Abstract
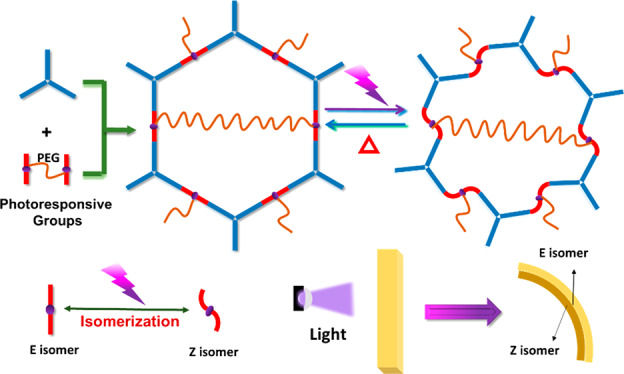
Seeking new photoresponsive materials with high energy conversion efficiency, good mechanical properties, as well as well-defined photoactuation mechanisms is of paramount significance. To address these challenges, we first introduced crystalline covalent organic frameworks (COFs) into the photoactuator field and created a facile fabrication strategy to directly install photoresponsive functional groups (i.e., acylhydrazone) on the skeletons of COFs. Herein, an approach to use polyethylene glycol (PEG) cross-linked dimers as the building blocks of the COF-42 platform was developed and afforded a series of uniform and freestanding membranes (PEG-COF-42) with outstanding mechanical properties (e.g., high flexibility and mechanical strength). Notably, these membranes possessed a fast mechanical response (e.g., bending) to UV light and good reversibility upon blue light or heating. After an in-depth investigation of the photoactuation mechanism via various techniques, we proposed a mechanism for the photoresponsive performance of PEG-COF-42: configurational change of acylhydrazone (i.e., E ↔ Z isomerization) accompanied by an excited-state intramolecular proton transfer (ESIPT) process intramolecularly transferring hydrogens from hydrogen donors (N—H) to hydrogen acceptors (oxygen in PEG). Moreover, attributed to the PEG moieties, PEG-COF-42 also demonstrated a vapor-responsive performance. This study not only broadens the application scopes of COFs but also provides new opportunities for the construction of multi-stimuli-responsive materials.
Short abstract
We created a new class of smart COFs, PEG-COFs, which possess high crystallinity and mechanical properties, and exhibit a photomechanical property and vapor-triggered artificial muscle performance.
Introduction
Smart materials that can convert various forms of stimuli (e.g., heat, light, electric field, magnetic field, pH, moisture) to mechanical motions are currently attracting intense attention.1−4 Among all stimuli, light possesses intrinsic advantages such as wireless control, abundant light sources with variable wavelengths, cost-effectiveness, cleanliness, and safety.5 Photomechanical smart materials have demonstrated great potential for applications as diverse as information storage, sensors, actuators, artificial muscles, self-healing materials, and so forth.6−8 Until now, photomechanical smart materials have been mostly concentrated on carbon-based materials9 and polymeric materials including organic polymers,10 liquid-crystalline elastomers,11 hydrogels,12 and biopolymers.13 However, these materials usually possess no or low crystallinity that unavoidably lead to low intrinsic energy conversion efficiencies, impeding their application scopes. In other words, the arrangement of photoresponsive chromophores (e.g., azobenzene,14−16 anthracenes,17−20 diarylethenes21) in material structures is not or partially in an order that often leads to low coupling efficiency between light and mechanical energy.22 Thus, developing new photoresponsive materials with high energy conversion efficiency, well-defined photoactuation mechanisms, and robust mechanical properties is of high importance and in urgent demand.
Recently, molecular crystals have demonstrated great promise as smart materials due to their advantages such as well-defined crystal structures, faster response time, and shorter recovery time.23 The energy transfer between stimuli and active groups is more efficient in crystalline structures with less energy dissipation. However, molecular crystals possess intrinsic disadvantages such as brittleness, low processability, and small size that seriously hinder their practical applications such as performing useful work (motion) or fabricating large devices. Thanks to the pioneering work of Yaghi in 2005,24 covalent organic frameworks (COFs) have emerged as a new generation of crystalline polymeric materials that combine the benefits of molecular crystals and organic polymers, such as high crystallinity,25 precisely determined structures,26 high porosity,27 tunable pore size,28 good processability,29−31 customizable functionality, and so on.32−40 Ascribed to these advantages, COFs can serve as an ideal class of materials for photomechanical applications. Nevertheless, to the best of our knowledge, there has been no report using COFs as photomechanical smart materials yet.
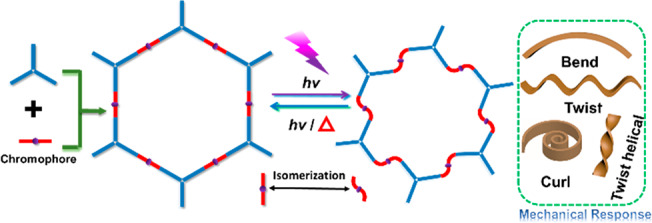
In order to prepare photomechanical COFs, a facile fabrication strategy is to directly install photoresponsive functional groups (e.g., azobenzene,41 hydrazone42) on COFs’ skeletons (Scheme 1). The hydrazone functional group, especially acylhydrazone, is perfectly suited to realize this strategy due to the following concerns.42 (i) A light stimulus can highly efficiently activate the E ↔ Z isomerization (i.e., configurational switching) of acylhydrazones, accompanied by a reversible change of materials’ properties such as volume, shape, and fluorescence.43−45 (ii) There are some classic COFs (e.g., COF-42, COF-43)46 based on acylhydrazone linkages that exhibit superior advantages such as rapid and scalable synthesis,47 high stability, and facile membrane formation.48 In this study, we applied COF-42 as a platform and engineered COF-42 with polyethylene glycol (PEG) bridging linkers that afforded a series of uniform, freestanding, and defect-free membranes. The formed PEG-COF-42 membranes exhibited an excellent mechanical property (high flexibility and good mechanical strength) and a reversible photoresponsive and vapor-responsive performance. This study paves a new avenue to prepare multi-stimuli-responsive materials based on COFs.
Scheme 1. Schematic Illustration of the Construction Approach of Photomechanical COFs.
Results and Discussion
Synthesis and Characterization of PEG-COF-42 Membranes
Forming membranes with good mechanical properties (e.g., high flexibility and mechanical strength) is highly desirable for mechanically responsive smart materials since materials need to hold the internal or external mechanical forces without fracture and destruction. According to the literature, the COF-42 membrane possessed a very weak mechanical property (e.g., easily broke upon mechanical force) (Figure 1 and Figure S1).49 To improve the mechanical properties of COF-42, recently, our group has developed a “polyCOF” strategy via introducing PEGylated chain polymers as the building blocks of COF-42.49 It was found that introducing chain polymers into COF structures not only can significantly improve the membrane formation ability and quality, and greatly enhance the mechanical performance, but also can endow new properties to COFs, such as a vapor-responsive performance. However, this strategy still exhibited some drawbacks: (i) a synthesis challenge of polymeric building blocks; (ii) relatively high polydispersity index (PDI) of polymers; and (iii) small monomers required to serve as co-building blocks to obtain a membrane with high crystallinity.
Figure 1.
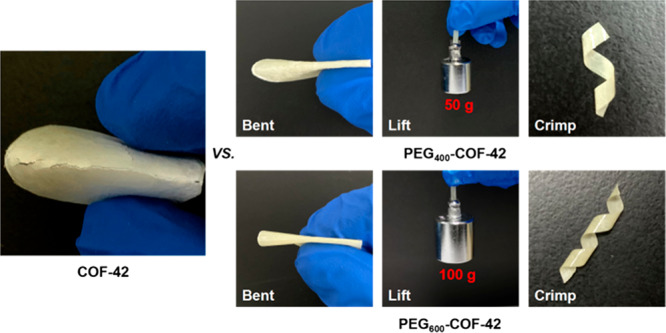
Comparison of the mechanical performance of PEG-COF-42 (intact upon bending, lifting, and crimping operations) vs COF-42 (fracture upon bending) membranes.
To address these issues, in this contribution, we developed a more feasible and simpler approach using 2,5-diethoxy-terephthalohydrazide (DTH) dimer with PEG bridging linkers (DTH-dimer-x, x = molecular weight of PEG) as the monomer of COF-42. Uniform and freestanding PEG-COF-42 membranes were harvested via interfacial polycondensation of 1,3,5-benzenetricarboxaldehyde (TB) with DTH-dimer-x at room temperature (Figure 2a and Figure S1). Further investigation revealed that the appropriate length of PEG linkers is crucial for the formation of crystalline membranes. Powder X-ray diffraction (PXRD) data showed that PEGx-COF-42 (x = 400 or 600) membranes possessed high crystallinity with the characteristic peak at 3.4°, 7.0°, and 26.9° as pristine COF-42 (Figure 2b).46 By contrast, PEG linkers with a molecule weight of 150 or 800 g/mol afforded amorphous materials due to the mismatching of PEG size and pore space (Figures S2 and S3). These results indicate that PEG linkers with the appropriate length would form interlayer or intralayer cross-linking in the COF-42 skeleton (Figure S3) without affecting the pristine crystallinity of COFs. We also found that the porosity and pore size of PEG-COF-42 significantly decreased due to the blockage of COFs’ channels by PEG moieties, and the longer PEG linker induced a lower porosity (pore volume and Langmuir surface area of 0.32 cm3/g and 782 m2/g, 0.15 cm3/g and 101 m2/g, and 0.01 cm3/g and 22 m2/g for COF-42, PEG400-COF-42, and PEG600-COF-42, respectively) (Figure S4).
Figure 2.
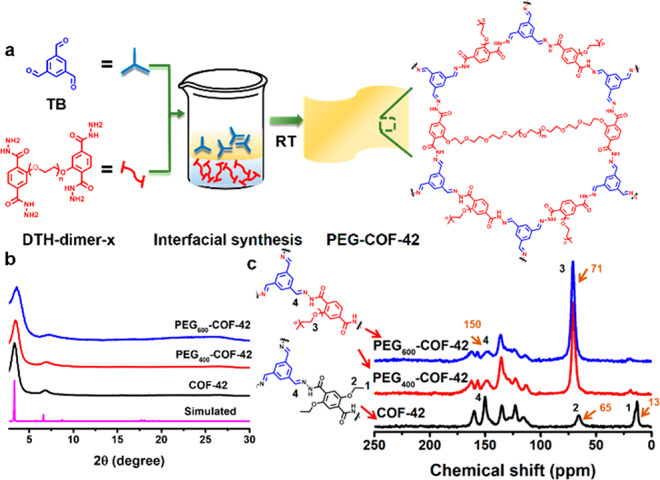
(a) Illustration of the approach to fabricate PEG-COF-42 membrane using interfacial synthesis. PXRD patterns (b) and solid-state 13C NMR (c) of COF-42 and PEG-COF-42.
Various solid-state characterization techniques were then applied to characterize PEG-COF-42 membranes. Fourier transform infrared (FT-IR) spectra exhibited new vibration bands at 1619 and 1221 cm–1 attributed to the formation of the C=N bonds. Meanwhile, the increase of characteristic peaks of C—H (2870 cm–1) and C—O (1100 cm–1) indicated the existence of PEG chains (Figure S4c).50 The 13C CP-MAS NMR result further validated the formation of PEG400-COF-42 and PEG600-COF-42. The characteristic signal at 150 ppm is ascribed to the chemical shift of the C=N bonds for PEG-COF-42. With the introduction of PEG, the peaks of ethyl groups (13 and 65 ppm) in COF-42 disappeared, and a new peak (71 ppm) assigned to carbons in PEG chains was observed in PEG-COF-42 (Figure 2c).
The quality of PEG-COF-42 membrane is evaluated using scanning electron microscopy (SEM) and stress–strain tests. SEM images showed that PEG-COF-42 presented as homogeneous, cohesive, and freestanding membrane structures (Figure 3 and Figure S5). Cross-sectional SEM images further revealed a uniform, compact particle packing nature (thickness of PEG400-COF-42, 12 μm; PEG600-COF-42, 14 μm) (Figure 3c and Figure S5). In order to protrude the advantages of PEG-COF-42, we also prepared a COF-42 membrane via the same procedure (i.e., interfacial polycondensation of TB and DTH) as PEG-COF-42. SEM images revealed that the COF-42 membrane exhibited a rough surface with loosely packed particles (Figure S5). Additionally, stress–strain experiments revealed that the toughness and Young’s modulus of PEG400-COF-42 and PEG600-COF-42 membranes were greatly improved, compared with the pristine COF-42 membranes (Figure 3d and Table S1). For instance, PEG600-COF-42 achieved the highest elongation of 12%, almost 5 times as much as the COF-42 membrane (2.6%) and 2 times better than our previously reported poly3/6COF-42 membrane (6.5%). The elongations at break of PEG400-COF-42 and PEG600-COF-42 are even higher than some commercial or classic polymeric membranes (e.g., PIM-1, PVDF, poly3/6COF-42) (Figure 3e). Furthermore, the PEG-COF-42 membrane can proceed repeatedly with deformations (e.g., bent, twisted, or stretched) without damage (Figure 1). Notably, PEG600-COF-42 membranes possessed the highest toughness to lift a steel object of 100 g. Overall, attributed to the cross-linking effect of PEG bridging linkers, the mechanical performance of PEG-COF-42 membranes was much better than the pristine COF-42 membranes.
Figure 3.
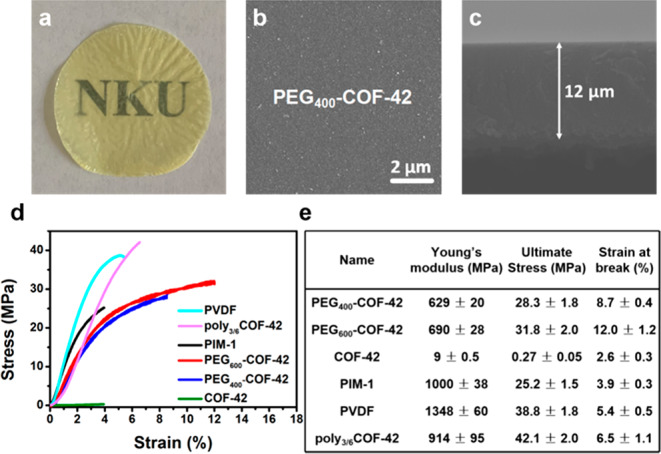
(a) Photograph of transparent PEG400-COF-42 membrane. (b) Top view and (c) cross-section SEM images of PEG400-COF-42. (d) Stress–strain curves for PEG-COF-42 vs COF-42, PIM-1, PVDF, and poly3/6COF-42 membranes. (e) Mechanical analysis of the stress–strain curves.
Evaluation of the Mechanically Responsive Performance of PEG-COF-42
The E ↔ Z isomerization of the acylhydrazone group triggered by light is always accompanied by a reversible change of materials’ properties such as volume and shape. Noteworthily, we found that PEG400-COF-42 and PEG600-COF-42 membranes were photoresponsive to UV light with reversible bending behaviors (Figure S6). Herein, PEG400-COF-42 membrane was selected as a representative to demonstrate the stimuli-responsive behavior. UV/vis spectroscopy unveiled absorption bands of λmax = 375 nm for PEG400-COF-42 in the UV region (Figure S7). Thus, we selected UV light with λirr = 370 nm (288 mW/cm2) to trigger the E ↔ Z isomerization of acylhydrazone groups in PEG400-COF-42. PEG400-COF-42 membranes were cut into a strip (12 mm × 3 mm) fixed on a glass fiber and irradiated with unilateral UV light in the vertical direction. We used the displacement (D) and bending angle (θ) to evaluate the degree of the mechanical response process (Figure 4a). It was found that the PEG400-COF-42 strip gradually bent toward the light to reach a maximum bending angle of θ = 30° in as short as 20 s. This photoresponsive bending performance is better than some state-of-the-art materials (e.g., trans-1).51 Notably, through irradiation with 440 nm blue light (288 mW/cm2) from the same direction for 3 min at room temperature or thermal treatment at 100 °C for 1 min, membranes can bend back to the initial position (Figure 4b). This reversible bending performance can be repeated for many cycles with alternating UV light and thermal treatment (Figure 4c). As mentioned in the literature,42,43 the E state of acylhydrazone-based compounds is thermal dynamically more stable than the Z state, hence leading to the E → Z transformation upon heat. Moreover, the behavior of bending toward light indicates that the side of PEG-COF-42 membrane close to light conducted a volume shrinkage during the light irradiation.
Figure 4.
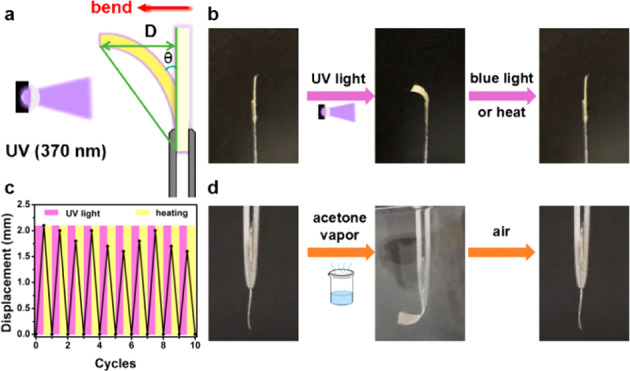
(a) Schematic illustration of the defined displacement (D) and bending angle (θ) in the mechanically responsive process. (b) Photoresponsive bending behavior of the PEG400-COF-42 membrane. (c) Plot of the reversible cycles of PEG400-COF-42 via UV irradiation and heating. (d) Vapor-responsive bending behavior of the PEG400-COF-42 membrane.
PEG-COF-42 membranes also can inherit the property of PEG-based building blocks.49 When exposed to the acetone vapor, PEG-COF-42 membranes showed a fast bending response in 2 s to achieve a bending angle of θ = 40° and can rapidly recover back to the initial position once exposed to air (Figure 4d). Notably, this reversible bending process can undergo >20 cycles without fatigue (Figures S8 and S9). As reported in our previous work, pure COF-42 membrane did not exhibit vapor-responsive behavior due to the lack of PEG moieties, while the pure PEG membrane exhibited a reversible vapor-responsive performance. Thus, we can conclude that the vapor-responsive property of PEG-COF-42 membranes originated from PEG moieties which can reversibly change their conformation upon a vapor stimulus. Taking advantage of the vapor-responsive and photoresponsive behaviors of PEG-COF-42 membranes, fabrication of multi-stimuli-responsive actuators to perform complicated motions is ongoing in our lab.
Investigating the Mechanism of the Photomechanical Response of PEG-COF-42
Various solid-state characterization techniques were applied to unveil the mechanism behind the photomechanical performance of PEG-COF-42. Herein, we further chose PEG400-COF-42 as a representative. PEG400-COF-42 before light irradiation exhibits the E isomer (named as PEG400-COF-42(E)).43 After irradiation with 370 nm UV light for 12 h, a broader UV spectrum band centered around λmax = 400 nm appeared (Figure 5a), indicating the isomerization to PEG400-COF-42(Z). SEM and 13C NMR spectra before and after light irradiation showed no obvious changes (Figures S10 and S11), indicative of no bond breaking occurring during the UV light irradiation. The PXRD pattern after UV irradiation showed the same pattern as pristine PEG400-COF-42, but with a decrease of peak intensity (Figure S12). It is possibly due to the partial loss of coherence of molecular building blocks in COFs. Upon irradiation to light, local disorientation is likely to take place, which makes the molecular building blocks deviate from their original lattice positions, hence leading to local incoherence, while their overall positions are maintained to afford almost identical peak positions but with lower intensity.
Figure 5.
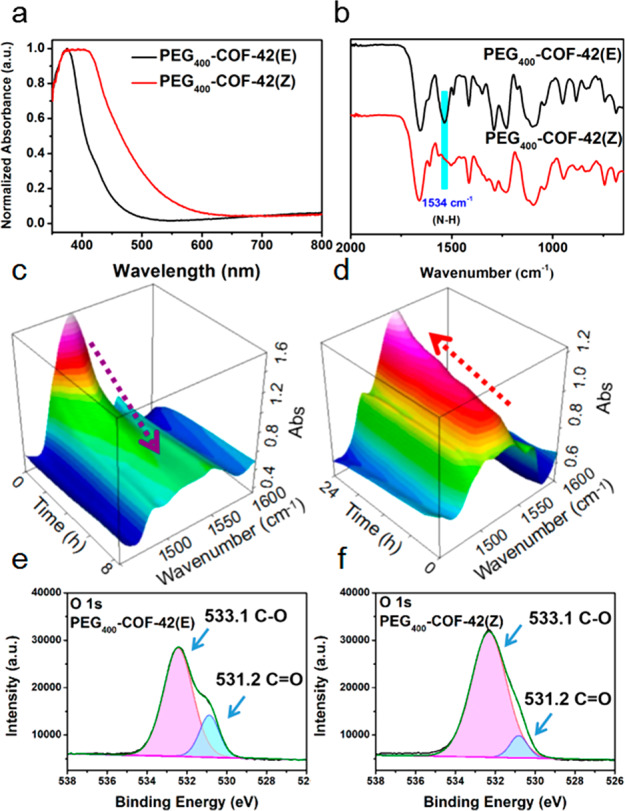
(a) Solid-state UV–vis absorption spectra of PEG400-COF-42 before and after UV irradiation. (b) FT-IR spectra of PEG400-COF-42 before and after UV irradiation. (c) In situ FT-IR spectra of the PEG400-COF-42 membrane under UV irradiation with different times. (d) In situ FT-IR spectra of the PEG400-COF-42 membrane at 100 °C. XPS O 1s signals for PEG400-COF-42 before (e) and after (f) UV irradiation.
The fluorescent spectrum of PEG400-COF-42(E) possessed a characteristic emission peak at 470 nm upon excitation at 365 nm (Figure S13). According to the literature, the fluorescent property of PEG400-COF-42(E) can be attributed to the excited-state intramolecular proton transfer (ESIPT) mechanism which is a process of intramolecularly transferring protons from donor groups (i.e., N—H) to proton acceptor groups (i.e., oxygen– in PEG) under light irradiation.52,53 After UV irradiation, the intensity of the characteristic emission peak decreased in PEG400-COF-42(Z), possibly due to the configurational change (i.e., E ↔ Z isomerization) of acylhydrazone that hindered the ESIPT process.52 As reported by Lehn,54 Aprahamian,42 and Hecht,43 acylhydrazone switches triggered by stimuli conducted isomerization through either an out-of-plane rotation of the double bond or in-plane nitrogen inversion of C=N. In PEG-COF-42, due to the stacking of 2-dimentional (2D) COF layers, out-of-plane rotation is forbidden. Thus, the isomerization is possibly through an in-plane nitrogen inversion of C=N. Moreover, the Z isomer can be stabilized through noncovalent interactions in PEG-COF-42, such as π···π interaction and intralayer/interlayer hydrogen bonding.47
In situ FT-IR, Raman and X-ray photoelectron spectroscopy (XPS) were further used to study the photoreactions that occurred during the light irradiation. As shown in the FT-IR spectra (Figure 5c), the intensity of the characteristic peak at 1540 cm–1 ascribed to the bending vibration of the N—H group in acylhydrazone gradually decreased as irradiation time extended. We also observed that the decreasing rate of peak intensity is faster in the initial stage and then gradually slowed down (Figure 5c and Figure S14). The Raman spectra clearly showed the disappearance of the peak at 1560 cm–1, which is also ascribed to the change of the N—H group (Figure S15). Interestingly, when the PEG400-COF-42(Z) membrane obtained after UV irradiation was placed in a 100 °C oven, FT-IR spectra revealed that the bending vibration at 1540 cm–1 gradually increased, indicative of the reversible nature of the proton transfer process (Figure 5d and Figure S16). The XPS O 1s signals of PEG400-COF-42 before and after UV irradiation are compared in Figure 5e,f. It was found that the relative intensity of C=O (531.2 eV) compared with C—O (533.1 eV) dramatically decreased after UV irradiation.45 The C 1s signals of PEG400-COF-42 did not show significant change before and after UV irradiation (Figure S17). These results indicate that a tautomerism (e.g., enolate-keto) exists in the ESIPT process (Figure 6a).
Figure 6.
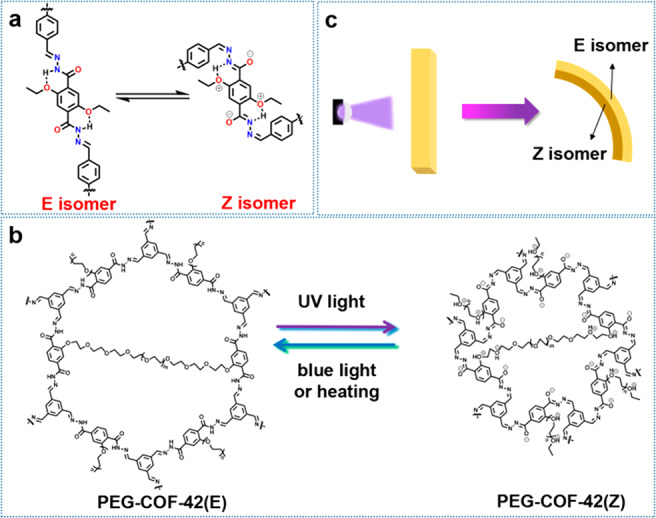
(a) Illustration of the possible mechanism of the E ↔ Z isomerization. (b) Mechanism to explain the bending toward light behavior of PEG-COF-42 membranes. (c) Proposed structure changes of PEG-COF-42 under UV irradiation.
According to the ESIPT mechanism, the photoexcited molecule can tautomerize through proton transfer. This agrees well with the result of XPS, FT-IR, and Raman spectra data. As illustrated in Figure 6a, the N—H group in the skeleton of PEG-COF-42(E) can form intramolecular hydrogen bonding with oxygen from alkoxy moieties in a six-membered ring fashion. After the photoreaction, the proton acceptor (i.e., oxygens in PEG) can capture a hydrogen from the adjacent N—H group to form a six-membered ring with adjacent nitrogen. In order to prove the crucial role of alkoxy moieties to the proton transferring reaction, we further prepared COF-42 and its analogues (Figures S18–S20) as a comparison. TB-Th COF was synthesized via the reaction of TB and terephthalohydrazide (TPHD). Due to the absence of the alkoxy group, the ESIPT process cannot occur in TB-Th COF, proven by FT-IR data with no change before and after UV irradiation for 12 h (Figure S21). DFDM-THz COF was synthesized via the reaction of benzene-1,3,5-tricarbohydrazide (THz) and 2,5-dimethoxyterephthalaldehyde (DFDM). Although the alkoxy group exists in DFDM-THz COF, its position is not close to the N—H group, hindering the proton transferring from N—H to the alkoxy group. This is verified by the FT-IR spectra with no change after UV irradiation for 12 h (Figure S22). These results strongly suggest that the existence of the alkoxy moiety and its position play important roles to the ESIPT process and the isomerization of acylhydrazone in COFs. In addition, we also tested the model compound of PEG-COF-42, which did not show any change for the FT-IR spectrum. This result suggests that the framework structure of COFs is necessary for the photoinduced isomerization (Figure S23).
As predicted, COF-42 containing the same skeleton as PEG-COF-42 should show a photoswitch property. UV/vis, FT-IR, XPS, and fluorescence spectra revealed results similar to those of PEG-COF-42. After irradiation for 12 h, it was observed the UV band of λmax = 375 nm disappeared, and a new UV band (λmax = 365 nm) appeared (Figure S24), indicating the isomerization from COF-42(E) to COF-42(Z).43 FT-IR spectra showed the characteristic peak at 1535 cm–1 ascribed to the bending vibration of the N—H group of acylhydrazone having gradually disappeared as irradiation time was extended (Figure S25). The intensity of the characteristic emission peak decreased after UV irradiation (Figure S26), and the intensity of C=O (531.2 eV) in XPS O 1s spectra decreased as well (Figure S27). These results strongly suggest that COF-42 has demonstrated an acylhydrazone photoswitch behavior under UV irradiation. Based on the above results and analyses, we can conclude that the configurational change of acylhydrazone (i.e., E ↔ Z isomerization) in COF-42 and PEG-COF-42 is accompanied by an ESIPT process intramolecularly transferring hydrogen from hydrogen donors of N—H to hydrogen acceptors of alkoxy moieties (Figure 6a). During light irradiation, the hexagonal channels of COFs can perform a volume shrink that induced the photomechanical behavior (Figure 6b,c). Surprisingly, COF-42 membranes exhibited no photomechanical motion under UV irradiation even after 12 h (Figure S28). As aforementioned, because the COF-42 membrane was formed by loosely packed particles (Figure S5), it possessed a very weak mechanical property and was easily broken under mechanical force. Therefore, due to the lack of a strong connection among particles in COF-42, the mechanical motion of independent particles cannot be aggregated together to perform macroscopic motions. These results indicate that the PEG linkage in PEG-COF-42 plays a key role to the stimuli-responsive behavior of PEG-COF-42. PEG linkers can cross-link COF particles to form integral membrane structures and transfer the microscopic motions of COFs or PEG moieties to macroscopic motions. Moreover, PEG linkers can function as “springs” that can help to stabilize the Z isomer and meanwhile promote the reversibility of the volume change of COFs.
Conclusion
In conclusion, we created a new approach to introduce PEG linkers to the COF-42 platform and successfully fabricated a new type of uniform, highly flexible, freestanding pure COF membranes (PEG-COF-42). These membranes exhibited outstanding mechanical properties, comparable to some commercial or classic polymeric membranes (e.g., PIM-1 and PVDF). Notably, PEG-COF-42 membranes possessed a fast mechanical response (e.g., bending) to UV light, and the bending process is reversible upon thermal treatment or blue light irradiation. Various techniques such as in situ FT-IR, Raman, and XPS spectra were applied to unveil the mechanism behind the photoresponsive behavior. All results and analyses point out an unprecedented mechanism for the photoresponsive performance: configurational change of acylhydrazone (i.e., E ↔ Z isomerization) accompanied by an ESIPT process intramolecularly transferring hydrogen from the hydrogen donor groups of N—H in acylhydrazone to hydrogen acceptor groups (i.e., oxygen in PEG). Attributed to the 2D COF layer stacking structure, the Z isomer formed after UV irradiation can be stabilized through noncovalent interactions such as π···π stacking interaction, and the cross-linking effect of PEG linkers. In addition, we found that PEG-COF-42 membranes can inherit the property of PEG-based building blocks, i.e., vapor-responsive behavior. By contrast, the pure COF-42 membrane without PEG moieties exhibited no response to UV light or vapor. These interesting results further validate the crucial role of PEG in PEG-COF-42: (i) cross-linking COF particles to form integral membrane structures; (ii) serving as proton acceptors during the ESIPT process; (iii) transferring the microscopic motions of COFs or PEG moieties to macroscopic motions; and (iv) functioning as “springs” to stabilize the Z isomer and promoting the reversibility of volume change of the COF structure. This study not only points out a new application direction (i.e., photoresponsive artificial muscle) for COF materials but also unveiled a new mechanism for the photoswitch performance of acylhydrazone-based materials.
Experimental Section
Syntheses of PEG-COF-42
PEG-COF-42 membranes were synthesized in a 25 mL glass beaker. First, 0.0375 mmol of DTH-dimer-x was dissolved in the mixture solvent of 1.0 mL of H2O and 1.0 mL of dioxane was added as a bottom layer in the beaker. TB (0.025 mmol, 4.0 mg) and 525 μL of CH3COOH were dissolved into 3.0 mL of mesitylene and then slowly added on the top of the hydrazine layer. The system was kept at room temperature for 48 h without disturbance. Membranes were formed at the interface and then transferred into CH3OH. The membranes were washed with CH3OH in a Soxhlet extractor for 48 h and then dried by a supercritical carbon dioxide dryer.
Safety Statement
No unexpected or unusually high safety hazards were encountered.
Acknowledgments
The authors acknowledge the National Natural Science Foundation of China (21971126) and 111 Project (B12015).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acscentsci.0c00260.
Detailed experimental procedures, materials, FT-IR, PXRD, and SEM (PDF)
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Lv J.-A.; Liu Y.; Wei J.; Chen E.; Qin L.; Yu Y. Photocontrol of Fluid Slugs in Liquid Crystal Polymer Microactuators. Nature 2016, 537, 179–184. 10.1038/nature19344. [DOI] [PubMed] [Google Scholar]
- Cao J.; Zhou C.; Su G.; Zhang X.; Zhou T.; Zhou Z.; Yang Y. Arbitrarily 3D Configurable Hygroscopic Robots with a Covalent-Noncovalent Interpenetrating Network and Self-Healing Ability. Adv. Mater. 2019, 31, 1900042. 10.1002/adma.201900042. [DOI] [PubMed] [Google Scholar]
- Jiang Z.-C.; Xiao Y.-Y.; Tong X.; Zhao Y. Selective Decrosslinking in Liquid Crystal Polymer Actuators for Optical Reconfiguration of Origami and Light-Fueled Locomotion. Angew. Chem., Int. Ed. 2019, 58, 5332–5337. 10.1002/anie.201900470. [DOI] [PubMed] [Google Scholar]
- Ghosh S.; Mishra M. K.; Ganguly S.; Desiraju G. R. Dual Stress and Thermally Driven Mechanical Properties of the Same Organic Crystal: 2,6-Dichlorobenzylidene-4-fluoro-3-nitroaniline. J. Am. Chem. Soc. 2015, 137, 9912–9921. 10.1021/jacs.5b05324. [DOI] [PubMed] [Google Scholar]
- Yu Q.; Aguila B.; Gao J.; Xu P.; Chen Q.; Yan J.; Xing D.; Chen Y.; Cheng P.; Zhang Z.; Ma S. Photomechanical Organic Crystals as Smart Materials for Advanced Applications. Chem. - Eur. J. 2019, 25, 5611–5622. 10.1002/chem.201805382. [DOI] [PubMed] [Google Scholar]
- Han D.-D.; Zhang Y.-L.; Ma J.-N.; Liu Y.-Q.; Han B.; Sun H.-B. Light-Mediated Manufacture and Manipulation of Actuators. Adv. Mater. 2016, 28, 8328–8343. 10.1002/adma.201602211. [DOI] [PubMed] [Google Scholar]
- Duan Y.; Semin S.; Tinnemans P.; Cuppen H.; Xu J.; Rasing T. Robust thermoelastic microactuator based on an organic molecular crystal. Nat. Commun. 2019, 10, 4573. 10.1038/s41467-019-12601-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong X.; del Campo A.; Cui J. Photoresponsive Polymers. Smart Polymers and their Applications 2019, 87–153. 10.1016/B978-0-08-102416-4.00004-1. [DOI] [Google Scholar]
- Yang M.; Yuan Z.; Liu J.; Fang Z.; Fang L.; Yu D.; Li Q. Photoresponsive Actuators Built from Carbon-Based Soft Materials. Adv. Opt. Mater. 2019, 7, 1900069. 10.1002/adom.201900069. [DOI] [Google Scholar]
- Ma S.; Li X.; Huang S.; Hu J.; Yu H. A Light-Activated Polymer Composite Enables On-Demand Photocontrolled Motion: Transportation at the Liquid/Air Interface. Angew. Chem., Int. Ed. 2019, 58, 2655–2659. 10.1002/anie.201811808. [DOI] [PubMed] [Google Scholar]
- Priimagi A.; Shimamura A.; Kondo M.; Hiraoka T.; Kubo S.; Mamiya J.-I.; Kinoshita M.; Ikeda T.; Shishido A. Location of the Azobenzene Moieties within the Cross-Linked Liquid-Crystalline Polymers Can Dictate the Direction of Photoinduced Bending. ACS Macro Lett. 2012, 1, 96–99. 10.1021/mz200056w. [DOI] [PubMed] [Google Scholar]
- Takashima Y.; Hatanaka S.; Otsubo M.; Nakahata M.; Kakuta T.; Hashidzume A.; Yamaguchi H.; Harada A. Expansion-Contraction of Photoresponsive Artificial Muscle Regulated by Host-Guest Interactions. Nat. Commun. 2012, 3, 1–8. 10.1038/ncomms2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero de Espinosa L.; Meesorn W.; Moatsou D.; Weder C. Bioinspired Polymer Systems with Stimuli-Responsive Mechanical Properties. Chem. Rev. 2017, 117, 12851–12892. 10.1021/acs.chemrev.7b00168. [DOI] [PubMed] [Google Scholar]
- Yu H.-T.; Tang J.-W.; Feng Y.-Y.; Feng W. Structural Design and Application of Azo-based Supramolecular Polymer Systems. Chin. J. Polym. Sci. 2019, 37, 1183–1199. 10.1007/s10118-019-2331-z. [DOI] [Google Scholar]
- Luo W.; Feng Y.; Cao C.; Li M.; Liu E.; Li S.; Qin C.; Hu W.; Feng W. A High Energy Density Azobenzene/Graphene Hybrid: A Nano-Templated Platform for Solar Thermal Storage. J. Mater. Chem. A 2015, 3, 11787–11795. 10.1039/C5TA01263E. [DOI] [Google Scholar]
- Si Q.; Feng Y.; Yang W.; Fu L.; Yan Q.; Dong L.; Long P.; Feng W. Controllable and Stable Deformation of a Self-Healing Photo-Responsive Supramolecular Assembly for an Optically Actuated Manipulator Arm. ACS Appl. Mater. Interfaces 2018, 10, 29909–29917. 10.1021/acsami.8b08025. [DOI] [PubMed] [Google Scholar]
- Kim T.; Zhu L.; Mueller L. J.; Bardeen C. J. Mechanism of Photoinduced Bending and Twisting in Crystalline Microneedles and Microribbons Composed of 9-Methylanthracene. J. Am. Chem. Soc. 2014, 136, 6617–6625. 10.1021/ja412216z. [DOI] [PubMed] [Google Scholar]
- Abendroth J. M.; Bushuyev O. S.; Weiss P. S.; Barrett C. J. Controlling Motion at the Nanoscale: Rise of the Molecular Machines. ACS Nano 2015, 9, 7746–7768. 10.1021/acsnano.5b03367. [DOI] [PubMed] [Google Scholar]
- Zhu L.; Al-Kaysi R. O.; Bardeen C. J. Photoinduced Ratchet-Like Rotational Motion of Branched Molecular Crystals. Angew. Chem., Int. Ed. 2016, 55, 7073–7076. 10.1002/anie.201511444. [DOI] [PubMed] [Google Scholar]
- Yu Q.; Yang X.; Chen Y.; Yu K.; Gao J.; Liu Z.; Cheng P.; Zhang Z.; Aguila B.; Ma S. Fabrication of Light-Triggered Soft Artificial Muscles via a Mixed-Matrix Membrane Strategy. Angew. Chem., Int. Ed. 2018, 57, 10192–10196. 10.1002/anie.201805543. [DOI] [PubMed] [Google Scholar]
- Morimoto M.; Irie M. A Diarylethene Cocrystal that Converts Light into Mechanical Work. J. Am. Chem. Soc. 2010, 132, 14172–14178. 10.1021/ja105356w. [DOI] [PubMed] [Google Scholar]
- Kim T.; Zhu L.; Al-Kaysi R. O.; Bardeen C. J. Organic photomechanical materials. ChemPhysChem 2014, 15, 400–414. 10.1002/cphc.201300906. [DOI] [PubMed] [Google Scholar]
- Naumov P.; Chizhik S.; Panda M. K.; Nath N. K.; Boldyreva E. Mechanically Responsive Molecular Crystals. Chem. Rev. 2015, 115, 12440–12490. 10.1021/acs.chemrev.5b00398. [DOI] [PubMed] [Google Scholar]
- Côté A. P.; Benin A. I.; Ockwig N. W.; O’Keeffe M.; Matzger A. J.; Yaghi O. M. Porous, Crystalline, Covalent Organic Frameworks. Science 2005, 310, 1166–1170. 10.1126/science.1120411. [DOI] [PubMed] [Google Scholar]
- Chen X.; Geng K.; Liu R.; Tan K. T.; Gong Y.; Li Z.; Tao S.; Jiang Q.; Jiang D. Covalent Organic Frameworks: Chemical Approaches to Designer Structures and Built-In Functions. Angew. Chem., Int. Ed. 2020, 59, 5050–5091. 10.1002/anie.201904291. [DOI] [PubMed] [Google Scholar]
- Guan X.; Chen F.; Fang Q.; Qiu S. Design and Applications of Three Dimensional Covalent Organic Frameworks. Chem. Soc. Rev. 2020, 49, 1357–1384. 10.1039/C9CS00911F. [DOI] [PubMed] [Google Scholar]
- Zhang J.; Han X.; Wu X.; Liu Y.; Cui Y. Multivariate Chiral Covalent Organic Frameworks with Controlled Crystallinity and Stability for Asymmetric Catalysis. J. Am. Chem. Soc. 2017, 139, 8277–8285. 10.1021/jacs.7b03352. [DOI] [PubMed] [Google Scholar]
- Fan H.; Mundstock A.; Feldhoff A.; Knebel A.; Gu J.; Meng H.; Caro J. Covalent Organic Framework-Covalent Organic Framework Bilayer Membranes for Highly Selective Gas Separation. J. Am. Chem. Soc. 2018, 140, 10094–10098. 10.1021/jacs.8b05136. [DOI] [PubMed] [Google Scholar]
- Shao P.; Li J.; Chen F.; Ma L.; Li Q.; Zhang M.; Zhou J.; Yin A.; Feng X.; Wang B. Flexible Films of Covalent Organic Frameworks with Ultralow Dielectric Constants under High Humidity. Angew. Chem., Int. Ed. 2018, 57, 16501–16505. 10.1002/anie.201811250. [DOI] [PubMed] [Google Scholar]
- Evans A. M.; Parent L. R.; Flanders N. C.; Bisbey R. P.; Vitaku E.; Kirschner M. S.; Schaller R. D.; Chen L. X.; Gianneschi N. C.; Dichtel W. R. Seeded growth of single-crystal two-dimensional covalent organic frameworks. Science 2018, 361, 52–57. 10.1126/science.aar7883. [DOI] [PubMed] [Google Scholar]
- Dey K.; Pal M.; Rout K. C.; Kunjattu H S.; Das A.; Mukherjee R.; Kharul U. K.; Banerjee R. Selective Molecular Separation by Interfacially Crystallized Covalent Organic Framework Thin Films. J. Am. Chem. Soc. 2017, 139, 13083–13091. 10.1021/jacs.7b06640. [DOI] [PubMed] [Google Scholar]
- Zeng Y.; Zou R.; Luo Z.; Zhang H.; Yao X.; Ma X.; Zou R.; Zhao Y. Covalent Organic Frameworks Formed with Two Types of Covalent Bonds Based on Orthogonal Reactions. J. Am. Chem. Soc. 2015, 137, 1020–1023. 10.1021/ja510926w. [DOI] [PubMed] [Google Scholar]
- Vyas V. S.; Haase F.; Stegbauer L.; Savasci G.; Podjaski F.; Ochsenfeld C.; Lotsch B. V. A Tunable Azine Aovalent Organic Framework Platform for Visible Light-induced Hydrogen Generation. Nat. Commun. 2015, 6, 8508. 10.1038/ncomms9508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S.; Cheng G.; Guo L.; Wang N.; Tan B.; Jin S. Strong-Base-Assisted Synthesis of a Crystalline Covalent Triazine Framework with High Hydrophilicity via Benzylamine Monomer for Photocatalytic Water Splitting. Angew. Chem. 2020, 132, 6063–6070. 10.1002/ange.201914424. [DOI] [PubMed] [Google Scholar]
- Hu Y.; Dunlap N.; Wan S.; Lu S.; Huang S.; Sellinger I.; Ortiz M.; Jin Y.; Lee S.-h.; Zhang W. Crystalline Lithium Imidazolate Covalent Organic Frameworks with High Li-ion Conductivity. J. Am. Chem. Soc. 2019, 141, 7518–7525. 10.1021/jacs.9b02448. [DOI] [PubMed] [Google Scholar]
- Zhang L.; Wang S.; Zhou Y.; Wang C.; Zhang X.-Z.; Deng H. Covalent Organic Frameworks as Favorable Constructs for Photodynamic Therapy. Angew. Chem. 2019, 131, 14351–14356. 10.1002/ange.201909020. [DOI] [PubMed] [Google Scholar]
- Sun T.; Wei L.; Chen Y.; Ma Y.; Zhang Y.-B. Atomic-Level Characterization of Dynamics of a 3D Covalent Organic Framework by Cryo-Electron Diffraction Tomography. J. Am. Chem. Soc. 2019, 141, 10962–10966. 10.1021/jacs.9b04895. [DOI] [PubMed] [Google Scholar]
- Park S.; Liao Z.; Ibarlucea B.; Qi H.; Lin H.-H.; Becker D.; Melidonie J.; Zhang T.; Sahabudeen H.; Baraban L.; Baek C.-K.; Zheng Z.; Zschech E.; Fery A.; Heine T.; Kaiser U.; Cuniberti G.; Dong R.; Feng X.. Two-Dimensional Boronate Ester Covalent Organic Framework Thin Films with Large Single Crystalline Domains for Neuromorphic Memory Device. Angew. Chem. 2020, in press. 10.1002/ange.201916595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascherl L.; Sick T.; Margraf J. T.; Lapidus S. H.; Calik M.; Hettstedt C.; Karaghiosoff K.; Döblinger M.; Clark T.; Chapman K. W.; Auras F.; Bein T. Molecular docking sites designed for the generation of highly crystalline covalent organic frameworks. Nat. Chem. 2016, 8, 310–316. 10.1038/nchem.2444. [DOI] [Google Scholar]
- Wang Y.; Liu Y.; Li H.; Guan X.; Xue M.; Yan Y.; Valtchev V.; Qiu S.; Fang Q. Three-Dimensional Mesoporous Covalent Organic Frameworks through Steric Hindrance Engineering. J. Am. Chem. Soc. 2020, 142, 3736–3741. 10.1021/jacs.0c00560. [DOI] [PubMed] [Google Scholar]
- Das G.; Prakasam T.; Addicoat M. A.; Sharma S. K.; Ravaux F.; Mathew R.; Baias M.; Jagannathan R.; Olson M. A.; Trabolsi A. Azobenzene-Equipped Covalent Organic Framework: Light-Operated Reservoir. J. Am. Chem. Soc. 2019, 141, 19078–19087. 10.1021/jacs.9b09643. [DOI] [PubMed] [Google Scholar]
- Su X.; Aprahamian I. Hydrazone-Based Switches, Metallo-Assemblies and Sensors. Chem. Soc. Rev. 2014, 43, 1963–1981. 10.1039/c3cs60385g. [DOI] [PubMed] [Google Scholar]
- van Dijken D. J.; Kovaříček P.; Ihrig S. P.; Hecht S. Acylhydrazones as Widely Tunable Photoswitches. J. Am. Chem. Soc. 2015, 137, 14982–14991. 10.1021/jacs.5b09519. [DOI] [PubMed] [Google Scholar]
- Gupta P.; Panda T.; Allu S.; Borah S.; Baishya A.; Gunnam A.; Nangia A.; Naumov P.; Nath N. K. Crystalline Acylhydrazone Photoswitches with Multiple Mechanical Responses. Cryst. Growth Des. 2019, 19, 3039–3044. 10.1021/acs.cgd.8b01860. [DOI] [Google Scholar]
- Cvrtila I.; Fanlo-Virgós H.; Schaeffer G.; Santiago G. M.; Otto S. Redox Control over Acyl Hydrazone Photoswitches. J. Am. Chem. Soc. 2017, 139, 12459–12465. 10.1021/jacs.7b03724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uribe-Romo F. J.; Doonan C. J.; Furukawa H.; Oisaki K.; Yaghi O. M. Crystalline Covalent Organic Frameworks with Hydrazone Linkages. J. Am. Chem. Soc. 2011, 133, 11478–11481. 10.1021/ja204728y. [DOI] [PubMed] [Google Scholar]
- Li X.; Qiao J.; Chee S. W.; Xu H.-S.; Zhao X.; Choi H. S.; Yu W.; Quek S. Y.; Mirsaidov U.; Loh K. P. Rapid, Scalable Construction of Highly Crystalline Acylhydrazone Two-dimensional Covalent Organic Frameworks via Dipole-Induced Antiparallel Stacking. J. Am. Chem. Soc. 2020, 142, 4932–4943. 10.1021/jacs.0c00553. [DOI] [PubMed] [Google Scholar]
- Ding S.-Y.; Cui X.-H.; Feng J.; Lu G.; Wang W. Facile Synthesis of -C=N- Linked Covalent Organic Frameworks under Ambient Conditions. Chem. Commun. 2017, 53, 11956–11959. 10.1039/C7CC05779B. [DOI] [PubMed] [Google Scholar]
- Wang Z.; Yu Q.; Huang Y.; An H.; Zhao Y.; Feng Y.; Li X.; Shi X.; Liang J.; Pan F.; Cheng P.; Chen Y.; Ma S.; Zhang Z. PolyCOFs: A New Class of Freestanding Responsive Covalent Organic Framework Membranes with High Mechanical Performance. ACS Cent. Sci. 2019, 5, 1352–1359. 10.1021/acscentsci.9b00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh M. I.; Kusrini E.; Saad B.; Fun H.-K.; Yamin B. M. Coordination of Trivalent Lanthanum with Polyethylene Glycol in the Presence of Picrate Anion: Spectroscopic and X-ray Structural Studies. J. Alloys Compd. 2009, 474, 428–440. 10.1016/j.jallcom.2008.06.101. [DOI] [Google Scholar]
- Koshima H.; Ojima N.; Uchimoto H. Mechanical motion of azobenzene crystals upon photoirradiation. J. Am. Chem. Soc. 2009, 131, 6890–6891. 10.1021/ja8098596. [DOI] [PubMed] [Google Scholar]
- Li X.; Gao Q.; Wang J.; Chen Y.; Chen Z. H.; Xu H. S.; Tang W.; Leng K.; Ning G. H.; Wu J.; Xu Q. H.; Quek S. Y.; Lu Y.; Loh K. P. Tuneable Near White-Emissive Two-Dimensional Covalent Organic Frameworks. Nat. Commun. 2018, 9, 2335. 10.1038/s41467-018-04769-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coman A. G.; Paun A.; Popescu C. C.; Hădade N. D.; Anghel C. C.; Mădălan A. M.; Ionită P.; Matache M. Conformation-Induced Light Emission Switching of N-Acylhydrazone Systems. New J. Chem. 2018, 42, 14111–14119. 10.1039/C8NJ01880D. [DOI] [Google Scholar]
- Lehn J.-M. Conjecture: Imines as Unidirectional Photodriven Molecular Motors-Motional and Constitutional Dynamic Device. Chem. - Eur. J. 2006, 12, 5910–5915. 10.1002/chem.200600489. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


