Abstract
Cerebellar cortex, which is cytoarchitectonically homogenous, can be functionally differentiated by connectivity differences across the cerebral cortex. The cerebral cortical dorsal attention network exhibits strong, selective connectivity with a set of cerebellar circuits, including lobule VIIb/VIIIa. Recent findings demonstrate that lobule VIIb/VIIIa exhibits functional properties characteristic of the cortical dorsal attention network: Task-specific activation; working memory load-dependent responses; and the representation of visuospatial location. Moreover, functional cortico-cerebellar subnetworks exhibit topographic specialization for different aspects of visual attentional processing. Thus, cerebellar lobule VIIb/VIIIa, rather than simply supporting motor functions, appears to be an integral part of the brain’s visual attentional circuitry. More generally, these findings suggest that parallel cortico-cerebellar networks may play highly specific functional roles in a broad range of cognitive processes.
Introduction
Our understanding of the neural substrates of attention and working memory has greatly expanded in recent years. This includes a greater appreciation of subcortical contributions to attentional processing. A limited set of subcortical areas have historically been implicated in visual attention, namely the superior colliculus and pulvinar nucleus [1–3]. The majority of subcortical structures, however, have not been traditionally associated with attentional functions. The cerebellum, in particular, has received little consideration as a locus of attentional control. Rather, substantial evidence primarily links the cerebellum with motor control and coordination [4–8]. However, recent evidence suggests that cerebellar cortex exhibits considerable heterogeneity in its anatomical and functional connections with extracerebellar structures [9,10]. Interestingly, a substantial portion of the cerebellum is found to be connected with portions of cerebral cortex associated with non-motor functions [10]. Consequently, a number of studies have found evidence for cerebellar contributions to a variety of non-motor functions, including attention and working memory (see [11] for an in-depth review of cerebellar involvement in cognitive function more generally; [12–19]). Here, we will review converging evidence from lesion and functional imaging studies for cerebellar contributions to attention and working memory function. In particular, we will summarize recent research indicating that a functional brain network responsible for directing visual attention and working memory processes includes cerebellar structures.
Cerebellar Anatomy and Connectivity
The cerebellum can be grossly subdivided into two hemispheres and a midline region known as the vermis. Each of these structures can be further subdivided into 10 lobules (Figure 1a) [20]. Cerebellar lobules are organized into an anterior lobe (lobule I–V), a posterior lobe (lobule VI–IX), and a flocculonodular lobe (lobule X). All connections between cerebral cortex and cerebellum are polysynaptic. Afferent fibers originating from the cerebral cortex reach cerebellar cortex via the pons or the inferior olivary nucleus. Efferent connections are relayed to the cerebral cortex through the cerebellar nuclei and then the thalamus [4,21–23]. Thus, both afferent and efferent limbs of the cerebrocerebellar circuit consist of two stages.
Figure 1.
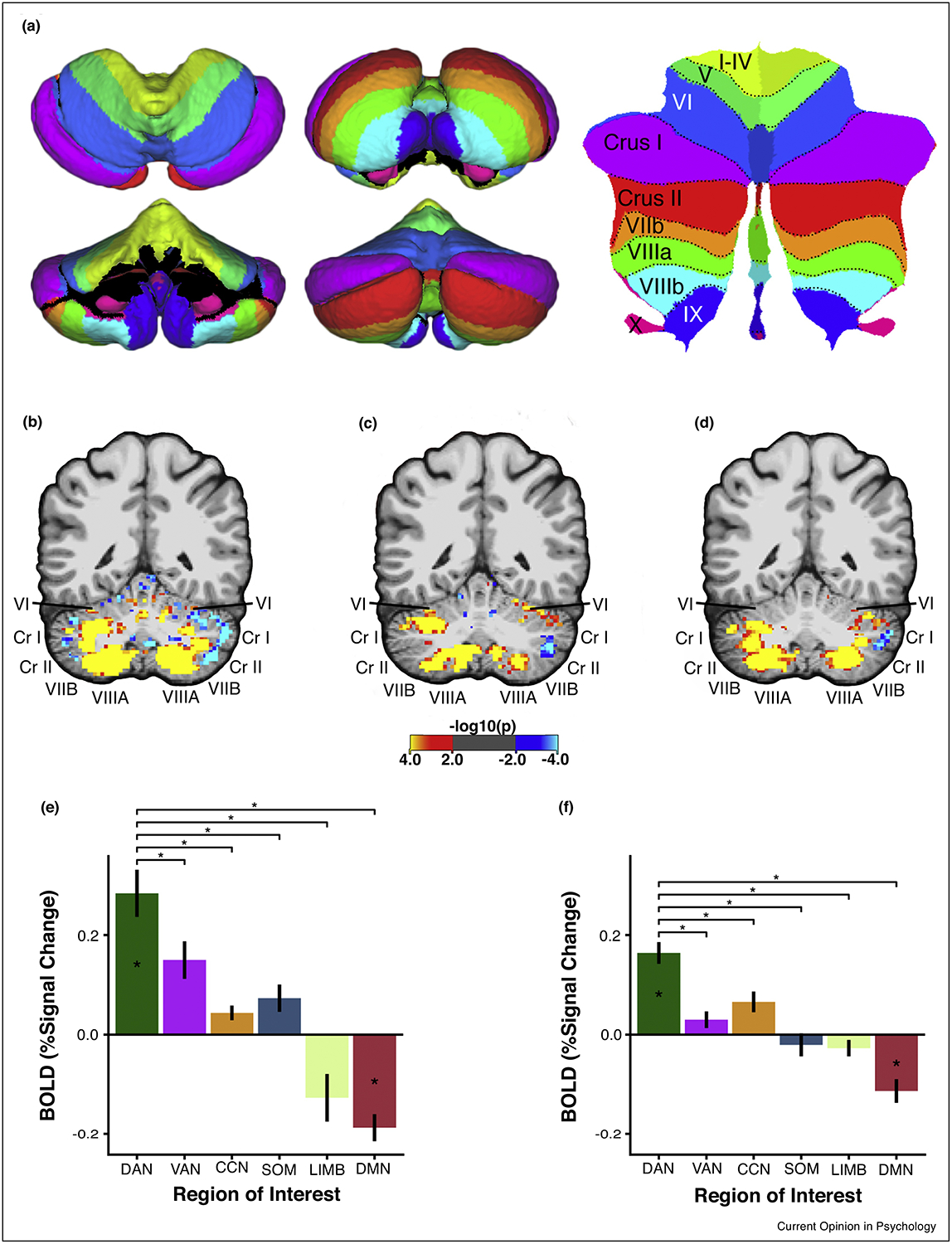
Functional MRI evidence for a cerebellar node of the dorsal attention network. (a) Pial surface representation of the cerebellum from superior (top left), anterior (bottom left), inferior (top middle), and posterior (bottom middle) views. Colors denote lobular boundaries. Flatmap representation of the cerebellum [82] is shown on the right with corresponding lobular labels. (b) A single subject’s pattern of resting-state functional connectivity with the cortical dorsal attention network. Color map reflects p-value of the t-statistic produced by regressing the time course of each cerebellar voxel on the average time course of the cortical DAN. (c) The same subject’s activation pattern for a visual attention multiple object tracking task (contrast of set size 4 vs. set size 0). The task required participants to covertly track multiple moving target items among identical distractors. Color map reflects p-value of t-statistic for task activation contrast via GLM. (d) Cerebellar activation pattern elicited by a contrast of high load (set size 4) working memory with low load (set size 1) working memory. Participants were required to maintain the identity of varying numbers of items over short delays in order to detect the presence or absence of a change in the identity of one of the stored items. (e) Cerebellar region-of-interest (ROI) analysis shows that covert attentional tracking selectively recruits portions of the cerebellar that are functionally coupled with the cortical DAN. Cerebellar ROIs were defined by winner-take-all procedure which assigned cerebellar voxels to the cortical network with which they had the strongest correlation. Other networks shown: Ventral Attention Network (VAN), Cognitive Control Network (CCN), Somatomotor Network (SOM), Limbic Network (LIMB), and Default Mode Network (DMN). (f) Cerebellar network ROI analysis for the visual working memory load contrast (set size 4 > set size 1). Adapted from Ref. [17]
Cerebellum and Cognition
The effect of cerebellar injury on cognitive impairment has been the subject of considerable debate. Cerebellar cognitive affective syndrome (CCAS), resulting from injury to the cerebellar posterior lobe, is characterized by deficits across a diverse array of cognitive and affective domains [24,25]. Furthermore, a number of studies have reported covert attentional deficits resulting from cerebellar damage [26–29]. However, a competing literature suggests that injury to the cerebellum produces considerable motor deficits with little to no cognitive deficits [30–35]. One explanation for these divergent findings is the use of heterogeneous patient groups, which vary substantially in the location and extent of cerebellar damage, as well as the source of cerebellar abnormality (lesion or degeneration). A more precise symptom-lesion mapping of attention deficits revealed that only a subset of cerebellar lesion patients exhibited abnormal reaction times in a covert attention task and that lesions in these patients tended to be localized to vermal lobule VI and Crus I [36]. A later study found that lesions located in Crus II, lobule VIIb and lobule VIIIa also produced attentional impairments [37]. These findings provide evidence for functional specialization within the cerebellum, with specific cerebellar structures associated with attention deficits. Functional imaging studies provide further evidence for the recruitment of specific cerebellar structures by attention and working memory tasks [12,38,39]. In one notable study, Allen and colleagues [12] found evidence for a double dissociation whereby attention activated an area located within lobule VI and Crus I, and a motor task activated a distinct area within the anterior lobe (lobule III–V). Another study showed that shifts of attention elicited robust activation of Crus I, as well as a midline region within lobule VII [38]. There is also substantial evidence that specific portions of the cerebellum are modulated by working memory load [13,14,17,40,41]. For instance, one study found that parametrically increasing the number of to-be-remembered letter stimuli in a Sternberg task produced linear load-dependent increases in activation in both lobule VI/Crus I and lobule VIIb/VIIIa [14]. These cerebellar findings set the stage for network-level examinations of cortico-cerebellar interactions supporting attention and working memory function.
Dorsal Attention Network Extends to the Cerebellum
Considerable evidence demonstrates that a network of frontal and parietal cerebral cortical areas referred to as the dorsal attention network (DAN) supports visual attentional function [42–45]. The DAN is typically considered to include the intraparietal sulcus and superior parietal lobule within the parietal lobe, the superior and inferior pre-central sulcus within lateral frontal cortex, and area MT within occipito-temporal cortex [42,46–51]. A substantial body of research has detailed three characteristic properties of functional areas belonging to the visual DAN: 1) recruitment by sustained attention and working memory paradigms when contrasted with a sensorimotor control condition e.g. [42,47,52,53]; 2) load-dependent activity that increases parametrically with the number of items successfully attended or maintained in working memory e.g. [53–55]; and 3) the representation of the spatial locus of attention and working memory. For instance, persistent activity in fronto-parietal cortex has been shown to accurately predict the location of a stimulus covertly attended or maintained in working memory [56,57]. This representational specificity corresponds with the location of topographic maps of the visual field identified via retinotopic mapping procedures [56,58,59].
Recent work from our laboratory demonstrates that a specific portion of the cerebellum, dorsomedial lobule VIIb/VIIIa, is functionally connected with the visual DAN and that it exhibits these three characteristic functional properties of the DAN [17,18]. Multiple cerebellar sites have been shown to exhibit robust and specific intrinsic connectivity with cortical DAN areas, including lobules VI, Crus I, VIIb, VIIIa and IX [10,17,60]. We [17] observed that a visual attention multiple object tracking paradigm robustly recruited areas of the cerebellum that exhibit intrinsic functional connectivity with the cortical DAN (Figure 1b–c). This confluence of DAN connectivity and attention recruitment was found to be primarily located within cerebellar lobules VIIb and VIIIa within the posterior cerebellum. This task recruitment was specific to cerebellar areas coupled with the cortical DAN as opposed to other networks (Figure 1e). Further, we [17] observed that cerebellar lobule VIIb/VIIIa was sensitive to working memory and working memory load, indicating that this recruitment reflected cognitive aspects of the task (Figure 1d & f). Additionally, we found preferential suppression of cerebellar areas that were functionally connected with the cortical default mode network, mirroring the suppressed responses of cortical default network regions during goal-directed task performance [61,62] (Figure 1e–f).
Cerebellar Visual Field Representations
The cortical DAN contains multiple spatial maps of the visual field, which are theorized to constitute attentional priority maps that represent prioritized portions of visual space in service of attention and working memory [56,57]. We [18] recently observed visual field representations within cerebellar lobule VIIb/VIIIa across two experiments. The first experiment employed a lateralized visual working memory change detection paradigm in which stimuli were presented bilaterally, but working memory target items were restricted to one visual hemifield (as shown in Figure 3a). Voxels located in the dorsomedial portion of lobule VIIb/VIIIa were selective for the ipsilateral visual hemifield. This ipsilateral bias mirrors the well-documented contralateral bias observed throughout the cortical DAN [47,53,56,58,59], and is consistent with the crossing of fiber tracts connecting the cerebral cortex and cerebellum at the cerebellar peduncles [63,64]. The second experiment performed population receptive field (pRF) mapping analysis (Figure 2a) and confirmed the existence of an ipsilateral visual hemifield representation within the dorsomedial portion of lobule VIIb/VIIIa (Figure 2b–c). Thus, dorsomedial cerebellar lobule VIIb/VIIIa accurately encodes the visual field locus of attention in a similar manner to cortical attention areas.
Figure 3.
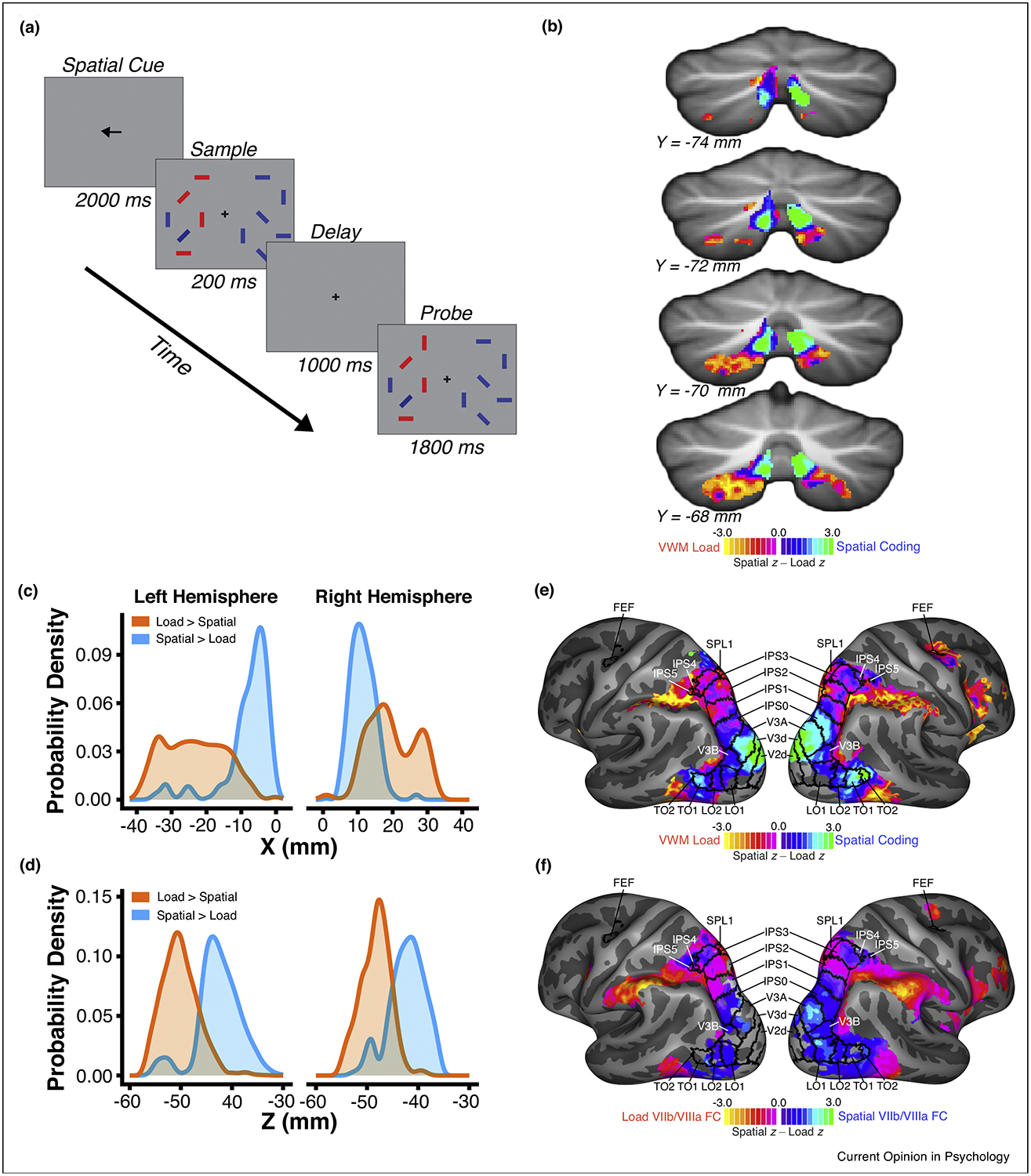
Fine-scale topography of cortico-cerebellar networks for visual attention and working memory. (a) Task Configuration. Participants held central fixation while covertly performing a spatially lateralized visual working memory task, in which they were asked to encode the orientation of 1 or 4 target stimuli (red) and to report whether the orientation of any bar changed (one bar changed on 50% of trials, no change on other 50% of trials) across a brief delay interval. Target stimuli alternated visual hemifields across blocks of trials. (b) Normalized comparison of spatial location coding and VWM load coding revealed complementary gradients, for which spatial coding is more robust dorsomedially and VWM load coding is more robust ventrolaterally. (c, d) Probability density curves for VWM load coding (orange) and for spatial coding (blue) showed separable profiles in the X- and Z-dimensions (MNI coordinates). (e) Normalized comparison of spatial coding and VWM load coding in the cerebral cortex revealed a gradient in parieto-occipital cortical regions. Within parietal cortex, dorsomedial retinotopic areas IPS0–5 exhibited varying degrees of spatial bias, while the ventrolateral portion was biased for VWM load coding (f) Contrast between resting-state functional connectivity of cerebellar lobule VIIb/VIIIa spatial coding and VWM load coding seeds accurately reflected the functional gradient observed in the task data of panel e. Adapted from Ref. [18].
Figure 2.
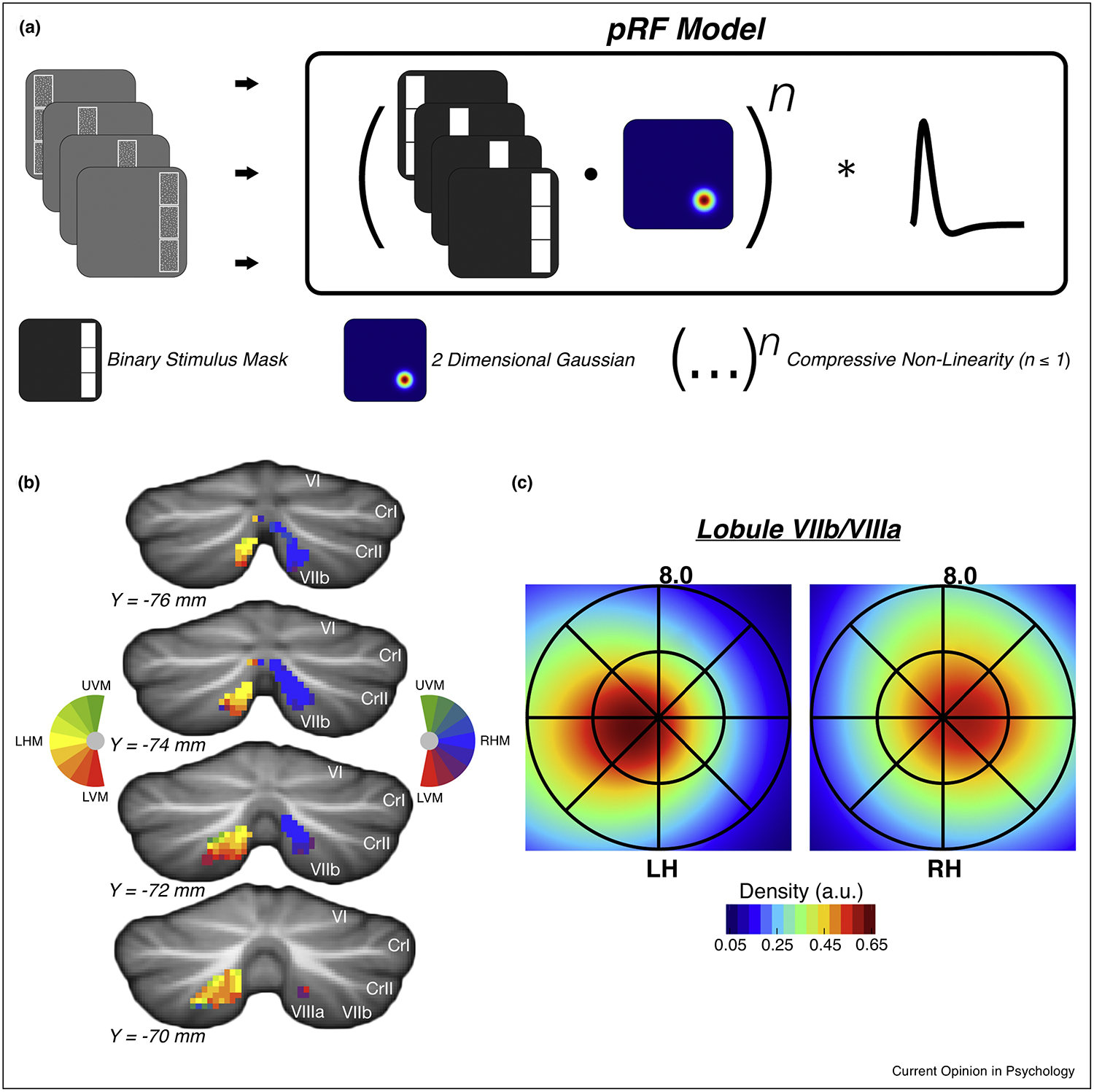
Cerebellar lobule VIIb/VIIIa visual field representations. (a) Schematic of population receptive field (pRF) mapping procedure. Participants held central fixation while bar-like apertures containing moving dot stimuli were slowly swept across the visual field in each of four cardinal directions. The task was to report which of the two outer sections possessed dot motion in the same direction as the inner section. This task was repeated for each step in the visual field sweep. The pRF modeling procedure consists of converting trial stimulus images to 2D binary masks and then computing the dot product between the binary mask and a 2D Gaussian (representing a predicted pRF) for each timepoint. A static power-law non-linearity is then applied to account for subadditive spatial summation. Lastly, the generated time course is convolved with a hemodynamic response function. (b) Polar angle visual field location preferences produced by a pRF mapping analysis for a single subject. Colors reflect visual field polar angle relative to central fixation; LHM – left horizontal meridian (yellow), RHM – right horizontal meridian (blue), UVM – upper vertical meridian (green), LVM – lower vertical meridian (red). (c) Group-average (n=5) visual field coverage maps for left and right hemisphere lobule VIIb/VIIIa. Coverage maps are produced by averaging the pRFs (scaled to peak at 1) of above-threshold (cross-validated predicted-actual correlation > 0.2) lobule VIIb/VIIIa voxels within and across subjects. Adapted from Ref. [18]
Functional topography of visual attention and working memory processing within cerebellum
The cerebral cortex is characterized by a high degree of functional specialization. Current evidence suggests the cerebral cortex contains upwards of 200 specialized areas in each hemisphere e.g. [65,66]. The functional specificity of cerebral cortex can in part be explained by differences in cytoarchitecture [67]. The microstructural organization of the cerebellum, on the other hand, is relatively homogenous [68,69]. Hence, the cerebellum is traditionally viewed as functionally invariant. However, both task and connectivity measures provide evidence for the topographic arrangement of function within the cerebellum. It has been shown that distinct portions of the cerebellum are reciprocally connected with motor and association cortex respectively [9,10]. Furthermore, a wide range of tasks have been shown to elicit non-overlapping patterns of activation within cerebellar cortex, including working memory, attention, language, executive function and affective tasks [16,19,70,71]. Yet, these studies do not provide sufficient evidence to suggest that the cerebellum possesses a similar level of functional specificity as cerebral cortex. We [18] examined the specificity of cerebellar recruitment by closely related aspects of visual attention and working memory tasks by comparing effect size estimates for spatial coding (attend left vs. right) and visual working memory load (set size 4 vs. 1) within lobule VIIb/VIIIa (Figure 3b). Different portions of lobule VIIb/VIIIa were found to be selective for spatial coding and working memory load. The dorsomedial portion of lobule VIIb/VIIIa was more strongly recruited by spatial coding, and an area shifted ventolaterally was more strongly activated by visual working memory load (Figure 3c & d). This functional organization was shown to mirror the organization of these processes in cerebral cortex (Figure 3e). Furthermore, we observed that the specificity of task recruitment could be explained by fine-scale differences in connectivity between cortex and cerebellum. Resting-state functional connectivity of space- and load-selective portions of lobule VIIb/VIIIa with cerebral cortex differed and this differential connectivity accurately predicted differences in functional selectivity across occipito-parietal cortex (Figure 3f). This evidence indicates that, at least in the attentional domain, cerebellar cortex exhibits fine-scale functional specialization similar to the degree of specialization observed in cerebral cortex. Furthermore, the observed specificity is reflected by fine-scale patterns of cortico-cerebellar functional connectivity.
Conclusions
The research findings discussed here demonstrate that visual attention and visual working memory functions are supported by dorsomedial cerebellar lobule VIIb/VIIIa, but numerous questions remain for future research (Box 1). More broadly, we note that the current evolution in the field’s understanding of the cerebellum’s role in cognitive processes can likened to a similar progression in our view of basal ganglia function. That is to say, we suggest that “the cerebellum is the new basal ganglia.” A few decades ago, the basal ganglia were widely thought to be limited to providing support for motor control functions; however, today the basal ganglia are understood to make major contributions to a vast array of emotional and cognitive processes [72–75]. The cerebellum, despite containing half of the brain’s neurons, has been largely neglected by much of the cognitive neuroscience community, outside of those studying motor control. Due to the homogeneity of cerebellar cortical circuit organization and the lack of monosynaptic connections between the cerebral cortex and cerebellum, progress in understanding the functional specificity of the cerebellum has long been stalled. However, recent advances in resting-state fMRI connectivity analysis and in polysynaptic anatomical tracing find evidence for fine-scale specificity in cortico-cerebellar networks similar to the specificity observed in cortico-striatal networks [9,10,18,76]. We argue that each cerebral cortical network may functionally extend to a specific cerebellar component. The anatomical regularity of the cerebellar circuitry suggests each cerebellar region may play a consistent computational role in parallel cortico-cerebellar networks, and that functional differences between cerebellar regions may arise from variation in the specific cortical sites with which they are connected [77]. Future cognitive neuroscience studies across a broad range of topic areas should further investigate cerebellar contributions to functions previously thought to be predominantly, if not exclusively, mediated by cerebral cortical regions.
Box 1. Questions for Future Research.
Cerebellar Specificity for Working Memory Processes. Although lobule VIIb/VIIIa exhibits load-dependent recruitment during visual working memory (VWM) task performance, it is not clear if this reflects processes specific to VWM, such as delay-period activation or stimulus-specific VWM responses, or rather reflects more general attentional processes.
Attention Contributions from regions other than VIIb/VIIIa. In addition to lobule VIIb/VIIIa, smaller regions located within lobules V/VI and VIIIb/IX also exhibit functional connectivity with the cortical DAN [10]; finer-scale investigations of the possible attentional contributions of these regions should be performed.
Distinct Cerebellar Contributions to DAN. The results summarized in this paper focus on the functional similarities of cerebellar regions to regions of the cortical dorsal attention network. Future studies should strive to isolate the unique functional contributions of cerebellar regions to visual attention and VWM.
Influence of Microsaccades on Cerebellar Responses. In-scanner eye tracking demonstrates that the cerebellar attention results described here do not reflect eye position or the rate of saccadic eye movements [17,18]; however, it remains unclear if any cerebellar regions contribute to the suppression of microsaccades that occurs during visual attentional encoding [78,79].
Cerebellar Specificity for Attended Sensory Modality.While attention to different sensory modalities recruits different cortical networks [80], it is unclear if these modality-specific attention networks extend to cerebellum. One study observed that visual and auditory motion stimuli drive the cerebellum differently, but that task-difficulty increases in each modality drove only a shared cerebellar region [81].
Acknowledgements
This work was supported by the National Institutes of Health (NIH R01EY022229, NIH R21EY027703 to D.C.S.), and National Science Foundation (NSF Graduate Research Fellowship DGE-1247312 to J.A.B.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
Nothing declared.
References
- [1].Petersen SE, Robinson DL, Morris JD, Contributions of the pulvinar to visual spatial attention, Neuropsychologia. 25 (1987) 97–105. [DOI] [PubMed] [Google Scholar]
- [2].Ignashchenkova A, Dicke PW, Haarmeier T, Thier P, Neuron-specific contribution of the superior colliculus to overt and covert shifts of attention, Nature Neuroscience. 7 (2004) 56–64. doi: 10.1038/nn1169. [DOI] [PubMed] [Google Scholar]
- [3].Müller JR, Philiastides MG, Newsome WT, Microstimulation of the superior colliculus focuses attention without moving the eyes, Pnas 102 (2005) 524–529. doi: 10.1073/pnas.0408311101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Evarts EV, Thach WT, Motor mechanisms of the CNS: cerebrocerebellar interrelations, Annu. Rev. Physiol 31 (1969) 451–498. doi: 10.1146/annurev.ph.31.030169.002315. [DOI] [PubMed] [Google Scholar]
- [5].Gilbert PF, Thach WT, Purkinje cell activity during motor learning, Brain Research. 128 (1977) 309–328. [DOI] [PubMed] [Google Scholar]
- [6].Brooks VB, Thach WT, Cerebellar Control of Posture and Movement, 2nd ed., John Wiley & Sons, Inc, Hoboken, NJ, USA, 2011. doi: 10.1002/cphy.cp010218. [DOI] [Google Scholar]
- [7].Ito M, The cerebellum and neural control, Raven Pr, 1984. [Google Scholar]
- [8].Llinás R, Functional Significance of the Basic Cerebellar Circuit in Motor Coordination, in: Cerebellar Functions, Springer Berlin Heidelberg, Berlin, Heidelberg, 1985: pp. 170–185. doi: 10.1007/978-3-642-69980-1_13. [DOI] [Google Scholar]
- [9].Kelly RM, Strick PL, Cerebellar Loops with Motor Cortex and Prefrontal Cortex of a Nonhuman Primate, Journal of Neuroscience. 23 (2003) 8432–8444. doi: 10.1002/cne.902860306. [DOI] [PMC free article] [PubMed] [Google Scholar]; Using transneuronal tracing techniques, the authors examined the topographic arrangement of circuits connecting the cerebellum with either primary motor cortex (M1) or area 46 within dorsolateral prefrontal cortex. They found that distinct portions of cerebellar cortex formed closed-loop circuits with M1 and area 46, respectively. These results provide anatomical evidence for cerebellar contributions to both cognitive and motor processing.
- [10].Buckner RL, Krienen FM, Castellanos A, Diaz JC, Yeo BTT, The organization of the human cerebellum estimated by intrinsic functional connectivity, Journal of Neurophysiology. 106 (2011) 2322–2345. doi: 10.1152/jn.00339.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]; Buckner et al. used resting state fMRI data from a 1,000 subjects to examine the organization of cortico-cerebellar networks. A cluster analysis revealed that the proportion of the cerebellum preferentially coupled with each cortical network is proportional to that network’s extent in the cerebral cortex. Remarkably, it was shown that the majority of the cerebellum maps to cortical association networks.
- [11].Schmahmann JD, Guell X, Stoodley CJ, Halko MA, The Theory and Neuroscience of Cerebellar Cognition, Annu. Rev. Neurosci (2019). doi: 10.1146/annurev-neuro-070918-050258. [DOI] [PubMed] [Google Scholar]
- [12].Allen G, Buxton RB, Wong EC, Courchesne E, Attentional activation of the cerebellum independent of motor involvement, Science. 275 (1997) 1940–1943. [DOI] [PubMed] [Google Scholar]; One of the first fMRI studies to examine cerebellar contributions to attentional processes. Showed that attention and motor task components recruit distinct portions of the cerebellum.
- [13].Chen SA, Desmond JE, Cerebrocerebellar networks during articulatory rehearsal and verbal working memory tasks, NeuroImage. 24 (2005) 332–338. doi: 10.1016/j.neuroimage.2004.08.032. [DOI] [PubMed] [Google Scholar]
- [14].Kirschen MP, Chen SA, Schraedley-Desmond P, Desmond JE, Load- and practice-dependent increases in cerebro-cerebellar activation in verbal working memory: an fMRI study, NeuroImage. 24 (2005) 462–472. doi: 10.1016/j.neuroimage.2004.08.036. [DOI] [PubMed] [Google Scholar]
- [15].Stoodley CJ, Schmahmann JD, Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies, NeuroImage. 44 (2009) 489–501. doi: 10.1016/j.neuroimage.2008.08.039. [DOI] [PubMed] [Google Scholar]
- [16].Stoodley CJ, Valera EM, Schmahmann JD, Functional topography of the cerebellum for motor and cognitive tasks: An fMRI study, NeuroImage. 59 (2012) 1560–1570. doi: 10.1016/j.neuroimage.2011.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]; Stoodley et al. examined the functional topography of various cognitive and motor functions within the cerebellum using fMRI. It was shown that distinct portions of cerebellar cortex were recruited by motor, language, spatial and working memory tasks.
- [17].Brissenden JA, Levin EJ, Osher DE, Halko MA, Somers DC, Functional Evidence for a Cerebellar Node of the Dorsal Attention Network, J. Neurosci 36 (2016) 6083–6096. doi: 10.1523/JNEUROSCI.0344-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]; Brissenden et al. examined the relationship between cortico-cerebellar dorsal attention network functional connectivity and recruitment by visual attention and visual working memory tasks. They found that a region located within cerebellar lobules VIIb and VIIIa was robustly activated by a sustained attention multiple object tracking paradigm and a visual working memory change detection paradigm in a load-dependent fashion. These results provide evidence indicating the dorsal attention network functionally extends to portion of the cerebellum.
- [18].Brissenden JA, Tobyne SM, Osher DE, Levin EJ, Halko MA, Somers DC, Topographic Cortico-cerebellar Networks Revealed by Visual Attention and Working Memory, Curr. Biol 28 (2018) 3364–3372.e5. doi: 10.1016/j.cub.2018.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]; Brissenden et al. (2018) examined the specificity of cerebellar recruitment by different aspects of visual attention and working memory tasks. It was shown that spatial attention and visual working memory load recruited dissociable portions of the lobule VIIb/VIIIa. The observed functional specialization was further shown to be predicted by fine-scale differential functional connectivity with cerebral cortex. This work highlights the existence of high specific cortico-cerebellar networks dedicated to different attentional functions.
- [19].Guell X, Gabrieli JDE, Schmahmann JD, Triple representation of language, working memory, social and emotion processing in the cerebellum: Convergent evidence from task and seed-based resting-state fMRI analyses in a single large cohort, NeuroImage. (2018). doi: 10.1016/j.neuroimage.2018.01.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Schmahmann JD, Doyon J, McDonald D, Holmes C, Lavoie K, Hurwitz AS, et al. , Three-dimensional MRI atlas of the human cerebellum in proportional stereotaxic space, NeuroImage. 10 (1999) 233–260. doi: 10.1006/nimg.1999.0459. [DOI] [PubMed] [Google Scholar]
- [21].Kemp JM, Powell TP, The connexions of the striatum and globus pallidus: synthesis and speculation, Philosophical Transactions of the Royal Society B: Biological Sciences. 262 (1971) 441–457. doi: 10.1098/rstb.1971.0106. [DOI] [PubMed] [Google Scholar]
- [22].Strick PL, How do the basal ganglia and cerebellum gain access to the cortical motor areas? Behavioural Brain Research. 18 (1985) 107–123. [DOI] [PubMed] [Google Scholar]
- [23].Schmahmann JD, Pandya DN, The cerebrocerebellar system, Int. Rev. Neurobiol 41 (1997) 31–60. [DOI] [PubMed] [Google Scholar]; Schmahmann & Sherman described a constellation of cognitive and affective deficits observed in patients with lesions confined to the cerebellum. Cognitive impairments were found to be most prominent in patients with lesions located within the posterior lobe of the cerebellum.
- [24].Schmahmann JD, Sherman JC, The cerebellar cognitive affective syndrome, Brain. 121 (Pt 4) (1998) 561–579. [DOI] [PubMed] [Google Scholar]
- [25].Levisohn L, Cronin-Golomb A, Schmahmann JD, Neuropsychological consequences of cerebellar tumour resection in children, Brain. 123 (2014) 1041–1050. [DOI] [PubMed] [Google Scholar]
- [26].Akshoomoff NA, Courchesne E, A new role for the cerebellum in cognitive operations, Behav. Neurosci 106 (1992) 731–738. [DOI] [PubMed] [Google Scholar]
- [27].Akshoomoff NA, Courchesne E, ERP Evidence for a Shifting Attention Deficit in Patients with Damage to the Cerebellum, Journal of Cognitive Neuroscience. 6 (1994) 388–399. doi: 10.1162/jocn.1994.6.4.388. [DOI] [PubMed] [Google Scholar]
- [28].Courchesne E, Townsend J, Akshoomoff NA, Saitoh O, Yeung-Courchesne R, Lincoln AJ, et al. , Impairment in shifting attention in autistic and cerebellar patients, Behav. Neurosci 108 (1994) 848–865. [DOI] [PubMed] [Google Scholar]
- [29].Townsend J, Courchesne E, Covington J, Westerfield M, Harris NS, Lyden P, et al. , Spatial attention deficits in patients with acquired or developmental cerebellar abnormality, Journal of Neuroscience. 19 (1999) 5632–5643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Dimitrov M, Grafman J, Kosseff P, Wachs J, Alway D, Higgins J, et al. , Preserved cognitive processes in cerebellar degeneration, Behavioural Brain Research. 79 (1996) 131–135. [DOI] [PubMed] [Google Scholar]
- [31].Yamaguchi S, Tsuchiya H, Kobayashi S, Visuospatial attention shift and motor responses in cerebellar disorders, Journal of Cognitive Neuroscience. 10 (1998) 95–107. [DOI] [PubMed] [Google Scholar]
- [32].Ravizza SM, Ivry RB, Comparison of the basal ganglia and cerebellum in shifting attention, Journal of Cognitive Neuroscience. 13 (2001) 285–297. [DOI] [PubMed] [Google Scholar]
- [33].Golla H, Thier P, Haarmeier T, Disturbed overt but normal covert shifts of attention in adult cerebellar patients, Brain. 128 (2005) 1525–1535. doi: 10.1093/brain/awh523. [DOI] [PubMed] [Google Scholar]
- [34].Frank B, Schoch B, Richter S, Frings M, Karnath H-O, Timmann D, Cerebellar lesion studies of cognitive function in children and adolescents - limitations and negative findings, Cerebellum. 6 (2007) 242–253. doi: 10.1080/14734220701297432. [DOI] [PubMed] [Google Scholar]
- [35].Haarmeier T, Thier P, The attentive cerebellum - myth or reality? Cerebellum. 6 (2007) 177–183. doi: 10.1080/14734220701286187. [DOI] [PubMed] [Google Scholar]
- [36].Baier B, Dieterich M, Stoeter P, Birklein F, Müller NG, Anatomical correlate of impaired covert visual attentional processes in patients with cerebellar lesions, J. Neurosci 30 (2010) 3770–3776. doi: 10.1523/JNEUROSCI.0487-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]; Baier et al. tested patients with right hemisphere cerebellar lesions on a covert visual attention task and explicitly related cerebellar lesion location to the presentation of attentional deficits. Compared with non-impaired patients, attentionally impaired patients were more likely to possess lesions of cerebellar midline structures. These results suggest that specific areas within the cerebellum influence covert attention.
- [37].Striemer CL, Cantelmi D, Cusimano MD, Danckert JA, Schweizer TA, Deficits in reflexive covert attention following cerebellar injury, Front Hum Neurosci. 9 (2015) 428. doi: 10.3389/fnhum.2015.00428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Le TH, Pardo JV, Hu X, 4 T-fMRI Study of Nonspatial Shifting of Selective Attention: Cerebellar and Parietal Contributions, Journal of Neurophysiology. 79 (1998) 1535–1548. doi: 10.1152/jn.1998.79.3.1535. [DOI] [PubMed] [Google Scholar]
- [39].Striemer CL, Chouinard PA, Goodale MA, de Ribaupierre S, Overlapping neural circuits for visual attention and eye movements in the human cerebellum, Neuropsychologia. 69 (2015) 9–21. doi: 10.1016/j.neuropsychologia.2015.01.024. [DOI] [PubMed] [Google Scholar]
- [40].Desmond JE, Gabrieli JD, Wagner AD, Ginier BL, Glover GH, Lobular patterns of cerebellar activation in verbal working-memory and finger-tapping tasks as revealed by functional MRI, Journal of Neuroscience. 17 (1997) 9675–9685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Chen SA, Desmond JE, Temporal dynamics of cerebro-cerebellar network recruitment during a cognitive task, Neuropsychologia. 43 (2005) 1227–1237. doi: 10.1016/j.neuropsychologia.2004.12.015. [DOI] [PubMed] [Google Scholar]
- [42].Corbetta M, Shulman GL, Control of goal-directed and stimulus-driven attention in the brain, Nat. Rev. Neurosci 3 (2002) 201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- [43].Ptak R, The Frontoparietal Attention Network of the Human Brain, The Neuroscientist. 18 (2012) 502–515. doi: 10.1177/1073858411409051. [DOI] [PubMed] [Google Scholar]
- [44].Scolari M, Seidl-Rathkopf KN, Kastner S, Functions of the human frontoparietal attention network: Evidence from neuroimaging, Curr Opin Behav Sci 1 (2015) 32–39. doi: 10.1016/j.cobeha.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Ptak R, Schnider A, Fellrath J, The Dorsal Frontoparietal Network: A Core System for Emulated Action, Trends in Cognitive Sciences. 21 (2017) 589–599. doi: 10.1016/j.tics.2017.05.002. [DOI] [PubMed] [Google Scholar]
- [46].Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME, The human brain is intrinsically organized into dynamic, anticorrelated functional networks, Pnas 102 (2005) 9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Szczepanski SM, Konen CS, Kastner S, Mechanisms of Spatial Attention Control in Frontal and Parietal Cortex, Journal of Neuroscience. 30 (2010) 148–160. doi: 10.1523/JNEUROSCI.3862-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, et al. , Functional Network Organization of the Human Brain, Neuron. 72 (2011) 665–678. doi: 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Ptak R, Schnider A, The attention network of the human brain: Relating structural damage associated with spatial neglect to functional imaging correlates of spatial attention, Neuropsychologia. 49 (2011) 3063–3070. doi: 10.1016/j.neuropsychologia.2011.07.008. [DOI] [PubMed] [Google Scholar]
- [50].Yeo BTT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, et al. , The organization of the human cerebral cortex estimated by intrinsic functional connectivity, Journal of Neurophysiology. 106 (2011) 1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Gao W, Lin W, Frontal parietal control network regulates the anti-correlated default and dorsal attention networks, Hum. Brain Mapp 33 (2012) 192–202. doi: 10.1002/hbm.21204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Culham JC, Brandt SA, Cavanagh P, Kanwisher NG, Dale AM, Tootell RB, Cortical fMRI activation produced by attentive tracking of moving targets, Journal of Neurophysiology. 80 (1998) 2657–2670. doi: 10.1152/jn.1998.80.5.2657. [DOI] [PubMed] [Google Scholar]
- [53].Sheremata SL, Bettencourt KC, Somers DC, Hemispheric asymmetry in visuotopic posterior parietal cortex emerges with visual short-term memory load, J. Neurosci 30 (2010) 12581–12588. doi: 10.1523/JNEUROSCI.2689-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Culham JC, Cavanagh P, Kanwisher NG, Attention response functions: characterizing brain areas using fMRI activation during parametric variations of attentional load, Neuron 32 (2001) 737–745. [DOI] [PubMed] [Google Scholar]
- [55].Todd JJ, Marois R, Capacity limit of visual short-term memory in human posterior parietal cortex, Nature. 428 (2004) 751–754. doi: 10.1038/nature02466. [DOI] [PubMed] [Google Scholar]
- [56].Jerde TA, Merriam EP, Riggall AC, Hedges JH, Curtis CE, Prioritized maps of space in human frontoparietal cortex, J. Neurosci 32 (2012) 17382–17390. doi: 10.1523/JNEUROSCI.3810-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Sprague TC, Serences JT, Attention modulates spatial priority maps in the human occipital, parietal and frontal cortices, Nature Neuroscience. 16 (2013) 1879–1887. doi: 10.1038/nn.3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Swisher JD, Halko MA, Merabet LB, McMains SA, Somers DC, Visual topography of human intraparietal sulcus, J. Neurosci 27 (2007) 5326–5337. doi: 10.1523/JNEUROSCI.0991-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Mackey WE, Winawer J, Curtis CE, Visual field map clusters in human frontoparietal cortex, Elife. (2017) 1–23. doi: 10.7554/eLife.22974.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Ramanoel S, York E, Habas C, Participation of the caudal cerebellar lobule IX to the dorsal attentional network, Cerebellum & Ataxias 2018 5:1. 5 (2018) 9. doi: 10.1186/s40673-018-0088-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Shulman GL, Fiez JA, Corbetta M, Buckner RL, Miezin FM, Raichle ME, et al. , Common Blood Flow Changes across Visual Tasks: II. Decreases in Cerebral Cortex, Journal of Cognitive Neuroscience. 9 (1997) 648–663. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- [62].Gusnard DA, Raichle ME, Searching for a baseline: functional imaging and the resting human brain, Nat. Rev. Neurosci 2 (2001) 685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- [63].Schmahmann JD, Pandya DN, Anatomic organization of the basilar pontine projections from prefrontal cortices in rhesus monkey, Journal of Neuroscience. 17 (1997) 438–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Middleton FA, Strick PL, Cerebellar projections to the prefrontal cortex of the primate, J. Neurosci 21 (2001) 700–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Glasser MF, Coalson TS, Robinson EC, Hacker CD, Harwell J, Yacoub E, et al. , A multi-modal parcellation of human cerebral cortex, Nature. 536 (2016) 171–178. doi: 10.1038/nature18933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Gordon EM, Laumann TO, Adeyemo B, Huckins JF, Kelley WM, Petersen SE, Generation and Evaluation of a Cortical Area Parcellation from Resting-State Correlations, Cereb. Cortex 26 (2016) 288–303. doi: 10.1093/cercor/bhu239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Zilles K, Amunts K, Centenary of Brodmann’s map--conception and fate., Nature Publishing Group, 2010. doi: 10.1038/nrn2776. [DOI] [PubMed] [Google Scholar]
- [68].Eccles JC, The Cerebellum as a Neuronal Machine, Springer Science & Business Media, Berlin, Heidelberg, 2013. doi: 10.1007/978-3-662-13147-3. [DOI] [Google Scholar]
- [69].Bloedel JR, Functional heterogeneity with structural homogeneity: How does the cerebellum operate? in: Movement Control, Cambridge University Press, 1994: pp. 64–76. doi: 10.1017/CBO9780511529788.007. [DOI] [Google Scholar]
- [70].Stoodley CJ, Schmahmann JD, Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies, NeuroImage 44 (2009) 489–501. doi: 10.1016/j.neuroimage.2008.08.039. [DOI] [PubMed] [Google Scholar]
- [71].Guell X, Schmahmann JD, Gabrieli JD, Ghosh SS, Functional gradients of the cerebellum, Elife. 7 (2018). doi: 10.7554/eLife.36652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Graybiel AM, The basal ganglia and cognitive pattern generators, Schizophr Bull 23 (1997) 459–469. [DOI] [PubMed] [Google Scholar]
- [73].Brown LL, Schneider JS, Lidsky TI, Sensory and cognitive functions of the basal ganglia, Curr. Opin. Neurobiol 7 (1997) 157–163. [DOI] [PubMed] [Google Scholar]
- [74].Middleton FA, Strick PL, Basal ganglia output and cognition: evidence from anatomical, behavioral, and clinical studies, Brain Cogn 42 (2000) 183–200. doi: 10.1006/brcg.1999.1099. [DOI] [PubMed] [Google Scholar]
- [75].Frank MJ, Loughry B, O’Reilly RC, Interactions between frontal cortex and basal ganglia in working memory: a computational model, Cognitive, Affective, & Behavioral Neuroscience. 1 (2001) 137–160. [DOI] [PubMed] [Google Scholar]
- [76].Middleton FA, Strick PL, Basal-ganglia “projections” to the prefrontal cortex of the primate, Cereb. Cortex 12 (2002) 926–935. [DOI] [PubMed] [Google Scholar]
- [77].Ramnani N, The primate cortico-cerebellar system: anatomy and function, Nat. Rev. Neurosci 7 (2006) 511–522. doi: 10.1038/nrn1953. [DOI] [PubMed] [Google Scholar]
- [78].Hafed ZM, Alteration of visual perception prior to microsaccades, Neuron 77 (2013) 775–786. doi: 10.1016/j.neuron.2012.12.014. [DOI] [PubMed] [Google Scholar]
- [79].Arnstein D, Junker M, Smilgin A, Dicke PW, Thier P, Microsaccade control signals in the cerebellum, J. Neurosci 35 (2015) 3403–3411. doi: 10.1523/JNEUROSCI.2458-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Michalka SW, Kong L, Rosen ML, Shinn-Cunningham BG, Somers DC, Short-Term Memory for Space and Time Flexibly Recruit Complementary Sensory-Biased Frontal Lobe Attention Networks, Neuron 87 (2015) 882–892. doi: 10.1016/j.neuron.2015.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Baumann O, Mattingley JB, Scaling of neural responses to visual and auditory motion in the human cerebellum, J. Neurosci 30 (2010) 4489–4495. doi: 10.1523/JNEUROSCI.5661-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Diedrichsen J, Zotow E, Surface-Based Display of Volume-Averaged Cerebellar Imaging Data, Plos One. 10 (2015) e0133402. doi: 10.1371/journal.pone.0133402. [DOI] [PMC free article] [PubMed] [Google Scholar]


