Abstract
Rechargeable Li metal batteries are one of the most attractive energy storage systems due to their high energy density. However, the hostless nature of Li, the excessive dendritic growth, and the accumulation of nonactive Li induce severe volume variation of Li anodes. The volume variation can give rise to a fracture of solid electrolyte interphase, continuous consumption of Li and electrolytes, low Coulombic efficiency, fast performance degradation, and finally short cycle life. This Outlook provides a comprehensive understanding of the origin and consequences of Li volume variation. Recent strategies to address this challenge are reviewed from liquid to gel to solid-state electrolyte systems. In the end, guidelines for structural design and fabrication suggestions for future long-life Li composite anodes are presented.
Short abstract
This Outlook focuses on the recent progress in stabilizing the electrode volume of the Li anode from liquid to gel to solid-state electrolyte systems.
1. Introduction
Lithium-ion batteries (LIBs) as an efficient energy storage system dominate in portable electronics and electric vehicles mainly due to their unique advantages, such as high safety, long cyclic life, low cost, and environmental friendliness.1 With the ever-growing demand for longer driving range (>500 km) of battery electric vehicles, LIBs based on intercalation chemistry have been constrained by energy density limitations.2 Therefore, researchers have focused on the exploration of rechargeable lithium metal batteries (LMBs) with high capacity and high energy density. A typical LMB is composed of a Li metal anode, an electrolyte, and a cathode. In contrast to commercial LIBs based on the intercalation mechanism of graphite anodes, LMBs realize energy output based on a continuous plating/stripping mechanism of Li anodes. As a key component of LMBs, Li metal anodes contribute to the high energy density of 2600 W h kg–1 (refers to Li–S battery) by delivering a remarkable theoretical capacity of 3860 mA h g–1 and a reasonable operating voltage of −3.04 V (vs standard hydrogen electrode).3 Therefore, as an anode material, metallic Li not only serves as an alternative to graphite but also plays an indispensable role in next-generation energy storage systems of Li–air and Li–S batteries.4,5
Despite its advantages, metallic Li is still not practical because of its dramatic volume variation and related performance degradation.6 The intrinsic hostless feature of Li deposition, Li dendritic growth, and accumulation of inactive Li debris are three main reasons for severe Li volume variation. The plating/stripping of Li is a “hostless” behavior. This brings uncontrolled Li volume expansion/contraction during repeated charge/discharge processes. Driven by the thermodynamic factor of a high diffusion barrier, Li atoms tend to aggregate as several isolated atomic groups and grow into dendritic shapes, which form a porous structure after cycling. The prolonged Li dendrites may also detach from the current collector. The Li debris that loses electrochemical activity is called “dead Li”. The continuous accumulation of “dead Li” also contributes to the volume expansion of Li. Notably, Li volume variation also has a connection with interfacial issues. Poor interfacial stability aggravates the excessive formation and growth of Li dendrites and finally results in Li volume expansion.
Since it induces low Coulombic efficiency, severe safety hazards, and significant battery capacity degradation, the Li volume variation issue hinders the application of Li metal anodes in the future high-energy sector.7 This Outlook aims at addressing this critical issue by presenting recent achievements in Li metal anodes with three types of electrolyte systems containing liquid, gel, and solid-state electrolyte systems (Figure 1). Additionally, the challenges and opportunities with ongoing technologies are presented, along with the elucidation of the structure–performance relationship, providing a perspective for rational design and fabrication of a hybrid Li anode.
Figure 1.
Schematic illustration of developing low-volume-change Li metal anodes for safe LMBs.
2. Recent Advances in the Liquid Electrolyte Systems
2.1. SEI-Reinforced Li Metal Anodes
As an indispensable component of the battery, electrolytes have a great influence on the cycling stability of Li metal anodes. Since metallic Li has a low reduction potential and a high reactive activity, it can easily react with the liquid electrolytes to form a solid-electrolyte interphase (SEI) layer on the anode surface. SEI stability is strongly correlated with electrode volume stability. The interface fluctuation can induce the generation of cracks, which aggravates the growth of Li dendrites and large morphological variation of the plated/stripped Li. Consequently, SEI reinforcement is needed to accommodate Li volume change.
An ideal SEI film should be a thin, dense, uniform, ion-conductive but electronically insulating layer. Such SEI film is expected to uniformize Li+ distribution, guide even Li nucleation and growth, and alleviate excessive volume changes of Li metal. Reinforcement of SEI film can be an effective approach, for example, adjusting the electrolyte composition or introducing electrolyte additives, such as polysulfides,8 LiNO3,9 H2O,10 AlCl3,11 vinylene carbonate,12 and fluoroethylene carbonate.13 However, the suppression effect is not fully sustainable because of the unceasing consumption of electrolytes and additives. Moreover, the as-formed SEI layers are usually too brittle to maintain their shape when faced with huge surface fluctuations. Hence, SEI cracks and continuous growth of Li dendrites are inevitable.
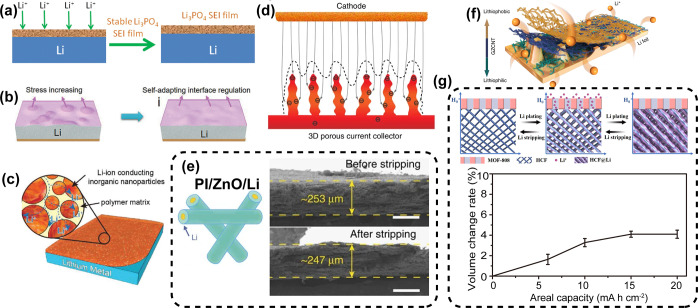
As an alternative, mechanically stable artificial SEI coatings, including inorganic or polymer blocking layers, have been developed to restrain Li dendrite growth and mitigate the volume change of Li anodes. Inorganic SEI films such as Li3PO4,14 LiF,15 Li3N,16 LIPON,17 and Al2O318,19 films have been devised as efficient blocking layers, exhibiting high Li+ conductivity, excellent chemical stability, and a high Young’s modulus. For instance, Guo et al. demonstrated a layer of Li3PO4 on the surface of Li foil suppressed the formation of Li dendrites and reduced the side reaction between Li metal and electrolyte (Figure 2a).14 In addition to the inorganic SEI films, polymeric coatings have also been developed as blocking layers due to their good flexibility or elasticity. SEI films with proper flexibility and elasticity can effectively adapt to the huge electrode volume fluctuation during cycling. For practical batteries, areal capacities ≥3 mA h cm–2 of Li are needed, corresponding to a thickness variation of the Li anode of more than 15 μm. Because of its rigidity, a thin inorganic SEI layer is not likely to contact the Li metal anode conformally on a microscopic scale without cracks. In this context, poly(vinyl alcohol) (PVA),20 poly(ethylene oxide) (PEO)@ureido-pyrimidinone (UPy),21 poly(dimethylsiloxane) (PDMS) film,22 Li-Nafion,23 and poly(acrylic acid) (LiPAA)24 have been utilized to obtain a flat and dense Li deposition. Specifically, silly putty, a dynamically cross-linked polymer, exhibited an excellent adaptivity toward the dynamic volume changes of the Li anode.25 In particular, Guo et al. demonstrated Li stability and battery safety improvements by coating a LiPAA layer on a Li disk with high elasticity and a good self-adaptive ability (Figure 2b).24 Other than pure inorganic or polymeric coatings, a combination of two types of materials can be a feasible way to fabricate SEI films with high Young’s modulus, good flexibility, and high Li+ conductivity (Figure 2c). Following this principle, a series of SEI composites, including Cu3N/SBR,26 PVDF-HFP/Al2O3,27 Si-interlinked OOCOR molecules,28 silica@poly(methyl methacrylate) (SiO2@PMMA) nanospheres,29 and poly(vinylsulfonyl fluoride-ran-2-vinyl-1,3-dioxolane)-graphene oxide nanosheets,30 have been explored as strong physical barriers and Li-ion conductive media.
Figure 2.
(a) Li3PO4 coated Li anode.14 (b) Flexible LiPAA layer modified Li anode.24 (c) Li3N/SBR polymer hybrid artificial SEI coated Li anode.26 (d) Illustration of electrochemical Li plating on 3D Cu.31 (e) Cross-sectional SEM images of the PI/ZnO/Li electrode before and after Li stripping.63 (f) Schematic presentation of Li plating on Li foil anchored with a lithiophilic–lithiophobic gradient interfacial layer.68 (g) Schematic of the Li plating/stripping process on MOF-HCF and the volume change rate of MOF-HCF@Li anode after plating various areal capacities of Li.69 Images reprinted with permission from refs (14, 24, 26, 31, 63, 68, and 69). Copyright 2016, 2017, 2018 Wiley-VCH, 2016 and 2018 Nature Publishing Group, 2019 Elsevier.
2.2. Construction of 3D Li Anodes
The current collector structure influences initial Li nucleation, effective current density, electric field distribution, and morphology of the deposited Li. Considering the hostless nature of Li, planar Cu foil as a conventional current collector cannot restrain the Li volume expansion/shrinkage during the Li plating/stripping process. Therefore, a three-dimensional (3D) Li anode realized by encaging Li into a 3D host featuring high pore volume is probably the most ideal type of anode configuration. The 3D structure with a large specific surface area can decrease the effective current density, retard dendritic Li growth, and provide accommodation for deposited Li during cycling. In this section, recent progress on 3D current collectors and their Li composites will be discussed.
In 2015, a 3D porous copper current collector consisting of numerous protuberant tips was first designed as a Li host (Figure 2d).31 The protuberant tips served as the nucleation sites to help direct the deposition of Li with nanosized lumps inside the 3D structure without remarkable volume changes at a certain plating capacity. The groundbreaking work inspired worldwide research on 3D current collectors, such as 3D Cu and 3D Ni.32−36 Besides, 3D current collectors together with effective coatings, including Ni foam anchored with graphene,37,38 graphitic carbon nitride,39 graphitized spherical C granules,40 well-arranged ladderlike carbon nanoarray membrane,41 or transition metal oxides,42 have been developed to accommodate Li volume change and enhance interfacial stability simultaneously. Notably, the introduction of metallic skeletons is not advantageous for the improvement of practical capacity and energy density of batteries due to their large density.
Compared with metallic current collectors, carbon-based current collectors have attracted great attention due to their unique advantages of light weight, high specific surface area, large pore volume, high electronic conductivity, high mechanical strength, stable chemical stability, ecofriendliness, and low cost.43,44 Recently, considerable efforts have been made to prove the feasibility of porous carbon skeletons to improve Li plating/stripping electrochemistry and alleviate the volume fluctuations of the Li anode during cycling. The first reported carbon-fiber papers with an anisotropic spatially heterogeneous structure exhibiting improved Li plating/stripping electrochemistry have greatly surpassed the pristine carbon papers by insulating the electrolyte-facing surface while maintaining conductivity in the other parts.45 Additionally, graphitized carbon fiber,46 free-standing 3D hollow carbon fiber,47 nitrogen-doped graphitic carbon foams,48 carbon nanotube,49 and carbonized wood,50 with high electronic conductivity, high electroactive surface area, and high pore volume, have also been developed to alleviate the huge volume changes and dendritic propagation of metallic Li. Similarly, the connected graphite microtubes were employed as a firmly conductive matrix to stabilize the Li anodes.51 Graphene features a layered structure, favorable charge-transport mobility, and high surface area and has been designed as an efficient Li host, since it can effectively reduce local current density, induce uniform deposition of Li metal, suppress the growth of Li dendrites, and relieve the huge volume expansion. In this context, Koratkar et al. developed a free-standing porous graphene network with prevalent divacancy defects via a facile thermal shock approach.52 The defects in the graphene lattice served as seed points that could direct the subsequent plating of Li metal within the interior of the porous graphene network, leading to small dimensional electrode variation. Very recently, a metallically conductive 2D transition-metal carbides and carbonitrides (MXene) paper has been developed as an efficient Li host due to its light weight, high conductivity, and favorable flexibility.53 Besides being a host, C-based materials, such as interconnected carbon nanosphere thin films, graphene, and MXene, have been developed as interfacial coatings to improve the intrinsic heterogeneity and rough surface of the current collectors.54,55
Despite these significant achievements, the application of the above composite Li anodes is restricted by the predeposited technology, involving predeposited Li followed by disassembly and reassembly of the packed cells. Therefore, a thermal infusion strategy, relating to fusion followed by infiltration, has been developed to obtain the composite Li anodes. In this context, Cui et al. reported a composite Li anode by encapsulating molten Li into a 3D conducting C scaffold via a melt infusion strategy.56 Compared with the bare Li foil anode, the Li/C composite anode maintained a relatively intact and stable surface by demonstrating little shape and volume changes due to good confinement of the rigid C backbone on Li metal during cycling, which might result in enhanced safety. Further, a layered Li–rGO composite anode was successfully prepared through a “spark” reaction between the densely stacked GO film and molten Li, which exhibited significant suppression of dendritic Li and minor electrode dimensional change of ∼20% attributable to the porous stable lithiophilic scaffold of rGO.57 Following this discovery, a lithiophilic 3D nitrogen-doped graphene with uniform and large nanopores was used as a Li framework.58 Benefiting from the considerable porosity, high surface area, and high conductivity of the N-doped graphene, dendrite-free Li deposits were plated into the interior of the graphene network, demonstrating a sustainable electrode volume change. Similarly, the Cu–Ni core–shell nanowire network and Cu–CuO–Ni hybrid structures based on Ni foam as a 3D porous substrate were immersed by molten Li.59,60 From the perspective of practical application, the thermal infusion strategy outperforms the predeposited approach in preparing composite Li anodes. However, most 3D porous hosts show poor affinity with molten Li. In this regard, nanomaterials that have certain solubilities in Li attract tremendous interest in improving lithiophilicity. The most common and effective lithiophilic coatings are related to noble metals or Li-rich composite alloys, particularly Au,61 Ag,62 Li–Zn,63 Li–Sn,64 and lithium silicide.65 The seminal work in developing Au as seeds for selective Li metal deposition was conducted by Cui and co-workers.61 Of particular interest, Hu et al. reported that ultrafine silver (Ag) nanoparticles, which were prepared with the aid of a novel rapid Joule method, can act as nanoseeds to regulate the even deposition of Li within the 3D host materials.62 Specifically, the polyimide (PI)–ZnO hosted Li anode can maintain an average thickness range of 247–253 μm before and after stripping, corresponding to only ∼2.4% of the electrode volume change (Figure 2e).63 More recently, Guo et al. utilized a lithiophilic binary alloy phase-lithium aluminum layer to direct metallic Li nucleation and growth.66 Furthermore, Guo and co-workers reported that the relatively negative Gibbs formation energy and the newly formed chemical bonds arising from the reaction between molten Li and functional organic coatings or elemental additives will act cooperatively to drive liquid Li drops to spread onto the lithiophobic substrates.67
Due to the interconnected Li-ion network within a 3D framework and the homogeneity of hosts in structure and property, the emergence of Li dendrites on the surfaces of skeletons is inevitable during prolonged battery operation. The construction of a 3D host that features lithiophilic–lithiophobic gradient properties has proven to be a feasible method to direct dense Li deposition from the bottom (Figure 2f).68 The SEI on 3D nanostructured Li anodes shows undesirable durability in long-term cycling, which might lead to the formation of Li dendrites on their surface over the prolonged cycling. Therefore, a 3D Li host that features a high ionically conductive interface layer is probably the most ideal type of anode configuration, which is expected to address the problems of dendritic growth and enormous volume variation. In this regard, a hybrid host featuring a 3D conducting scaffold with a coating layer of metal–organic frameworks (MOFs) was engineered to accommodate Li (Figure 2g).69 The effective pore volume of the bottom 3D scaffold was up to 1.43 cm3 cm–2, and the resultant 3D host can store 29 mA h cm–2 of Li. Additionally, the topmost porous MOFs layer acted as an “ion sieve”, which can uniformize the distribution of Li+ and regulate the even deposition of Li. Moreover, its high Young’s modulus (>32 GPa) can arrest dendrite propagation. Benefiting from the synergistic effects of the MOF coating and the 3D scaffold, the as-obtained Li anode exhibited extremely low dimensional variation (<5%) at high areal capacity, maintaining electrode stability over 1000 h in symmetric cells and over 200 cycles in full cells.
3. Recent Advances in the Gel Electrolyte Systems
Gel polymer electrolytes (GPEs), another class of promising electrolytes, have attracted worldwide attention for rechargeable LMBs. In general, GPEs mainly consist of polymer matrixes and immobilized liquid electrolytes. Because of the partial substitution of liquid solvents by polymers, GPEs can reduce the parasitic reactions between Li metal and electrolytes, lower the risk of the flammable liquid leakage, and retain superior electrode/electrolyte interfacial characteristics.70 In addition, their features of flexibility and elasticity enable them to adapt to the volume change of the Li anode.
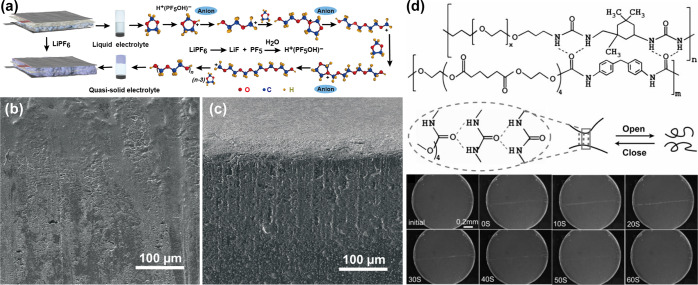
The seminal work of introducing organic solvents into the polymer–salt binary system to generate GPEs was performed by Feuillade and co-workers in 1975.71 Subsequently, various efficient GPEs have been developed by solution casting, inverted phase, and electrospinning technology for LMBs. Polymer matrixes such as PEO, PAN, PVDF, poly(vinylidene fluoride-co-hexafluoropropylene) (PVDF-HFP), and poly(methyl methacrylate) (PMMA) have been introduced as the skeleton of the GPEs. Three types of electrolytes in terms of ester-based electrolytes, ether-based electrolytes, and ionic liquids are generally utilized as the liquid plasticizers. Nevertheless, a nonconformal solid–solid contact originating from the simple physical placement of ex situ formed GPEs onto the Li anode might induce large interface resistance, uneven Li deposition and growth, and volume change of Li anode during prolonged cycling. Therefore, in situ polymerizations including thermal-, radiation-, and electrochemical-initiated methods were proposed to enhance the interfacial contact. For example, an in situ free-radical polymerization of vinyl monomers in ionic liquids has been explored to increase the ionic conductivity and cation transference number, as well as to improve the flexibility of the polymer electrolyte film and interfacial contact with the Li anode.72 However, the introduction of initiators and residual monomers during polymerization as impurities can deteriorate the battery performance.73 In this context, Guo and co-workers have reported an in situ gelation strategy based on Li salt of LiPF6 and ether-based electrolyte of DOL/DME (Figure 3a).74 Due to the improved interfacial compatibility between gel and Li and the good confinement of the liquid phase in the polymer framework, interfacial side reactions were remarkably reduced. Meanwhile, the uniform distribution of Li+ on the surface of Li was realized. As a result, a dense Li anode was maintained without pulverization and dendritic morphology during cycling (Figure 3b,c).
Figure 3.
(a) Schematic illustration of the in situ polymerization mechanism of DOL induced by LiPF6.74 (b) Top-view and (c) Cross-sectional SEM images of a cycled Li anode in the GPE system. (d) Supramolecular structure for self-healable SPE and corresponding self-healing process.89 Images reprinted with permission from refs (74 and 89). Copyright 2018 American Association for the Advancement of Science, 2019 Wiley-VCH.
In order to suppress the growth of Li dendrites, GPEs featuring high ionic conductivity and high mechanical modulus have been explored. Chazalviel and co-workers have supposed that the higher ionic conductivity and higher tLi+ of the electrolytes can efficiently alleviate anion-depletion-induced large electric fields difference, and thus regulate the even nucleation of Li, avoiding the forming of dendrites.75 The Monroe and Newman model pointed out that the electrolytes with high shear modulus doubled compared to that of Li metal could act as a blocking layer to arrest dendrite propagation.76 Targeted at the above issues of GPEs, numerous efforts have been explored, including building a 3D cross-linked polymer network, introducing inorganic nanoparticles (e.g., SiO2, Al2O3, and TiO2) into GPEs, and constructing double-network gels.77−80
4. Recent Advances in the Solid-State Electrolyte Systems
Solid-state electrolytes (SSEs) have been utilized to construct high-performance LMBs free of safety issues. Compared to the aforementioned organic liquid/gel electrolytes, they are of great interest due to their high mechanical modulus, distinctive nonflammability, and antileakage. However, the rigid solid–solid contact brings an uneven distribution of Li+ ions through the interface.81 The uneven electric field distribution at the interface leads to large interfacial impedances and poor electrode/electrolyte compatibility, thus accelerating uncontrollable growth of Li dendrites.82 During a continuous Li plating/stripping process, a portion of the Li dendrites might break off and get isolated to form “dead Li”. The excessive dendritic growth and accumulation of “dead Li” give rise to unavoidable electrode volume variation of Li metal anodes, large interfacial gaps, and, sometimes, Li detachment from SSEs, which shorten the service lives of batteries.
Targeting the volume variation that arises from Li dendrites, attempts to build stable interfacial contact have proven feasible. Stable interfacial contact plays an important role in guiding uniform Li deposition and alleviating excessive volume change. In situ polymerizations have been applied to enhance the polymer electrolyte/Li anode interfacial contacts. Inorganic solid electrolytes, exhibiting high rigidity and fragility, show poor contact with electrodes. Various lithiophilic interphases including both inorganic and organic interphases have been developed to help eliminate the interfacial problem, maximize the interfacial contact, and maintain stable interfacial morphology. Inorganic interphases, such as Au,83 Ag,84 and Mg,85 that have certain solubilities in Li have been developed to improve the wettability of molten Li toward solid electrolytes by generating a Li-rich alloy layer and constructing a robust solid electrolyte/Li integral structure. Organic polymer coatings, for example, tris(2,2,2-trifluoroethyl)orthoformate (TFEO) polymer,86 polypropylene carbonate@poly(ethylene oxide) (PC@PEO),87 and poly(acrylamide-2-methyl-1-propane-sulfonate)@poly(ethylene oxide) (PAS@PEO),88 have been considered as the other kind of ideal interphase in solid-state batteries. With high flexibility and high viscoelasticity, they can tightly stick Li anodes on the solid electrolyte and ensure interfacial integrity. Recently, a solid polymeric electrolyte (SPE) featuring fast self-healing rates, rigid–soft coexisting stability, and high ion conductivity has been confirmed to effectively and quickly repair the cracks arising from morphology variations in Li metal (Figure 3d).89
Although the engineered interfaces alleviate Li volume problems by suppressing the formation/growth of Li dendrites, the Li volume change cannot be completely avoided when the Li stripping/plating process proceeds. As stated, a series of 3D Li composites have shown efficacy in addressing both the dendrite formation and volume change issues of Li metal. However, two critical issues relating to the 3D Li composite anodes in SSEs should be emphasized: (i) high Li-ion conductive frameworks are needed to ensure Li deposition inside the structure instead of on the surface of Li; (ii) completely conformal solid–solid contact is beneficial for further decreasing interfacial impedances.
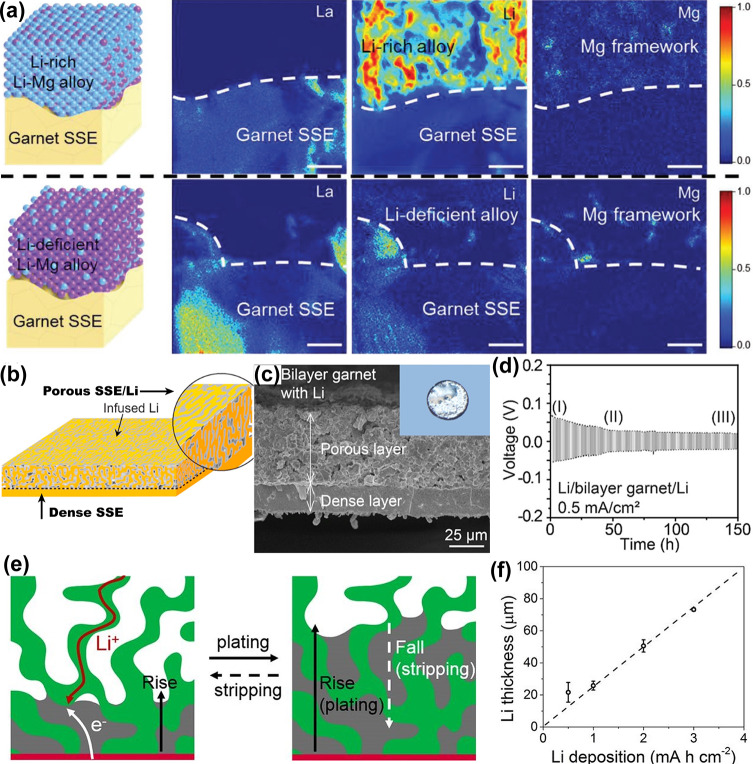
In this context, Hu and co-workers first proposed to improve the solid-state Li-metal anode performance by using a Li-ion and electron dual-conductive framework prepared by dealloying a Li–Mg alloy anode on a garnet electrolyte (Figure 4a).90 The Li-rich Li–Mg alloy has intentionally reserved a Li reservoir to offset any Li loss upon cycling. Particularly, the Li–Mg alloy can still maintain a stable framework structure and ensure strong binding between garnet SSE and the Li anode even if a high areal of Li is stripped. Constructing an integral 3D Li anode featuring a continuous ionic contact with solid electrolytes has been considered as another effective way to improve the interfacial contact and alleviate huge volume variations. Of particular interest, a garnet-based asymmetric 3D framework with a porous-dense bilayer structure was designed as a solid-state Li metal host for safe and high-energy-density Li metal batteries (Figure 4b).91 On one hand, the porous layer filled with molten Li not only ensures highly conductive and continuous ionic pathways for Li-ion transport due to a continuous and firm contact between Li and electrolyte but also preserves the electrode dimensional stability because of the good accommodation of Li within the ion-conductive host (Figure 4c). Conversely, the dense layer with high mechanical properties and electrochemical stability acts as a separator to arrest dendrite propagation. With this unique bilayer structural design, a solid-state Li metal battery with smaller overpotential and higher gravimetric/volumetric energy was achieved (Figure 4d). However, during Li deposition, the Li ions migrate through the 3D ion-conductive skeleton to the surface while the electrons transfer through the battery steel shell, resulting in the inevitable deposition of metallic Li outside of the framework. In this context, a trilayer garnet structural Li host with a bottom-coated Cu has been devised to construct a safe and dendrite-free solid Li metal anode.92 During the Li deposition process, Li grows along the ionic-conductive garnet framework when the bottom Cu layer induces the initial Li nucleation (Figure 4e). Particularly, the middle dense layer has intentionally been designed as a blocking layer to prohibit dendritic Li propagation. This structural design keeps the battery from short-circuit risks with nearly zero volume change (Figure 4f).
Figure 4.
(a) ToF-SIMS elemental mapping of the alloy–garnet interface of the Li-rich Li–Mg alloy before cell cycling and the Li-deficient Li–Mg alloy after stripping Li.90 (b) An illustration of the Li infiltration into a garnet-based asymmetric 3D framework with a porous-dense bilayer structure.91 (c) Cross-sectional SEM image of Li and bilayer garnet. (d) Voltage profiles of metallic Li plating/stripping in the Li/bilayer garnet/Li symmetric cell. (e) Schematic diagrams of Li plating/stripping processes on the trilayer garnet framework.92 (f) The thickness of the hosted Li anode with various capacities of Li. Images reprinted with permission from refs (90−92). Copyright 2018 Wiley-VCH, 2018 Elsevier, 2018 National Academy of Sciences.
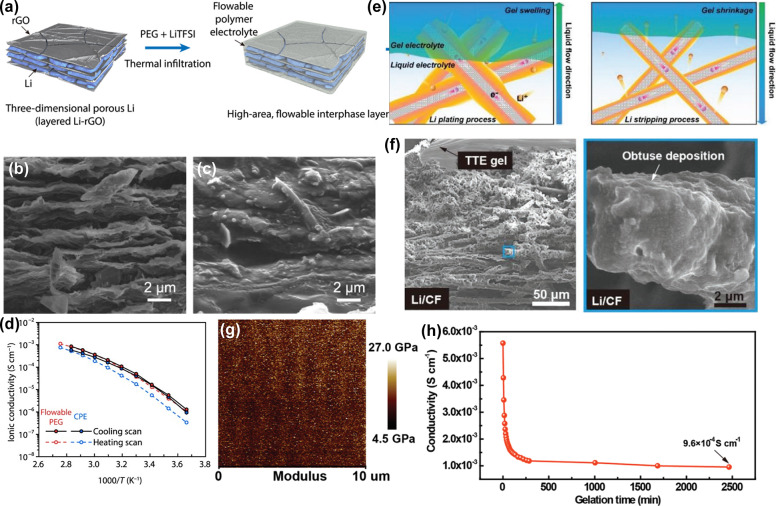
Even so, the limited interfacial contact area arising from solid–solid contact results in large interfacial resistance and low Li utilization. Incorporating GPEs into the three-dimensional Li framework to form a hybrid Li anode will give rise to a continuous and flowable ionic contact between Li and electrolyte and achieve improved electrochemical performance. A remarkable example was a rational design and fabrication of layered reduced graphene oxide (rGO) as an efficient host for metallic Li with embedded microscale Li metal to form the 3D anode.93 Then, poly(ethylene glycol) plasticized by bis(trifluoromethane)sulfonimide Li salt (LiTFSI) was cast into the as-obtained 3D Li anode to generate a hybrid Li anode (Figure 5a). Since metallic Li is sandwiched by rGO, the Li anode with micrometer-scale volume changes was realized (Figure 5b,c). Due to a continuous and integrated structure, continuous adhesive contact between the Li anode and electrolyte is achieved. However, the limited porosity and low mechanical strength of rGO cannot adapt to the Li electrode volume change during the prolonged deposition. In addition, the ionic conductivity of PEG-LiTFSI is merely the order of 10–4 S cm–1 at 40 °C (Figure 5d). Therefore, it is imperative to engineer a hybrid Li anode with characteristics of appropriate porosity, high mechanical strength, and favorable ionic conductivity at room temperature. In this context, an integral anode has been realized by encapsulating both a 3D Li/CF composite anode and liquid electrolyte into an in situ polymerized gel (Figure 5e).94 Due to the good confinement of the 3D scaffold on Li, deposited Li was accommodated into the networks, free from dendrites risk and huge electrode volume change (Figure 5f). The autopolymerized gel electrolyte that implanted trace liquid electrolyte exhibited a high Young’s modulus (∼13.3 GPa), stable electrochemical window (∼5 V vs Li+/Li), and high ionic conductivity (9.6 × 10–4 S cm–1), which help build continuous contacts with both electrodes and arrest Li dendrite propagation (Figure 5g,h). Gradient polymerization of solid electrolytes is encouraged to achieve a configuration featuring a flowable ionic contact within the 3D frameworks and a solid stiff surface for Li dendrite suppression.95
Figure 5.
(a) Schematic presentation of incorporating liquid-like PEG-LiTFSI into 3D Li-rGO composite.93 Cross-sectional SEM images of 3D Li-rGO composite before (b) and after (c) thermal infiltration of the flowable PEG. (d) Comparison of the ionic conductivities of different electrolyte systems. (e) Schematic diagrams of Li plating/stripping processes on the 3D Li/CF composite anode with optimized Li+ ionic transport in TTE gel electrolyte.94 (f) SEM images of Li/CF in TTE gel electrolyte. (g) The Young’s modulus mapping of TTE gel film. (h) The ionic conductivity of the TTE gel vs gelation time plot. Images reprinted with permission from refs (93 and 94). Copyright 2017 American Association for the Advancement of Science, 2020 Wiley-VCH.
5. Conclusions and Future Outlook
Rechargeable LMBs have attracted fast-growing attention, where performance degradation and safety hazards originating from dramatic volume changes hinder the commercial applications of LMBs. This Outlook focuses on an in-depth analysis of the causes and challenges of the volume change issue of Li anodes. Advanced strategies have also been summarized to stabilize the electrode volume of the Li anode and, thus, improve the electrochemical performance of LMBs from liquid to solid-state electrolyte systems. Intrinsic hostless features of Li deposition, Li dendritic growth, and accumulation of inactive Li debris are three main factors for large volume variations in Li metal. During the plating/stripping process, the endless volume change induces huge internal stress fluctuations, which can impose crack formation at the electrode/separator interface. The exposed fresh Li at the cracks aggravates the nonuniform Li deposition and the parasitic reaction with the electrolyte. Even worse, the large volume variation problem of the Li anode also challenges the packing technique of practical batteries.
Devising SEI films with good flexibility, high mechanical modulus, and favorable ionic conductivity has become one of the most powerful means to arrest the growth of Li dendrites and alleviate Li volume change. However, the poor binding affinity of inorganic SEI films to the Li surface, low mechanical modulus, and poor electrochemical/chemical stability of the organic SEI films make the suppression effect not fully durable and efficient during prolonged cycling. An exciting direction is to design the composite SEI layer where inorganic particle composites are integrated with the polymeric matrix. The SEI composite inherits the merits of the inorganic and polymer materials, including high ionic conductivity, good electrochemical/chemical stability, high Young’s modulus, and excellent elasticity. Functional polymers featuring highly elastic (polyrotaxanes) and self-healing ability (polymers with a dynamic hydrogen bonding network) may be developed as potential SEI films. Nevertheless, nearly all previous studies on SEI films were based on planar Li foils, so that the huge volume change of the Li anode during Li plating/stripping remains a crucial yet unsolved issue.
Research into current collectors has made great progress and should continue. Encaging Li in 3D hosts is beneficial in taking advantage of the porous structure to control the volume change brought by deposited Li. Hosts with appropriate pore size/structure are encouraged to accommodate Li with high reversibility and to alleviate Li volume change with sufficient space. In addition, the features of ultrathin, ultrastrong, and flexible for hosts should be emphasized. To improve the affinity of a matrix to Li metal, surface-modification strategies in terms of chemical reaction or physical interaction (such as capillary force) attract tremendous interest. However, due to the good flowable ion contact within the 3D interconnected network and the uniformity of substrates in composition and conductivity, metallic Li tends to deposit on the surface of skeletons upon cycling. When batteries are operated at high current densities, the terminals of overcharge, or low operation temperatures, safety hazards, including uncontrollable dendrites, fires, or even explosions, may occur. Designing 3D hosts with a lithiophilic–lithiophobic gradient or hybrid structure is another possible direction, which keeps Li deposition away from separators. A 3D Li host coated by a layer with high ion conductivity is probably the most ideal type of future anode configuration, which is expected to address the problems of dendritic growth and enormous volume variation.
However, seen from the present, an all-solid-state battery is a promising candidate for a next-generation energy storage system. Designing a Li anode with negligible volume variation is the key to realize long-term service life of batteries. Ideally, the anode composites consisting of both electrolytes and Li within a 3D host can provide ample space to accommodate Li and continuous ionic contact for efficient Li-ion transport. Improving the affinity between solid-state electrolytes and Li anodes via interfacial engineering is pivotal. The design of the multilayer structure for solid electrolytes is highly suggested for the development of LMBs toward safe and high energy density. Future endeavors are welcomed in developing flame-retardant materials, which can intrinsically avoid the risk of fire and improve battery safety.
Acknowledgments
We are grateful for financial support from the Basic Science Center Project of NSFC (51788104), the National Key R&D Program of China (Grant No. 2016YFA0202500), the National Natural Science Foundation of China (Grant Nos. 21773264, 21805105, 21975091, and 21773078), the Fundamental Research Funds for the Central Universities of China (2662017QD028), the Science and Technology Department of Hubei Province (2018FB238), China Postdoctoral Science Foundation (Grant No. 2018M640185), and Special Financial Grant from China Postdoctoral Science Foundation (Grant No. 2019T120136).
Author Contributions
∥ H.Y. and Y.Z. contributed equally to this work.
The authors declare no competing financial interest.
References
- Tarascon J.-M.; Armand M. Issues and Challenges Facing Rechargeable Lithium Batteries. Nature 2001, 414, 359–367. 10.1038/35104644. [DOI] [PubMed] [Google Scholar]
- Manthiram A. An Outlook on Lithium Ion Battery Technology. ACS Cent. Sci. 2017, 3, 1063–1069. 10.1021/acscentsci.7b00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin D. C.; Liu Y. Y.; Cui Y. Reviving the Lithium Metal Anode for High-Energy Batteries. Nat. Nanotechnol. 2017, 12, 194. 10.1038/nnano.2017.16. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Zuo T.-T.; Popovic J.; Lim K.; Yin Y.-X.; Maier J.; Guo Y.-G. Towards Better Li Metal Anodes: Challenges and Strategies. Mater. Today 2020, 33, 56. 10.1016/j.mattod.2019.09.018. [DOI] [Google Scholar]
- Bruce P. G.; Freunberger S. A.; Hardwick L. J.; Tarascon J.-M. Li-O2 and Li-S Batteries with High Energy Storage. Nat. Mater. 2012, 11, 19–29. 10.1038/nmat3191. [DOI] [PubMed] [Google Scholar]
- Cheng X.-B.; Zhang R.; Zhao C.-Z.; Zhang Q. Toward Safe Lithium Metal Anode in Rechargeable Batteries: A Review. Chem. Rev. 2017, 117, 10403–10473. 10.1021/acs.chemrev.7b00115. [DOI] [PubMed] [Google Scholar]
- Yang C.; Fu K.; Zhang Y.; Hitz E.; Hu L. Protected Lithium-Metal Anodes in Batteries: From Liquid to Solid. Adv. Mater. 2017, 29, 1701169. 10.1002/adma.201701169. [DOI] [PubMed] [Google Scholar]
- Li W. Y.; Yao H. B.; Yan K.; Zheng G. Y.; Liang Z.; Chiang Y.-M.; Cui Y. The Synergetic Effect of Lithium Polysulfide and Lithium Nitrate to Prevent Lithium Dendrite Growth. Nat. Commun. 2015, 6, 7436. 10.1038/ncomms8436. [DOI] [PubMed] [Google Scholar]
- Xiong S. Z.; Xie K.; Diao Y.; Hong X. B. Characterization of the Solid Electrolyte Interphase on Lithium Anode for Preventing the Shuttle Mechanism in Lithium-Sulfur Batteries. J. Power Sources 2014, 246, 840–845. 10.1016/j.jpowsour.2013.08.041. [DOI] [Google Scholar]
- Qian J. F.; Xu W.; Bhattacharya P.; Engelhard M.; Henderson W. A.; Zhang Y. H.; Zhang J.-G. Dendrite-Free Li Deposition Using Trace-Amounts of Water as an Electrolyte Additive. Nano Energy 2015, 15, 135–144. 10.1016/j.nanoen.2015.04.009. [DOI] [Google Scholar]
- Ye H.; Yin Y.-X.; Zhang S.-F.; Shi Y.; Liu L.; Zeng X.-X.; Wen R.; Guo Y.-G.; Wan L.-J. Synergism of Al-Containing Solid Electrolyte Interphase Layer and Al-Based Colloidal Particles for Stable Lithium Anode. Nano Energy 2017, 36, 411–417. 10.1016/j.nanoen.2017.04.056. [DOI] [Google Scholar]
- Wang Y. X.; Nakamura S.; Tasaki K.; Balbuena P. B. Theoretical Studies to Understand Surface Chemistry on Carbon Anodes for Lithium-Ion Batteries: How Does Vinylene Carbonate Play Its Role as an Electrolyte Additive?. J. Am. Chem. Soc. 2002, 124, 4408–4421. 10.1021/ja017073i. [DOI] [PubMed] [Google Scholar]
- Michan A. L.; Parimalam B. S.; Leskes M.; Kerber R. N.; Yoon T.; Grey C. P.; Lucht B. L. Fluoroethylene Carbonate and Vinylene Carbonate Reduction: Understanding Lithium-Ion Battery Electrolyte Additives and Solid Electrolyte Interphase Formation. Chem. Mater. 2016, 28, 8149–8159. 10.1021/acs.chemmater.6b02282. [DOI] [Google Scholar]
- Li N.-W.; Yin Y.-X.; Yang C.-P.; Guo Y.-G. An Artificial Solid Electrolyte Interphase Layer for Stable Lithium Metal Anodes. Adv. Mater. 2016, 28, 1853–1858. 10.1002/adma.201504526. [DOI] [PubMed] [Google Scholar]
- Liu Q.-C.; Xu J.-J.; Yuan S.; Chang Z.-W.; Xu D.; Yin Y.-B.; Li L.; Zhong H.-X.; Jiang Y.-S.; Yan J.-M.; Zhang X.-B. Artificial Protection Film on Lithium Metal Anode toward Long-Cycle-Life Lithium-Oxygen Batteries. Adv. Mater. 2015, 27, 5241–5247. 10.1002/adma.201501490. [DOI] [PubMed] [Google Scholar]
- Li Y. B.; Sun Y. M.; Pei A.; Chen K. F.; Vailionis A.; Li Y. Z.; Zheng G. Y.; Sun J.; Cui Y. Robust Pinhole-Free Li3N Solid Electrolyte Grown from Molten Lithium. ACS Cent. Sci. 2018, 4, 97–104. 10.1021/acscentsci.7b00480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudney N. J. Addition of a Thin-Film Inorganic Solid Electrolyte (LiPON) as a Protective Film in Lithium Batteries with a Liquid Electrolyte. J. Power Sources 2000, 89, 176–179. 10.1016/S0378-7753(00)00427-4. [DOI] [Google Scholar]
- Kozen A. C.; Lin C.-F.; Pearse A. J.; Schroeder M. A.; Han X.; Hu L.; Lee S.-B.; Rubloff G. W.; Noked M. Next-Generation Lithium Metal Anode Engineering Via Atomic Layer Deposition. ACS Nano 2015, 9, 5884–5892. 10.1021/acsnano.5b02166. [DOI] [PubMed] [Google Scholar]
- Kazyak E.; Wood K. N.; Dasgupta N. P. Improved Cycle Life and Stability of Lithium Metal Anodes through Ultrathin Atomic Layer Deposition Surface Treatments. Chem. Mater. 2015, 27, 6457–6462. 10.1021/acs.chemmater.5b02789. [DOI] [Google Scholar]
- Zhao Y. M.; Wang D. W.; Gao Y.; Chen T. H.; Huang Q. Q.; Wang D. H. Stable Li Metal Anode by a Polyvinyl Alcohol Protection Layer Via Modifying Solid-Electrolyte Interphase Layer. Nano Energy 2019, 64, 103893. 10.1016/j.nanoen.2019.103893. [DOI] [Google Scholar]
- Wang G.; Chen C.; Chen Y. H.; Kang X. W.; Yang C. H.; Wang F.; Liu Y.; Xiong X. H. Self-Stabilized and Strongly Adhesive Supramolecular Polymer Protective Layer Enables Ultrahigh-Rate and Large-Capacity Lithium-Metal Anode. Angew. Chem., Int. Ed. 2020, 59, 2055–2060. 10.1002/anie.201913351. [DOI] [PubMed] [Google Scholar]
- Zhu B.; Jin Y.; Hu X.; Zheng Q.; Zhang S.; Wang Q.; Zhu J. Poly(Dimethylsiloxane) Thin Film as a Stable Interfacial Layer for High-Performance Lithium-Metal Battery Anodes. Adv. Mater. 2017, 29, 1603755. 10.1002/adma.201603755. [DOI] [PubMed] [Google Scholar]
- Song J.; Lee H.; Choo M.-J.; Park J.-K.; Kim H.-T. Ionomer-Liquid Electrolyte Hybrid Ionic Conductor for High Cycling Stability of Lithium Metal Electrodes. Sci. Rep. 2015, 5, 14458. 10.1038/srep14458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N.-W.; Shi Y.; Yin Y.-X.; Zeng X.-X.; Li J.-Y.; Li C.-J.; Wan L.-J.; Wen R.; Guo Y.-G. A Flexible Solid Electrolyte Interphase Layer for Long-Life Lithium Metal Anodes. Angew. Chem., Int. Ed. 2018, 57, 1505–1509. 10.1002/anie.201710806. [DOI] [PubMed] [Google Scholar]
- Liu K.; Pei A.; Lee H. R.; Kong B.; Liu N.; Lin D.; Liu Y.; Liu C.; Hsu P.-C.; Bao Z.; Cui Y. Lithium Metal Anodes with an Adaptive “Solid-Liquid” Interfacial Protective Layer. J. Am. Chem. Soc. 2017, 139, 4815–4820. 10.1021/jacs.6b13314. [DOI] [PubMed] [Google Scholar]
- Liu Y. Y.; Lin D. C.; Yuen P. Y.; Liu K.; Xie J.; Dauskardt R. H.; Cui Y. An Artificial Solid Electrolyte Interphase with High Li-Ion Conductivity, Mechanical Strength, and Flexibility for Stable Lithium Metal Anodes. Adv. Mater. 2017, 29, 1605531. 10.1002/adma.201605531. [DOI] [PubMed] [Google Scholar]
- Lee D. J.; Lee H.; Kim Y.-J.; Park J.-K.; Kim H.-T. Sustainable Redox Mediation for Lithium-Oxygen Batteries by a Composite Protective Layer on the Lithium-Metal Anode. Adv. Mater. 2016, 28, 857–863. 10.1002/adma.201503169. [DOI] [PubMed] [Google Scholar]
- Zhao Q.; Tu Z.; Wei S.; Zhang K.; Choudhury S.; Liu X.; Archer L. A. Building Organic/Inorganic Hybrid Interphases for Fast Interfacial Transport in Rechargeable Metal Batteries. Angew. Chem., Int. Ed. 2018, 57, 992–996. 10.1002/anie.201711598. [DOI] [PubMed] [Google Scholar]
- Liu W.; Li W. Y.; Zhuo D.; Zheng G. Y.; Lu Z.; Liu K.; Cui Y. Core-Shell Nanoparticle Coating as an Interfacial Layer for Dendrite-Free Lithium Metal Anodes. ACS Cent. Sci. 2017, 3, 135–140. 10.1021/acscentsci.6b00389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y.; Yan Z.; Gray J. L.; He X.; Wang D.; Chen T.; Huang Q.; Li Y. C.; Wang H.; Kim S. H.; Mallouk T. E.; Wang D. Polymer-Inorganic Solid-Electrolyte Interphase for Stable Lithium Metal Batteries under Lean Electrolyte Conditions. Nat. Mater. 2019, 18, 384–389. 10.1038/s41563-019-0305-8. [DOI] [PubMed] [Google Scholar]
- Yang C. P.; Yin Y. X.; Zhang S. F.; Li N. W.; Guo Y. G. Accommodating Lithium into 3D Current Collectors with a Submicron Skeleton Towards Long-Life Lithium Metal Anodes. Nat. Commun. 2015, 6, 8058. 10.1038/ncomms9058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L.-L.; Ge J.; Yang J.-N.; Chen S.-M.; Yao H.-B.; Zhou F.; Yu S.-H. Free-Standing Copper Nanowire Network Current Collector for Improving Lithium Anode Performance. Nano Lett. 2016, 16, 4431–4437. 10.1021/acs.nanolett.6b01581. [DOI] [PubMed] [Google Scholar]
- Li Q.; Zhu S. P.; Lu Y. Y. 3D Porous Cu Current Collector/Li-Metal Composite Anode for Stable Lithium-Metal Batteries. Adv. Funct. Mater. 2017, 27, 1606422. 10.1002/adfm.201606422. [DOI] [Google Scholar]
- Wang S.-H.; Yin Y.-X.; Zuo T.-T.; Dong W.; Li J.-Y.; Shi J.-L.; Zhang C.-H.; Li N.-W.; Li C.-J.; Guo Y.-G. Stable Li Metal Anodes Via Regulating Lithium Plating/Stripping in Vertically Aligned Microchannels. Adv. Mater. 2017, 29, 1703729. 10.1002/adma.201703729. [DOI] [PubMed] [Google Scholar]
- Wang C.; Wang D. L.; Dai C. S. High-Rate Capability and Enhanced Cyclability of Rechargeable Lithium Batteries Using Foam Lithium Anode. J. Electrochem. Soc. 2008, 155, A390–A394. 10.1149/1.2894205. [DOI] [Google Scholar]
- Chi S.-S.; Liu Y.; Song W.-L.; Fan L.-Z.; Zhang Q. Prestoring Lithium into Stable 3D Nickel Foam Host as Dendrite-Free Lithium Metal Anode. Adv. Funct. Mater. 2017, 27, 1700348. 10.1002/adfm.201700348. [DOI] [Google Scholar]
- Ren F.; Lu Z.; Zhang H.; Huai L.; Chen X.; Wu S.; Peng Z.; Wang D.; Ye J. Pseudocapacitance Induced Uniform Plating/Stripping of Li Metal Anode in Vertical Graphene Nanowalls. Adv. Funct. Mater. 2018, 28, 1805638. 10.1002/adfm.201805638. [DOI] [Google Scholar]
- Xie K.; Wei W.; Yuan K.; Lu W.; Guo M.; Li Z.; Song Q.; Liu X.; Wang J.-G.; Shen C. Toward Dendrite-Free Lithium Deposition Via Structural and Interfacial Synergistic Effects of 3D Graphene@Ni Scaffold. ACS Appl. Mater. Interfaces 2016, 8, 26091–26097. 10.1021/acsami.6b09031. [DOI] [PubMed] [Google Scholar]
- Lu Z.; Liang Q. H.; Wang B.; Tao Y.; Zhao Y. F.; Lv W.; Liu D. H.; Zhang C.; Weng Z.; Liang J. C.; Li H.; Yang Q.-H. Graphitic Carbon Nitride Induced Micro-Electric Field for Dendrite-Free Lithium Metal Anodes. Adv. Energy Mater. 2019, 9, 1803186. 10.1002/aenm.201803186. [DOI] [Google Scholar]
- Ye H.; Xin S.; Yin Y. X.; Li J. Y.; Guo Y. G.; Wan L. J. Stable Li Plating/Stripping Electrochemistry Realized by a Hybrid Li Reservoir in Spherical Carbon Granules with 3D Conducting Skeletons. J. Am. Chem. Soc. 2017, 139, 5916–5922. 10.1021/jacs.7b01763. [DOI] [PubMed] [Google Scholar]
- Liu L.; Yin Y.-X.; Li J.-Y.; Guo Y.-G.; Wan L.-J. Ladderlike Carbon Nanoarrays on 3D Conducting Skeletons Enable Uniform Lithium Nucleation for Stable Lithium Metal Anodes. Chem. Commun. 2018, 54, 5330–5333. 10.1039/C8CC02672F. [DOI] [PubMed] [Google Scholar]
- Zhao F.; Zhou X.; Deng W.; Liu Z. Entrapping Lithium Deposition in Lithiophilic Reservoir Constructed by Vertically Aligned Zno Nanosheets for Dendrite-Free Li Metal Anodes. Nano Energy 2019, 62, 55–63. 10.1016/j.nanoen.2019.04.087. [DOI] [Google Scholar]
- Ye H.; Xin S.; Yin Y.-X.; Guo Y.-G. Advanced Porous Carbon Materials for High-Efficient Lithium Metal Anodes. Adv. Energy Mater. 2017, 7, 1700530. 10.1002/aenm.201700530. [DOI] [Google Scholar]
- Liu H.; Liu X. X.; Li W.; Guo X.; Wang Y.; Wang G. X.; Zhao D. Y. Porous Carbon Composites for Next Generation Rechargeable Lithium Batteries. Adv. Energy Mater. 2017, 7, 1700283. 10.1002/aenm.201700283. [DOI] [Google Scholar]
- Ji X. L.; Liu D.-Y.; Prendiville D. G.; Zhang Y. C.; Liu X. N.; Stucky G. D. Spatially Heterogeneous Carbon-Fiber Papers as Surface Dendrite-Free Current Collectors for Lithium Deposition. Nano Today 2012, 7, 10–20. 10.1016/j.nantod.2011.11.002. [DOI] [Google Scholar]
- Zuo T. T.; Wu X. W.; Yang C. P.; Yin Y. X.; Ye H.; Li N. W.; Guo Y. G. Graphitized Carbon Fibers as Multifunctional 3D Current Collectors for High Areal Capacity Li Anodes. Adv. Mater. 2017, 29, 1700389. 10.1002/adma.201700389. [DOI] [PubMed] [Google Scholar]
- Liu L.; Yin Y.-X.; Li J.-Y.; Li N.-W.; Zeng X.-X.; Ye H.; Guo Y.-G.; Wan L.-J. Free-Standing Hollow Carbon Fibers as High-Capacity Containers for Stable Lithium Metal Anodes. Joule 2017, 1, 563–575. 10.1016/j.joule.2017.06.004. [DOI] [Google Scholar]
- Liu L.; Yin Y. X.; Li J. Y.; Wang S. H.; Guo Y. G.; Wan L. J. Uniform Lithium Nucleation/Growth Induced by Lightweight Nitrogen-Doped Graphitic Carbon Foams for High-Performance Lithium Metal Anodes. Adv. Mater. 2018, 30, 1706216. 10.1002/adma.201706216. [DOI] [PubMed] [Google Scholar]
- Sun Z.; Jin S.; Jin H.; Du Z.; Zhu Y.; Cao A.; Ji H.; Wan L.-J. Robust Expandable Carbon Nanotube Scaffold for Ultrahigh-Capacity Lithium-Metal Anodes. Adv. Mater. 2018, 30, 1800884. 10.1002/adma.201800884. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Luo W.; Wang C. W.; Li Y. J.; Chen C. J.; Song J. W.; Dai J. Q.; Hitz E. M.; Xu S. M.; Yang C. P.; Wang Y. B.; Hu L. B. High-Capacity, Low-Tortuosity, and Channel-Guided Lithium Metal Anode. Proc. Natl. Acad. Sci. U. S. A. 2017, 114, 3584. 10.1073/pnas.1618871114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin S.; Sun Z. W.; Guo Y. L.; Qi Z. K.; Guo C. K.; Kong X. H.; Zhu Y. W.; Ji H. X. High Areal Capacity and Lithium Utilization in Anodes Made of Covalently Connected Graphite Microtubes. Adv. Mater. 2017, 29, 1700783. 10.1002/adma.201700783. [DOI] [PubMed] [Google Scholar]
- Mukherjee R.; Thomas A. V.; Datta D.; Singh E.; Li J. W.; Eksik O.; Shenoy V. B.; Koratkar N. Defect-Induced Plating of Lithium Metal within Porous Graphene Networks. Nat. Commun. 2014, 5, 3710. 10.1038/ncomms4710. [DOI] [PubMed] [Google Scholar]
- Zhang X.; Lv R.; Wang A.; Guo W.; Liu X.; Luo J. Mxene Aerogel Scaffolds for High-Rate Lithium Metal Anodes. Angew. Chem. 2018, 130, 15248–15253. 10.1002/ange.201808714. [DOI] [PubMed] [Google Scholar]
- Zheng G. Y.; Lee S. W.; Liang Z.; Lee H.-W.; Yan K.; Yao H. B.; Wang H. T.; Li W. Y.; Chu S.; Cui Y. Interconnected Hollow Carbon Nanospheres for Stable Lithium Metal Anodes. Nat. Nanotechnol. 2014, 9, 618–623. 10.1038/nnano.2014.152. [DOI] [PubMed] [Google Scholar]
- Zhang D.; Wang S.; Li B.; Gong Y.; Yang S. Horizontal Growth of Lithium on Parallelly Aligned Mxene Layers Towards Dendrite-Free Metallic Lithium Anodes. Adv. Mater. 2019, 31, 1901820. 10.1002/adma.201901820. [DOI] [PubMed] [Google Scholar]
- Liang Z.; Lin D. C.; Zhao J.; Lu Z. D.; Liu Y. Y.; Liu C.; Lu Y. Y.; Wang H. T.; Yan K.; Tao X. Y.; Cui Y. Composite Lithium Metal Anode by Melt Infusion of Lithium into a 3D Conducting Scaffold with Lithiophilic Coating. Proc. Natl. Acad. Sci. U. S. A. 2016, 113, 2862–2867. 10.1073/pnas.1518188113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin D. C.; Liu Y. Y.; Liang Z.; Lee H.-W.; Sun J.; Wang H. T.; Yan K.; Xie J.; Cui Y. Layered Reduced Graphene Oxide with Nanoscale Interlayer Gaps as a Stable Host for Lithium Metal Anodes. Nat. Nanotechnol. 2016, 11, 626. 10.1038/nnano.2016.32. [DOI] [PubMed] [Google Scholar]
- Zhang R.; Chen X.-R.; Chen X.; Cheng X.-B.; Zhang X.-Q.; Yan C.; Zhang Q. Lithiophilic Sites in Doped Graphene Guide Uniform Lithium Nucleation for Dendrite-Free Lithium Metal Anodes. Angew. Chem. 2017, 129, 7872–7876. 10.1002/ange.201702099. [DOI] [PubMed] [Google Scholar]
- Lu L.-L.; Zhang Y.; Pan Z.; Yao H.-B.; Zhou F.; Yu S.-H. Lithiophilic Cu-Ni Core-Shell Nanowire Network as a Stable Host for Improving Lithium Anode Performance. Energy Storage Mater. 2017, 9, 31–38. 10.1016/j.ensm.2017.06.004. [DOI] [Google Scholar]
- Wu S. L.; Zhang Z. Y.; Lan M. H.; Yang S. R.; Cheng J. Y.; Cai J. J.; Shen J. H.; Zhu Y.; Zhang K. L.; Zhang W. J. Lithiophilic Cu-CuO-Ni Hybrid Structure: Advanced Current Collectors toward Stable Lithium Metal Anodes. Adv. Mater. 2018, 30, 1705830. 10.1002/adma.201705830. [DOI] [PubMed] [Google Scholar]
- Yan K.; Lu Z. D.; Lee H.-W.; Xiong F.; Hsu P.-C.; Li Y. Z.; Zhao J.; Chu S.; Cui Y. Selective Deposition and Stable Encapsulation of Lithium through Heterogeneous Seeded Growth. Nat. Energy 2016, 1, 16010. 10.1038/nenergy.2016.10. [DOI] [Google Scholar]
- Yang C. P.; Yao Y. G.; He S. M.; Xie H.; Hitz E.; Hu L. B. Ultrafine Silver Nanoparticles for Seeded Lithium Deposition toward Stable Lithium Metal Anode. Adv. Mater. 2017, 29, 1702714. 10.1002/adma.201702714. [DOI] [PubMed] [Google Scholar]
- Liu Y. Y.; Lin D. C.; Liang Z.; Zhao J.; Yan K.; Cui Y. Lithium-Coated Polymeric Matrix as a Minimum Volume-Change and Dendrite-Free Lithium Metal Anode. Nat. Commun. 2016, 7, 10992. 10.1038/ncomms10992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu H. L.; Tang T. Y.; Asif M.; Li W.; Zhang T.; Hou Y. L. Stable Lithium Metal Anode Enabled by Lithium Metal Partial Alloying. Nano Energy 2019, 65, 103989. 10.1016/j.nanoen.2019.103989. [DOI] [Google Scholar]
- Tang W.; Yin X.; Kang S.; Chen Z.; Tian B.; Teo S. L.; Wang X.; Chi X.; Loh K. P.; Lee H.-W.; Zheng G. W. Lithium Silicide Surface Enrichment: A Solution to Lithium Metal Battery. Adv. Mater. 2018, 30, 1801745. 10.1002/adma.201801745. [DOI] [PubMed] [Google Scholar]
- Ye H.; Zheng Z.-J.; Yao H.-R.; Liu S.-C.; Zuo T.-T.; Wu X.-W.; Yin Y.-X.; Li N.-W.; Gu J.-J.; Cao F.-F.; Guo Y.-G. Guiding Uniform Li Plating/Stripping through Lithium-Aluminum Alloying Medium for Long-Life Li Metal Batteries. Angew. Chem., Int. Ed. 2019, 58, 1094–1099. 10.1002/anie.201811955. [DOI] [PubMed] [Google Scholar]
- Wang S. H.; Yue J. P.; Dong W.; Zuo T. T.; Li J. Y.; Liu X. L.; Zhang X. D.; Liu L.; Shi J. L.; Yin Y.-X.; Guo Y.-G. Tuning Wettability of Molten Lithium Via a Chemical Strategy for Lithium Metal Anodes. Nat. Commun. 2019, 10, 4930. 10.1038/s41467-019-12938-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H. M.; Liao X. B.; Guan Y. P.; Xiang Y.; Li M.; Zhang W. F.; Zhu X. Y.; Ming H.; Lu L.; Qiu J. Y.; Huang Y. Q.; Cao G. P.; Yang Y. S.; Mai L. Q.; Zhao Y.; Zhang H. Lithiophilic-Lithiophobic Gradient Interfacial Layer for a Highly Stable Lithium Metal Anode. Nat. Commun. 2018, 9, 3729. 10.1038/s41467-018-06126-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z.-J.; Su Q.; Zhang Q.; Hu X.-C.; Yin Y.-X.; Wen R.; Ye H.; Wang Z.-B.; Guo Y.-G. Low Volume Change Composite Lithium Metal Anodes. Nano Energy 2019, 64, 103910. 10.1016/j.nanoen.2019.103910. [DOI] [Google Scholar]
- Zhou D.; Shanmukaraj D.; Tkacheva A.; Armand M.; Wang G. X. Polymer Electrolytes for Lithium-Based Batteries: Advances and Prospects. Chem. 2019, 5, 2326–2352. 10.1016/j.chempr.2019.05.009. [DOI] [Google Scholar]
- Feuillade G.; Perche P. Ion-Conductive Macromolecular Gels and Membranes for Solid Lithium Cells. J. Appl. Electrochem. 1975, 5, 63–69. 10.1007/BF00625960. [DOI] [Google Scholar]
- Susan M. A. B. H.; Kaneko T.; Noda A.; Watanabe M. Ion Gels Prepared by in Situ Radical Polymerization of Vinyl Monomers in an Ionic Liquid and Their Characterization as Polymer Electrolytes. J. Am. Chem. Soc. 2005, 127, 4976–4983. 10.1021/ja045155b. [DOI] [PubMed] [Google Scholar]
- Lee K.-H.; Lim H. S.; Wang J. H. Effect of Unreacted Monomer on Performance of Lithium-Ion Polymer Batteries Based on Polymer Electrolytes Prepared by Free Radical Polymerization. J. Power Sources 2005, 139, 284–288. 10.1016/j.jpowsour.2004.07.007. [DOI] [Google Scholar]
- Liu F.-Q.; Wang W.-P.; Yin Y.-X.; Zhang S.-F.; Shi J.-L.; Wang L.; Zhang X.-D.; Zheng Y.; Zhou J.-J.; Li L.; Guo Y.-G. Upgrading Traditional Liquid Electrolyte Via in Situ Gelation for Future Lithium Metal Batteries. Sci. Adv. 2018, 4, eaat5383 10.1126/sciadv.aat5383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chazalviel J. Electrochemical Aspects of the Generation of Ramified Metallic Electrodeposits. Phys. Rev. A: At., Mol., Opt. Phys. 1990, 42, 7355–7367. 10.1103/PhysRevA.42.7355. [DOI] [PubMed] [Google Scholar]
- Monroe C.; Newman J. The Impact of Elastic Deformation on Deposition Kinetics at Lithium/Polymer Interfaces. J. Electrochem. Soc. 2005, 152, A396. 10.1149/1.1850854. [DOI] [Google Scholar]
- Lu Q. W.; He Y.-B.; Yu Q. P.; Li B. H.; Kaneti Y. V.; Yao Y. W.; Kang F. Y.; Yang Q.-H. Dendrite-Free, High-Rate, Long-Life Lithium Metal Batteries with a 3D Cross-Linked Network Polymer Electrolyte. Adv. Mater. 2017, 29, 1604460. 10.1002/adma.201604460. [DOI] [PubMed] [Google Scholar]
- Lu Y. Y.; Das S. K.; Moganty S. S.; Archer L. A. Ionic Liquid-Nanoparticle Hybrid Electrolytes and Their Application in Secondary Lithium-Metal Batteries. Adv. Mater. 2012, 24, 4430–4435. 10.1002/adma.201201953. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Lee J. Y.; Hong L. In Situ Preparation of Poly(Ethylene Oxide)-SiO2 Composite Polymer Electrolytes. J. Power Sources 2004, 129, 303–311. 10.1016/j.jpowsour.2003.11.026. [DOI] [Google Scholar]
- Yu L.; Guo S.; Lu Y.; Li Y.; Lan X.; Wu D.; Li R.; Wu S.; Hu X. Highly Tough, Li-Metal Compatible Organic-Inorganic Double-Network Solvate Ionogel. Adv. Energy Mater. 2019, 9, 1900257. 10.1002/aenm.201900257. [DOI] [Google Scholar]
- Tsai C.-L.; Roddatis V.; Chandran C. V.; Ma Q.; Uhlenbruck S.; Bram M.; Heitjans P.; Guillon O. Li7La3Zr2O12 Interface Modification for Li Dendrite Prevention. ACS Appl. Mater. Interfaces 2016, 8, 10617–10626. 10.1021/acsami.6b00831. [DOI] [PubMed] [Google Scholar]
- Cheng X.-B.; Zhao C.-Z.; Yao Y.-X.; Liu H.; Zhang Q. Recent Advances in Energy Chemistry between Solid-State Electrolyte and Safe Lithium-Metal Anodes. Chem. 2019, 5, 74–96. 10.1016/j.chempr.2018.12.002. [DOI] [Google Scholar]
- Tsai C. L.; Roddatis V.; Chandran C. V.; Ma Q.; Uhlenbruck S.; Bram M.; Heitjans P.; Guillon O. Li7La3Zr2O12 Interface Modification for Li Dendrite Prevention. ACS Appl. Mater. Interfaces 2016, 8, 10617–10626. 10.1021/acsami.6b00831. [DOI] [PubMed] [Google Scholar]
- Feng W.; Dong X.; Li P.; Wang Y.; Xia Y. Interfacial Modification of Li/Garnet Electrolyte by a Lithiophilic and Breathing Interlayer. J. Power Sources 2019, 419, 91–98. 10.1016/j.jpowsour.2019.02.066. [DOI] [Google Scholar]
- Fu K. K.; Gong Y.; Fu Z.; Xie H.; Yao Y.; Liu B.; Carter M.; Wachsman E.; Hu L. Transient Behavior of the Metal Interface in Lithium Metal-Garnet Batteries. Angew. Chem., Int. Ed. 2017, 56, 14942–14947. 10.1002/anie.201708637. [DOI] [PubMed] [Google Scholar]
- Cao X.; Ren X.; Zou L.; Engelhard M. H.; Huang W.; Wang H.; Matthews B. E.; Lee H.; Niu C.; Arey B. W.; Cui Y.; Wang C.; Xiao J.; Liu J.; Xu W.; Zhang J.-G. Monolithic Solid-Electrolyte Interphases Formed in Fluorinated Orthoformate-Based Electrolytes Minimize Li Depletion and Pulverization. Nat. Energy 2019, 4, 796–805. 10.1038/s41560-019-0464-5. [DOI] [Google Scholar]
- Yang H.; Zhang Y.; Tennenbaum M. J.; Althouse Z.; Ma Y.; He Y.; Wu Y.; Wu T. H.; Mathur A.; Chen P.; Huang Y.; Fernandez-Nieves A.; Kohl P. A.; Liu N. Polypropylene Carbonate-Based Adaptive Buffer Layer for Stable Interfaces of Solid Polymer Lithium Metal Batteries. ACS Appl. Mater. Interfaces 2019, 11, 27906–27912. 10.1021/acsami.9b08285. [DOI] [PubMed] [Google Scholar]
- Zhou W.; Zhu Y.; Grundish N.; Sen X.; Wang S.; You Y.; Wu N.; Gao J.; Cui Z.; Li Y.; Goodenough J. B. Polymer Lithium-Garnet Interphase for an All-Solid-State Rechargeable Battery. Nano Energy 2018, 53, 926–931. 10.1016/j.nanoen.2018.09.004. [DOI] [Google Scholar]
- Wu N.; Shi Y.-R.; Lang S.-Y.; Zhou J.-M.; Liang J.-Y.; Wang W.; Tan S.-J.; Yin Y.-X.; Wen R.; Guo Y.-G. Self-Healable Solid Polymeric Electrolytes for Stable and Flexible Lithium Metal Batteries. Angew. Chem., Int. Ed. 2019, 58, 18146–18149. 10.1002/anie.201910478. [DOI] [PubMed] [Google Scholar]
- Yang C. P.; Xie H.; Ping W. W.; Fu K.; Liu B. Y.; Rao J. C.; Dai J.; Wang C. W.; Pastel G.; Hu L. B. An Electron/Ion Dual-Conductive Alloy Framework for High-Rate and High-Capacity Solid-State Lithium-Metal Batteries. Adv. Mater. 2019, 31, 1804815. 10.1002/adma.201804815. [DOI] [PubMed] [Google Scholar]
- Liu B. Y.; Zhang L.; Xu S. M.; McOwen D. W.; Gong Y. H.; Yang C. P.; Pastel G. R.; Xie H.; Fu K.; Dai J. Q.; Chen C. J.; Wachsman E. D.; Hu L. B. 3D Lithium Metal Anodes Hosted in Asymmetric Garnet Frameworks toward High Energy Density Batteries. Energy Storage Mater. 2018, 14, 376–382. 10.1016/j.ensm.2018.04.015. [DOI] [Google Scholar]
- Yang C. P.; Zhang L.; Liu B. Y.; Xu S. M.; Hamann T.; McOwen D.; Dai J. Q.; Luo W.; Gong Y. H.; Wachsman E. D.; Hu L. B. Continuous Plating/Stripping Behavior of Solid-State Lithium Metal Anode in a 3D Ion-Conductive Framework. Proc. Natl. Acad. Sci. U. S. A. 2018, 115, 3770. 10.1073/pnas.1719758115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y. Y.; Lin D. C.; Jin Y.; Liu K.; Tao X. Y.; Zhang Q. H.; Zhang X. X.; Cui Y. Transforming from Planar to Three-Dimensional Lithium with Flowable Interphase for Solid Lithium Metal Batteries. Sci. Adv. 2017, 3, eaao0713 10.1126/sciadv.aao0713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.; Shi Y.; Hu X.-C.; Wang W.-P.; Wen R.; Xin S.; Guo Y.-G. A 3D Lithium/Carbon Fiber Anode with Sustained Electrolyte Contact for Solid-State Batteries. Adv. Energy Mater. 2020, 10, 1903325. 10.1002/aenm.201903325. [DOI] [Google Scholar]
- Dong W.; Zeng X.-X.; Zhang X.-D.; Li J.-Y.; Shi J.-L.; Xiao Y.; Shi Y.; Wen R.; Yin Y.-X.; Wang T.-S.; Wang C.-R.; Guo Y.-G. Gradiently Polymerized Solid Electrolyte Meets with Micro-/Nanostructured Cathode Array. ACS Appl. Mater. Interfaces 2018, 10, 18005–18011. 10.1021/acsami.8b05288. [DOI] [PubMed] [Google Scholar]