to statistical analysis using in-house Diffprot software (Malinowska et al. 2012). All software used is accessible at http://proteom.ibb.waw.pl.
Results and Discussion
Alterations in OMVs production. In our experiments, the presence of polymyxin B significantly increased OMVs production by C. jejuni 81–176 strain, based on total OMVs’ protein content corrected for CFU measurement (Fig. 1). We did not assess the size and shape of OMVs. Polymyxin B is a cyclic cationic antimicrobial peptide (AMPs), which penetrates into the bacterial cell, integrates into the outer leaflet of the outer membrane and impairs the structure of OM forming pores, altering the membrane permeability. Our results are consistent with the previous observations.
Fig. 1.
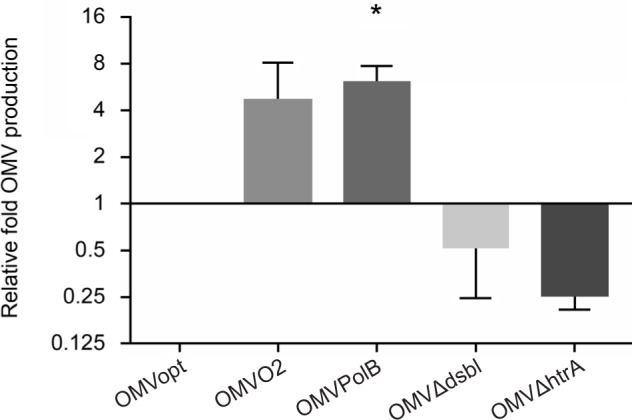
Comparison of the OMVs production.
The concentration of OMVs, isolated from C. jejuni 81–176 grown under optimal and stress conditions (15% O2 and polymyxin B) and from C. jejuni 81–176 dsbI and htrA mutants, was measured by BCA assay. The concentration of OMVs produced by the strain grown under optimal conditions was marked as 1. A statistical analysis was performed using GraphPad Prism 6 (GraphPad Software). Statistical analysis was carried out using multifactorial (one-way) ANOVA followed by Tukey’s multiple comparisons test. An asterisk indicates significant differences (p < 0.05) between analyzed groups and the control group.
Many studies have revealed that the levels of OMVs production depend largely on the growth conditions of bacteria (oxygen stress, antibiotic pressure, and envelope stress) (Mcbroom et al. 2007; Kulp et al. 2015). It was observed that stress (physical and chemical) increases the number of secreted OMVs. The E. coli hyper-vesiculating yieM mutant (ΔyieM) grown on the medium containing polymyxin B exhibited survival rate higher than the wild type strain, (Manning and Kuehn 2011, Manning and Kuehn 2013). Mac-Donald and Kuehn also concluded that polymyxin B treatments increased OMVs formation by P. aeruginosa PA14 strain (MacDonald and Kuehn 2013).
OMVs release was also increased under oxygen stress (15% O2 instead of a physiological level of 6% O2), albeit nonsignificantly. Our results are consistent with the data obtained for other bacterial species. P. aeruginosa cells, treated with ciprofloxacin (an antibiotic that leads to DNA damage and, as a consequence, to activation of the of SOS system), responded with increased production of OMVs. Moreover, MacDonald and Kuehn (2013) found that OMVs production significantly increased after hydrogen peroxide treatment. Also, physiological stress increased OMVs production. The same authors have also shown that temperature change and oxidative stress-stimulated OMVs production by P. aeruginosa (MacDonald and Kuehn 2013).
We further explored the relationship between OMVs production level and mutations in the htrA and dsbI genes of C. jejuni. Surprisingly, we haven’t noticed any significant changes (Fig. 1).
Qualitative content of OMV proteome. We have made the identification and quantitative analysis of the protein contents of C. jejuni OMVs using the LC-MS-MS/MS analysis. OMVs were isolated from the wild-type strain 81–176 grown in optimal and under the stress conditions (increased oxygen content, presence of polymyxin B). We also analyzed the OMVs from C. jejuni strains with mutations in the htrA and dsbI genes. To ensure reproducibility, each analysis was repeated four times.
Irrespectively of the factors used (polymyxin B, oxygen content, ΔhtrA and ΔdsbI mutations), the qualitative protein composition of the OMVs in our study matched the previously presented for the unstressed wt strain (Elmi et al. 2012). However, we discovered numerous quantitative differences described and discussed below.
Polymyxin B influence on the quantitative composition of OMVs. C. jejuni exhibits intrinsic resistance to polymyxin B, presumably due to the absence of the appropriate targets and/or low-affinity binding to targets (Iovine 2013). We used a relatively high concentration of antibiotic to induce the stress (~15 times higher concentration than in Campylobacter Selective Supplement). We noticed that four proteins exhibited increased level within OMVs and one showed a decrease (Table II). The highest, nine-fold increase was noted for the major outer membrane protein (MOMP) encoded by the porA gene. Overexpression of MOMP alters membrane permeability and plays a role in Campylobacter resistance against antibiotics, probably also against polymyxin B. MOMP elevation in OMVs was observed also under aerobic stress, consistent with the study demonstrating upregulation of MOMP expression in Campylobacter subjected to low and high oxygen level (1.88% and > 15%, respectively) (Guccione et al. 2017).
Table II.
Up- and downregulated proteins under the polymyxin B and oxidative stress and in the Δdsbl mutant background (the protein numeration of C. jejuni NCTC11168 strain).
| Protein / Gene | Number of peptides | q-value | Fold* |
|---|---|---|---|
| Strain 81–176 wt vs. strain 81–176 grown under oxidative stress conditions | |||
| Major outer membrane protein (porA, Cj1259) | 56 | 0.00038 | +1.96 |
| Flagellin A (flaA, Cj1339c) | 25 | 0.00494 | +2.18 |
| Iron ABC transporter, periplasmic iron-binding protein (Cj0175c) | 29 | 0.00507 | +1.83 |
| Putative lipoprotein (Cj1090c) | 7 | 0.00627 | −4.07 |
| Strain 81–176 wt vs. strain 81–176 grown under the polymyxin B stress | |||
| Flagellin B (flaB, Cj1338c) | 83 | 0.00021 | +4.57 |
| Major outer membrane protein (porA, Cj1259) | 60 | 0.00021 | +9.09 |
| Serine protease, protease DO (htrA, Cj1228c) | 55 | 0.01684 | −3.14 |
| Flagellar hook protein (flgE, Cj1729c) | 34 | 0.019 | +4.67 |
| Putative pyridoxamine 5-phosphate oxidase (Cj1613c) | 7 | 0.08 | +6.16 |
| Strain 81–176 wt vs. strain 81–176 dsbI– | |||
| 60 kDa chaperonin (groL, Cj1221) | 95 | 0.00040 | −4.45 |
| Fagellar hook-associated protein (flgL, Cj0887c) | 20 | 0.003 | +8.81 |
| Enolase (Cj1672c) | 27 | 0.02 | −2.16 |
| Coproporphyrinogen-III oxidase (hemN, Cj0992c) | 10 | 0.038 | +10.68 |
| Flagellar basal body rod protein (flgC, Cj0527c) | 18 | 0.042 | −2,49 |
| Flagellar hook protein (flgE, Cj1729c) | 33 | 0.059 | +2.7 |
| Anthranilate synthase subunit I (trpE, Cj0345) | 18 | 0.06 | −2.7 |
| Fumarate hydratase class II (fumC, Cj1364c) | 34 | 0.09 | −2.3 |
| Strain 81–176 wt vs. strain 81–176 htrA– | |||
| No significant changes noted | |||
Positive values correspond to the fold of higher abundance in the stress conditions and negative values correspond to the fold of lower abundance in the stress conditions for each spot.
Another highly upregulated protein (over 6-fold) was a putative pyridoxamine 5’-phosphate oxidase (Cj1613c), predicted to catalyze the terminal step in de novo vitamin B6 synthesis: oxidation of pyridoxamine-5-P (PMP) and pyridoxine-5-P (PNP) to pyridoxal-5-P (https://www.ebi.ac.uk/interpro/entry/IPR024029, InterPro, Protein sequence analysis & classification). PMP is a coenzyme required for the biosynthesis of deoxysugars, which attach to lipopolysaccharides (LPS), the major integral components of the outer membrane of Gram-negative bacteria (Romo and Liu 2011). Thus, the overexpression of Cj1613c may be linked to the destabilization of the bacterial envelope. Also, vitamin B6 itself was implicated in oxidative stress responses (Bilski et al. 2000).
Additionally, two flagellar proteins (FlaB and FlgE) were overrepresented. The destabilization of bacterial envelope may affect the integrity of flagella and result in an elevation of flagellar proteins in OMV. Of note, synthesis of flagella proteins and the ability to form flagella affects the production of OMVs by E. coli W3110 (Manabe et al. 2013).
The underrepresented protein was identified as HtrA, which is surprising and resonates with the observed lack of effect of htrA mutation on the OMVs production and composition (see ‘Genetic factors...’ for a joint discussion).
Oxygen influence on the quantitative composition of OMVs. Oxidative stress resulted in an elevation of the level of three proteins and a decrease of the level of one within Campylobacter OMVs (Table II). MOMP and flagellar proteins were again among overrepresented, perhaps embodying a general response to the environmental stress. The third upregulated protein was the periplasmic iron-binding protein (Cjj81176_0211). While the iron uptake should be tightly controlled to avoid the iron-associated oxidative-stress-mediated damage to the cell, the changes in the Cjj81176_0211 expression were subtle and more studies are needed to unequivocally claim their impact (Holmes et al. 2005).
Genetic factors and the quantitative composition of OMVs. The mechanism of OMVs biogenesis remains unclear, but many studies suggest that the vesiculation has a genetic basis. Recently nearly 150 genes were implicated in the E. coli OMVs production, leading to the conclusion that the surface-exposed oligosaccharides negatively affect the vesiculation and an intact oxidative stress response is required for the wild type vesiculation (Kulp et al. 2015). Roier et al. (2016) have recently shown the role of the vacJ/yrb genes in the OMVs biogenesis. They found that mutations within these genes that make up ABC (ATP-binding cassette) transport system increase the OMVs production in Haemophilus influenzae and Vibrio cholerae. The disruption of both or one of these genes entails phospholipid accumulation in the outer leaflet of OM which initiates an outward bulging of OM. Further accumulation supports the budding of the OM, which leads to the formation and release of OMVs (Roier et al. 2016).
One of the first identified over-vesiculating mutants was the E. coli degP mutant. The degP homolog of C. jejuni, htrA, is vital for heat tolerance, bacterial invasion, and transmigration (Backert et al. 2018). It encodes a protein with proteolytic and chaperone activity, known to participate in periplasmic protein quality control in stress response (Boehm et al. 2018) (see also ‘Introduction’). Unexpectedly, HtrA was underrepresented in OMVs from Campylobacter upon the polymyxin B stress (Table II). It thus seems that HtrA is not involved in the envelope stress response, warranting further investigations. Moreover, the htrA mutation neither significantly increased the OMVs production (Fig. 1) nor altered the OMV composition (Table II). In contrast, DegP in Vibrio cholerae was shown to affect the level of nine proteins in OMVs (Altindis et al. 2014). However, most of these proteins were involved in biofilm formation, a phenomenon not observed in the present study due to the culture conditions.
Interestingly, C. jejuni and Helicobacter pylori have recently been shown to actively secrete HtrA to cleave host cell junctional proteins such as E-cadherin (Hoy et al. 2010; Boehm et al. 2018). Others and we (Elmi et al. 2012) identified HtrA protein inside OMVs and the 11168H htrA mutant exhibited reduced proteolytic activity of OMVs (Elmi et al. 2018). In light of the current study, the latter may be largely due to the lack of HtrA protein itself.
The second gene we analyzed was dsbI, a rare variant of the DsbB paralog identified by our group (Raczko et al. 2005) and absent in E. coli. It belongs to C. jejuni Dsb (disulfide bond) system, which catalyzes the formation of disulfide bridges in extracytoplasmic proteins, stabilizing their tertiary and quaternary structures. Disturbances of Dsb cause protein misfolding and formation of insoluble aggregates accumulating in the periplasm. Under such circumstances, cells may increase the formation of vesicles as a protective mechanism. We observed that DsbI activity in C. jejuni manifests only if DsbB is inactivated, but still none of the DsbI substrates or redox partners are known. We hypothesized that DsbI is more specific than DsbB and acts only on a subset of DsbB substrates (Raczko et al. 2005). So, we were looking for substrates, which are affected by DsbI itself. We showed that mutation of dsbI (as well as htrA) of C. jejuni does not alter the amount of OMVs produced (Fig. 1), but our study revealed eight proteins, whose content in OMVs is significantly altered by dsbI mutation (Table II). Most of them possess one or more cysteine residues and may be able to form intra- or intermolecular bridges. Particularly noteworthy is the increase in the level of flagellar proteins (FlgC, FlgE and FlgL), observed also in response to the environmental stress. Our previous data revealed that double ΔdsbIΔdsbB mutant was completely nonmotile, whereas motility of a ΔdsbI mutant was comparable to the wild strain (Raczko et al. 2005).
Further two alterations comprised decreased content of 60 kDa GroL chaperonin and enolase, two of 12 most immunoreactive proteins possibly involved in the development of Guillain-Barré syndrome (GBS) (Loshaj-Shala et al. 2018). GroL and enolase reside in the cytoplasm, therefore OMVs may provide for their export. Further, we saw an overrepresentation of coproporphyrinogen-III oxidase, which catalyzes the conversion of coproporphyrinogen-III to protoporphyrinogen-IX during the heme biosynthesis. These three changes remain completely unclear.
Further studies are required to fully explain the production and function of OMVs and to provide us with new methods to control bacterial growth and host invasion.
Summarizing, we characterized how the proteome of secreted OMVs changed when the C. jejuni 81–176 strain was exposed to stress induced by the antibiotic polymyxin B or by increased oxygen levels. Our study is one of the few presenting such a comprehensive approach to the problem. Other studies in this field focused on particular genes and proteins, showing the interdependence of their levels, the total OMVs production and stress conditions. Thanks to our complete methodology, we identified all proteins, whose level in OMVs is altered by the conditions applied.
Several of the proteins identified, mostly upregulated ones, were components of the flagella apparatus. Presence of flagella protein in OMVs is not extraordinary. Manabe et al. (2013) reported that both the synthesis of flagella proteins and the ability to form flagella affects the production of OMVs by E. coli W3110.
In the study similar and somewhat complementary to ours, Chan et al. analyzed the OMV proteome of two clinical extraintestinal pathogenic E. coli (ExPEC) isolates under the stress conditions of the limited iron availability and the presence of the antibiotic gentamicin. Low iron environment resulted in major changes in the OMV proteome, including the upregulation of several stress response proteins (Hsp100/Clp proteins, ClpX and ClpA, the AAA+ protease, ClpP, which they interact with, and also ClpB) (Chan et al. 2017).
At present, we can propose neither causes nor consequences of up- or downregulation of certain proteins (coproporphyrinogen-III oxidase (hemN Cj0992c) or unknown putative lipoprotein), indicating that further research in this field is needed.
Further investigation of the regulation of C. jejuni OMVs’ composition combined with their role in virulence will allow us to better understand the pathomechanisms of the infection.
Acknowledgements
We thank Dr. L. Trzeciak for his critical reading of the manuscript.
Footnotes
Funding
This work was supported by the PARENT BRIDGE programme of the Foundation for Polish Science, co-financed from the European Union under the European Regional Development Fund [project POMOST/2012-6/4].
Conflict of interest
The authors do not report any financial or personal connections with other persons or organizations, which might negatively affect the contents of this publication and/or claim authorship rights to this publication.
ORCID
Renata Godlewska 0000-0001-7810-8482
Literature
- Altindis E, Fu Y, Mekalanos JJ.. Proteomic analysis of Vibrio cholerae outer membrane vesicles. Proc Natl Acad Sci USA. 2014. Apr 15;111(15):E1548–E1556. doi: 10.1073/pnas.1403683111 Medline [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backert S, Bernegger S, Skórko-Glonek J, Wessler S.. Extracellular HtrA serine proteases: an emerging new strategy in bacterial pathogenesis. Cell Microbiol. 2018. Jun;20(6):e12845. doi: 10.1111/cmi.12845 Medline [DOI] [PubMed] [Google Scholar]
- Bakun M, Karczmarski J, Poznanski J, Rubel T, Rozga M, Malinowska A, Sands D, Hennig E, Oledzki J, Ostrowski J, et al.. An integrated LC-ESI-MS platform for quantitation of serum peptide ladders. Application for colon carcinoma study. Proteomics Clin Appl. 2009. Aug;3(8):932–946. doi: 10.1002/prca.200800111 Medline [DOI] [PubMed] [Google Scholar]
- Bilski P, Li MY, Ehrenshaft M, Daub ME, Chignell CF.. Vitamin B6 (pyridoxine) and its derivatives are efficient singlet oxygen quenchers and potential fungal antioxidants. Photochem Photobiol. 2000;71(2):129–134. doi: Medline [DOI] [PubMed] [Google Scholar]
- Boehm M, Simson S, Escher U, Schmidt A-M, Bereswill S, Tegtmeyer N, Backert S, Heimesaat MM.. Function of serine protease HtrA in the lifecycle of the foodborne pathogen Campylobacter jejuni. Eur J Microbiol Immunol. 2018. Jul 17;8(3):70–77. doi: 10.1556/1886.2018.00011 Medline [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brøndsted L, Andersen MT, Parker M, Jørgensen K, Ingmer H.. The HtrA protease of Campylobacter jejuni is required for heat and oxygen tolerance and for optimal interaction with human epithelial cells. Appl Environ Microbiol. 2005. Jun 01;71(6):3205–3212. doi: 10.1128/AEM.71.6.3205-3212.2005 Medline [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan KW, Shone C, Hesp JR.. Antibiotics and iron-limiting conditions and their effect on the production and composition of outer membrane vesicles secreted from clinical isolates of extraintestinal pathogenic E. coli. Proteomics Clin Appl. 2017. Jan;11(1-2):1600091. doi: 10.1002/prca.201600091 Medline [DOI] [PubMed] [Google Scholar]
- Chutkan H, MacDonald I, Manning A, Kuehn MJ.. Quantitative and qualitative preparations of bacterial outer membrane vesicles. Methods Mol Biol. 2013;966:259–272. doi: 10.1007/978-1-62703-245-2_16 Medline [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmi A, Dorey A, Watson E, Jagatia H, Inglis NF, Gundogdu O, Bajaj-Elliott M, Wren BW, Smith DGE, Dorrell N.. The bile salt sodium taurocholate induces Campylobacter jejuni outer membrane vesicle production and increases OMV-associated proteolytic activity. Cell Microbiol. 2018. Mar;20(3):e12814. doi: 10.1111/cmi.12814 Medline [DOI] [PubMed] [Google Scholar]
- Elmi A, Watson E, Sandu P, Gundogdu O, Mills DC, Inglis NF, Manson E, Imrie L, Bajaj-Elliott M, Wren BW, et al.. Campylobacter jejuni outer membrane vesicles play an important role in bacterial interactions with human intestinal epithelial cells. Infect Immun. 2012. Dec;80(12):4089–4098. doi: 10.1128/IAI.00161-12 Medline [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godlewska R, Kuczkowski M, Wyszyńska A, Klim J, Derlatka K, Woźniak-Biel A, Jagusztyn-Krynicka EK.. Evaluation of a protective effect of in ovo delivered Campylobacter jejuni OMVs. Appl Microbiol Biotechnol. 2016. Oct;100(20):8855–8864. doi: 10.1007/s00253-016-7699-x Medline [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guccione EJ, Kendall JJ, Hitchcock A, Garg N, White MA, Mulholland F, Poole RK, Kelly DJ.. Transcriptome and proteome dynamics in chemostat culture reveal how Campylobacter jejuni modulates metabolism, stress responses and virulence factors upon changes in oxygen availability. Environ Microbiol. 2017. Oct;19(10): 4326–4348. doi: 10.1111/1462-2920.13930 Medline [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes K, Mulholland F, Pearson BM, Pin C, McNicholl-Kennedy J, Ketley JM, Wells JM.. Campylobacter jejuni gene expression in response to iron limitation and the role of Fur. Microbiology. 2005. Jan 01;151(1):243–257. doi: 10.1099/mic.0.27412-0 Medline [DOI] [PubMed] [Google Scholar]
- Hoy B, Löwer M, Weydig C, Carra G, Tegtmeyer N, Geppert T, Schröder P, Sewald N, Backert S, Schneider G, et al.. Helicobacter pylori HtrA is a new secreted virulence factor that cleaves E-cadherin to disrupt intercellular adhesion. EMBO Rep. 2010. Oct;11(10):798–804. doi: 10.1038/embor.2010.114 Medline [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iovine NM.. Resistance mechanisms in Campylobacter jejuni. Virulence. 2013. Apr;4(3):230–240. doi: 10.4161/viru.23753 Medline [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulp AJ, Sun B, Ai T, Manning AJ, Orench-Rivera N, Schmid AK, Kuehn MJ.. Genome-wide assessment of outer membrane vesicle production in Escherichia coli. PLoS One. 2015. Sep 25;10(9): e0139200. doi: 10.1371/journal.pone.0139200 Medline [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loshaj-Shala A, Colzani M, Brezovska K, Poceva Panovska A, Suturkova L, Beretta G.. Immunoproteomic identification of antigenic candidate Campylobacter jejuni and human peripheral nerve proteins involved in Guillain-Barré syndrome. J Neuroimmunol. 2018. Apr;317:77–83. doi: 10.1016/j.jneuroim.2018.01.006 Medline [DOI] [PubMed] [Google Scholar]
- MacDonald IA, Kuehn MJ.. Stress-induced outer membrane vesicle production by Pseudomonas aeruginosa. J Bacteriol. 2013. Jul 01; 195(13): 2971–2981. doi: 10.1128/JB.02267-12 Medline [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinowska A, Kistowski M, Bakun M, Rubel T, Tkaczyk M, Mierzejewska J, Dadlez M.. Diffprot – software for non-parametric statistical analysis of differential proteomics data. J Proteomics. 2012. Jul; 75(13):4062–4073. doi: 10.1016/j.jprot.2012.05.030 Medline [DOI] [PubMed] [Google Scholar]
- Manabe T, Kato M, Ueno T, Kawasaki K.. Flagella proteins contribute to the production of outer membrane vesicles from Escherichia coli W3110. Biochem Biophys Res Commun. 2013. Nov;441(1):151–156. doi: 10.1016/j.bbrc.2013.10.022 Medline [DOI] [PubMed] [Google Scholar]
- Manning AJ, Kuehn MJ.. Contribution of bacterial outer membrane vesicles to innate bacterial defense. BMC Microbiol. 2011; 11(1):258. doi: 10.1186/1471-2180-11-258 Medline [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning AJ, Kuehn MJ.. Functional advantages conferred by extracellular prokaryotic membrane vesicles. J Mol Microbiol Biotechnol. 2013;23(1-2):131–141. doi: 10.1159/000346548 Medline [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBroom AJ, Johnson AP, Vemulapalli S, Kuehn MJ.. Outer membrane vesicle production by Escherichia coli is independent of membrane instability. J Bacteriol. 2006. Aug 01;188(15):5385–5392. doi: 10.1128/JB.00498-06 Medline [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBroom AJ, Kuehn MJ.. Release of outer membrane vesicles by Gram-negative bacteria is a novel envelope stress response. Mol Microbiol. 2007. Jan;63(2):545–558. doi: 10.1111/j.1365-2958.2006.05522.x Medline [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raczko AM, Bujnicki JM, Pawlowski M, Godlewska R, Lewandowska M, Jagusztyn-Krynicka EK.. Characterization of new DsbB-like thiol-oxidoreductases of Campylobacter jejuni and Helicobacter pylori and classification of the DsbB family based on phylogenomic, structural and functional criteria. Microbiology. 2005. Jan 01;151(1):219–231. doi: 10.1099/mic.0.27483-0 Medline [DOI] [PubMed] [Google Scholar]
- Roier S, Zingl FG, Cakar F, Durakovic S, Kohl P, Eichmann TO, Klug L, Gadermaier B, Weinzerl K, Prassl R, et al.. A novel mechanism for the biogenesis of outer membrane vesicles in Gram-negative bacteria. Nat Commun. 2016. Dec;7(1):10515. doi: 10.1038/ncomms10515 Medline [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romo AJ, Liu H.. Mechanisms and structures of vitamin B6-dependent enzymes involved in deoxy sugar biosynthesis. Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics. 2011. Nov; 1814(11):1534–1547. doi: 10.1016/j.bbapap.2011.02.003 Medline [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwechheimer C, Kuehn MJ.. Outer-membrane vesicles from Gram-negative bacteria: biogenesis and functions. Nat Rev Microbiol. 2015. Oct;13(10):605–619. doi: 10.1038/nrmicro3525 Medline [DOI] [PMC free article] [PubMed] [Google Scholar]


