Abstract
Naturally occurring alterations in estradiol influence food intake in females. However, how motivational responses to food cues are affected by the estrous cycle or ovarian hormones is unknown. In addition, while individual susceptibility to obesity is accompanied by enhanced incentive motivational responses to food cues and increased NAc intrinsic excitability in males, studies in females are absent. Therefore, we examined basal differences in intrinsic NAc excitability of obesity-prone vs. obesity-resistant females and determined how conditioned approach (a measure of cue-triggered motivation), food intake, and motivation for food vary with the cycle in naturally cycling female obesity-prone, obesity-resistant, and outbred Sprague-Dawley rats. Finally, we used ovariectomy followed by hormone treatment to determine the role of ovarian hormones in cue-triggered motivation in selectively-bred and outbred female rats. We found that intrinsic excitability of NAc MSNs and conditioned approach are enhanced in female obesity-prone vs. obesity-resistant rats. These effects were driven by greater MSN excitability and conditioned approach behavior during metestrus/diestrus vs. proestrus/estrus in obesity-prone but not obesity-resistant rats, despite similar regulation of food intake and food motivation by the cycle in these groups. Furthermore, estradiol and progesterone treatment reduced conditioned approach behavior in obesity-prone and outbred Sprague-Dawley females. To our knowledge, these data are the first to demonstrate cycle- and hormone-dependent effects on the motivational response to a food cue, and the only studies to date to determine how individual susceptibility to obesity influences NAc excitability, cue-triggered food-seeking, and differences in the regulation of these neurobehavioral responses by the estrous cycle.
1. Introduction
Naturally occurring fluctuations in ovarian hormones influence food consumption and food preference in both humans and rodents (Eckel, 2011; Hirschberg, 2012). For example, in rats, food intake is lower during estrus compared to metestrus and diestrus (Tarttelin and Gorski, 1971). This effect of the cycle on food intake is mediated in part by central actions of estrogens (see Asarian and Geary, 2013 for review). Consistent with this, the removal of ovarian hormones via ovariectomy produces significant increases in food intake that are accompanied by weight gain (Blaustein and Wade, 1976; Yu et al., 2011). This increase can be normalized by treating the ovariectomized rats with estradiol (Tarttelin and Gorski, 1971; Wade, 1972; Asarian and Geary, 2002, 2006; Yu et al., 2011). Thus, ovarian hormones influence food intake.
In overweight women, stimuli that predict food availability (i.e., food cues) elicit stronger activations in brain regions that influence motivation including the Nucleus Accumbens (NAc; Stoeckel et al., 2008) compared to lean women. Furthermore, the magnitude of food cue elicited NAc activations predicts future weight gain in healthy weight females and future inability to lose weight after obesity (Murdaugh et al., 2012; Demos et al., 2012). These human studies suggest that enhanced neurobehavioral responses to food cues may promote the development of obesity. However, identifying pre-existing differences is not feasible in human studies and there is limited understanding of how ovarian hormones influence brain mechanisms governing cue-triggered food seeking (see Alonso-Caraballo et al., 2018 for additional discussion).
Consistent with a role for pre-existing differences, preclinical studies from our lab have shown that motivational responses to food cues are stronger in obesity-prone compared to obesity-resistant male rats (Derman and Ferrario, 2018a; Robinson et al., 2015). These behavioral differences in males are mediated by the NAc (Derman and Ferrario, 2018a) and intrinsic excitability of medium spiny neurons (MSNs) in the NAc is enhanced in obesity-prone vs. obesity-resistant males (Oginsky et al., 2016a). The risk for obesity is greater in females compared to males, and ovarian hormones influence NAc activity and plasticity (Cyr et al., 2001; Le Saux et al., 2006; Peterson et al., 2015; Cao et al., 2018). However, it is not known whether similar enhancements in motivational responses to food cues are also found in female obesity-prone vs. obesity-resistant rats. Furthermore, neural activations in response to monetary rewards or to visual food cues vary with the menstrual cycle in women, with higher activations observed in corticolimbic areas during the luteal phase compared to the follicular phase (Dreher et al., 2007; Frank et al., 2010; Arnoni-Bauer et al., 2017). This suggests that motivational responses to food cues may be influenced by ovarian hormones; however, no studies to date have directly examined this possibility.
In the current study, we first examined basal differences in intrinsic excitability of NAc MSNs of obesity-prone vs. obesity-resistant females across the cycle. Next, we determined how motivational responses to Pavlovian food cues and motivation for food itself vary with the cycle in naturally cycling female obesity-prone and obesity-resistant rats. Finally, we used ovariectomy followed by hormone treatment to determine the role of ovarian hormones in cue-triggered motivation in selectively-bred and outbred female rats.
2. Materials and methods
2.1. Subjects
Female selectively-bred obesity-prone (OP) and obesity-resistant (OR) rat lines were originally developed by Barry Levin (Levin et al., 1997) and were bred in house. Female outbred Sprague-Dawley rats were purchased from Charles River Breeding Labs (Portage, MI). All rats were approximately 70 days old at the time of behavioral testing and electrophysiological recordings. Rats were housed on a reverse 12-h light/dark cycle, had free access to food and water throughout, and were group housed unless otherwise noted. All behavioral testing was conducted approximately 4–6 h into the dark cycle. For studies involving ovariectomy, estrogen-free bedding (7090 Teklad sani-chips) and estrogen-free chow (Harlan 2916: 3 kcal/g; 4% fat; 16.4% protein; 48.5% protein; % of caloric content) were used. Rats were weighed 3–4 times per week unless otherwise specified. Procedures were approved by The University of Michigan Committee on the Use and Care of Animals in accordance with AAALAC and AVMA guidelines. See also https://sites.google.com/a/umich.edu/ferrario-lab-public-protocols/ for additional details.
2.2. Monitoring the estrous cycle
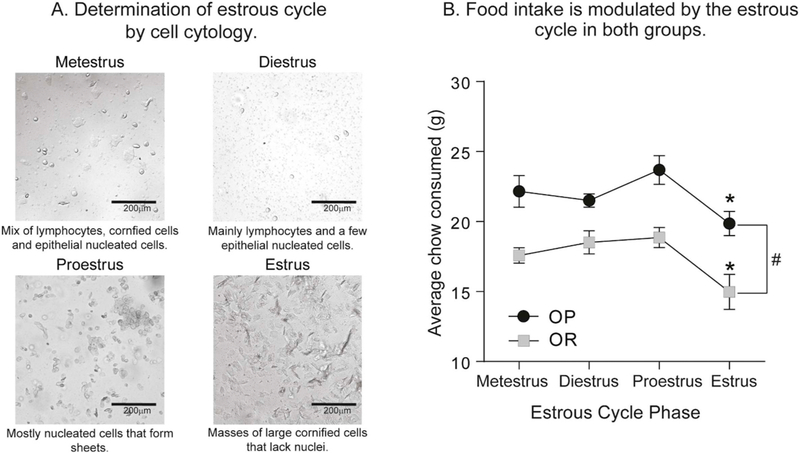
Estrous cycle phase was determined by daily observations of vaginal epithelial cell cytology, precopulatory, and copulatory behaviors (Marcondes et al., 2002). Epithelial cells were collected daily by vaginal lavage (during the dark phase) and visualized using an inverted light microscope (Olympus CKX53) under bright-field. Estrous cycle cell pictures were taken at 20× magnification with an EVOS XL Cell Imaging System (Thermo Fisher Scientific). Cell morphology was then used to determine cycle phase with metestrus characterized by a mix of lymphocytes, cornified cells and epithelial nucleated cells, diestrus by lymphocytes and a little to any epithelial nucleated cells, proestrus by nucleated cells that form sheets, and estrus by masses of large cornified cells that lack nuclei (see Fig. 2A). Body weight, food intake, precopulatory and copulatory behaviors (such as ear wiggling, darting, and lordosis) were also used to further verify the estrous cycle phase.
Fig. 2.
Home cage food intake decreases during estrus in both obesity-prone and obesity-resistant female rats. A) Representative pictures from vaginal lavages at each phase of the estrous cycle in female rats. B) Home cage chow consumption across the cycle. Home-cage chow consumption was greater in obesity-prone (OP) vs. obesity-resistant (OR) rats, and was reduced during estrus in both groups. * = planned post-hoc comparisons, # = obesity-prone vs. obesity-resistant rats, p < 0.05.
2.3. Behavioral training and testing
For all behavioral studies, rats were trained and tested hungry (chow removed from cages, 5–6 h prior to training/testing and replaced following training/testing). All training and testing occurred in standard operant boxes (Med Associates, St. Albans City, VT) housed within sound attenuating chambers. Testing occurred at the same time each day, approximately 6–8 h after the start of the dark cycle.
2.3.1. Instrumental procedures
To evaluate motivation for food in obesity-prone and obesity-resistant females, we used instrumental training followed by progressive ratio testing. First, rats underwent two magazine training sessions in which 20 sucrose pellets (45 mg TestDiet; cat. #1811251) were delivered into the food cup on a variable interval of 60 s (VI 60). Next, they were trained to press one lever (active) that resulted in the delivery of one sucrose pellet. Pressing a second lever (inactive) had no consequences but was recorded. No discrete cues were paired with pellet delivery. Left/right position of the active and inactive levers relative to the food cup was counterbalanced. Rats were trained in three sessions in which each response on the active lever resulted in delivery of a food pellet (i.e., fixed ratio 1 [FR1], 60 min/session or until 50 pellets were earned). This was followed by three sessions in which five responses on the active lever were required to receive one food pellet (FR5, 60 min/session or until 50 pellets were earned). Progressive ratio (PR) testing was then used to determine motivation to obtain food. During PR testing the number of lever presses required to obtain each subsequent sucrose pellet increased exponentially (5e(0.2*delivery+1)-5; adapted from (Richardson and Roberts, 1996). The PR session ended when rats did not meet the next ratio requirement within 60 min (i.e., breakpoint). The number of active and inactive lever presses, pellets earned, and final break point achieved were recorded.
2.3.2. Pavlovian procedures
First rats underwent two magazine training sessions in which 20 sucrose pellets were delivered into the food cup on a VI 60 schedule. During Pavlovian conditioning sessions, one auditory cue (CS+; 2 min) was paired with the delivery of 4 sucrose pellets (US), whereas a second auditory cue (CS−; 2 min) was never paired with sucrose (CS+/CS− tone or white noise counterbalanced). Sucrose pellets were delivered on a VI of 20 s during CS+ presentation. A total of 4 CS+ and 4 CS− trials separated by a variable 5-min inter-trial-interval (ITI; range 2–7 min) were given per session (5–8 sessions, 60 min per session, 1 session/day). Subsequent testing was identical to initial conditioning except that no sucrose was given. Food cup entries during the ITI, CS+ and CS− periods were recorded throughout.
2.4. Electrophysiology
Whole-cell patch clamp recordings of MSNs in the NAc core were conducted in naturally cycling obesity-prone and obesity-resistant rats (P70-P80) during the metestrus/diestrus or proestrus/estrus phases using established procedures (e.g., Oginsky et al., 2016b). Briefly, rats were anesthetized with chloral hydrate (400 mg/kg, i.p.) prior to slice preparation, brains were rapidly removed and placed in ice-cold oxygenated (95% O2–5% CO2) aCSF containing (in mM): 125 NaCl, 25 NaHCO3, 12.5 glucose, 1.25 NaH2PO4, 3.5 KCl, 1 L-ascorbic acid, 0.5 CaCl2, 3 MgCl2, pH 7.4, 305 mOsm. A vibratome (Leica Biosystems, Buffalo Grove, IL, USA) was used to make 300 μm coronal slices containing the NAc. Before recording, slices were allowed to rest in oxygenated aCSF for at least 40 min at 35 °C, followed by a 10-minute recovery time at room temperature. For the recording aCSF, CaCl2 was increased to 2.5 mM and MgCl2, was decreased to 1 mM. Patch pipettes were pulled from 1.5 mm borosilicate glass capillaries (WPI, Sarasota, FL; 4–7 MΩ resistance) with a horizontal puller (model P97, Sutter Instruments, Novato, CA, USA) and filled with a solution containing (in mM): 130 K-gluconate, 10 KCl, 1 EGTA, 2 Mg2+-ATP, 0.6 Na+-GTP and 10 HEPES, pH 7.45, 285 mOsm. Recordings were conducted in the presence of the GABAA receptor antagonist, picrotoxin (50 μM). For all recordings, cells were allowed to stabilize for 2 min after break in and prior to any current injection. After this time, resting membrane potential and input resistance were measured. MSNs in the NAc core were identified based on their resting membrane potential and their distinctive characteristics including a slowly developing ramp depolarization, delayed action potential firing, and non-linear inward rectification with current injection (Nisenbaum and Wilson, 1995; Wilson & Kawaguchi 1996). For data analysis, only cells with a leak of < 50 pA and access resistance of < 30 pA were used. These cell parameters were recorded before at the start and end of data collection and only cells with < 20% change between the start and end of recordings were included in analyses. I/V plots and action potential number were generated from the average of three sets of current injections (each consisting of a 200 ms injection of −200 to 200 pA in 25 pA increments), with each set of current injections separated by 30 s. I/V relationships were determined by calculating the difference between the baseline voltage and the voltage 200 ms after initial current injections. Input resistance was determined by the change in voltage from −50 pA to +50 pA current injections. The number of action potentials elicited by each depolarizing current injection was used to determine neuronal excitability. Rheobase was defined as the minimum amount of current injection to elicit an action potential. The maximum second derivative method (Sekerli et al., 2004) was used to determine the action potential threshold.
2.5. Ovariectomy and hormone treatment
Rats were bilaterally ovariectomized under isoflurane anesthesia (2.5–5%, inhalation) via a single back incision. Before surgery rats were given the analgesic Carprofen (3 mg/kg, s.c.; Rimadyl®, Henry Schein). The incision was closed using dissolvable sutures internally (Reli Redi Gut Chromic Suture), and 9 mm wound clips externally (Reflex 9 mm wound clips, VWR international). In addition, carprofen was given again 48 h after surgery. Rats were allowed to recover for at least 10 days prior to any behavioral testing. Vaginal lavages were taken beginning 7 days after surgery and throughout the remainder of the study.
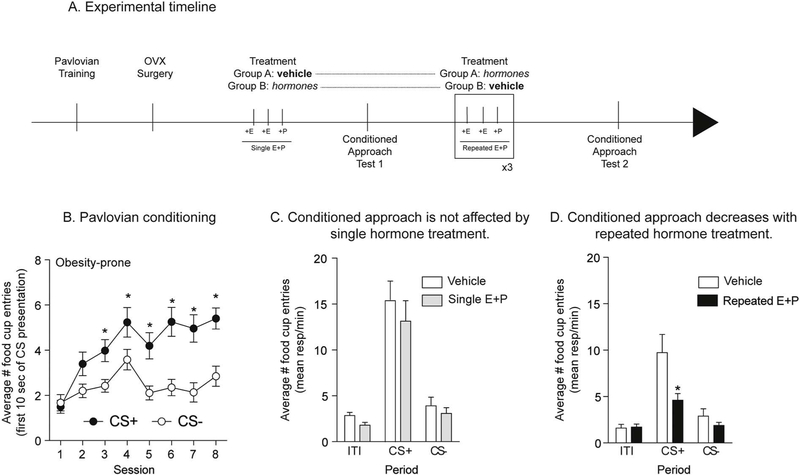
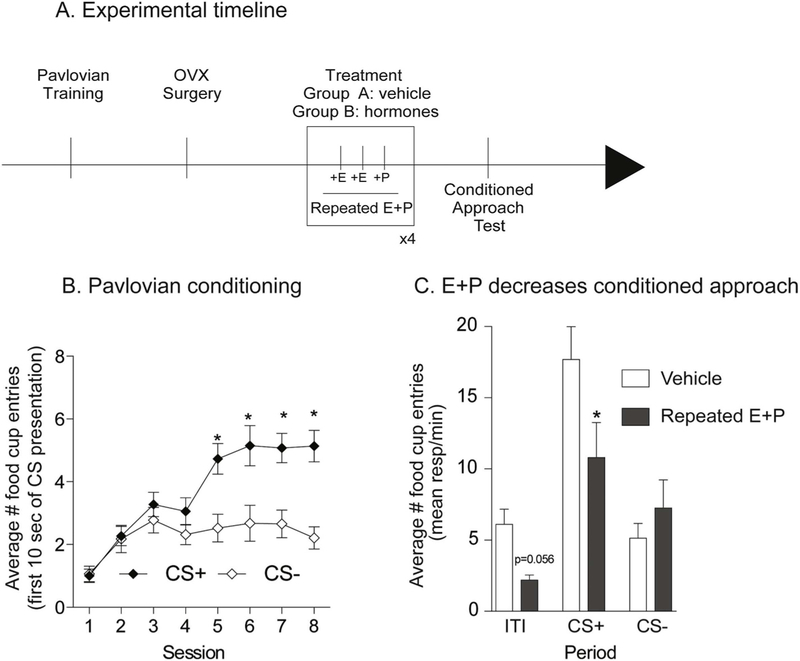
The effect of three different hormone treatments was evaluated. Hormone treatment consisted of subcutaneous injections of 17β-estradiol benzoate (estradiol; Sigma-Aldrich, E8515) for two consecutive days (5 μg in 0.1 ml of peanut oil, 24 h apart), followed by a single progesterone injection on the third day (500 μg in 0.1 ml of peanut oil, Sigma-Aldrich, P0130). Doses and treatment regimen were based on (Cummings and Becker, 2012 and Yu et al., 2011). For repeated hormone treatment this cycle was repeated 3 or 4 times (see additional details below). Controls were given the same number of vehicle injections (0.1 ml of peanut oil, s.c.). For studies of estradiol or progesterone alone, the same total number of injections were given, with progesterone or estradiol injection replaced by vehicle as appropriate. Finally, for all studies involving progesterone, the final progesterone injection was given 4–6 h prior to testing (see also timelines in Figs. 6A and 7A, D).
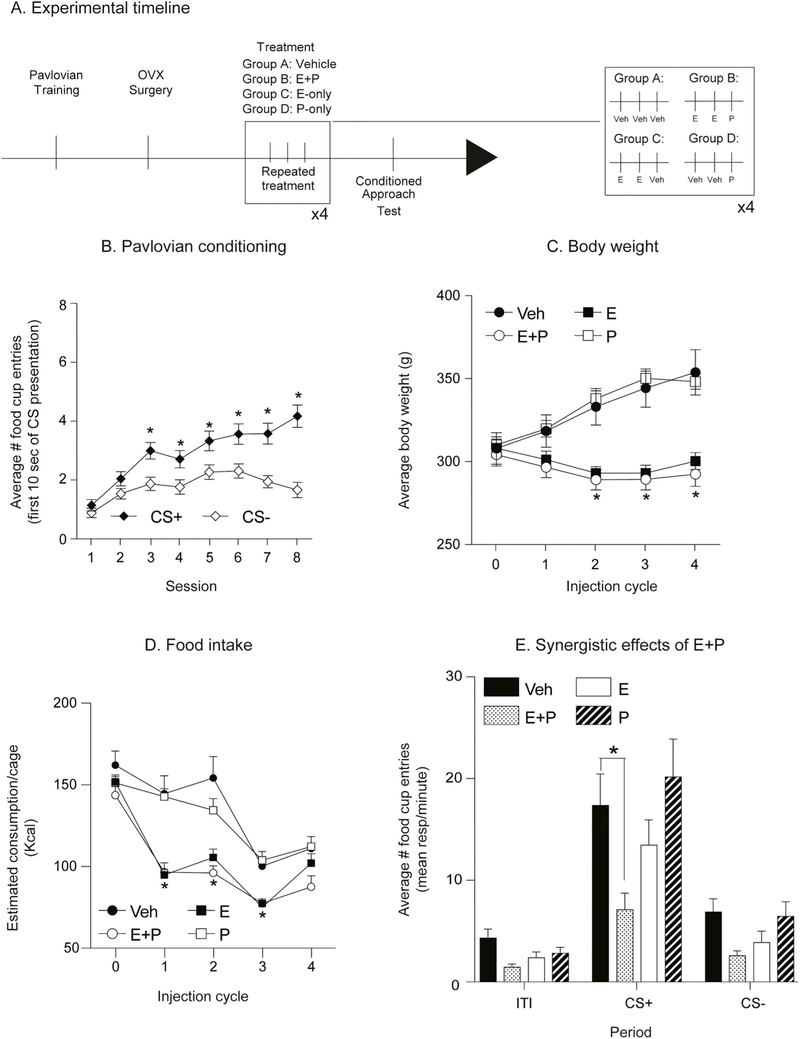
Fig. 6.
Repeated treatment with estradiol and progesterone decreased conditioned approach in obesity-prone females. A) Schematic of experimental timeline. Rats received 8 sessions of Pavlovian conditioning, followed by ovariectomy surgery (OVX) and 10 days of recovery before being treated with estradiol (+E) and progesterone (+P) or vehicle. 4–6 h after the last progesterone injection rats were tested in extinction conditions. B) Average number of food cup entries during the first 10 s of CS presentation in obesity-prone rats (OP). Obesity-prone rats learned to discriminate between the CS+ and CS−. C) Average number of food cup entries during the CS+, CS− and inter-trial interval (ITI) in vehicle and hormone treated groups. A single cycle of hormone treatment is not sufficient to decrease conditioned approach. D) Average number of food cup entries during the CS+, CS− and inter-trial interval (ITI) in vehicle and hormone treated groups. Repeated estradiol and progesterone treatment decreased conditioned approach compared to vehicle treated controls, although repeated testing reduced the magnitude of conditioned approach in both treatment groups. * = p < 0.05.
Fig. 7.
Repeated treatment with estradiol and progesterone decreased conditioned approach in outbred females. A) Schematic of experimental timeline. Rats received 8 sessions of Pavlovian conditioning, followed by ovariectomy surgery (OVX) and 10 days of recovery before being treated with estradiol (E) and progesterone (P) or vehicle. 4–6 h after the last progesterone injection rats were tested in extinction conditions. B) Average number of food cup entries during the first 10 s of CS presentation. Outbred rats learned to discriminate between the CS + and the CS−. C) Average number of food cup entries during the CS+, CS− and inter-trial interval (ITI) in vehicle and hormone treated groups. Four cycles of estradiol and progesterone treatment decreased conditioned approach in outbred rats.* = p < 0.05.
2.6. Statistics and data analysis
Two-tailed t-tests, two-way or three-way ANOVAs and Sidak’s post-hoc multiple comparisons were used (Prism 8, GraphPad, San Diego, CA). Electrophysiology data were analyzed using both Clampfit 10.4 (Molecular Devices) and MATLAB (R2018b, Mathworks Software). Post-hoc calculation of effect size was conducted for all analyses, as per the journal’s request. For t-tests, Cohen’s d was used to determine effect size for studies in which the number of subjects was equal between groups, while Hedge’s g was used for studies with unequal number of subjects between groups (Supplemental Table 1). For comparisons using ANOVAs we provide the eta squared (η2) and partial η2 for each comparison (Supplemental Table 2). The following formulas were used for each of these calculations:
Cohen’s d:
Hedge’s g:
η2:
Partial η2:
3. Experimental design
3.1. Experiment 1: Experiment 1: verification of female obesity-prone and obesity-resistant phenotypes and effects of the cycle on food intake and motivation for food
We first verified differences in body weight, adiposity and food intake between obesity-prone (OP) and obesity-resistant (OR) females and evaluated effects of the cycle on food intake and motivation for food (Levin and Govek, 1998). Rats were maintained on either standard lab chow (Lab Diet 5001: 4 kcal/g; 4.5% fat, 23% protein, 48.7% carbohydrates; % of caloric content) or a junk-food diet (JF) made in house (Robinson et al., 2015; Oginsky et al., 2016a, 2016b). The junk-food diet was a mash of Ruffle potato chips (40 g), Chips Ahoy (130 g), Nesquik (130 g), Jiff peanut butter (130 g), Lab Diet 5001 (200 g) and 180 ml of water (19.6% fat, 14% protein, and 58% carbohydrates; 4.5 kcal/g). Before junk-food diet exposure, body composition was determined by nuclear magnetic resonance spectroscopy (NMR; Minispec LF90II, Bruker Optics) conducted by the University of Michigan Animal Phenotyping Core in female obesity-prone (n = 16) and obesity-resistant (n = 16) rats. The rats were then assigned to chow or junk-food groups (counter balanced by initial weight and fat mass within obesity-prone and obesity-resistant groups) in order to verify weight-gain phenotypes (n = 8 per group: OP-chow, OP-JF, OR-chow, and OR-JF). Food intake and body weight were monitored throughout the 4 weeks of junk-food vs. chow consumption and NMR measures were made again at the end of this period.
In a separate set of rats, we determined how food intake varied across the estrous cycle in naturally-cycling obesity-prone and obesity-resistant rats. Home cage food intake, body weight, and estrous cycle phase were measured for 13 days in singly-housed naturally cycling obesity-prone (n = 6) and obesity-resistant (n = 6) rats and estrous cycle phase was determined as described above. Finally, we also determined estrous cycle effects on motivation to work for food using PR testing. Naturally-cycling obesity-prone (n = 10) and obesity-resistant (n = 10) rats were trained to lever press for a sucrose pellet and motivation to obtain this food was then determined using the progressive ratio procedure described above. In order to determine potential differences in break point across the cycle, all rats were given 8 PR test sessions and data were analyzed according to cycle phase during testing. This allowed us to make within subject comparisons of motivation to work for food during metestrus/diestrus vs. proestrus/estrus phases.
3.2. Experiment 2: differences in NAc core MSN intrinsic excitability between obesity-prone and obesity-resistant female rats
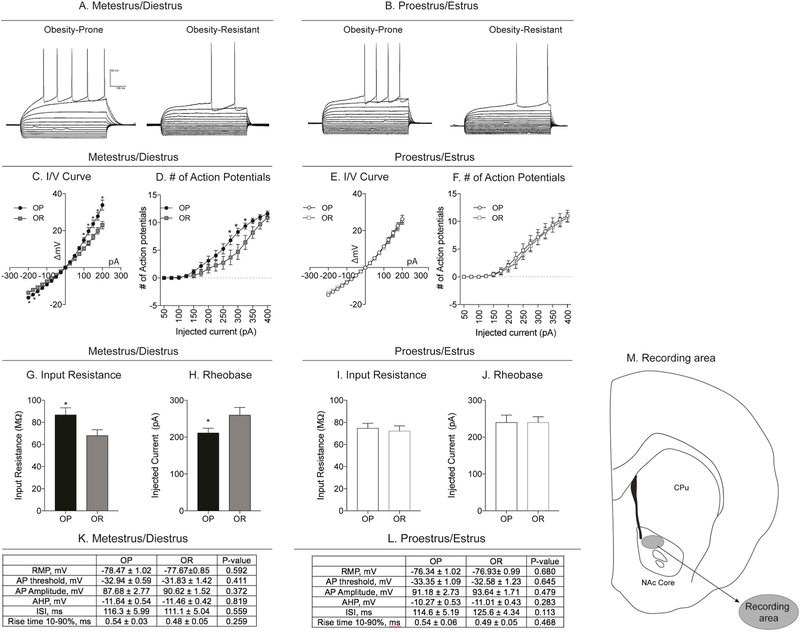
Potential differences in intrinsic excitability of MSNs in the NAc core between obesity-prone and obesity-resistant rats were determined in naturally cycling females. Estrous cycle phase was monitored for 5–7 days as described above and reconfirmed 1 h before slice preparation and electrophysiological recordings (OP-M + D n = 6 rats, 22 cells; OP-P + E n = 5 rats, 13 cells; OR-M + D n = 5 rats, 13 cells; OR-P + E n = 5 rats, 15 cells). A map of the recording area is depicted in Fig. 4M.
Fig. 4.
NAc core MSN intrinsic excitability is enhanced in obesity-prone vs. obesity-resistant rats during metestrus/diestrus. A) Example traces of current-clamp recordings from MSNs in slices from obesity-prone (OP) and obesity-resistant (OR) rats prepared during metestrus/diestrus. B) Example traces of current-clamp recordings from MSNs in slices from obesity-prone and obesity-resistant rats prepared during proestrus/estrus. C, D) Current/voltage (I/V) relationship and number of action potentials in obesity-prone and obesity-resistant from slices made during metestrus/diestrus. I/V relationships are shifted to the right in in obesity-prone vs. obesity-resistant rats and the same current injection intensity elicits more action potentials in MSNs from obesity-prone vs. obesity-resistant rats. E, F) Current/voltage (I/V) relationship and number of action potentials in obesity-prone and obesity-resistant from slices made during proestrus/estrus. I/V relationships and the number of action potentials fired are similar between obesity-prone and obesity-resistant rats in proestrus/estrus. G, H) Input resistance and rheobase: Obesity-prone rats have a higher input resistance and lower rheobase vs. obesity-resistant rats. I, J) Input resistance and rheobase during proestrus/estrus: No differences were observed in input resistance or rheobase between obesity-prone and obesity-resistant rats. K, L) Cell parameters from recordings conducted in slices prepared during metestrus/diestrus and proestrus/estrus. No differences in resting membrane potential (RMP), action potential threshold, action potential amplitude (AP), after-hyperpolarization amplitude (AHP), inter-spike interval (ISI) or rise time were observed between groups or cycle phase metestrus/diestrus or proestrus/estrus; * = p < 0.05. M) Map of NAc core MSN recording area.
3.3. Experiment 3: modulation of cue-triggered motivation by the estrous cycle in obesity-prone and obesity-resistant rats
We determined whether there were differences in cue-triggered motivation (as measured by conditioned approach) between obesity-prone and obesity-resistant rats and the role of the estrous cycle in modulating this behavior. Naturally-cycling obesity-prone (n = 31) and obesity-resistant (n = 31) rats underwent 5 sessions of Pavlovian training followed by a single session of testing in extinction conditions. Vaginal lavages were taken after the end of each session to confirm cycle phase on the day of testing.
3.4. Experiment 4: effects of single and repeated estradiol-progesterone treatment on cue-triggered motivation in obesity-prone female rats
Here we determined the effect of estradiol and progesterone treatment on cue-triggered motivation in obesity-prone rats (obesity-resistant rats were not included because there were no effects of the cycle on cue-triggered motivation in this group, see results below). Naturally cycling obesity-prone rats (n = 20) underwent 8 sessions of Pavlovian training followed by ovariectomy and 10 days of recovery as described above. Some rats were then given one cycle of estradiol and progesterone treatment (see above) followed by a single test in extinction conditions (n = 10), while others received vehicle treatment prior to the extinction test. Next, rats in the vehicle group were treated with 3 cycles of hormone treatment while the rats that initially received one cycle of treatment were given repeated vehicle injections before undergoing an additional test in extinction conditions.
3.5. Experiment 5: effects of repeated hormone treatment on cue-triggered motivation in outbred female rats
Here we determined whether repeated hormone treatment with both estradiol and progesterone, or repeated treatment with each hormone alone were sufficient to alter cue-triggered motivation in outbred Sprague-Dawley female rats. To examine effects of dual hormone treatment, naturally cycling outbred rats (n = 20) were trained and ovariectomized as described above. After recovery from surgery, they were assigned to vehicle (n = 10) or repeated hormone treatment (n = 10) groups, counterbalanced by post-operative weight. They received 4 consecutive cycles of either vehicle or hormone treatment followed by a single extinction test (see Fig. 7A).
To assess the effects of each hormone on its own, a separate set of naturally-cycling outbred rats (n = 40) underwent 8 sessions of Pavlovian training followed by ovariectomy and recovery as described above; 2 rats were excluded from studies due to complications during ovariectomy surgery. Rats were then assigned to vehicle (Veh; n = 9), estradiol alone (E; n = 10), progesterone alone (P; n = 10) or estradiol and progesterone treated groups (E + P; n = 9), counterbalanced by post-operative weight. Body weight and food intake were measured throughout the hormone injection cycles.
4. Results
4.1. Experiment 1a: verification of female obesity-prone and obesity-resistant phenotypes
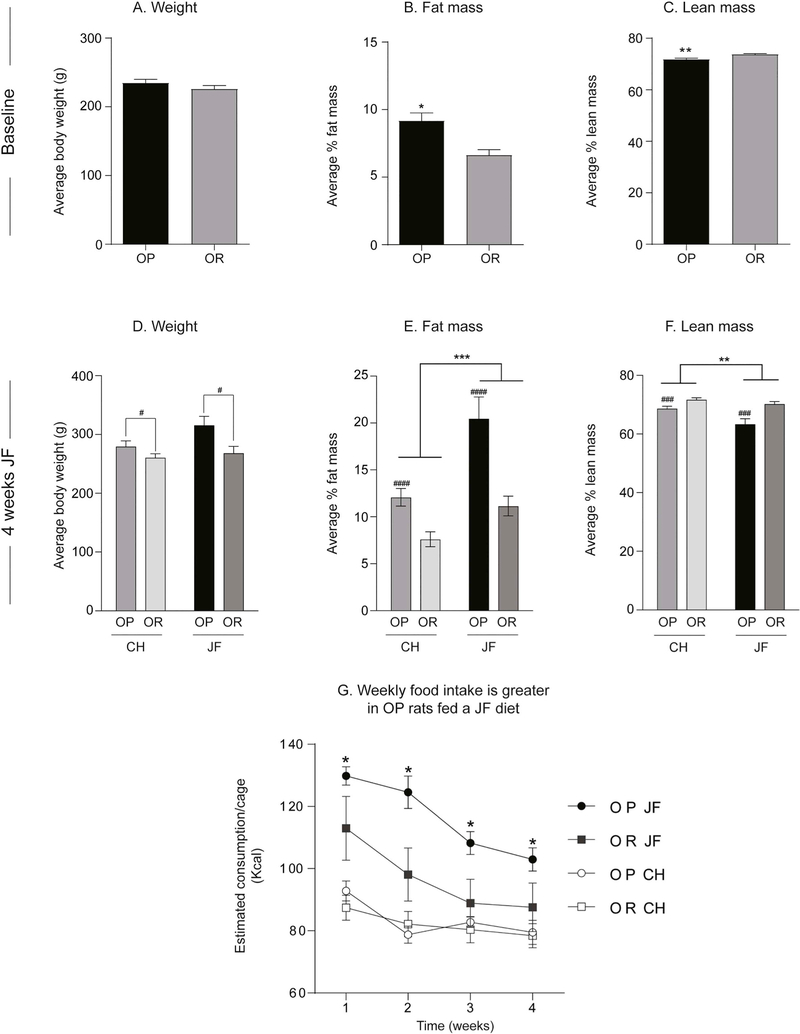
Fig. 1 shows weight, adiposity and food intake of obesity-prone and obesity-resistant female rats at baseline (Fig. 1A-C) and after 4 weeks of junk-food exposure (Fig. 1D-F). Although body weight was similar between groups (Fig. 1A), fat mass was significantly greater in female obesity-prone vs. obesity-resistant groups at baseline (Fig. 1B: t30 = 3.593, p < 0.01). This was accompanied by significantly lower lean mass in obesity-prone vs. obesity-resistant groups (Fig. 1C: t30 = 3.642, p < 0.01). After 4 weeks of free access to junk-food or chow, obesity-prone rats remained heavier than obesity-resistant rats (Fig. 1D: Two-way ANOVA main effect of group: F(1,28) = 9.05, p < 0.01, no diet x group interaction). Measurements of adiposity revealed that junk-food consumption significantly increased fat mass in both groups (Fig. 1E: Two-way ANOVA main effect of diet: F(1, 28) = 18.09, p < 0.001) and reduced lean mass (Fig. 1F: Two-way ANOVA main effect of diet: F(1, 28) = 9.78, p < 0.01) compared to chow-fed groups. However, as expected, the effect of junk-food on fat mass was more pronounced in obesity-prone groups (Fig. 1E: Average change from chow OP: 16.3 ± 4.2; OR: 9.4 ± 1.8). Fig. 1G shows food intake during the 4-week diet manipulation. As expected, junk-food consumption was greater than chow consumption in both groups, with obesity-prone rats consuming more junk-food than obesity-resistant rats (Fig. 1G: Three-way ANOVA main effect of time: F(3,36) = 54.07, p < 0.0001; main effect of diet: F(1,12) = 22.5, p = 0.0005; main effect of group: F(1,12) = 4.33, p = 0.06; and group ×diet × time interaction: F(3, 36) = 3.31, p = 0.03). In addition, chow consumption was similar between obesity-prone and obesity-resistant (Fig. 1G, open symbols) even though obesity-prone rats were heavier and had more fat mass than obesity-resistant rats. These data are consistent with previous reports in males (Vollbrecht et al., 2015) and confirm obesity-prone and obesity-resistant phenotypes in females.
Fig. 1.
Verification of female obesity-prone and obesity-resistant phenotype. A-C) Weight and adiposity measures at baseline: Although body weight is similar between groups, obesity-prone rats (OP) have greater fat mass and lower lean mass vs. obesity-resistant (OR) rats. D-F) Weight and adiposity measures after 4 weeks of junk-food diet (JF) or chow (CH) consumption: Obesity-prone rats remain heavier than obesity-resistant rats, and have greater fat mass and lower lean mass vs. obesity-resistant rats. Junk-food increases fat mass and reduces lean mass in both groups, but the magnitude of this effect is stronger in obesity-prone rats. G) Home cage food intake during 4 weeks of junk-food or chow consumption: Junk-food consumption was greater than chow consumption in both groups, with obesity-prone rats consuming more junk-food than obesity-resistant rats. All data are shown as mean ± SEM unless otherwise noted. # = differences between obesity-prone and obesity-resistant rats, * = differences between chow and junk-food, p < 0.05.
4.2. Experiment 1b: food intake and motivation to obtain sucrose are reduced during estrus in obesity-prone and obesity-resistant females
Home cage food intake and responding during progressive ratio testing were examined in each phase of the cycle in naturally cycling females. Fig. 2A shows example images capturing cell cytology distinctive of each phase of the cycle (20× magnification). Consistent with the existing literature, home cage food intake was reduced during the estrus phase compared to other phases of the cycle in both groups (Fig. 2B: Two-way RM ANOVA main effect of estrous phase F(3,30) = 8.34, p < 0.001; planned Sidak’s multiple comparisons proestrus vs. estrus, p < 0.05). In addition, food intake was again greater in obesity-prone vs. obesity-resistant rats, regardless of estrous phase (Fig. 2B: Two-way RM ANOVA main effect of group: F(1,10) = 28.40, p = 0.0003). Importantly, there were no differences in number of cycles completed between obesity-prone vs. obesity-resistant groups across 13 days (data not shown).
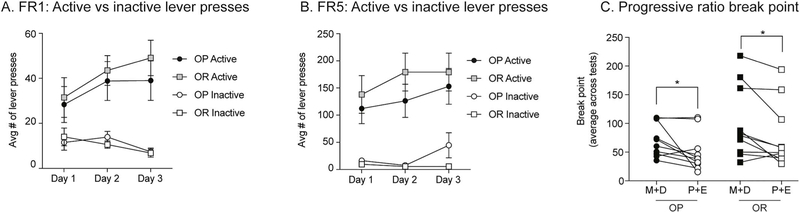
We next determined how motivation to obtain sucrose changes across the cycle. Fig. 3 shows behavior during initial fixed ratio training (Fig. 3A, B) and subsequent progressive ratio testing (Fig. 3C). Acquisition of instrumental responding for sucrose was similar between groups, with all rats preferentially responding on the active vs. inactive lever during FR1 (Fig. 3A: Three-way ANOVA main effect of lever F(1,108) = 0.08, p < 0.0001) and FR5 training (Fig. 3B: Three-way ANOVA main effect of lever F(1,108) = 137.9, p < 0.0001). In addition, the magnitude of responding was similar between obesity-prone and obesity-resistant groups. Progressive ratio testing was conducted across 8 consecutive days and analyzed by averaging break points within subjects (OP n = 10; OR n = 10) tested during metestrus/diestrus and proestrus/estrus. Break point was significantly lower during proestrus/ estrus vs. metestrus/diestrus in both obesity-prone and obesity-resistant groups (Fig. 3C: Two-way RM ANOVA main effect of estrous cycle phase: F(1,18) = 12.05, p < 0.01). Although some visual trends were present, no significant differences in average break point between obesity-prone and obesity-resistant groups were observed (Fig. 3C: Two-way RM ANOVA effect of group: F(1,18) = 2.0, p = 0.2). These data show that despite the differences in home cage food intake between obesity-prone and obesity-resistant rats, motivation to obtain sucrose pellets is similar between groups. Additionally, the estrous cycle affects motivation to obtain food and food intake similarly in both groups.
Fig. 3.
Motivation to work for a sucrose pellet decreases during proestrus/estrus in both obesity-prone and obesity-resistant rats. A, B) Average number of active and inactive lever presses across training. Acquisition of instrumental responding for sucrose was similar between groups. All rats preferentially responded on the active vs. inactive lever during fixed ratio 1 (FR1) and fixed ratio 5 (FR5) sessions. C) Average break point reached during progressive ratio testing. Break point was significantly lower during proestrus/estrus (P + E) vs. metestrus/diestrus (M + D) in both groups. No differences between obesity-prone (OP) and obesity-resistant (OR) groups were observed; * = p < 0.05.
4.3. Experiment 2: NAc core MSN intrinsic excitability is enhanced in obesity-prone vs. obesity-resistant rats during metestrus/diestrus
Recordings were made in both obesity-prone and obesity-resistant rats in metestrus/diestrus (Fig. 4 left graphs: OP n = 22 cells from 6 rats; OR n = 13 from 5 rats) or proestrus/estrus (Fig. 4 right graphs: OP n = 13 cells from 5 rats; OR n = 15 cells from 5 rats) to examine differences in MSN intrinsic excitability in the NAc core between groups and across the estrous cycle. Consistent with our previous results in males (Oginsky et al., 2016b), the I/V curved showed a greater change in membrane potential in response to positive and negative current injections in MSNs from obesity-prone vs. obesity-resistant groups (Fig. 4C: Two-way RM ANOVA group × current injection interaction: F(16,528) = 6.348, p < 0.0001; main effect of group F(1,33) = 5.683, p = 0.02; main effect of current injection F(16,528) = 263.9, p < 0.0001; Sidak’s multiple comparison p < 0.05). Similarly, during metestrus/diestrus, the number of action potentials elicited across current injections was greater in obesity-prone vs. obesity-resistant groups (Fig. 4D: Two-way RM ANOVA group x current injection interaction F(14,462) = 3.04, p = 0.0002; main effect of current injection F(14,462) = 129.1, p < 0.0001; main effect of group F(1,33) = 3.67, p = 0.06; Sidak’s multiple comparison p < 0.05). In contrast, when comparisons were made during the proestrus/estrus phases, I/V relationships and the number of action potentials elicited by current injection were similar between groups (Fig. 4E, F). Consistent with group differences found during metestrus/diestrus, input resistance was greater in obesity-prone rats (Fig. 4G: Two-tailed unpaired t-test, t(33) = 2.09, p = 0.04) and rheobase was reduced (Fig. 4H: Two-tailed unpaired t-test, t(33) = 2.15, p = 0.04) compared to obesity-resistant rats during metestrus/diestrus. However, these group differences were absent when comparisons were made during the proestrus/estrus phases (Fig. 4I, J). We did not observe differences in basal cell parameters including resting membrane potential (RMP), action potential threshold, amplitude, rise time (10–90%), after-hyperpolarization (AHP) amplitude, or duration of the first inter-spike interval between recordings made during metestrus/diestrus in obesity-prone and obesity-resistant rats (Fig. 4K) or proestrus/estrus (Fig. 4L) phases. Based on the number of cells in this study we are powered to see large effect sizes (power (1 – β) = 0.6). However, most of the effect sizes for data in Fig. 4K and L range between 0.02 and 0.69. Thus, any potential differences that we might be missing are likely to be very small. In sum, these data show that similar to male obesity-prone rats, basal intrinsic excitability is enhanced in female obesity-prone vs. obesity-resistant rats, but these differences are only apparent when recordings are made during the metestrus/diestrus phase of the estrous cycle.
4.4. Experiment 3: cue-triggered motivation is modulated by the estrous cycle in obesity-prone, but not obesity-resistant rats
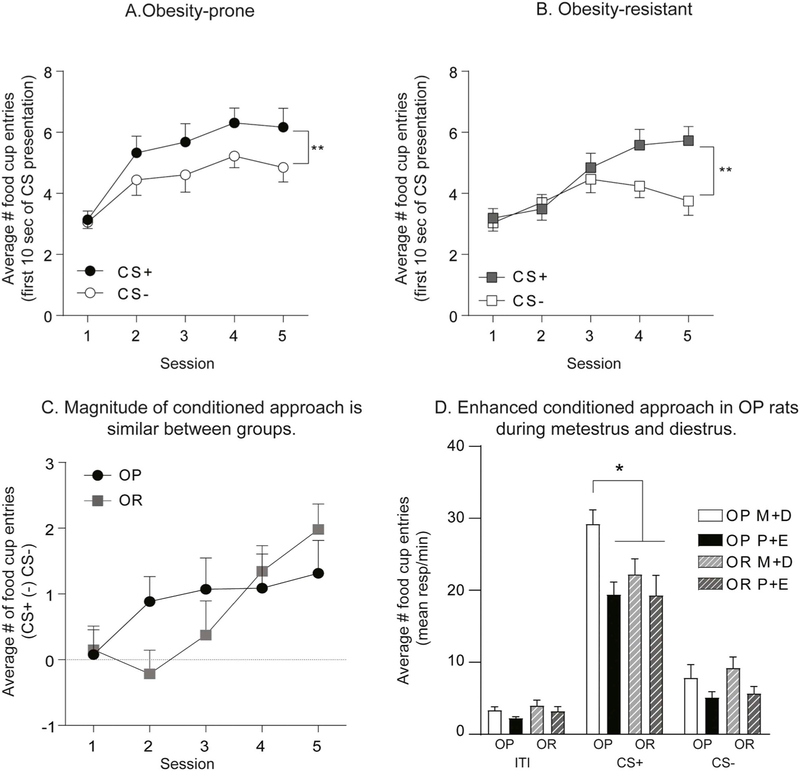
Here, we determined how cue-triggered motivation in the form of conditioned approach varies across the cycle in naturally cycling obesity-prone and obesity-resistant rats. Fig. 5 shows behavior during initial conditioning (Fig. 5A–C) and subsequent testing in extinction conditions (Fig. 5D). Behavior during initial Pavlovian conditioning was similar between groups, with both obesity-prone and obesity-resistant rats acquiring a similar magnitude of discrimination between the CS+ and CS− (Fig. 5A OP: Two-way RM ANOVA: main effect of CS F(1,30) = 9.79 p < 0.01; Fig. 5B OR: Two-way RM ANOVA: main effect of CS F(1,30) = p < 0.01; Fig. 5C OP vs. OR: main effect of session: F(4,240) = 4.98, p < 0.001; no effect of group F(1,60) = 0.17, p = 0.7; no group × session interaction F(4,120) = 1.78, p = 0.14). Rats were then tested for conditioned approach in extinction conditions and data were analyzed according to which phase of the cycle rats were in during testing (Fig. 5D). The CS+ elicited strong conditioned approach in all groups (Fig. 5D: Two-way RM ANOVA period (ITI, CS+, CS−) × group interaction F(6,116) = 3.1, p = 0.007; main effect of group F(3,58) = 3.85, p = 0.01; main effect of period F(2,116) = 222.7, p < 0.0001; Sidak’s multiple comparison: CS+ vs. ITI and CS+ vs. CS−, P < 0.0001). However, the magnitude of conditioned approach in response to the CS+ was significantly greater in obesity-prone rats tested during metestrus/diestrus compared to all other groups (Fig. 5D; Sidak’s multiple comparison, p < 0.05). Furthermore, differences in CS+ responding between obesity-prone and obesity-resistant groups were driven entirely by enhanced approach behavior during metestrus/ diestrus vs. proestrus/estrus in obesity-prone, but not obesity-resistant rats (Fig. 5D: Sidak’s multiple comparison CS+: OP-M + D vs. OP-P + E, p = 0.0001; CS+: OR-M + D vs. OR-P + E, p = 0.73). No significant differences in responding during the ITI or CS− were found between groups or across cycle phase. Thus, in obesity-prone, but not obesity resistant rats, cue-triggered motivation varied across the cycle and was stronger in obesity-prone vs. obesity-resistant rats tested in metestrus/diestrus phases. Taken with results from progressive ratio testing above, data show a dissociation between the ability of the cycle to influence motivation for food (Fig. 3) but not motivation triggered by a food cue in obesity-resistant rats (Fig. 5D).
Fig. 5.
Cue-triggered motivation is modulated by the estrous cycle in obesity-prone but not in obesity-resistant rats. A) Average number of food cup entries during the first 10 s of CS presentation in obesity-prone rats (OP). Obesity-prone rats discriminate between the CS+ and the CS− during initial Pavlovian conditioning. B) Average number of food cup entries during the first 10 s of CS presentation in obesity-resistant rats (OR). Obesity-resistant rats discriminate between the CS+ and the CS− during initial Pavlovian conditioning. C) Average difference in the number of food cup entries during the first 10 s of CS+ vs. CS− presentation. No difference in magnitude of conditioned approach between obesity-prone and obesity-resistant rats was observed. D) Extinction Test: Average number of food cup entries during the CS+, CS− and inter-trial interval (ITI) in obesity-prone and obesity-resistant rats tested in metestrus/diestrus (M + D) and proestrus/estrus (P + E). Conditioned approach is stronger during metestrus/diestrus vs proestrus/estrus in obesity-prone, but not obesity-resistant rats. In addition, the magnitude of conditioned approach is greater in obesity-prone vs. obesity-resistant rats tested during the metestrus/diestrus phase. * = p < 0.05, ** = p < 0.01.
4.5. Experiment 4: repeated treatment with estradiol and progesterone is sufficient to decrease cue-triggered motivation in obesity-prone rats
Given that the estrous cycle modulates cue-triggered motivation in obesity-prone but not obesity-resistant rats (Exp. 3), we next determined whether single vs. repeated treatment with estradiol and progesterone in ovariectomized rats is sufficient to replicate this effect in obesity-prone rats (Fig. 6A: experimental timeline). As expected, obesity-prone rats learned to discriminate between the CS+ and the CS− during Pavlovian training (Fig. 6B: Two-way RM ANOVA main effect of CS F(1,19) = 25.6, p < 0.0001; significant session × CS interaction F(7,133) = 3.74, p = 0.001; Sidak’s post-test p < 0.05). A single cycle of estradiol and progesterone treatment was not sufficient to alter conditioned approach behavior compared to vehicle treated controls (Fig. 6C). However, repeated estradiol and progesterone treatment (repeated E + P), decreased conditioned approach compared to controls (Fig. 6D: Two-way RM ANOVA; main effect of treatment, p < 0.05; significant period (ITI, CS+ and CS−) x treatment interaction, p < 0.05; Sidak’s multiple comparisons CS+: vehicle vs. hormone treatment, p = 0.002). It should be noted that the same rats were tested twice (see timeline Fig. 6A). Thus, it is not surprising that the magnitude of conditioned approach was lower overall during the second test conducted under extinction conditions (Fig. 6D vs. C). Nonetheless, these data show that single hormone treatment does not affect conditioned approach, whereas repeated hormone treatment reduces it in obesity-prone rats.
4.6. Experiment 5a: repeated treatment with estradiol and progesterone is sufficient to decrease cue-triggered motivation in outbred rats
Here, we determined if the decrease in cue-triggered motivation with repeated E + P observed in ovariectomized obesity-prone rats also extend to ovariectomized outbred Sprague-Dawley females (see Fig. 7A for experiment timeline). As expected, outbred females learned to discriminate between the CS+ and the CS− during Pavlovian conditioning (Fig. 7B: Two-way RM ANOVA: main effect of CS F(1,19) = 13.83, p = 0.002; significant session x CS interaction F(7,133) = 7.78, p < 0.0001, Sidak’s multiple comparisons p < 0.05). Consistent with effects in selectively-bred rats, repeated estradiol and progesterone treatment resulted in a decrease in conditioned approach behavior to the CS+ (Fig. 7C: Two-way RM ANOVA main effect of treatment p < 0.05; significant period × treatment interaction p = 0.05; Sidak’s multiple comparisons CS+: vehicle vs. hormone treated, p < 0.05). There was a slight effect of hormone treatment on food cup entries during the ITI (Fig. 7C; vehicle vs. hormone treated p = 0.056) but not during the CS− (Fig. 7C vehicle vs. hormone treated p = 0.83). However, when comparing across all three separate experiments (see below), there do not appear to be consistent effects of hormone replacement on responding during the ITI. In sum, data from outbred females replicate the primary effect of hormone treatment found in obesity-prone rats, supporting a role for ovarian hormones in reductions in conditioned approach behavior during metestrus/diestrus.
4.7. Experiment 5b: estradiol and progesterone act synergistically to decrease cue-triggered motivation in outbred rats
Above we demonstrate that repeated estradiol and progesterone treatment decreases cue-triggered motivation in both obesity-prone and outbred females. Here we evaluated the ability of repeated estradiol or progesterone alone to reduce cue-triggered motivation in outbred rats (see Fig. 8A for experimental timeline). We also included an additional experimental group that was given repeated treatment with both hormones in order to replicate effects above in a separate group of rats. After acquisition of Pavlovian discrimination (Fig. 8B: main effect of CS F(1,38) = 26.9, p < 0.0001; significant session × CS interaction F(7,266) = 6.29, p < 0.0001, Sidak’s multiple comparisons, p < 0.05), rats were separated into 4 treatment groups [vehicle (veh), estradiol and progesterone (E + P), estradiol alone (E) and progesterone alone (P)], counterbalanced for behavior during the last Pavlovian training session.
Fig. 8.
Estradiol and progesterone act synergistically to decrease cue-triggered motivation in outbred rats. A) Schematic of experimental timeline. Outbred rats received 8 sessions of Pavlovian conditioning, followed by ovariectomy surgery (OVX) and 10 days of recovery. Rats were the counterbalanced by weight and divided into 4 groups: vehicle, estradiol and progesterone (E + P), estradiol alone (E) and progesterone alone (P). B) Average number of food cup entries during the first 10 s of CS presentation. Outbred rats learned to discriminate between the CS+ and the CS−. C, D) Average body weight and food intake across the treatment period. Estradiol treatment (E or E + P) reduced body weight and food intake compared to the vehicle (Veh) and progesterone (P) groups. E) Average number of food cup entries during the CS+, CS− and inter-trial interval (ITI) in vehicle and hormone treated groups. Estradiol or progesterone alone did not affect conditioned approach. However, repeated estradiol and progesterone treatment decreased conditioned approach in outbred rats. Thus, although estradiol is sufficient to reduce weight and food intake, both estradiol and progesterone are needed to reduce conditioned approach. * = p < 0.05.
Throughout hormone treatment we measured body weight and food intake. We found that body weight and food intake were reduced in both estradiol treated groups (E and E + P; Fig. 8C body weight: Two-way RM ANOVA: main effect of injection cycle F(4,144) = 45.63, p < 0.0001; main effect of group F(3,36) = 8.43, p = 0.0002; significant interaction injection cycle × group F(12,144) = 45.21, Sidak’s multiple comparisons p < 0.05; Fig. 8D food intake: Two-way RM ANOVA: main effect of injection cycle F(4,64) = 70.14, p ≤0.0001; main effect of group F(3,16) = 17.88, p < 0.0001; significant injection cycle x group F(12,64) = 3.73, p = 0.0003, Sidak’s multiple comparison, p < 0.05). Consistent with experiment 5a above, repeated E + P treatment significantly decreased conditioned approach to the CS+ (Fig. 8E: Two-way RM ANOVA significant period × treatment interaction F(6,68) = 2.6; main effect of period F(2,68) = 62.5, p < 0.0001; main effect of treatment F(3,34) = 5.4, p = 0.004; Planned Sidak’s multiple comparisons CS+: vehicle vs. E + P, p = 0.0008), with no effect of treatment on responding during the CS− or ITI (vehicle vs. E + P; p = 0.84, 0.46 respectively). However, treatment with estradiol or progesterone alone was not sufficient to decrease conditioned approach in outbred rats (Fig. 8E: Sidak’s multiple comparisons CS+: Veh vs. E, p = 0.54; Fig. 8E: Sidak’s multiple comparisons CS+: Veh vs. P, p = 0.84). Together these data suggest a synergistic effect of repeated estradiol and progesterone treatment on cue-triggered motivation, even though estradiol is sufficient to decrease home-cage food intake and body weight.
5. Discussion
Naturally occurring alterations in estradiol influence food intake in females. However, how motivational responses to food cues are affected by the estrous cycle or elevations in ovarian hormones is unknown. In addition, while individual susceptibility to obesity is accompanied by enhanced incentive motivational responses to food cues and increased intrinsic excitability of MSNs within the NAc of males, studies in females are lacking (see Introduction and Alonso-Caraballo et al., 2018). Here, we show that intrinsic excitability of NAc MSNs and conditioned approach behavior are enhanced in female obesity-prone vs. obesity-resistant rats when measured during metestrus/diestrus. However, neural and behavioral responses between these groups were similar when measured during proestrus/estrus. The emergence of group differences during metestrus/diestrus were due to effects of the cycle on NAc excitability and cue-triggered motivation within obesity-prone, but not obesity-resistant rats. Additionally, we found that estradiol and progesterone treatment in ovariectomized females reduced conditioned approach behavior in obesity-prone and outbred Sprague-Dawley rats. To our knowledge, these data are the first to demonstrate cycle- and hormone-dependent effects on the motivational response to a food cue, and the only studies to date to determine how individual susceptibility to obesity influences NAc excitability, cue-triggered food-seeking, and differences in the regulation of these neurobehavioral responses by the cycle.
5.1. Effects of the cycle on weight, food intake, and motivation for sucrose in obesity-prone and obesity-resistant females
We began by verifying obesity-prone and obesity-resistant phenotypes in females. As expected, female obesity-prone rats were heavier, ate more, and had greater fat mass and less lean mass than obesity-resistant females (Fig. 1). In addition, weight gain induced by a junk-food diet was more pronounced in obesity-prone vs. obesity-resistant females. Instrumental responding for a sucrose pellet during FR1, FR5 or progressive ratio testing was similar between groups (Fig. 3), despite differences in home cage food consumption. In males break point is modestly elevated in obesity-prone vs. obesity-resistant rats working for sucrose (Vollbrecht et al., 2015). In the current study, pressing on the active lever resulted in the delivery of a sucrose pellet but no discrete cue. However, in our previous study of males, active lever presses resulted in the presentation of an auditory cue in addition to the delivery of the sucrose pellet. Thus, it’s possible that inclusion of a discrete cue added salience to the motivation to work for food in our previous study using males, and that the absence of cue presentation in the current study contributed to similar break points in female obesity-prone and resistant groups. However, the absence of strong differences in break point between obesity-prone and obesity-resistant females here did not impede our ability to observe effects of the cycle on this behavior, discussed further below. Overall, obesity-prone and -resistant phenotypes of females are similar to those previously reported for males of these selectively-bred lines (Vollbrecht et al., 2015, 2016). Additionally, within naturally cycling obesity-prone and obesity-resistant females, food intake and body weight were reduced during estrus (Fig. 2B). Furthermore, motivation to obtain sucrose was lower when rats were tested during proestrus/estrus vs. metestrus/diestrus (Fig. 3C), regardless of susceptibility to obesity. These changes in motivation to obtain sucrose in naturally cycling females are consistent with variations in home cage food intake across the cycle found here and with established roles for ovarian hormones in the regulation of food intake (Tarttelin and Gorski, 1971; Asarian and Geary, 2013).
5.2. Differences in NAc MSN intrinsic excitability in obesity-prone vs. obesity-resistant rats
The NAc is comprised predominantly of MSNs which integrate both dopaminergic and glutamatergic inputs to ultimately influence behavioral responses to reinforces like food, sex, and drugs as well as cues paired with them (Schultz, 1997; Berridge and Robinson, 1998; Schultz, 2013; Wolf, 2002; Wolf et al., 2003). One factor that strongly influences the output of the NAc is the intrinsic excitability of MSNs, i.e., how readily they can be depolarized and fire action potentials (Nicola et al., 2000; Hu, 2007). We found that excitability of MSNs in the NAc core is enhanced in obesity-prone vs. obesity-resistant females when recordings are made during metestrus/diestrus, but that this group difference is not apparent when recordings are made during proestrus/estrus (Fig. 4). This was due to cycle effects on MSN excitability in obesity-prone, but not obesity-resistant rats. Specifically, in obesity-prone rats changes in voltage at positive current injections were significantly greater during metestrus/diestrus vs. proestrus/estrus. This is similar to effects of the cycle on NAc excitability in outbred Sprague-Dawley females (Proaño et al., 2018). To our knowledge this is the only other study to specifically examine effects of the estrous cycle on MSN excitability, and the mechanisms by which ovarian hormones influence NAc excitability have not been determined. However, there is evidence for sex differences in MSN excitability (Cao et al., 2018), suggesting a potential role for hormonal regulation. In addition, cycle-dependent changes in dopaminergic transmission, which indirectly influence MSN excitability (Perez et al., 2006; Azdad et al., 2009; Podda et al., 2010; Zhao et al., 2016), have been observed in the NAc. Furthermore, stimulated dopamine release in the NAc increases during the estrus vs. diestrus phase in female mice (Calipari et al., 2017), suggesting that hormonal regulation of dopamine transmission could contribute to changes in excitability across the cycle. Additionally, NAc glutamatergic transmission increases during proestrus and estrus in female rats (Proaño et al., 2018), and can be influenced by neonatal exposure to estradiol (Cao et al., 2016). Although not well understood, reductions in excitatory transmission often co-occur with increases in intrinsic excitability, and vice versa (see Oginsky and Ferrario, 2019 for additional discussion). Thus, future studies should examine how dopamine and glutamate transmission may contribute to the observed effects of the cycle on MSN intrinsic excitability.
MSN excitability is largely determined by the number and distribution of voltage-gated potassium channels, with inwardly-rectifying K+ currents (IKir) dominating at negative membrane potentials (< −90 mV) and A-type K+ currents (IA) dominating at positive membrane potentials (> −40 mV; Nisenbaum and Wilson, 1995; Perez et al., 2006). Differences during metestrus/diestrus between obesity-prone and obesity-resistant females were present across a wide range of current injections, but were most pronounced at positive potentials. This, in combination with a lower rheobase and absence of differences in resting membrane potential, suggests that group differences may be due to lower IA in obesity-prone vs. obesity-resistant females. These basal group differences in excitability during metestrus/diestrus are similar to those seen in male obesity-prone vs. obesity-resistant rats where differences were found across the I/V curve and were accompanied by a lower rheobase and an absence of differences in resting membrane potential (Oginsky et al., 2016b).
The reduction in excitability during proestrus/estrus vs. metestrus/diestrus within obesity-prone rats is consistent with the ability of estradiol to enhance IA, thereby reducing excitability in cultured hippocampal neurons (Zhang et al., 2015). Progesterone also increases during the proestrus phase, and relatively high concentrations of exogenous progesterone can result in decreased neuronal excitability in part through altering the activity of voltage-gated sodium and calcium channels (Kelley and Mermelstein, 2011). However, we did not observe changes in the threshold for action potential firing or action potential rise time, which are mediated by Na+ currents, nor did we find changes in after hyperpolarization amplitude, which are mainly influenced by Ca+ channels. Thus, it seems less likely that alterations in excitability observed across the cycle in obesity-prone rats are mediated by progesterone. Of course, many hormones change across the cycle, and effects observed here could be either direct, or indirect. Nonetheless, these data demonstrate that in the NAc core MSN excitability is enhanced in obesity-prone rats during phases of the cycle when motivational responses to food cues are also elevated (see below for additional discussion of this relationship).
5.3. Basal differences in cue-triggered motivation in obesity-prone vs. obesity-resistant females
Food intake is not only regulated by homeostatic feedback, but external signals including food cues also influence the motivation to eat independent of hunger state (Derman and Ferrario, 2018a, 2018b; Holland, 1977). These cue-triggered urges to seek out and consume food contribute to opportunistic eating that drives obesity (Ferrario et al., 2016; Ferrario, 2017; Stice et al., 2013) and are more pronounced in male obesity-susceptible vs. -resistant populations (Robinson et al., 2015; Derman and Ferrario, 2018a; Alonso-Caraballo et al., 2018). Conditioned approach behavior was stronger in female obesity-prone vs. obesity-resistant rats (Fig. 5D). This is consistent with data from males, where the magnitude of Pavlovian conditioned approach, conditioned reinforcement, and Pavlovian to instrumental transfer is greater in obesity-susceptible vs. –resistant populations (Robinson et al., 2015; Derman and Ferrario, 2018a). Taken as a whole, these data support the idea enhanced responsivity to the motivational properties of food cues is likely a phenotypic, neurobehavioral difference in those individuals that are more vs. less susceptible to diet-induced weight gain.
Difference in conditioned approach between obesity-prone and obesity-resistant females was apparent when naturally cycling rats were tested during the metestrus/diestrus phases of the cycle, but the magnitude of conditioned approach was similar across groups when tested during proestrus/estrus. Specifically, in obesity-prone rats conditioned approach was elevated during metestrus/diestrus compared to proestrus/estrus (Fig. 5), consistent with shifts in home cage food intake and break point across the cycle. In contrast, in obesity-resistant females, conditioned approach behavior was stable across the cycle, even though motivation for food itself and food consumption rose and fell with the cycle in a manner comparable to that found in obesity-prone rats (Fig. 5). This dissociation between motivation for food and motivational responses triggered by a food cue does not appear to be due to deficits in Pavlovian learning in obesity-resistant rats, as behavior during acquisition and the degree of discrimination between the CS+ and CS− were similar to that seen in the obesity-prone group (Fig. 5). Indeed, obesity-resistant rats show quite reliable conditioned approach and discrimination between the CS+ and CS− during testing. Thus, the lack of cycle dependent shifts in conditioned approach, despite shifts in food intake and break point, suggest a potential mechanistic dissociation between appetitive and consummatory behaviors (see Berridge et al., 2010 for review). Although speculative, given the role of the NAc core in motivational responses to food cues (Kelley et al., 2005; Aitken et al., 2016) it’s possible that the cycle-dependent fluctuations in MSN excitability in obesity-prone rats discussed above, could contribute to differences in the modulation of conditioned approach across the cycle in obesity-prone but not obesity-resistant rats. This should be examined in future studies.
5.4. Ovarian hormones modulate cue-triggered motivation in obesity-prone and outbred females
Given that the magnitude of conditioned approach varied across the cycle in intact obesity-prone rats, we also determined the degree to which estradiol and progesterone treatment of ovariectomized rats reduces conditioned approach behavior. A single cycle of estradiol and progesterone treatment did not alter the expression of conditioned approach behavior (Fig. 6C), whereas repeated cycles of this treatment were sufficient to reduce conditioned approach in three separate experiments (Figs. 6D, 7C, 8E). Importantly, this effect was found in both obesity-prone and outbred Sprague-Dawley females; thus, it is not unique to our selectively-bred rat strain. Similarities across obesity-prone and outbred rats are not surprising given that obesity-susceptible rats can be identified in the outbred population (e.g., Levin et al., 1997; Robinson et al., 2015; Madsen et al., 2010).
The fact that repeated hormone treatment was needed in order to recapitulate effects seen in naturally cycling rats may in part be due to effects of ovariectomy. Following Pavlovian conditioning rats were ovariectomized and hormone replacement did not begin until rats failed to enter estrus within an 8–10 days’ period. The removal of the ovaries could itself produce neuroadaptations, such as changes in progesterone and estradiol receptor expression. Thus, repeated cycles of estradiol and progesterone may have been needed in order to compensate for adaptations induced by ovariectomy itself. Nonetheless, these data demonstrate that ovarian hormones strongly influence conditioned approach elicited by a food cue. Furthermore, repeated treatment with estradiol or progesterone alone was not sufficient to reduce conditioned approach behavior (Fig. 5). In contrast, repeated treatment with estradiol was sufficient to decrease home cage food intake and body weight, consistent with previous reports (Blaustein and Wade, 1976). However, it was only when estradiol and progesterone were co-administered that a decrease in conditioned approach was observed. This again points to dissociable mechanisms through which ovarian hormones regulate food intake vs. food-seeking.
To our knowledge, this is the first demonstration that ovarian hormones influence cue-triggered food-seeking. There have been many studies examining how ovarian hormones influence motivation for primary reinforcers like food, sex, and potentially addictive substances like cocaine (Yoest et al., 2018; Yoest et al., 2014; Rivera and Stincic, 2018; Frye, 2007). Although the mechanisms underlying these behaviors are not well understood, ovarian hormones do influence both dopaminergic and glutamatergic transmission within the NAc core in ways that are consistent with the behavioral effects found here (Micevych and Meisel, 2017; Tonn Eisinger et al., 2018; Yoest et al., 2018). In addition to effects of dopamine on NAc intrinsic excitability (discussed above), estradiol treatment also decreases dendritic spine density (an indirect measure of excitatory synapses) in the NAc (Peterson et al., 2015), and reduces AMPA receptor specific binding (Cyr et al., 2001) and GluA2 AMPAR subunit mRNA in the NAc (Le Saux et al., 2006). Thus, reductions in the motivational responses to food cues may be related to the effects of estradiol on excitatory transmission in the NAc. However, it’s important to keep in mind that effects on conditioned approach required both estradiol and progesterone treatment, suggesting a more complex mechanism.
6. Conclusions & future directions
In sum, we find that in females individual susceptibly to obesity is associated with enhanced motivational responses to food cues and increased intrinsic excitability of NAc core MSNs, consistent with overall patterns found in males. In addition, both estradiol and progesterone are needed to reduce conditioned approach behavior in obesity-prone rats, whereas estradiol treatment alone is sufficient to reduce food intake and motivation for food. These data suggest dissociations between hormonal regulation of food-seeking vs. consumption. Furthermore, interactions between individual susceptibility to obesity and the regulation of incentive motivation by ovarian hormones likely represent phenotypic differences that may be mediated by altered NAc responsivity. Future studies of the precise mechanisms involved, as well as the effects of diet-induced obesity and consumption of sugary, fatty foods are needed in order to understand the fundamental neurobiological mechanisms of food-seeking in the non-obese and obese state. Finally, effects of ovarian hormones on conditioned approach were similar in obesity-prone and outbred females, suggesting that neurobehavioral effects of ovarian hormones observed here extend to outbred populations.
Acknowledgements
This work was supported by NIH including R01DK106188-02-S1 and 1F99NS108549-01 to YAC, and R01DK106188; 1R01DK115526-01, and R21DA045277 to CRF. Additionally, we thank Ms. Sophia Dunlap, Ms. Limmy Kim and Ms. Liana Dunietz for their help with behavioral portions of this study, Dr. Johnny Sexton for the use of his light microscope and Dr. Arif Hamid for helping write MATLAB code for electrophysiology data analysis.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.yhbeh.2019.104583.
References
- Aitken TJ, Greenfield VY, Wassum KM, 2016. Nucleus accumbens core dopamine signaling tracks the need-based motivational value of food-paired cues. J. Neurochem. 136 (5), 1026–1036. 10.1111/jnc.13494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso-Caraballo Y, Jorgensen ET, Brown TE, Ferrario CR, 2018. Functional and structural plasticity contributing to obesity: roles for sex, diet, and individual susceptibility. Curr. Opin. Behav. Sci 23, 160–170. 10.1016/j.cobeha.2018.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnoni-Bauer Y, Bick A, Raz N, Imbar T, Amos S, Agmon O, Marco L, Levin N, Weiss R, 2017. Is it me or my hormones? Neuroendocrine activation profiles to visual food stimuli across the menstrual cycle. J. Clin. Endocrinol. Metab 102 (9), 3406–3414. 10.1210/jc.2016-3921. [DOI] [PubMed] [Google Scholar]
- Asarian L, Geary N, 2002. Cyclic estradiol treatment normalizes body weight and restores physiological patterns of spontaneous feeding and sexual receptivity in ovariectomized rats. Horm. Behav. 42 (4), 461–471. 10.1006/hbeh.2002.1835. [DOI] [PubMed] [Google Scholar]
- Asarian L, Geary N, 2006. Modulation of appetite by gonadal steroid hormones. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 361 (1471), 1251–1263. 10.1098/rstb.2006.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asarian L, Geary N, 2013. Sex differences in the physiology of eating. Am. J. Physiol. Regul. Integr. Comp. Physiol 305 (11), R1215–R1267. 10.1152/ajpregu.00446.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azdad K, Chàvez M, Don Bischop P, Wetzelaer P, Marescau B, De Deyn PP, Gall D, Schiffmann SN, 2009. Homeostatic plasticity of striatal neurons intrinsic excitability following dopamine depletion. PLoS One 4, e6908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE, 1998. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res. Rev 10.1016/S0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Ho CY, Richard JM, Difeliceantonio AG, 2010. The tempted brain eats: pleasure and desire circuits in obesity and eating disorders. Brain Res. 10.1016/j.brainres.2010.04.003. NIH Public Access. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein JD, Wade GN, 1976. Ovarian influences on the meal patterns of female rats. Physiol. Behav. 17 (2), 201–208. 10.1016/0031-9384(76)90064-0. [DOI] [PubMed] [Google Scholar]
- Calipari ES, Juarez B, Morel C, Walker DM, Cahill ME, Riberio E, Deisseroth K, Han MH, Nestler EJ, 2017. Dopaminergic dynamics underlying sex-specific cocaine reward. Nat. Commun. 8, 13877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Dorris DM, Meitzen J, 2016. Neonatal masculinization blocks increased excitatory synaptic input in female rat nucleus accumbens core. Endocrinology 157, 3181–3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Willett JA, Dorris DM, Meitzen J, 2018. Sex differences in medium spiny neuron excitability and glutamatergic synaptic input: heterogeneity across striatal regions and evidence for estradiol-dependent sexual differentiation. Front. Endocrinol. 10.3389/fendo.2018.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings JA, Becker JB, 2012. Quantitative assessment of female sexual motivation in the rat: hormonal control of motivation. J. Neurosci. Methods 204 (2), 227–233. 10.1016/j.jneumeth.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyr M, Ghribi O, Thibault C, Morissette M, Landry M, Di Paolo T, 2001. Ovarian steroids and selective estrogen receptor modulators activity on rat brain NMDA and AMPA receptors. Brain Res. Rev. 37, 153–161. 10.1016/S0165-0173(01)00115-1. [DOI] [PubMed] [Google Scholar]
- Demos KE, Heatherton TF, Kelley WM, 2012. Individual differences in nucleus accumbens activity to food and sexual images predict weight gain and sexual behavior. J. Neurosci. 32 (16), 5549–5552. 10.1523/JNEUROSCI.5958-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derman RC, Ferrario CR, 2018a. Enhanced incentive motivation in obesity-prone rats is mediated by NAc core CP-AMPARs. Neuropharmacology 131, 326–336. 10.1016/j.neuropharm.2017.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derman RC, Ferrario CR, 2018b. Junk-food enhances conditioned food cup approach to a previously established food cue, but does not alter cue potentiated feeding; implications for the effects of palatable diets on incentive motivation. Physiol. Behav. 192, 145–157. 10.1016/j.physbeh.2018.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreher J-C, Schmidt PJ, Kohn P, Furman D, Rubinow D, Berman KF, 2007. Menstrual cycle phase modulates reward-related neural function in women. Proc. Natl. Acad. Sci 104 (7), 2465–2470. 10.1073/pnas.0605569104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckel LA, 2011. The ovarian hormone estradiol plays a crucial role in the control of food intake in females. Physiol. Behav. 104 (4), 517–524. 10.1016/j.physbeh.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario CR, 2017. Food Addiction and Obesity. pp. 42. 10.1038/npp.2016.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario CR, Labouèbe G, Liu S, Nieh EH, Routh VH, Xu S, O’Connor EC, 2016. Homeostasis meets motivation in the battle to control food intake. J. Neurosci. 36 (45), 11469–11481. 10.1523/JNEUROSCI.2338-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank TC, Kim GL, Krzemien A, Van Vugt DA, 2010. Effect of menstrual cycle phase on corticolimbic brain activation by visual food cues. Brain Res. 1363, 81–92. 10.1016/j.brainres.2010.09.071. [DOI] [PubMed] [Google Scholar]
- Frye CA, 2007. Progestins influence motivation, reward, conditioning, stress, and/or response to drugs of abuse. Pharmacol. Biochem. Behav 10.1016/j.pbb.2006.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschberg AL, 2012. Sex hormones, appetite and eating behaviour in women. Maturitas. 10.1016/j.maturitas.2011.12.016. [DOI] [PubMed] [Google Scholar]
- Holland PC, 1977. Conditioned stimulus as a determinant of the form of the Pavlovian conditioned response. J. Exp. Psychol. Anim. Behav. Process 3 (1), 77–104. 10.1037/0097-7403.3.1.77. [DOI] [PubMed] [Google Scholar]
- Hu XT, 2007. Cocaine withdrawal and neuro-adaptations in ion channel function. Mol. Neurobiol. 35 (1), 95–112. 10.1007/BF02700626. [DOI] [PubMed] [Google Scholar]
- Kelley BG, Mermelstein PG, 2011. Progesterone blocks multiple routes of ion flux. Mol. Cell. Neurosci 48 (2), 137–141. 10.1016/j.mcn.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley Ann E., Baldo Brian A., Pratt Wayne E., Will Matthew J., 2005. Corticostriatal-hypothalamic circuitry and food motivation: integration of energy, action and reward. In: Physiology and Behavior. vol. 86. pp. 773–795. 10.1016/j.physbeh.2005.08.066. [DOI] [PubMed] [Google Scholar]
- Le Saux M, Estrada-Camarena E, Di Paolo T, 2006. Selective estrogen receptor-alpha but not-beta agonist treatment modulates brain alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors. J. Neurosci. Res 84 (5), 1076–1084. 10.1002/jnr.21007. [DOI] [PubMed] [Google Scholar]
- Levin BE, Govek E, 1998. Gestational obesity accentuates obesity in obesity-prone progeny. Am. J. Phys 275 (4 Pt 2), R1374–R1379. Retrieved from. http://ajpregu.physiology.org/content/ajpregu/275/4/R1374.full.pdf. [DOI] [PubMed] [Google Scholar]
- Levin BE, Dunn-Meynell A, Balkan B, Keesey RE, 1997. Selective breeding for diet-induced obesity and resistance in Sprague-Dawley rats. Am. J. Phys 273, R725–R730. Retrieved from. http://www.physiology.org/doi/pdf/10.1152/ajpregu.1997.273.2.R725. [DOI] [PubMed] [Google Scholar]
- Madsen AN, Hansen G, Paulsen SJ, Lykkegaard K, Tang-Christensen M, Hansen HS, Vrang N, 2010. Long-term characterization of the diet-induced obese and dietresistant rat model: a polygenetic rat model mimicking the human obesity syndrome. J. Endocrinol. 206 (3), 287–296. 10.1677/JOE-10-0004. [DOI] [PubMed] [Google Scholar]
- Marcondes FK, Miguel KJ, Melo LL, Spadari-Bratfisch RC, 2002. Estrous cycle influences the response of female rats in the elevated plus-maze test. Physiol. Behav. 74 (4–5), 435–440. 10.1016/S0031-9384(01)00593-5. [DOI] [PubMed] [Google Scholar]
- Micevych PE, Meisel RL, 2017. Integrating neural circuits controlling female sexual behavior. Front. Syst. Neurosci 11, 42. 10.3389/fnsys.2017.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdaugh DL, Cox JE, Cook EW 3rd, Weller RE, 2012. fMRI reactivity to high-calorie food pictures predicts short- and long-term outcome in a weight-loss program. Neuroimage 59 (3), 2709–2721. Retrieved from. https://www.ncbi.nlm.nih.gov/pubmed/22332246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola SM, Surmeier DJ, Malenka RC, 2000. Dopaminergic modulation of neuronal excitability in the striatum and nucleus accumbens. Annu. Rev. Neurosci 23 (1), 185–215. 10.1146/annurev.neuro.23.1.185. [DOI] [PubMed] [Google Scholar]
- Nisenbaum ES, Wilson CJ, 1995. Potassium currents responsible for inward and outward rectification in rat neostriatal spiny projection neurons. J. Neurosci. 15 (6), 4449–4463. Retrieved from. http://www.jneurosci.org/content/jneuro/15/6/4449.full.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oginsky MF, Ferrario CR, 2019. Eating junk-food has opposite effects on intrinsic excitability of nucleus accumbens core medium spiny neurons in obesity-susceptible vs.-resistant rats. J. Neurophysiol. 10.1152/jn.00361.2019. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oginsky MF, Goforth PB, Nobile CW, Lopez-Santiago LF, Ferrario CR, 2016a. Eating “junk-food” produces rapid and long-lasting increases in NAc CP-AMPA receptors: implications for enhanced cue-induced motivation and food addiction. Neuropsychopharmacology 41 (13), 2977–2986. 10.1038/npp.2016.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oginsky MF, Maust JD, Corthell JT, Ferrario CR, 2016b. Enhanced cocaine-induced locomotor sensitization and intrinsic excitability of NAc medium spiny neurons in adult but not in adolescent rats susceptible to diet-induced obesity. Psychopharmacology 233 (5), 773–784. 10.1007/s00213-015-4157-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez MF, White FJ, Hu XT, 2006. Dopamine D 2 receptor modulation of K + channel activity regulates excitability of nucleus accumbens neurons at different membrane potentials. J. Neurophysiol. 96 (5), 2217–2228. 10.1152/jn.00254.2006. [DOI] [PubMed] [Google Scholar]
- Peterson BM, Mermelstein PG, Meisel RL, 2015. Estradiol mediates dendritic spine plasticity in the nucleus accumbens core through activation of mGluR5. Brain Struct. Funct. 220 (4), 2415–2422. 10.1007/s00429-014-0794-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podda MV, Riccardi E, D’Ascenzo M, Azzena GB, Grassi C, 2010. Dopamine D1-like receptor activation depolarizes medium spiny neurons of the mouse nucleus accumbens by inhibiting inwardly rectifying K+ currents through a cAMP-dependent protein kinase A-independent mechanism. Neuroscience 167, 678–690. [DOI] [PubMed] [Google Scholar]
- Proaño SB, Morris HJ, Kunz LM, Dorris DM, Meitzen J, 2018. Estrous cycle-induced sex differences in medium spiny neuron excitatory synaptic transmission and intrinsic excitability in adult rat nucleus accumbens core. J. Neurophysiol. 120 (3), 1356–1373. 10.1152/jn.00263.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson NR, Roberts DCS, 1996. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J. Neurosci. Methods 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Rivera H, Stincic T, 2018. Estradiol and the control of feeding behavior. Steroids 133, 44–52. 10.1016/j.steroids.2017.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MJF, Burghardt PR, Patterson CM, Nobile CW, Akil H, Watson SJ, Ferrario CR, 2015. Individual differences in cue-induced motivation and striatal systems in rats susceptible to diet-induced obesity. Neuropsychopharmacology 40 (9), 2113–2123. 10.1038/npp.2015.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, 1997. Dopamine neurons and their role in reward mechanisms. Curr. Opin. Neurobiol 7 (2), 191–197. 10.1016/S0959-4388(97)80007-4. [DOI] [PubMed] [Google Scholar]
- Schultz W, 2013. Updating dopamine reward signals. Curr. Opin. Neurobiol 23, 229–238. 10.1016/j.conb.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekerli M, Del Negro CA, Lee RH, Butera RJ, 2004. Estimating action potential thresholds from neuronal time-series: new metrics and evaluation of methodologies. IEEE Trans. Biomed. Eng 51 (9), 1665–1672. 10.1109/TBME.2004.827531. [DOI] [PubMed] [Google Scholar]
- Stice E, Figlewicz DP, Gosnell BA, Levine AS, Pratt WE, 2013. The contribution of brain reward circuits to the obesity epidemic. Neurosci. Biobehav. Rev 37 (9 Pt A), 2047–2058. 10.1016/j.neubiorev.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckel LE, Weller RE, Cook EW, Twieg DB, Knowlton RC, Cox JE, 2008. Widespread reward-system activation in obese women in response to pictures of high-calorie foods. NeuroImage 41 (2), 636–647. 10.1016/j.neuroimage.2008.02.031. [DOI] [PubMed] [Google Scholar]
- Tarttelin MF, Gorski RA, 1971. Variations in food and water intake in the normal and acyclic female rat. Physiol. Behav. 7 (6), 847–852. 10.1016/0031-9384(71)90050-3. [DOI] [PubMed] [Google Scholar]
- Tonn Eisinger KR, Larson EB, Boulware MI, Thomas MJ, Mermelstein PG, 2018. Membrane estrogen receptor signaling impacts the reward circuitry of the female brain to influence motivated behaviors. Steroids 133, 53–59. 10.1016/j.steroids.2017.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollbrecht PJ, Nobile CW, Chadderdon AM, Jutkiewicz EM, Ferrario CR, 2015. Pre-existing differences in motivation for food and sensitivity to cocaine-induced locomotion in obesity-prone rats. Physiol. Behav. 152 (Pt A), 151–160. 10.1016/j.physbeh.2015.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollbrecht PJ, Mabrouk OS, Nelson AD, Kennedy RT, Ferrario CR, 2016. Pre-existing differences and diet-induced alterations in striatal dopamine systems of obesity-prone rats. Obesity 24 (3), 670–677. 10.1002/oby.21411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade GN, 1972. Gonadal hormones and behavioral regulation of body weight. Physiol. Behav. 10.1016/0031-9384(72)90340-X. [DOI] [PubMed] [Google Scholar]
- Wolf ME, 2002. Addiction: making the connection between behavioral changes and neuronal plasticity in specific pathways. Mol. Interv. 2 (3), 146–157. 10.1124/mi.2.3.146. [DOI] [PubMed] [Google Scholar]
- Wolf ME, Mangiavacchi S, Sun X, 2003. Mechanisms by which dopamine receptors may influence synaptic plasticity. Ann. N. Y. Acad. Sci 10.1196/annals.1300.015. [DOI] [PubMed] [Google Scholar]
- Yoest KE, Cummings JA, Becker JB, 2014. Estradiol, dopamine and motivation. Cent Nerv Syst Agents Med Chem. Cent Nerv Syst Agents Med Chem 14 (2), 83–89. 10.14440/jbm.2015.54.A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoest KE, Quigley JA, Becker JB, 2018. Rapid effects of ovarian hormones in dorsal striatum and nucleus accumbens. Horm. Behav. 10.1016/j.yhbeh.2018.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z, Geary N, Corwin RL, 2011. Individual effects of estradiol and progesterone on food intake and body weight in ovariectomized binge rats. Physiol. Behav. 104 (5), 687–693. 10.1016/j.physbeh.2011.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Huang Y, Liu X, Wang G, Wang X, Wang Y, 2015. Estrogen suppresses epileptiform activity by enhancing Kv4.2-mediated transient outward potassium currents in primary hippocampal neurons. Int. J. Mol. Med 36 (3), 865–872. 10.3892/ijmm.2015.2287. [DOI] [PubMed] [Google Scholar]
- Zhao B, Zhu J, Dai D, Xing J, He J, Fu Z, Zhang L, Li Z, Wang W, 2016. Differential dopaminergic regulation of inwardly rectifying potassium channel mediated subthreshold dynamics in striatal medium spiny neurons. Neuropharmacology 107, 396–410. [DOI] [PubMed] [Google Scholar]