Abstract
Background
To our knowledge, the rate of positive intraoperative cultures in patients undergoing primary shoulder arthroplasty with prior ipsilateral nonarthroplasty shoulder surgery is unknown. The aim of this study was to determine the incidence and predictors of positive cultures in these patients.
Methods
We performed a retrospective review of patients with prior ipsilateral shoulder surgery with intraoperative cultures taken at the time of primary shoulder arthroplasty. We evaluated culture results, demographics, and number of prior surgeries. Regression analysis was used to determine patient-related risk factors that predict positive cultures.
Results
A total of 682 patients underwent primary shoulder arthroplasty, 83 had at least 1 prior ipsilateral shoulder surgery: 65.1% male, mean age 64.2 ± 10.9 years. For the cohort of 83 patients, an average of 3.2 ± 1.2 tissue samples were obtained for each patient, with a mean of 0.84 ± 1.14 tissue cultures being positive (range 0-5). Thirty-seven of the 83 patients (44.5%) had at least 1 positive culture, with Cutibacterium acnes the most frequent organism (31/37; 83.4%). An average of 1.9 ± 0.96 tissue cultures resulted positive (range 1-5) for the 37 patients who had positive cultures, 40.5% (15/37) had only 1 positive tissue culture (12/15 C acnes, 2/15 Staphylococcus epidermidis, and 1/15 vancomycin-resistant enterococcus). Male sex and history of prior shoulder infection were predictive of culture positivity (odds ratios: 2.5 and 20.9, respectively). Age, race, medical comorbidities, number of prior shoulder surgeries, and time from index shoulder surgery were not predictive of culture positivity.
Conclusion
About 45% of patients with no clinical signs of infection and a history of prior ipsilateral shoulder surgery undergoing primary shoulder arthroplasty grew positive intraoperative cultures. The significance of these findings remains unclear with regard to risk of periprosthetic infection and how these patients should be managed.
Keywords: Shoulder arthroplasty, Cutibacterium acnes, infection, tissue culture, periprosthetic shoulder infection, prevention
The implications of unexpected positive cultures (UPCs) in the setting of shoulder arthroplasty remains unclear.20,32 Several recent studies have reported the incidence of positive cultures with no preoperative clinical signs of infection at the time of revision shoulder arthroplasty and discussed their implications on clinical outcomes as well as implant survivorship.1
Cutibacterium (formerly Propionibacterium) acnes is the most frequent pathogen implicated in perioperative shoulder infections.7,9,12,15,29 An anaerobic gram-positive bacillus, C acnes typically resides in the pilosebaceous glands of the skin of the chest, back, and axillae.10,29 Given its location, the shoulder has an increased rate of colonization compared with other sites such as the hip and knee and is found in higher quantities in males than females.24 The rates of positive intraoperative cultures differ based on the location of the specimen, with the subdermal layer being shown in previous studies to produce the highest proportion of positive culture growth.4,12 Indolent infection with C acnes, when unrecognized or untreated, may explain postoperative findings of shoulder pain, stiffness, or implant loosening, even years after index shoulder arthroplasty.19
Among the challenges in diagnosing C acnes infection are the slow-growing nature of the bacterium and the limited number of reliable diagnostic tools for predicting infection preoperatively.2,30,31 In addition, there exists a relevant potential for contamination, with one study showing a 76.7% positive culture rate from the unprepared skin incision site preceding revision shoulder arthroplasty surgery.17
To date, previously published studies in the literature have reported intraoperative culture rates in otherwise presumed uninfected shoulders for primary shoulder arthroscopy,25,29 primary open shoulder surgery,12,21 primary shoulder arthroplasty,15,16 revision shoulder arthroscopy,8 and revision shoulder arthroplasty.5,7,9,11,13,19,23,26 To our knowledge, the rate of positive intraoperative cultures in patients undergoing primary shoulder arthroplasty who have had prior ipsilateral nonarthroplasty shoulder surgery has never been reported. The aim of this study was to determine the incidence and predictors (risk factors) of positive cultures in patients undergoing primary shoulder arthroplasty who have had prior nonarthroplasty shoulder surgery.
Methods
Participants
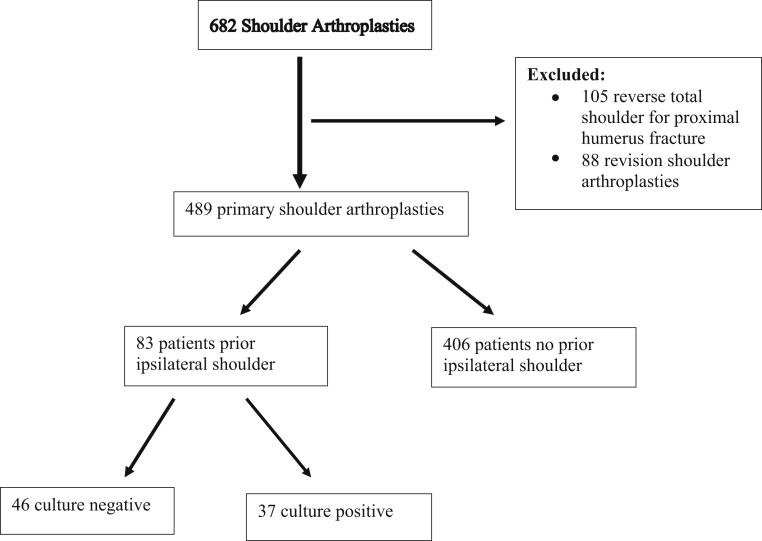
We performed a retrospective review of 682 consecutive patients undergoing shoulder arthroplasty surgery by a single surgeon at an academic university hospital over a 4-year period (January 1, 2015, to March 31, 2019). All arthroplasty surgeries were performed by the senior author, which included hemiarthroplasty, anatomic total shoulder arthroplasty, and reverse total shoulder arthroplasty. Patients undergoing revision shoulder arthroplasty (n = 88) or shoulder arthroplasty for fracture or malunion/nonunion (n = 105) were excluded. No patients had any documented clinical signs of ongoing shoulder or systemic infection. Of the remaining 489 patients undergoing primary shoulder arthroplasty, 83 had a history of prior ipsilateral shoulder surgery with intraoperative culture specimens taken at the time of primary shoulder arthroplasty (Fig. 1). Primary reverse total shoulder arthroplasty comprised the majority of procedures (n = 77), whereas the remaining included primary anatomic total shoulder arthroplasty (n = 5) and primary hemiarthroplasty (n = 2). Indications for surgical intervention included rotator cuff arthropathy (n = 66), glenohumeral arthritis (n = 13), and humeral head avascular necrosis (n = 4). The mean final follow-up for the cohort was 361.3 days (mean 12 months, range 1-43.5 months).
Figure 1.
Flow diagram for patient inclusion.
Procedures and measures
Tissue samples were taken in a standardized fashion with new clean instruments used for each tissue specimen. Tissue specimens for culture were taken at the same location in every case including subdeltoid space, tissue from the greater tuberosity at the site where sutures/suture anchors remained, and deep capsular layer adjacent to the anterior inferior glenoid rim. An average of 3.2 ± 1.2 (range 1-8) tissue specimens were taken from each shoulder joint at the time of surgery and sent to the microbiology laboratory. All patients received routine perioperative intravenous antibiotics 30 minutes before skin incision, which were continued for 24 hours postoperatively. Each sample was grown in 4 aerobic mediums (blood agar, chocolate agar, MacConkey agar, thioglycolate broth) and 3 anaerobic mediums (blood agar Centers for Disease Control formulation, biplate of bacteroides isolation agar with laked blood with kanamycin and vancomycin, anaerobic phenylethyl alcohol agar) using the standard protocol at this institution's microbiology lab. Cultures were held through the time of positive growth, or for a mean of 3.1 days for aerobic cultures and 12.2 days for anaerobic cultures that were negative for growth.
Outcome variables
The primary outcome variable was the presence of positive cultures during primary shoulder arthroplasty in the setting of patients with a history of prior ipsilateral shoulder surgery. Thus, positive culture growth was operationalized as a binary outcome, where those with at least 1 documented positive culture were considered positive. We modeled the probability of a positive culture. The total number of positive intraoperative cultures as a count variable was a secondary outcome measure.
Potential predictor variables
An initial pool of 10 characteristic variables was selected for analysis as potential predictors of presence of positive intraoperative culture. These variables were selected based on the results of previously published findings and other factors that have been associated with patients who had prior ipsilateral shoulder surgery.9,21,26 Covariates were selected a priori. The pool of potential predictors included age (years), sex (male vs. female), body mass index, type of prior shoulder surgery (open vs. arthroscopic), total number of concurrent medical problems, number of previous ipsilateral shoulder surgeries, and duration (years) between first shoulder surgery and first shoulder arthroplasty. Current smoking status, type 2 diabetes mellitus, and history of ipsilateral shoulder infection were binary indicators (yes vs. no) and also included in the pool of potential predictors.
Statistical analysis
Demographic and clinical characteristics for the sample of 83 patients who underwent ipsilateral shoulder arthroplasty were described using the sample mean and standard deviation for continuous variables and the frequency and percentage for categorical variables. To identify group differences on these characteristics between those with (n = 37) and without (n = 46) positive intraoperative cultures, we used the 2–independent sample t test with the Satterthwaite method for unequal variances (for continuous variables) and Fisher exact test (for categorical variables).
Starting with an initial pool of 10 variables, a filtering process was used to identify a subset of variables that seemed to contain discriminative or predictive power. The process was implemented using SAS's PROC HPREDUCE to perform supervised variable selection in the context of reducing dimensionality based on covariance and discriminant analysis, while minimizing the Bayesian information criterion (BIC) scores. The “best” subset selection of regressors (predictors) that emerged from this supervised variable selection (filtering) process included sex and history of ipsilateral shoulder infection. Next, and on the basis of the variable filtering process with sex (male vs. female) and history of ipsilateral shoulder infection (yes vs. no) included as regressors, a multiple logistic regression model, with penalized maximum likelihood estimation along with Firth's bias correction, was implemented to estimate the odds (or probability) of positive intraoperative culture, and a generalized negative binomial (Poisson) mixed model, with maximum likelihood estimation and robust standard errors (HC3 first-order residual empirical estimator), was used to estimate the number of positive cultures. Finally, we implemented 10,000 bootstrap samples on each of the above-mentioned regression models and reported the bootstrapped parameter estimates (mean and standard deviation) along with the 95% bootstrap confidence interval based on bootstrapped averages. For the 95% confidence interval (CI) that did not contain zero, the respective mean parameter estimate was statistically significant at alpha = 0.05 (2-tailed).
Statistical analyses were carried out using SAS software, version 9.4 (SAS Institute, Inc., Cary, NC, USA). The procedures of PROC HPREDUCE, PROC LOGISTIC, and PROC GLIMMIX in SAS software were used to conduct the analysis. The level of significance was set at α = 0.05 (2-tailed).
Results
Demographic and clinical characteristics of the 83 patients who underwent ipsilateral shoulder arthroplasty in the current study are shown in Table I. Of the 83 patients, 65.1% were male, and the mean age was 64.2 ± 10.9 years (range, 22-84 years). The mean number of ipsilateral shoulder surgeries was 1.4 ± 0.87 (range, 1-7), with an average of 9.7 ± 10.1 years between first shoulder surgery and primary shoulder arthroplasty. About 80% of patients had arthroscopic-only procedures compared with about 20% having at least 1 open procedure. Approximately 6% had a history of ipsilateral shoulder infection, all of whom grew at least 1 positive culture. History of prior ipsilateral shoulder postoperative infection was defined based on patient history detailing treatment with a course of either oral or intravenous antibiotics.
Table I.
Demographic and clinical characteristics of patients with and without positive cultures during shoulder arthroplasty
| Characteristic | Overall sample (N = 83) | Positive cultures (n = 37) | No positive cultures (n = 46) | P value |
|---|---|---|---|---|
| Demographics | ||||
| Age, yr, M (SD) | 64.20 (10.91) | 63.78 (12.84) | 64.54 (9.20) | .76 |
| Male sex, n (%) | 54 (65.06) | 27 (72.97) | 27 (58.70) | .25 |
| White, non-Hispanic, n (%) | 76 (91.57) | 33 (89.19) | 43 (93.48) | .67 |
| Patient factors | ||||
| Currently a smoker, n (%) | 11 (13.25) | 04 (10.81) | 07 (15.22) | .75 |
| BMI, M (SD) | 29.82 (5.06) | 30.07 (5.18) | 29.61 (5.01) | .69 |
| Total number of medical problems, M (SD) | 1.41 (1.25) | 1.48 (1.17) | 1.34 (1.32) | .62 |
| History of ipsilateral shoulder infection, n (%) | 05 (6.02) | 05 (13.51) | 0 (0.00) | .015∗ |
| Type of previous shoulder surgery, n (%) | .58 | |||
| Arthroscopic | 61 (79.22) | 25 (75.76) | 36 (81.82) | |
| Open | 16 (20.78) | 08 (24.24) | 08 (18.18) | |
| Number of ipsilateral shoulder surgeries, M (SD) | 1.43 (0.87) | 1.32 (0.53) | 1.52 (1.07) | .28 |
| Duration between surgeries, years, M (SD) | 9.69 (10.05) | 9.51 (9.48) | 9.85 (10.62) | .89 |
| Comorbidities, n (%) | ||||
| Type 2 diabetes | 22 (26.51) | 10 (27.03) | 12 (26.09) | >.99 |
| Rheumatoid arthritis | 05 (6.02) | 02 (5.41) | 03 (6.52) | >.99 |
| Thyroid disease | 14 (16.87) | 04 (10.81) | 10 (21.74) | .24 |
| Hepatitis C | 06 (7.23) | 03 (8.11) | 03(6.52) | >.99 |
| Heart disease | 17 (20.48) | 11 (29.73) | 06 (13.04) | .10 |
| Laboratory values, n (%) | ||||
| Elevated preoperative CRP | 11 (21.15) | 06 (25.00) | 05 (17.86) | .75 |
| Elevated preoperative ESR | 10 (23.26) | 05 (21.74) | 05 (25.00) | >.99 |
| Elevated preoperative WBC count | 02 (2.60) | 00 (0.00) | 02 (4.76) | .50 |
M, sample mean; SD, standard deviation; BMI, body mass index; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; WBC, white blood cell.
Positive culture was operationalized as the presence of at least 1 positive culture. The P value (2-tailed) is associated with the test of Group differences (positive cultures vs. no presence of positive cultures) on each characteristic; t test was used for continuous variables and Fisher exact test was used for categorical variables. Duration between surgeries = time (years) between first shoulder surgery and first shoulder arthroplasty.
False discovery rate = 0.27.
An average of 3.4 ± 1.2 tissue samples were sent for culture for each patient. Cultures were positive in at least 1 tissue sample in 44.5% (37/83) of patients. An average of 1.9 ± 0.96 tissue samples were positive (range 1-5) from the average 3.4 ± 1.5 samples taken in these 37 patients, accounting for 47 total positive cultures of the 88 total samples drawn (53.4%). C acnes was the most frequent organism, present in 83.4% (31/37) of the patients who grew at least 1 positive culture. The remaining pathogens included Staphylococcus epidermidis, diphtheroid bacilli, Staphylococcus saccharolyticus, Enterococcus faecalis, Enterococcus amnigenus, vancomycin-resistant enterococcus, and Cladosporium fungal species. The average time to final positive growth and speciation among the aerobic and anaerobic organisms was 2.3 and 7.4 days, respectively. Fifteen of 37 patients (40.5%) grew only 1 positive tissue culture (12 C acnes, 2 S epidermidis, and 1/15 vancomycin-resistant enterococcus), whereas the remainder grew 2 or more positive tissue samples.
Using a binary outcome of positive vs. negative cultures, bootstrapped results from the logistic regression model revealed that males had greater predicted odds of a positive culture during shoulder arthroplasty than females (mean odds ratio [OR] = 2.51, 95% bootstrap CI: 0.96, 7.57), while controlling for history of shoulder infection, albeit not significant. However, those with a history of shoulder infection had significantly greater predicted odds of a positive culture during shoulder arthroplasty than those without a history of shoulder infection (mean OR = 20.95, 95% bootstrap CI: 5.54, 67.22), while controlling for sex. The bootstrapped area under the curve (AUC) for this logistic regression model was 0.64 (Table II).
Table II.
Multiple logistic regression model for predictors of positive culture
| Model outcome and predictor variables∗ | Bootstrapped parameter estimates |
||||||
|---|---|---|---|---|---|---|---|
| Mean estimate | SD | 95% CI for estimate | Mean OR | 95% CI for OR | Standardized estimate | Mean AUC | |
| Positive culture (positive vs. negative) | 0.6468 | ||||||
| Intercept | –0.9999 | 0.4565 | –1.9938, –0.1910 | 0 | |||
| Sex (male vs. female) | 0.9239 | 0.5324 | –0.0369, 2.0254 | 2.5191 | 0.9637, 7.5791 | 0.2098 | |
| History of ipsilateral shoulder infection (yes vs. no) | 3.0423 | 0.6175 | 1.7135, 4.2081 | 20.9533 | 5.5483, 67.2287 | 0.4233 | |
SD, standard deviation; OR, odds ratio; AUC, area under the curve.
The parameter estimates were based on 10,000 bootstrap samples of the logistic regression model, with penalized maximum likelihood estimation along with Firth's bias correction. The mean and variance were estimated on the logarithmic scale and represent log odds; 95% CI for the mean parameter estimate. For the 95% CI for estimate that does not contain zero (0), the respective mean parameter estimate is statistically significant at alpha = 0.05. Observed sample, N = 83.
Predictor variables for the model were selected from a pool of 10 potential predictor variables via SAS's PROC HPREDUCE to perform supervised variable selection and then implemented in the context of a penalized logistic regression model that was based on 10,000 bootstrap samples.
Similar findings emerged for the count variable of number of positive cultures. The bootstrapped results from the negative binomial regression model revealed that the predicted or expected log odds of number of positive cultures during shoulder arthroplasty was significantly greater for males than for females (geometric mean = 2.43 positive cultures, 95% bootstrap CI = 1.25, 5.61), while controlling for a history of shoulder infection. And those with a history of shoulder infection had significantly greater predicted or expected log odds of number of positive cultures during shoulder arthroplasty than those without a history of shoulder infection (geometric mean = 3.49 positive cultures, 95% bootstrap CI = 2.08, 7.29), while controlling for sex. The bootstrapped results for the negative binomial regression model for number of positive cultures are shown in Table III.
Table III.
Multiple negative binomial regression model for predictors of number of positive cultures
| Model outcome and predictor variables∗ | Bootstrapped parameter estimates |
||||
|---|---|---|---|---|---|
| Mean estimate | SD | 95% CI for mean estimate | GM | 95% CI for GM | |
| Number of positive cultures | |||||
| Intercept | –0.9585 | 0.3852 | –1.7492, –0.3629 | 0.3834 | 0.1739, 0.6956 |
| Sex (male vs. female) | 0.8912 | 0.4089 | 0.2239, 1.7248 | 2.4381 | 1.2509, 5.6114 |
| History of ipsilateral shoulder infection (yes vs. no) | 1.2515 | 0.3451 | 0.7339, 1.9871 | 3.4955 | 2.0832, 7.2943 |
SD, standard deviation; CI, confidence interval; GM, geometric mean (natural antilogarithm of the ln mean estimate).
The parameter estimates were based on 10,000 bootstrap samples of the negative binomial model. The mean and variance were estimated on the logarithmic scale. For the 95% CI that does not contain zero, the respective mean parameter estimate is statistically significant at alpha = 0.05. Observed sample, N = 83.
Predictor variables for the model were selected from a pool of 10 potential predictor variables via SAS's PROC HPREDUCE to perform supervised variable selection and then implemented in the context of a negative binomial model that was based on 10,000 bootstrap samples.
At the time of final follow-up at 361.3 days (mean 12 months, range 1-43.5 months), no complications were reported related to infection, implant loosening/failure, or return to surgery. One patient did sustain a periprosthetic fracture during the postoperative course after a fall during an altercation, which was fixed with open reduction internal fixation and revision arthroplasty.
Discussion
The results of this investigation demonstrate a high rate of positive cultures in this patient population, with 44.5% of patients having at least 1 positive intraoperative culture at the time of their primary shoulder arthroplasty procedure. Postoperative shoulder infection may present as indolent shoulder pain, stiffness, or implant loosening, and poses a major challenge to patient outcomes and health care costs following shoulder arthroplasty surgery.15,19 Therefore, a focus on prevention and recognition is paramount. The goal of this study was to identify the incidence and risk factors of UPCs in the setting of primary shoulder arthroplasty in a cohort of patients with a history of prior ipsilateral shoulder surgery. To our knowledge, this is the first study examining the incidence of positive cultures found at the time of primary shoulder arthroplasty in patients who have undergone prior ipsilateral shoulder surgery. These findings are comparable to the rates of UPCs found at the time of revision shoulder arthroplasty.5,7, 8, 9,11, 12, 13,15,16,19,21,23,25,26,29 The ramification of these findings as well as how and if these cultures should be treated with antibiotics to prevent future periprosthetic infection is unknown.
UPC rates in revision shoulder arthroplasty have been well documented. Foruria et al5 found a 15% rate of UPCs in a cohort of 107 consecutive patients undergoing revision shoulder arthroplasty without any other cause for suspicion of infection. In other studies, the reported rates have been even higher, ranging from 23.9%-56.0% UPCs during revision shoulder arthroplasty.13,23,26 Although Foruria et al5 postulated that at least one-quarter of their positive cultures had no true clinical relevance, persistent infection was found in as many as 10% (11 of 107), from which the organisms matched the index intraoperative findings. They further discovered that the number of previous surgeries statistically correlated with the likelihood of true infection vs. contamination, a risk factor that did not reach statistical significance in predicting culture positivity in our study.5
In similarly conducted studies, Mook et al21 reported a 20.5% rate of UPCs in primary open shoulder surgery and Sethi et al29 reported a 56% rate of UPCs in primary shoulder arthroscopy. Levy et al15 reported a 41.8% rate (23/55 patients) of unexpected positive C acnes cultures in primary shoulder arthroplasty, although patients with previous ipsilateral shoulder surgery were excluded.
We found that 44.5% (37/83) of the patients in our cohort had at least 1 positive culture. Within this cohort of 37 patients with a UPC, 53.4% (47/88) of the intraoperative tissue samples drawn were positive. C acnes was by far the most frequent organism, present in 83.4% (31/37) of the patients who grew at least 1 positive culture. Fifteen of the 37 patients (40.5%) grew only 1 positive tissue culture. It is uncertain if there is any significance to these cultures given there is a 20% false positive culture rate for C acnes.20,22 Additionally, the incidence of positive C acnes cultures in native shoulders with no history of prior shoulder surgery is 9%-41% at the time of primary shoulder arthroplasty.12,15,18,27 Therefore, it is unknown if the percentage of patients who grew C acnes (80%) in this series is consistent with what has been shown previously as a result of pre-existing C acnes in the subdermal skin layer or as a result of the previous surgery.
We also found that male sex and those with a history of prior shoulder infection were predictive of culture positivity within this cohort and have greater predicted number of positive cultures. As demonstrated in several previous studies, we also found greater risk of positive cultures in male shoulders.4,9,12,14,19,21,26,28
The remainder of the potential risk factors had no statistically relevant prediction to the likelihood of having positive intraoperative cultures. These included patient age, race, smoking status, body mass index, number or type of comorbidities, total number of prior ipsilateral shoulder surgeries, and the proportion of open (vs. arthroscopic) procedures. The presence of elevated preoperative inflammatory markers (white blood cell count, erythrocyte sedimentation rate, C-reactive protein) was also not predictive of positive intraoperative cultures., which is similar to the previously reported notion that indolent C acnes infection or colonization can present with quite variable or even normal indices.3,30
At the time of final follow-up, no patients had any clinical evidence of infection, infection-related complications, or infection-related return to surgery. With a mean follow-up of 12 months, it is not possible to draw any definite conclusions from these findings, although that was not the primary purpose of the investigation. To substantiate long-term infection risks from this patient cohort, extended follow-up is necessary.
The retrospective nature of this study presents several limitations. First, it potentiates a selection bias. It has been standard practice of the senior surgeon to obtain tissue samples in this type of patient population who could be considered higher risk, although the lack of a well-defined prospective protocol obviated what could have made for a higher sample size in this investigation. A second limitation is the relatively small sample size, which does not allow for subgroup analysis of the patient-related factors, which could explain why only approximately half of the subjects had positive cultures, Patzer et al16, 25 found a higher prevalence of C acnes in the glenohumeral space compared with the subacromial space in patients undergoing primary shoulder arthroscopy, whereas Maccioni et al16, 25 highlighted the importance of strict tissue sample collection techniques to decrease contamination and positive cultures. Prospectively defining a protocol for reliable tissue collection sites and methods could mitigate any inconsistency.
Given surgeries within this patient cohort were performed at a single institution by a single surgeon, extrapolating data to other institutions could be limited by differences in specific lab protocols and contamination rates, a factor not specifically addressed by this study. Time to final growth and speciation of C acnes was 8.4 days in our study, comparable to the average duration in similar studies.2,6 Frangiamore et al6 found that the time to C acnes growth was significantly shorter in a probable-true positive culture group (5 days) compared with a probable contaminant group (9 days). The duration of time that samples were held for our study, therefore, was likely adequate, as the samples that never resulted in positive growth were kept for a mean of 3.1 and 12.2 days for aerobic and anaerobic cultures, respectively.
The results of this study help to better understand the likelihood of obtaining a positive culture result in the setting of primary shoulder arthroplasty in patients with a history of previous ipsilateral shoulder surgery. We found that 83.4% of these patients will have a positive culture for C acnes. Although further investigation is needed to determine its significance in regard to long-term clinical outcomes and implant survivorship, it does reinforce the importance of cautious awareness of sterile technique as well as the increased risk of C acnes bacterial biofilm in a patient population that is otherwise categorized as undergoing “primary” arthroplasty surgery. Additionally, what is unknown is if these cultures are a result of contamination from prior surgical procedures or identification of the already existing bacteria that is found in the subdermal layers.
Conclusions
About 45% of patients with no clinical signs of infection and a history of prior ipsilateral shoulder surgery undergoing primary shoulder arthroplasty grew positive intraoperative cultures for C acnes. Male sex and a history of prior shoulder infection were predictive of culture positivity within this cohort. Similar to UPCs found during revision shoulder arthroplasty, the significance of these findings remain unclear in regard to risk of progression to clinically meaningful infection and how these patients should be managed.
Disclaimer
Michael Khazzam is a consultant for Tornier/Wright Medical, but the consulting work is unrelated to the subject of this work.
The other authors, their immediate families, and any research foundations with which they are affiliated have not received any financial payments or other benefits from any commercial entity related to the subject of this article.
Footnotes
Institutional review board approval was received from the University of Texas Southwestern (CR00020726/ STU 062016-012).
References
- 1.Brolin T.J., Hackett D.J., Abboud J.A., Hsu J.E., Namdari S. Routine cultures for seemingly aseptic revision shoulder arthroplasty: are they necessary? J Shoulder Elbow Surg. 2017;26:2060–2066. doi: 10.1016/j.jse.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 2.Dodson C.C., Craig E.V., Cordasco F.A., Dines D.M., Dines J.S., Dicarlo E. Propionibacterium acnes infection after shoulder arthroplasty: a diagnostic challenge. J Shoulder Elbow Surg. 2010;19:303–307. doi: 10.1016/j.jse.2009.07.065. [DOI] [PubMed] [Google Scholar]
- 3.Dramis A., Aldlyami E., Grimer R.J., Dunlop D.J., O'Connell N., Elliott T. What is the significance of a positive Propionibacterium acnes culture around a joint replacement? Int Orthop. 2009;33:829–833. doi: 10.1007/s00264-008-0534-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Falconer T.M., Baba M., Kruse L.M., Dorrestijn O., Donaldson M.J., Smith M.M. Contamination of the surgical field with Propionibacterium acnes in primary shoulder arthroplasty. J Bone Joint Surg Am. 2016;98:1722–1728. doi: 10.2106/JBJS.15.01133. [DOI] [PubMed] [Google Scholar]
- 5.Foruria A.M., Fox T.J., Sperling J.W., Cofield R.H. Clinical meaning of unexpected positive cultures (UPC) in revision shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22:620–627. doi: 10.1016/j.jse.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 6.Frangiamore S.J., Saleh A., Grosso M.J., Alolabi B., Bauer T.W., Iannotti J.P. Early versus late culture growth of Propionibacterium acnes in revision shoulder arthroplasty. J Bone Joint Surg Am. 2015;97:1149–1158. doi: 10.2106/JBJS.N.00881. [DOI] [PubMed] [Google Scholar]
- 7.Grosso M.J., Sabesan V.J., Ho J.C., Ricchetti E.T., Iannotti J.P. Reinfection rates after 1-stage revision shoulder arthroplasty for patients with unexpected positive intraoperative cultures. J Shoulder Elbow Surg. 2012;21:754–758. doi: 10.1016/j.jse.2011.08.052. [DOI] [PubMed] [Google Scholar]
- 8.Horneff J.G., 3rd, Hsu J.E., Voleti P.B., O'Donnell J., Huffman G.R. Propionibacterium acnes infection in shoulder arthroscopy patients with postoperative pain. J Shoulder Elbow Surg. 2015;24:838–843. doi: 10.1016/j.jse.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 9.Hou C., Gupta A., Chen M., Matsen F.A., 3rd How do revised shoulders that are culture positive for Propionibacterium differ from those that are not? J Shoulder Elbow Surg. 2015;24:1427–1432. doi: 10.1016/j.jse.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 10.Hsu J.E., Bumgarner R.E., Matsen F.A., 3rd Propionibacterium in shoulder arthroplasty: what we think we know today. J Bone Joint Surg Am. 2016;98:597–606. doi: 10.2106/JBJS.15.00568. [DOI] [PubMed] [Google Scholar]
- 11.Hsu J.E., Gorbaty J.D., Whitney I.J., Matsen F.A., 3rd Single-stage revision is effective for failed shoulder arthroplasty with positive cultures for propionibacterium. J Bone Joint Surg Am. 2016;98:2047–2051. doi: 10.2106/JBJS.16.00149. [DOI] [PubMed] [Google Scholar]
- 12.Hudek R., Sommer F., Kerwat M., Abdelkawi A.F., Loos F., Gohlke F. Propionibacterium acnes in shoulder surgery: true infection, contamination, or commensal of the deep tissue? J Shoulder Elbow Surg. 2014;23:1763–1771. doi: 10.1016/j.jse.2014.05.024. [DOI] [PubMed] [Google Scholar]
- 13.Kelly J.D., 2nd, Hobgood E.R. Positive culture rate in revision shoulder arthroplasty. Clin Orthop Relat Res. 2009;467:2343–2348. doi: 10.1007/s11999-009-0875-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khan U., Torrance E., Townsend R., Davies S., Mackenzie T., Funk L. Low-grade infections in nonarthroplasty shoulder surgery. J Shoulder Elbow Surg. 2017;26:1553–1561. doi: 10.1016/j.jse.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 15.Levy O., Iyer S., Atoun E., Peter N., Hous N., Cash D. Propionibacterium acnes: an underestimated etiology in the pathogenesis of osteoarthritis? J Shoulder Elbow Surg. 2013;22:505–511. doi: 10.1016/j.jse.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 16.Maccioni C.B., Woodbridge A.B., Balestro J.C., Figtree M.C., Hudson B.J., Cass B. Low rate of Propionibacterium acnes in arthritic shoulders undergoing primary total shoulder replacement surgery using a strict specimen collection technique. J Shoulder Elbow Surg. 2015;24:1206–1211. doi: 10.1016/j.jse.2014.12.026. [DOI] [PubMed] [Google Scholar]
- 17.Matsen F.A., 3rd, Butler-Wu S., Carofino B.C., Jette J.L., Bertelsen A., Bumgarner R. Origin of propionibacterium in surgical wounds and evidence-based approach for culturing propionibacterium from surgical sites. J Bone Joint Surg Am. 2013;95:e1811–e1817. doi: 10.2106/JBJS.L.01733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsen F.A., 3rd, Russ S.M., Bertelsen A., Butler-Wu S., Pottinger P.S. Propionibacterium can be isolated from deep cultures obtained at primary arthroplasty despite intravenous antimicrobial prophylaxis. J Shoulder Elbow Surg. 2015;24:844–847. doi: 10.1016/j.jse.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 19.McGoldrick E., McElvany M.D., Butler-Wu S., Pottinger P.S., Matsen F.A., 3rd Substantial cultures of Propionibacterium can be found in apparently aseptic shoulders revised three years or more after the index arthroplasty. J Shoulder Elbow Surg. 2015;24:31–35. doi: 10.1016/j.jse.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 20.Mook W.R., Garrigues G.E. Diagnosis and management of periprosthetic shoulder infections. J Bone Joint Surg Am. 2014;96:956–965. doi: 10.2106/JBJS.M.00402. [DOI] [PubMed] [Google Scholar]
- 21.Mook W.R., Klement M.R., Green C.L., Hazen K.C., Garrigues G.E. The incidence of Propionibacterium acnes in open shoulder surgery: a controlled diagnostic study. J Bone Joint Surg Am. 2015;97:957–963. doi: 10.2106/JBJS.N.00784. [DOI] [PubMed] [Google Scholar]
- 22.Namdari S., Nicholson T., Parvizi J., Ramsey M. Preoperative doxycycline does not decolonize Propionibacterium acnes from the skin of the shoulder: a randomized controlled trial. J Shoulder Elbow Surg. 2017;26:1495–1499. doi: 10.1016/j.jse.2017.06.039. [DOI] [PubMed] [Google Scholar]
- 23.Padegimas E.M., Lawrence C., Narzikul A.C., Zmistowski B.M., Abboud J.A., Williams G.R. Future surgery after revision shoulder arthroplasty: the impact of unexpected positive cultures. J Shoulder Elbow Surg. 2017;26:975–981. doi: 10.1016/j.jse.2016.10.023. [DOI] [PubMed] [Google Scholar]
- 24.Patel A., Calfee R.P., Plante M., Fischer S.A., Green A. Propionibacterium acnes colonization of the human shoulder. J Shoulder Elbow Surg. 2009;18:897–902. doi: 10.1016/j.jse.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 25.Patzer T., Petersdorf S., Krauspe R., Verde P.E., Henrich B., Hufeland M. Prevalence of Propionibacterium acnes in the glenohumeral compared with the subacromial space in primary shoulder arthroscopies. J Shoulder Elbow Surg. 2018;27:771–776. doi: 10.1016/j.jse.2017.10.039. [DOI] [PubMed] [Google Scholar]
- 26.Pottinger P., Butler-Wu S., Neradilek M.B., Merritt A., Bertelsen A., Jette J.L. Prognostic factors for bacterial cultures positive for Propionibacterium acnes and other organisms in a large series of revision shoulder arthroplasties performed for stiffness, pain, or loosening. J Bone Joint Surg Am. 2012;94:2075–2083. doi: 10.2106/JBJS.K.00861. [DOI] [PubMed] [Google Scholar]
- 27.Rao A.J., Chalmers P.N., Cvetanovich G.L., O'Brien M.C., Newgren J.M., Cole B.J. Preoperative doxycycline does not reduce Propionibacterium acnes in shoulder arthroplasty. J Bone Joint Surg Am. 2018;100:958–964. doi: 10.2106/JBJS.17.00584. [DOI] [PubMed] [Google Scholar]
- 28.Richards J., Inacio M.C., Beckett M., Navarro R.A., Singh A., Dillon M.T. Patient and procedure-specific risk factors for deep infection after primary shoulder arthroplasty. Clin Orthop Relat Res. 2014;472:2809–2815. doi: 10.1007/s11999-014-3696-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sethi P.M., Sabetta J.R., Stuek S.J., Horine S.V., Vadasdi K.B., Greene R.T. Presence of Propionibacterium acnes in primary shoulder arthroscopy: results of aspiration and tissue cultures. J Shoulder Elbow Surg. 2015;24:796–803. doi: 10.1016/j.jse.2014.09.042. [DOI] [PubMed] [Google Scholar]
- 30.Shields M.V., Abdullah L., Namdari S. The challenge of Propionibacterium acnes and revision shoulder arthroplasty: a review of current diagnostic options. J Shoulder Elbow Surg. 2016;25:1034–1040. doi: 10.1016/j.jse.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 31.Topolski M.S., Chin P.Y., Sperling J.W., Cofield R.H. Revision shoulder arthroplasty with positive intraoperative cultures: the value of preoperative studies and intraoperative histology. J Shoulder Elbow Surg. 2006;15:402–406. doi: 10.1016/j.jse.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 32.Updegrove G.F., Armstrong A.D., Kim H.M. Preoperative and intraoperative infection workup in apparently aseptic revision shoulder arthroplasty. J Shoulder Elbow Surg. 2015;24:491–500. doi: 10.1016/j.jse.2014.10.005. [DOI] [PubMed] [Google Scholar]