Abstract
The gold standard in microbiological diagnostics of bacteremia is a blood culture in automated systems. This method may take several days and has low sensitivity. New screening methods that could quickly reveal the presence of bacteria would be extremely useful. The objective of this study was to estimate the effectiveness of these methods with respect to blood cultures in the context of antibiotic therapy. Blood samples from 92 children with sepsis were analyzed. Blood cultures were carried out in standard automated systems. Subsequently, FISH (Fluorescent In-Situ Hybridization) and nested multiplex-real-time-PCR (PCR) were performed. Blood cultures, FISH and PCR yielded positive results in 18%, 39.1%, and 71.7% of samples, respectively. Significant differences were found between the results obtained through culture before and after induction of antibiotherapy: 25.5% vs. 9.7%. There was no significant difference in FISH and PCR results in relation to antibiotics. The three methods employed demonstrated significant differences in detecting bacteria effectively. Time to obtain test results for FISH and PCR averaged 4–5 hours. FISH and PCR allow to detect bacteria in blood without prior culture. These methods had high sensitivity for the detection of bacteremia regardless of antibiotherapy. They provide more timely results as compared to automated blood culture, and may be useful as rapid screening tests in sepsis.
Key words: antibiotic therapy, FISH, PCR, sepsis
Introduction
Bacteremia is defined as a confirmed presence of one or more bacterial species in the blood (Albur et al. 2016). Sepsis is defined as a life-threatening organ dysfunction resulting from an impaired regulation of the response to an infection caused mainly by bacteremia. Criteria of organ dysfunction are based on Sepsis Related Organ Failure Assessment (SOFA) and quick Sepsis Related Organ Failure Assessment (qSOFA) scores. In the case of suspicion of a systemic infection, sepsis is diagnosed given ≥ 2 points in SOFA score or in the presence of ≥ 2 clinical signs in qSOFA score, including low blood pressure (SBP ≤ 100 mmHg), high respiratory rate (≥ 22 breaths/min) or altered mental status (Glasgow coma scale < 15). To give a diagnosis, systemic inflammatory response syndrome (SIRS) criteria still come as useful, including leucocytosis, and also CRP and procalcitonin level.
Current recommendations are unfortunately only related to adults (Singer et al. 2016). The pediatric criteria thus far, largely based on SIRS, met with a lot of criticism similarly to adult criteria, since sepsis in children differs when it comes to clinical signs and symptoms, lab results and management (Goldstein et al. 2005; Churpek et al. 2015; Kaukonen et al. 2015). Implementation of new sepsis recommendations in pediatrics needs standardization of organ failure depending on age, which is especially difficult owing to the specific character of childhood diseases.
At present, it is considered that sepsis is the main cause of death in children globally (Dugani and Kissoon 2017). Epidemiologic data are incomplete, but it is estimated that infectious conditions are responsible for approximately 40% of deaths in children below 5 years of age. In 2015, the World Health Organization (WHO) has announced four main causes of death due to infectious conditions in children: pneumonia (16%), diarrhea (9%), neonatal sepsis (7%), malaria (5%), followed by measles (1%) and HIV/AIDS (1%) (WHO 2017).
Owing to limitations of the diagnostic criteria, it is still prevalent to identify the blood infection microbiologically, in order to definitely confirm sepsis.
The current “gold standard” of microbiological sepsis diagnostics is blood culture in automated systems. This method is of relatively small efficiency due to the low number of pathogens in the blood and the fact that it is time-consuming (up to 72 h), with 15–20% of results being positive (Jamal et al. 2006). Microbial growth inhibitors may additionally impede or delay the detection of microorganisms; therefore, it is recommended to administer adequate antibiotic therapy after blood for microbiological testing has been collected, but only if such collection will not significantly delay treatment (> 45 min). Lack of specific pathogen identification yields empirical therapy. Ineffectiveness of such treatment may be life threatening or leads to permanent multi-organ dysfunction and failure to thrive.
There are ongoing efforts to improve the efficacy of microbial identification with alternative diagnostic techniques, independent of antibiotic therapy, such as serological (detection of lipopolysaccharide (LPS) of Gram-negative bacteria or mannan and galactomannan of fungi) or molecular methods FISH and PCR, detecting the DNA or RNA of pathogens in the blood. Unfortunately, molecular methods are costly, need special laboratory apparatuses but are quick (3–4 h) and sensitive (Klouche and Schröder 2008; Gosiewski, Jurkiewicz-Badacz, et al. 2014; Źródłowski et al. 2017). Identification of the etiological agent and prompt targeted therapy could lower mortality and permanent health consequences in patients.
The aim of the study was the evaluation of efficacy of FISH, PCR and blood culture in detection of the etiological factor of bacteremia in children and adolescents, before and after antibiotic treatment.
Experimental
Materials and Methods
Blood samples from 92 children and adolescents aged from 1 week to 18 years of age (mean age 4.7 years; standard deviations (SD) 3.54), with the clinical symptoms and blood lab results of sepsis, were analysed. Patients in the age group 2 to 5 years dominated (45%), followed by neonates (23.4%).
Blood samples originated from the Department of Neuroinfections and Pediatric Neurology as well as from the Department of Pediatric Infectious Diseases of the John Paul II Specialist Hospital in Cracow in the years 2010–2012 (30 months) according to SIRS criteria. The study was approved by the Bioethical Committee from the Regional Chamber of Physicians in Cracow, decision no. 30/KBL/OIL/2010 from 17 March 2010.
Inclusion criteria (at least two were to be met) (Levy et al. 2003) were as follows: i) body temperature over 38.5°C or below 36.0°C (rectal or oral); ii) leukocytosis over 2 SD for the given age; iii) clinical signs and symptoms or results of additional studies suggesting an infection (organ abscess, leukocytes present in physiologically sterile fluids, radiological evidence of pneumonia, hemorrhagic eruption), and iv) C-reactive protein (CRP) exceeding 50 IU/ml.
The patients excluded from the study group consisted of those that did not fulfil the inclusion criteria, or no permission was obtained from their lawful caretakers. Also excluded were the patients whose blood samples were of too small volume to conduct at least one of the test methods. Eventually, 92 patients were included into the study.
Microbiological examinations. Two blood samples were taken from each patient. The samples came from a separate puncture of a different vein. Sample volume depended on the patient’s age. In neonates: 1–2 ml of blood was collected, in children from 1 month to 2 years of age: 2–3 ml, in older children: 3–5 ml, in teenagers: 10 ml. Blood samples underwent standard microbiological diagnostics based on the BactAlert (bioMérieux, Marcy l’Etoile, France) automated blood culture system in the Microbiology Laboratory of the John Paul II Specialist Hospital in Cracow. The incubation time used for detection of bacteria in automated blood culture was 7 days.
Molecular studies. Parallel molecular studies were performed on the blood samples using FISH and PCR. In order to perform molecular studies, 1.5 ml of blood was collected to Vacutainer K3EDTA tubes (BectonDickinson, Warsaw, Poland) from all patients, irrespective of age. Samples were frozen to –70°C, and then transferred to the Chair of Microbiology of Jagiellonian University Medical College in Cracow.
Microbial DNA isolation from blood. With the aim of determining the sensitivity of the PCR method, microbial DNA was isolated from 1.5-ml blood samples. DNA isolation was carried out according to the method described by Gosiewski et al. with the employment of a ready-to-use Blood Mini (A&A Biotechnology) kit (Gosiewski, Szała, et al. 2014).
PCR amplification. All the processes of DNA amplification were performed with the use of the real-time PCR method (qPCR) in a CFX96 thermal cycler (Bio-Rad) by employing the species-specific starters and TaqMan probes (Table IV), according to the procedure by Gosiewski et al. (Gosiewski, Flis, et al. 2014). Additionally, β-actin gene detection was performed in every sample of DNA isolated from blood in order to verify whether PCR inhibition takes place (Gosiewski, Jurkiewicz-Badacz, et al. 2014).
Table IV.
Sequences of primers and probes (Genomed) utilized in this study.
| Amplification | Oligonucleotide | 5’-3’ | Origin | Target sequences |
|---|---|---|---|---|
| Bacteria | EXT_BAC_F | kGCGrACGGGTGAGTAA | (Gosiewski, Jurkiewicz-Badacz, et al. 2014) | 16S rRNA |
| EXT_BAC_R | CGCATTTCACCGCTA | |||
| *GN/GP_F | GACTCCTACGGGAGGC | (Bispo et al. 2011) | ||
| *GN/GP_R | GCGGCTGCTGGCAC | |||
| GP_Probe | Hex – CTGAyssAGCAACGCCGCG – TAMRA (Q) | |||
| GN_Probe | Cy5 – CCTGAysCAGCmATGCCGCG – BHQ-2 | |||
| β-actin gene (amplification inhibition control) | F | GCCAGTGCCAGAAGAGCCAA | (Valle Jr et al. 2010) | Human β-actin gene |
| R | TTAGGGTTGCCCATAACAGC | |||
| FISH | STA | CY3 – TCCTCCATATCTCTGCGC | (Kempf et al. 2000) | Staphylococcus spp. – 16S rRNA |
| ENT 183 | CY3-5’ – CTCTTTGGTCTTGCGACG | (Friedrich et al. 2003) | Enterobacteriaceae 16S rRNA | |
| EUB338 | FITC – GCTGCCTCCCGTAGGAGT – FITC | (Amann et al. 1990) | All bacteria – 16S rRNA |
FISH method. 200 µl of blood was subjected to preparation using 0.17 M of ammonium chloride solution (ICN Biomedicals) as in the case of preparing blood samples for DNA isolation until a pale pink pellet was obtained. The pellet was suspended in 20 µl of sterile deionized water from which 10 µl was transferred onto the surface of SuperFrost®Plus (Menzel-Glaser) microscope slide for hybridization in order to get a smear of approximately 10 mm in diameter. The preparation was dried under laminar flow and subsequently poured over with 500 µl of 4% paraformaldehyde (Sigma) solution and incubated for 20 min at 4°C. Then, the preparation was washed with PBS and poured over with 2 ml of 96% methanol (POCh). The whole specimen was kept under cover for 15 min at 20°C. Upon completion of the fixation process, methanol was washed off with warm (37°C) PBS solution and placed on 20 µl of diluted solution of lysozyme (1 mg/ml) (Sigma) and lysostaphin (0.05 mg/ml) (Sigma). It was incubated for 5 min at 37°C and then washed twice with sterile deionized water. Hybridization was performed with the use of single-chain oligonucleotide probes (Table IV) labelled with fluorochromes at 5’ ends, targeted at the 16S rRNA conservative fragment typical for the studied group of bacteria, according to the protocol published by Gosiewski et al. (Gosiewski, Flis, et al. 2014).
Statistical methods. Chi-squared test, Mann-Whitney U test and analysis of variance (ANOVA) were used to compare the effect of antibiotic therapy on the effectiveness of culture, PCR and FISH. Fisher’s exact test, two-tailed, was used to compare the effectiveness of methods (Gretl software ver. 1.9.4.). Verification of statistical hypotheses was performed at a significance level of p < 0.05.
Results
Data shown in Table I demonstrate that among the patients (n = 92) with blood samples collected and cultures performed before administration of antibiotics (n = 51), 25.5% of the samples were positive while 74.5% were negative. In the case of patients who had antibiotics administered prior to blood collection and blood cultures (n = 41), only 9.7% of the samples were positive and 90.3% negative – this difference was statistically significant. Gram-positive and Gram-negative bacteria constituted 47% (n = 8) and 53% (n = 9), respectively. In total, positive cultures were obtained from 18.5% (n = 17) of patients.
Table I.
Antibiotic therapy vs. blood culture results.
| Blood culture | Antibiotic therapy prior to blood collection | |
| n | % | |
| Negative | 37 | 90.3 |
| Positive | 4 | 9.7 |
| Total | 41 | 100.0 |
| Blood culture | Antibiotic therapy prior to blood collection | |
| n | % | |
| Negative | 38 | 74.5 |
| Positive | 13 | 25.5 |
| Total | 51 | 100.0 |
| Blood culture | Total | |
| n | % | |
| Negative | 75 | 81.5 |
| Positive | 17 | 18.5 |
| Total | 92 | 100.0 |
chi2 = 5.768; p = 0.024; Vc = 0.23; Results significant: p < 0.05
FISH results were positive in 36 out of 92 (39.1%) patients and demonstrated the presence of Gram-negative rods (n = 4), which constituted 11.1%, Gram negative coccus (n = 4) – 11.1% and Gram-positive coccus (n = 28) – 77.8% of all the bacteria detected.
Table II shows the relationship between antibiotic therapy and the FISH results. Chi-squared test did not show any statistically significant differences between the attributes studied.
Table II.
Antibiotic therapy vs. FISH results.
| FISH | Antibiotic therapy prior to blood collection | |
| n | % | |
| Negative | 31 | 63.3 |
| Positive | 18 | 36.7 |
| Total | 49 | 100.0 |
| FISH | Antibiotic therapy after blood collection | |
| n | % | |
| Negative | 25 | 58.1 |
| Positive | 18 | 41.9 |
| Total | 43 | 100.0 |
| FISH | Total | |
| n | % | |
| Negative | 56 | 60.9 |
| Positive | 36 | 39.1 |
| Total | 92 | 100.0 |
chi2 = 0.253; not significant
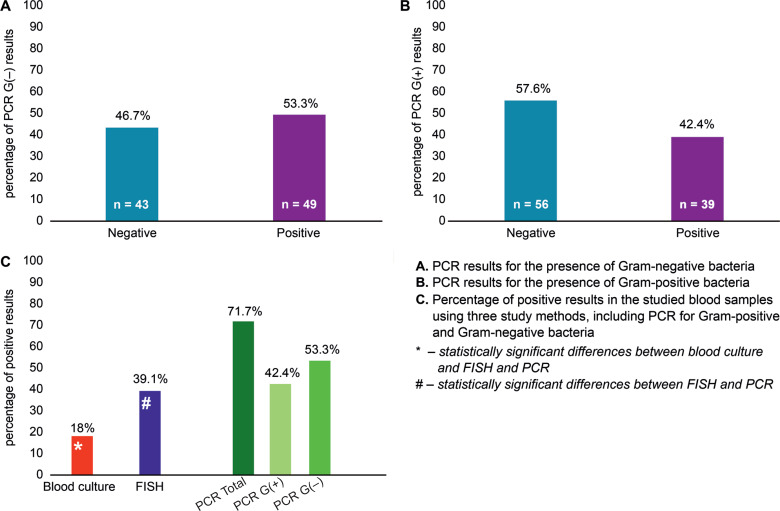
The PCR studies were performed for 92 patients in order to detect the presence of Gram-positive and Gram-negative bacteria. Amplification sensitivity was defined as the relation of the CT value, i.e. the number of reaction cycle in which the linear increase of the product cuts an established baseline 100 RFU (relative fluorescence unit) (Fig. 1). Table III shows the relationship between antibiotic therapy and PCR results. Chisquared test did not show any statistically significant differences between the attributes studied.
Fig. 1.
Results of detection of bacteria using blood culture, PCR and FISH.
Table III.
Antibiotic therapy vs. PCR results.
| PCR | Antibiotic therapy prior to blood collection | |
| n | % | |
| Negative | 12 | 24.5 |
| Positive | 37 | 75.5 |
| Total | 49 | 100.0 |
| PCR | Antibiotic therapy after blood collection | |
| n | % | |
| Negative | 14 | 32.6 |
| Positive | 29 | 67.4 |
| Total | 43 | 100.0 |
| PCR | Total | |
| n | % | |
| Negative | 26 | 28.3 |
| Positive | 66 | 71.7 |
| Total | 92 | 100.0 |
chi2 = 0.731; not significant
Figure 1 demonstrates positive results of the three methods used in this study: blood culture, FISH and PCR. Statistically significant differences were found between the results obtained after blood culture and FISH (F = 0.0, P < 0.05), FISH and PCR (F = 0.0029, P < 0.05) as well as blood culture and PCR (F = 0.0108, P < 0.01). Fungal microorganisms were not found with any of the methods. No significant differences in the effectiveness of the detection of the bacteria by three methods used depended on the age and sex of patients.
Repeatability of results using the three methods (culture, FISH and PCR) amounted to 21 samples (22.8%). Compatibility between blood culture and PCR involved 40 samples (43.5%), between blood culture and FISH: 71 samples (77.1%), while between FISH and PCR it was observed for 50 samples (54.3%).
Discussion
The American College of Critical Care Medicine/Pediatric Advanced Life Support (ACCM/PALS) published sepsis management guidelines. The “sepsis bundles” were proposed to improve treatment efficacy. The main objectives were: hemodynamic stability, treatment of infection and lowering oxygen demand (de Oliveira et al. 2008). According to recommendations, intravenous antimicrobial therapy should be administered as early as possible, i.e. within 1 h of suspecting sepsis or septic shock. In a retrospective study, Kumar et al. (2006) have shown that early administration of appropriate antibiotics improved survival rates in adult patients with septic shock. Weiss et al. (2014) obtained similar results for children.
The success of therapy depends to a big extent on the identification of the etiological factor (Weinstein et al. 1983; Ibrahim et al. 2000; MacArthur et al. 2004). Presently, the “gold standard” for diagnosis of sepsis is a classic blood culture. Surely, the advantages of this method are its simplicity, low cost and the possibility to determine drug sensitivity of pathogens. The disadvantages of the method are the time consumption and low sensitivity. The average time from the blood collection to results is 3–5 days, and the proportion of positive cultures to the total number of cultures performed varies from 15 to 30%, depending on the studies (Jamal et al. 2006; Gosiewski, Flis, et al. 2014). Microbial growth inhibitors in the form of antibiotics play a major role in the negative culture results, what was shown in our study. Positive culture results were obtained from 25.5% of patients without prior antibiotic therapy, and from only 9.7% after antibiotic use (Table I). Owing to such results, the search for new techniques to identify pathogens in the blood is ongoing. FISH and PCR are relatively well known molecular techniques. FISH is generally used to study post-culture media samples (Farina et al. 2012). Presently, the method of sample purification was worked out by Gosiewski et al. and a much clearer high-quality microscopic view was obtained without auto-fluorescence in the background, which allows the blood examination without the need for prior cultures (Gosiewski, Flis, Sroka, Kędzierska, et al. 2014). The PCR method is used, among others, in the commercially available Septi-Fast test (Roche). The study is based on the PCR technique and allows to identify 25 most common microorganisms responsible for over 90% of sepsis cases. In our study, a nested-multiplex real-time PCR method worked out by Gosiewski et al. was used (Gosiewski, Jurkiewicz-Badacz, et al. 2014; Gosiewski, Flis, et al. 2014).
In our study, both molecular methods turned out to be more sensitive and quicker than the classic culture and independent of antibiotic therapy. FISH and PCR were performed in 92 patients and positive results were obtained in 39.1% and 71.7% of patients, respectively, whereas using the classical blood culture approach – only in 18.5% the bacteria were detected In some samples analysed, DNA of Gram-negative and Gram-positive bacteria was isolated simultaneously (42.4% versus 53.3%, respectively). Hence, the sum of results is higher than the positive results of PCR (71.7%). The difference in sensitivity of molecular tests vs. blood cultures results from the fact that cultures detect only living bacteria, which are able to grow, but molecular methods also confirm the presence of DNA that may originate from dead cells (Farina et al. 2012). Repeatability of results using the three methods was 22.8%. The calculation takes into account both consistent positive results as well as consistent negative results. The highest result repeatability (77.1%) was obtained when comparing blood cultures with FISH; therefore, it can be assumed that this analytical method could be the best alternative to blood culture. On the other hand, it should be noted that FISH sensitivity is not high and amounts to circa 103 CFU/ml. Hence, it is possible that it was the reason of the highest compatibility with culture (Gosiewski et al. 2011). Furthermore, high percentage of Gram-positive bacteria may be due to contamination of the sample with skin bacteria during collection or processing, but non-template control (NTC) gave negative results in PCR. Unfortunately, we did not obtained consent of the bioethical commission, especially since the study also involved newborns. Our previous research showed that bacterial DNA could be detected by amplification method in the blood of healthy adult people but its taxonomic composition is completely different from the one seen in septic patients (Gosiewski et al. 2017). Also, these results are comparable to our previous studies (Gosiewski et al. 2005; Źródłowski et al. 2017). In the literature, there are no reports on using FISH to detect bacteria directly in the blood, except for the publications of our team; therefore, we cannot compare our results with other studies.
Molecular diagnostics of sepsis is a very hard task. Unfortunately, the methods of molecular biology encounter limitations while conducting microbiological diagnostic of the blood. The difficulty lies in isolating DNA matrix of a proper quality and of the highest possible concentration. Different species of bacteria, which may be biological agents inducing sepsis (sometimes polietiological) are characterized by varied susceptibility to cell lysis and, consequently, the propensity to efficient DNA isolation. An additional problem is constituted by the fact that the blood contains heme, which is a very strong inhibitor of DNA polymerases used in PCR (Opel et al. 2010). It is still the case that blood culture remains the basic diagnostic method, although theoretically a technical potential exists to detect pathogens with the molecular methods.
Acknowledgments
This study was supported by the Polish Ministry of Science and Higher Education within the framework of a project grant N N401 006739.
Footnotes
Conflict of interest
Author does not report any financial or personal connections with other persons or organizations, which might negatively affect the contents of this publication and/or claim authorship rights to this publication.
Literature
- Albur M, Hamilton F, MacGowan AP. 2016. Early warning score: a dynamic marker of severity and prognosis in patients with Gram-negative bacteraemia and sepsis. Ann Clin Microbiol Antimicrob. 15:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann RI, Binder BJ, Olson RJ, Chisholm SW, Devereux R, Stahl DA. 1990. Combination of 16S rRNA-targeted oligonucleotide probes with flow cytometry for analyzing mixed microbial populations. Appl Environ Microbiol. 56(6):1919–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bispo PJM, de Melo GB, Hofling-Lima AL, Pignatari ACC. 2011. Detection and gram discrimination of bacterial pathogens from aqueous and vitreous humor using real-time PCR assays. Invest Ophthalmol Vis Sci. 52(2):873–881. [DOI] [PubMed] [Google Scholar]
- Churpek MM, Zadravecz FJ, Winslow C, Howell MD, Edelson DP. 2015. Incidence and prognostic value of the systemic inflammatory response syndrome and organ dysfunctions in ward patients. Am J Respir Crit Care Med. 192(8):958–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugani S, Kissoon N. 2017. Global advocacy needed for sepsis in children. J Infect. 74 Suppl 1:S61–S65. [DOI] [PubMed] [Google Scholar]
- Farina C, Perin S, Andreoni S, Conte M, Fazii P, Lombardi G, Manso E, Morazzoni C, Sanna S. 2012. Evaluation of the peptide nucleic acid fluorescence in situ hybridisation technology for yeast identification directly from positive blood cultures: an Italian experience. Mycoses. 55(5):388–392. [DOI] [PubMed] [Google Scholar]
- Friedrich U, Van Langenhove H, Altendorf K, Lipski A. 2003. Microbial community and physicochemical analysis of an industrial waste gas biofilter and design of 16S rRNA-targeting oligonucleotide probes. Environ Microbiol. 5(3):183–201. [DOI] [PubMed] [Google Scholar]
- Goldstein B, Giroir B, Randolph A. 2005. International pediatric sepsis consensus conference: Definitions for sepsis and organ dysfunction in pediatrics*. Pediatr Crit Care Med. 6(1):2–8. [DOI] [PubMed] [Google Scholar]
- Gosiewski T, Flis A, Sroka A, Kędzierska A, Pietrzyk A, Kędzierska J, Drwiła R, Bulanda M. 2014. Comparison of nested, multiplex, qPCR; FISH; SeptiFast and blood culture methods in detection and identification of bacteria and fungi in blood of patients with sepsis. BMC Microbiol. 14:313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosiewski T, Jurkiewicz-Badacz D, Sroka A, Brzychczy-Włoch M, Bulanda M. 2014. A novel, nested, multiplex, real-time PCR for detection of bacteria and fungi in blood. BMC Microbiol. 14:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosiewski T, Kasprzyk A, Strus M. 2005. Comparision of the sensitivity of detection of bacteria in human blood using classic culture methods and molecular techniques: PCR and FISH. Med Dosw Mikrobiol. 57(3):319–325. [PubMed] [Google Scholar]
- Gosiewski T, Ludwig-Galezowska AH, Huminska K, Sroka-Oleksiak A, Radkowski P, Salamon D, Wojciechowicz J, Kus-Slowinska M, Bulanda M, Wolkow PP. 2017. Comprehensive detection and identification of bacterial DNA in the blood of patients with sepsis and healthy volunteers using next-generation sequencing method – the observation of DNAemia. Eur J Clin Microbiol Infect Dis. 36(2):329–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosiewski T, Pietrzyk A, Brzychczy-Wloch M, Heczko P. 2011. Use of PCR and FISH methods for rapid identification of bacterial bloodstream infections. Ann Acad Med Siles. 65(5–6):14–22. [Google Scholar]
- Gosiewski T, Szała L, Pietrzyk A, Brzychczy-Włoch M, Heczko PB, Bulanda M. 2014. Comparison of methods for isolation of bacterial and fungal DNA from human blood. Curr Microbiol. 68:149–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim EH, Sherman G, Ward S, Fraser VJ, Kollef MH. 2000. The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting. Chest. 118(1):146–155. [DOI] [PubMed] [Google Scholar]
- Jamal W, Tamaray G, Pazhoor A, Rotimi VO. 2006. Comparative evaluation of BacT/ALERT 3D and BACTEC systems for the recovery of pathogens causing bloodstream infections. Med Princ Pract. 15(3):223–227. [DOI] [PubMed] [Google Scholar]
- Kaukonen K-M, Bailey M, Pilcher D, Cooper DJ, Bellomo R. 2015. Systemic inflammatory response syndrome criteria in defining severe sepsis. N Engl J Med. 372(17):1629–1638. [DOI] [PubMed] [Google Scholar]
- Kempf VA, Trebesius K, Autenrieth IB. 2000. Fluorescent in situ hybridization allows rapid identification of microorganisms in blood cultures. J Clin Microbiol. 38(2):830–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klouche M, Schröder U. 2008. Rapid methods for diagnosis of bloodstream infections. Clin Chem Lab Med. 46(7):888–908. [DOI] [PubMed] [Google Scholar]
- Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, Suppes R, Feinstein D, Zanotti S, Taiberg L, et al. . 2006. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock*. Crit Care Med. 34(6):1589–1596. [DOI] [PubMed] [Google Scholar]
- Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent J-L, Ramsay G, et al. . 2003. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 31(4):1250–1256. [DOI] [PubMed] [Google Scholar]
- MacArthur RD, Miller M, Albertson T, Panacek E, Johnson D, Teoh L, Barchuk W. 2004. Adequacy of early empiric antibiotic treatment and survival in severe sepsis: Experience from the MONARCS Trial. Clin Infect Dis. 38(2):284–288. [DOI] [PubMed] [Google Scholar]
- de Oliveira CF, de Oliveira DSF, Gottschald AFC, Moura JDG, Costa GA, Ventura AC, Fernandes JC, Vaz FAC, Carcillo JA, Rivers EP, et al. . 2008. ACCM/PALS haemodynamic support guidelines for paediatric septic shock: an outcomes comparison with and without monitoring central venous oxygen saturation. Intensive Care Med. 34(6):1065–1075. [DOI] [PubMed] [Google Scholar]
- Opel KL, Chung D, McCord BR. 2010. A study of PCR inhibition mechanisms using real time PCR. J Forensic Sci. 55(1):25–33. [DOI] [PubMed] [Google Scholar]
- Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche J-D, Coopersmith CM, et al. . 2016. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 315(8):801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valle DL, Andrade JI, Cabrera EC, Rivera WL. 2010. Evaluation of buffy coat 16S rRNA PCR, buffy coat culture and whole blood PCR for detection of bacteraemia. Mem Inst Oswaldo Cruz. 105(2):117–122. [DOI] [PubMed] [Google Scholar]
- Weinstein MP, Reller LB, Murphy JR, Lichtenstein KA. 1983. The clinical significance of positive blood cultures: a comprehensive analysis of 500 episodes of bacteremia and fungemia in adults. I. Laboratory and epidemiologic observations. Rev Infect Dis. 5(1):35–53. [DOI] [PubMed] [Google Scholar]
- Weiss SL, Fitzgerald JC, Balamuth F, Alpern ER, Lavelle J, Chilutti M, Grundmeier R, Nadkarni VM, Thomas NJ. 2014. Delayed antimicrobial therapy increases mortality and organ dysfunction duration in pediatric sepsis*. Crit Care Med. 42(11):2409–2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO 2017. Causes of child mortality Geneva (Switzerland): World Health Organization. [Google Scholar]
- Źródłowski TW, Flis A, Ziętkiewicz M, Drwiła R, Gosiewski T. 2017. Fluorescent in situ hybridization and Gram-stained smears of whole blood as complementary screening tools in the diagnosis of sepsis. Polish Arch Intern Med. 127(2):122–124. [DOI] [PubMed] [Google Scholar]