Abstract
Aggregation-caused quenching (ACQ) and poor photostability in aqueous media are two common problems for organic fluorescence dyes which cause a dramatic loss of fluorescence imaging quality and photodynamic therapy (PDT) failure. Herein, a local hydrophobic cage is built up inside near-infrared (NIR) cyanine-anchored fluorescent silica nanoparticles (FSNPs) in which a hydrophobic silane coupling agent (n-octyltriethoxysilane, OTES) is doped into FSNPs for the first time to significantly inhibit the ACQ effect and inward diffusion of water molecules. Therefore, the obtained optimal FSNP-C with OTES-modification can provide hydrophobic repulsive forces to effectively inhibit the π–π stacking interaction of cyanine dyes and simultaneously reduce the formation of strong oxidizing species (•OH and H2O2) in reaction with H2O, resulting in the best photostability (fluorescent intensity remained at 90.1% of the initial value after 300 s of laser scanning) and a high PDT efficiency on two- and three-dimensional (spheroids) HeLa cell culture models. Moreover, through molecular engineering (including increasing covalent anchoring sites and steric hindrance groups of cyanine dyes), FSNP-C exhibits the highest fluorescent intensity both in water solution (12.3-fold improvement compared to free dye) and living cells due to the limitation of molecular motion. Thus, this study provides an effectively strategy by combining a local hydrophobic cage and molecular engineering for NIR FSNPs in long-term bright fluorescence imaging and a stable PDT process.
Short abstract
A local hydrophobic cage is built up inside fluorescent silica nanoparticles for enhancing the photophysical properties of organic dyes to overcome ACQ and poor photostability in aqueous media.
Introduction
Near infrared (NIR) cyanine dyes, such as indocyanine green (ICG, a FDA approved clinical agent), IR780, IR820, or other structurally modified cyanine dyes, have emerged as excellent NIR imaging agents and potential photosensitizers in the applications of cancer diagnosis and photodynamic therapy (PDT).1−8 The strong absorption and emission in the near-infrared region (650–900 nm), deep tissue penetration, and high photoconversion efficiency of these NIR cyanine dyes are inherent advantages in cancer diagnosis and treatment.9,10
Although traditional NIR cyanine dyes have been substantially studied, a notorious phenomenon in the physiological aqueous environment known as concentration quenching or aggregation-caused quenching (ACQ) is a big impediment to their extensive application.11,12 The ACQ effect in organic dyes is generally regarded as unfavorable, which has only allowed use of dilute solutions of fluorophores for biosensor applications13,14 To overcome the ACQ effect, some effective strategies based on molecular engineering have been proposed by some researchers. For example, Zhang et al. found that pentamethine cyanine dyes with a structure of benzothiopyrylium heterocycles obtained various antiquenching properties including high brightness and superior photostability in aqueous solution.15 Another effective strategy is to introduce bulky groups and polymer chains into the fluorophores, which can prevent π–π stacking of fluorophores in the polymeric matrix.16−19 However, these strategies require complicated synthetic efforts.
In addition, photostability in aqueous media is another critical index for cyanine dyes as fluorescent biolabels. Unfortunately, most cyanine dyes, such as Cy5 and Cy7, are prone to quick photobleaching by light irradiation, which does not allow long-term tracking of the biological processes.20 This is because reactive oxygen species (ROS, such as 1O2, O2–, H2O2, •OH, etc.) formed in situ by photosensitization of cyanine dyes lead to oxidative cleavage of the cyanine’s polyene linker, which is a well-defined photooxidation pathway.21−24 To address these deficiencies, researchers seek to rationally optimize the molecular structure of cyanine dyes to slow or even block photobleaching via molecular engineering. For example, certain cyanine dyes are modified through reducing the electron cloud density of the polyene chain or occupying the easily nucleophilic α-carbon of the polymethine chain to reduce the probability of C–C oxidative cleavage.25,26 The cyanine dyes are modified by the substituent groups with dendritic structure or steric hindrance groups of rigid structure, which are general strategies to significantly improve photostability.27,28 As is well-known, the excited triplet state of the photosensitizer is strongly linked to the production of ROS, which could sensitize 3O2 to 1O2. To reduce the species of triplet and radical states, some self-protective derivatives containing cyclooctate, trolox, or nitrobenzyl alcohol have also been developed.29,30 In addition, some supramolecular self-assembly strategies have been employed to provide better performance of photosensitizer molecules in PDT or bioimaging.31−36
All of the above effective strategies via molecular engineering have a positive effect on inhibiting ACQ and improving photostability in aqueous media. However, a variety of physiological microenvironment factors including the interaction between fluorophores and water molecules, pH, viscosity, and the localized concentration of the fluorophores can directly affect the photophysical properties of the fluorophores and further severely influence the effect of fluorescence bioimaging or PDT. Recently, nanomaterials, notably inorganic silica nanoparticles, have the advantages of good biocompatibility and easy surface functionalization, to be particularly suitable carriers of organic fluorescence dyes for biochemical analysis and tumor treatment.37,38 The fluorophores encapsulated in silica matrix can extend the luminescence lifetime and improve the photostability or fluorescence intensity due to the chemical inertness of the silicon-rich structure.39−42 However, deep inward diffusion of water is inevitable due to the porous structure and high hydrophilicity of silica nanoparticles. ACQ can still occur, and photostability can still be reduced due to the proximity of water.43 These two negative characteristics also dramatically hamper the application of fluorescent nanoparticles as imaging contrasts and PDT photosensitizers in the aqueous biological environment. How fluorescence intensity and photostability may be improved not only through molecular engineering or empirical efforts, but also through rationally regulating the fluorophore-microenvironment relationships to inhibit ACQ in the physiological aqueous media, is a challenging problem. In particular, preparing cyanine-anchored fluorescent silica nanoparticles with super fluorescence intensity, superior photostability, and high ROS generation in aqueous media remains a great challenge to date.
Herein, the hydrophobic organosilicone agent (n-octyltriethoxysilane, OTES), widely used in the hydrophobic modification of materials,44−47 is applied to construct a local hydrophobic cage inside FSNPs to inhibit the ACQ effect and inward diffusion of water molecules for the first time. The local hydrophobic cage can provide a nonpolar microenvironment for the doped cyanine dyes which is synchronously optimized via precise molecular engineering (including increasing covalent anchoring sites and steric hindrance groups). Such a collaborative strategy is expected to enhance the photophysical properties of the prepared FSNPs (Scheme 1). Accordingly, the effects of the combinatorial modification strategy on photophysical properties of FSNPs including fluorescence lifetime, fluorescence intensity, photostability, and the productive ability of 1O2 are systematically investigated. On this basis, the PDT effect, intracellular photostability, and intracellular anti-ROS ability of modified FSNPs are evaluated on 2D and 3D (spheroids) HeLa cell culture models.
Scheme 1. Schematic Representation of the Preparation of FSNP-A, -B, and -C.
Results and Discussion
Synthesis Strategy of FSNPs
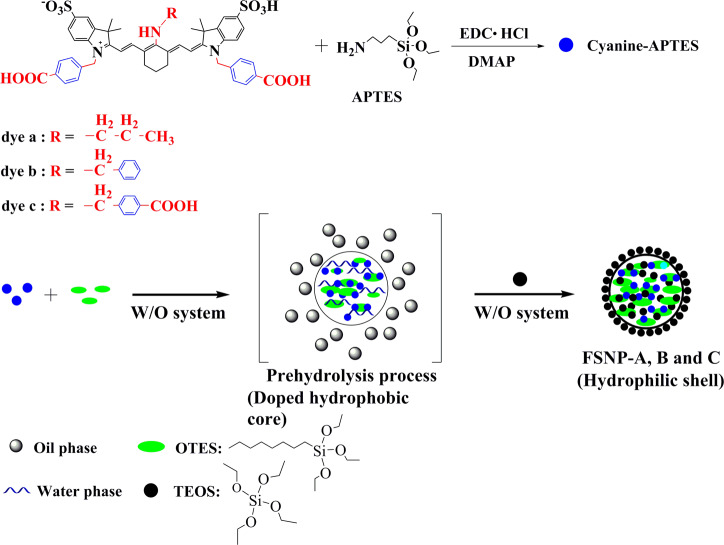
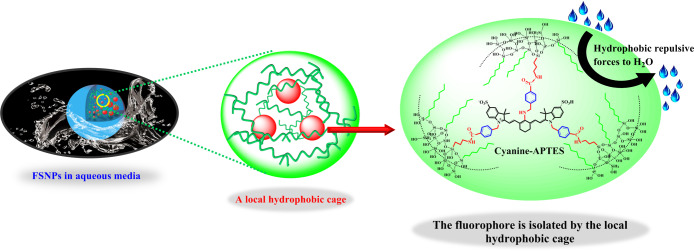
As shown in Scheme 1, three new heptamethine aminocyanine dyes (dye a, dye b, dye c) were designed and synthesized to form cyanine-APTES silane molecules as the precursor of nanoencapsulation.48 FSNP-A, -B, and -C were prepared by using modified water-in-oil (W/O) reverse microemulsion method based dyes a–c (Experiment Part in Supporting Information). Dyes a–c are expected to provide the prepared FSNPs better photostability and fluorescence brightness due to their bulky benzyl groups and two or three carboxyl acid anchoring sites. In particular, after the prehydrolysis process of cyanine-APTES with OTES for 12 h under alkaline condition before adding silica source (TEOS), the cyanine dyes were enclosed from all sides by hydrophobic alkyl chains of OTES so that they were “trapped”, as shown in Figure 1. Eventually, TEOS was hydrolyzed at the oil–water interface, encapsulating the monomer precursor (cyanine-APTES and OTES) in the interior to form a covalently cross-linked network through hydrolysis and polycondensation mechanism.
Figure 1.
Simplified representation of a local hydrophobic cage inside cyanine-anchored FSNPs.
It is worth noting that, when the modified coupling agents of different lengths of hydrophobic alkyl chains (C3-PTOS, C8-OTES, and C12-DTOS) were selected, their degree of hydrolysis in the W/O reverse microemulsion was completely different (details in Figure S1). After the terminated hydrolysis process using acetone and being left for 1 h, the modified nanoparticles by short hydrophobic chain (C3-PTOS and C8-OTES) could be successfully prepared, while the reaction system with long chain modification (C12-DTOS) was still a turbid emulsion without nanoparticle formation. Therefore, we speculate that the difference in the hydrolysis process may be closely related to the collision probability of oil-soluble silane coupling agents and water molecules (the schematic diagram of the hydrolysis process in Figure S1). In a water-in-oil system, aqueous ammonia is present in the aqueous phase, while TEOS and oil-soluble silane coupling agents are present in the oil phase. Undoubtedly, the hydrolysis of the silane coupling agents and TEOS is competitive. This is because the water phase is distributed in the oil phase in the form of nanoscale water droplets in this water-in-oil system in which the surfactant TX-100 working at the oil–water interface plays a stabilizing role. The relatively hydrophilic short chain silane coupling agent (C3-PTOS or C8-OTES) is more susceptible to hydrolysis at the oil–water interface than long chain silane coupling agents (C12-DTOS) in this water-in-oil system. Therefore, the presence of surfactant TX-100 makes it more difficult for hydrophobic long chain silane coupling agents (C12-DTOS) to hydrolyze in contact with the aqueous phase, eventually leading to the failure of the hydrolysis process and no nanoparticles formation (Figure S1c). As a result, the hydrophobic organosilicone agent OTES was chosen to provide the hydrophobic modification. Three kinds of FSNPs (FSNP-a, FSNP-b, and FSNP-c) without hydrophobic modification with OTES were also prepared as control groups through W/O reverse microemulsion method. The difference from the preparation of FSNP-A, FSNP-B and FSNP-C was that OTES was not added to the corresponding water-in-oil system.
Structural Characterization of FSNPs
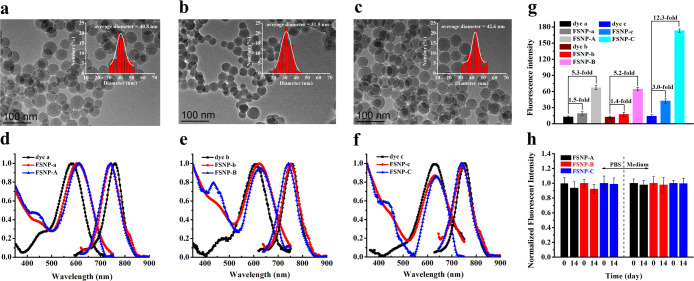
After the preparation of FSNP-A–C, their morphology characteristics were tested by TEM. FSNP-A–C (Figure 2a–c and Table S1) and control groups FSNP-a–c (Figure S2 and Table S2) have similar particle sizes (∼40 nm). Furthermore, FSNP-A–C remain almost the same pore diameter values with of pure SiO2. Zeta potential measurements of FSNP-A–C are also performed to provide surface parameters. The Zeta potential values of FSNP-A–C, and FSNP-a–c are very close to that of pure SiO2 (Table S1), which indicates that cyanine dyes encapsulation and hydrophobic modification are carried out inside of nanoparticles. Subsequently, we try to arrest the surface information on FSNP-A–C by FTIR (Figure S3). The pure SiO2 (black line) exhibits IR characteristic absorption peaks at the bands assigned to Si–O–Si bending (472 cm–1), Si–O–Si symmetric stretching (802 cm–1), external Si–OH groups (953 cm–1), Si–O–Si asymmetric stretching (1103 cm–1), and −OH antisymmetric stretching vibration of silanol with bound water (3417 cm–1). For OTES-doped FSNP-A–C, the absorption peak of the characteristic external Si–OH groups and −OH antisymmetric stretching vibration of silanol with bound water at 3417 and 953 cm–1 are same as pure SiO2 respectively, which ensures that the surface of modified FSNP-A–C is still hydrophilic. Meanwhile, the FSNP-A–C do not show additional bands, which can be assigned to the alkyl groups [−(CH2)n−] presented in OTES. Thus, OTES is most likely to be doped in the interior of FSNPs through the modified reverse microemulsion method while maintaining the hydrophilic property of FSNP-A–C.
Figure 2.
(a–c) TEM images of FSNP-A–C, respectively; (d–f) Normalized absorption and emission spectra of FSNP-A–C in water; (g) the fluorescent intensity of free dyes, FSNP-a–c and FSNP-A–C in vitro; (h) chemical durability of FSNP-A–C (0.5 mg mL–1) in phosphate buffer (pH = 7.4, 10 mM) and culture medium for 14 days. All samples are dispersed or dissolved in water to a final concentration with almost the same absorbance (0.01); error bars represent ± σ for triplicate measurements.
Photophysical Characteristics of FSNPs
Next, we systematically investigated the photophysical properties of FSNP-A–C, which may be affected by multiple modification factors. The absorption and emission spectra of FSNPs were recorded in Figure 2d–f. The spectra showed that FSNPs possessed the same large Stokes shift in water (>100 nm) as free dyes. This also indicated that the structure of cyanine was not destroyed after being encapsulated in the nanoparticles. As is well-known, most traditional polymethine cyanine dyes have a small Stokes shift. For example, the Stokes shift of ICG is only about 20 nm. Such a large overlap between absorption and emission spectra may cause fluorescence self-quenching. Especially in the case of a high concentration or nanoencapsulation, the fluorescence intensity will be significantly reduced. However, dye a, dye b, and dye c exhibit a large blue shift and a large Stokes shift (>100 nm) due to an excited-state intramolecular charge transfer (ICT) between the donor and acceptor in the heptamethine aminocyanine dyes.49 Therefore, the newly designed heptamethine aminocyanine dyes selected in this work can minimize the fluorescence intensity reduction caused by fluorescence self-quenching. Also, this is an important basis for constructing a local hydrophobic cage inside fluorescent silica nanoparticles (FSNPs) to further improve their fluorescence brightness.
It is well-known that the luminescent properties of the cyanine dyes are susceptible to an aqueous environment. Thus, the fluorescence intensity of FSNPs was measured in water through adjusting the absorbance to 0.01. It could be seen from Figure 2g that all the fluorescence intensity of FSNP-a–c and FSNP-A–C were improved after the cyanine dyes were encapsulated inside the silica frameworks. This is because cyanine was covalently anchored to the rigid silica matrix that could maximally restrict intramolecular rotation and restrain π–π stacking interaction, which can inhibit the ACQ effect and further reduce the nonradiative transition process. Additionally, we can clearly observe that the number of covalent anchoring sites had a positive effect on increasing fluorescence intensity (Figure 2g). When the number of anchoring sites was two, the fluorescence intensities of FSNP-a and FSNP-b were 1.5 and 1.4-fold than that of free dye a and b. Especially for FSNP-c, when the number of anchoring sites was increased to three, the fluorescence intensity was significantly improved (3.0-fold than that of free dye c). These results also confirmed that the increase in the number of anchoring sites could effectively limit intramolecular rotation, further reduce the energy loss of excited states, and eventually improve the fluorescence intensity, which were consistent with our previous work.50 Remarkably, the fluorescence intensities of FSNP-A–C were further significantly improved (the fluorescence intensities of FSNP-A and FSNP-B were up to 5.3- and 5.2-fold compared to that of free dye a and dye b, respectively), wherein the fluorescence intensity of FSNP-C reached to 12.3-fold that of free dye c. We speculated that it may result from OTES-doped FSNPs containing hydrophobic alkyl inside to provide a local hydrophobic cage (see Figure 1), which could efficiently inhibit the strong solvation of water molecules and the ACQ effect. So we further compared the fluorescence intensity of free dyes and FSNPs in absolute ethanol (Figure S4). As shown in Figure S4, the fluorescence intensities of free dyes in ethanol were higher than the corresponding fluorescence intensity in water. Also, it was observed that the increase in the number of anchoring sites could improve the fluorescence intensity. However, the enhanced fold of fluorescence intensities of OTES-doped FSNPs (FSNP-A, -B, and -C) compared with FSNP-a, -b, and -c in ethanol was not much higher than in water. This was attributed to the weaker solvation of ethanol compared to water, which did not effectively quench fluorescence. The above comparison experiment verified our presumption that doping of OTES could significantly improve the fluorescence intensity by constructing a local hydrophobic cage, especially in water. Thus, FSNP-C of hydrophobic OTES-doped containing three anchoring sites for embedding cyanine into silica matrices comprehensively resulted in FSNP-C with the highest fluorescence intensity.
Compared with fluorescence intensity, the data of fluorescence lifetime can prevent the possible measurement error of the quantity of used fluorophore. Also, the fluorescence lifetime can reflect the effect of the microenvironment of fluorophores.51 Therefore, the fluorescence lifetime of FSNPs in aqueous solution was measured (Table 1), to try to investigate the influence of the slight changes in the prepared FSNPs’ microenvironment on the fluorescence lifetime. The fluorescence lifetime measurements showed two lifetime components for all FSNPs (τ1 and τ2, Table 1; details in Figure S5) corresponding to the cyanine dyes being present in two different silica microenvironments according to the literature.52 The lifetime component (τ1) was assigned to solvent accessible dyes, and the lifetime component (τ2) was assigned to the dyes influenced by a restricted environment of inside of FSNPs. Therefore, free dye a–c had only one component (τ1) of fluorescence lifetime in aqueous solution. When free dyes were doped inside the nanoparticles, τ1 values of FSNP-a–c decreased significantly with the increase of τ2 values. After hydrophobic modification, both τ1 values and τ2 values of FSNP-A–C increased. Particularly, both τ1 values and the average lifetime (τ) of FSNP-a–c were shorter than that of corresponding free dyes. The reason was that Cy7 dyes tended to aggregate rather than disperse inside the nanoparticles, leading to the occurrence of ACQ and a short fluorescence lifetime because the permeability of water into FSNP-a–c and the presence of noncondensation Si–OH bonds in the silica matrix may greatly promote the occurrence of ACQ, also reducing the fluorescence lifetime. When cyanine dye molecules were separated from water molecules and the hydroxyl environment by the isolation effect of the local hydrophobic cage, the ACQ effect was inhibited, eventually leading to a larger τ2 value and a longer average lifetime (τ) of FSNP-A–C. The percentage of much bigger contribution (τ2) to the average lifetime (τ) of FSNPs significantly varied from 15.79% (FSNP-a) to 50.3% (FSNP-C). On the one hand, the τ1 values first decreased from free dye a–c to the FSNP-a–c due to ACQ enhancement. Then the τ1 values increased from FSNP-a–c to FSNP-A–C due to ACQ inhibition (blue bars in Figure 3). On the other hand, with the increase of the rotation limitation degree and the protection by OTES, the τ2 values increased significantly (green bars in Figure 3) from FSNP-a–c to FSNP-A–C. The upward trend of these percentages (τ2) indicated that the introduction of rigid hindered groups and an increased number of anchoring sites effectively inhibited the internal rotation or isomerization of the inner FSNPs’ cyanine dyes. Meanwhile, the hydrophobic alkyl of OTES may provide a relative nonpolar environment in the FSNPs’ hybrid network, which could effectively isolate Cy7 and inhibit the π–π stacking stack of Cy7. These positively comprehensive factors ultimately led to the longer fluorescence lifetime of FSNP-A–C than those of free dye a–c and FSNP-a–c in aqueous solution (rose bars in Figure 3). The longest fluorescence lifetime of FSNP-C indicates that the nonradiative decay can be reduced by increasing the number of anchoring sites, and the ACQ effect can be efficiently inhibited by the local hydrophobic cage.
Table 1. Photophysical Properties of the Prepared FSNPs.
| fluorecsence
lifetime/ns |
||||
|---|---|---|---|---|
| FSNPsa | τ1/ns (A1: contribution %) | τ2/ns (A2: contribution %) | τ/nsb | X2c |
| dye a | 0.452 ± 0.002 (100) | 0.452 ± 0.002 | 1.003 | |
| dye b | 0.452 ± 0.001 (100) | 0.452 ± 0.001 | 0.986 | |
| dye c | 0.496 ± 0.001 (100) | 0.496 ± 0.001 | 1.196 | |
| FSNP-a | 0.098 ± 0.001 (84.21) | 0.648 ± 0.001 (15.79) | 0.185 ± 0.001 | 1.120 |
| FSNP-b | 0.099 ± 0.002 (78.78) | 0.631 ± 0.003 (21.22) | 0.212 ± 0.001 | 1.128 |
| FSNP-c | 0.075 ± 0.002 (77.99) | 0.635 ± 0.001 (22.01) | 0.198 ± 0.002 | 1.026 |
| FSNP-A | 0.233 ± 0.002 (59.30) | 1.119 ± 0.005 (40.71) | 0.594 ± 0.002 | 1.079 |
| FSNP-B | 0.221 ± 0.003 (56.86) | 1.188 ± 0.003 (43.14) | 0.638 ± 0.003 | 0.972 |
| FSNP-C | 0.229 ± 0.001 (49.70) | 1.221 ± 0.002 (50.30) | 0.728 ± 0.002 | 0.920 |
All measurements performed in water.
The average lifetime τ = τ1 × A1 + τ2 × A2.
Represents the goodness of the fit. All lifetime values are based on triplicate measurements with the standard deviation given by ± value.
Figure 3.
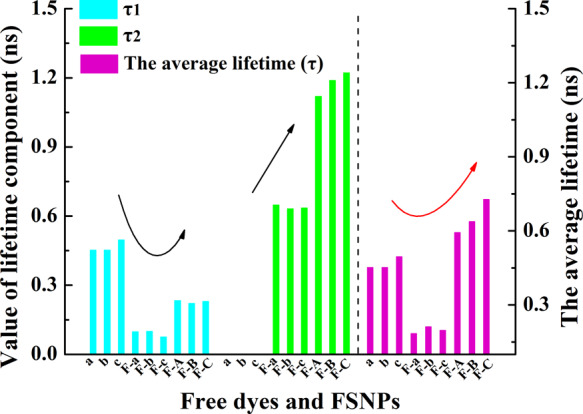
Synthetic histogram analysis for the fluorescence lifetime components (τ1 value and τ2 value) and average fluorescence lifetime (τ value) from Table 1. The a–c, F-a–c, and F-A–C in the bar chart represent free dye a–c, FSNP-a–c, and FSNP-A–C, respectively.
Chemical Durability and Physiological Stability of FSNPs
Maintaining the chemical stability of FSNPs under physiological condition is the prerequisite for biological applications. So we tested the chemical durability of FSNPs in phosphate buffer (pH = 7.4, 10 mM). As shown in Figure 2h, the fluorescence intensity of the prepared FSNPs did not fluctuate significantly during 2 weeks. These covalent linkages between organic dyes and inorganic frameworks can be an advantage over other weakly bonded materials,53 which can completely avoid the leaching of dyes. Furthermore, all FSNPs had a good stability in phosphate buffer over a wide pH range from 4.0 to 10.0 (Figure S6). Besides, the stability of FSNPs in complete medium (containing 10% of FBS) was also measured Figure 2h. As we expected, the fluorescence intensity of FSNPs had no significant change for 2 weeks, which ensured a robust biostability in the physiological environment. The effective covalent anchoring of Cy7 through three rigid hindrance anchor sites was the key to a good stability of FSNPs in solution. In addition, the isolated internal environment provided by the local hydrophobic cage could effectively prevent the collision between water molecules and Cy7.
Photostability Evaluation of FSNPs
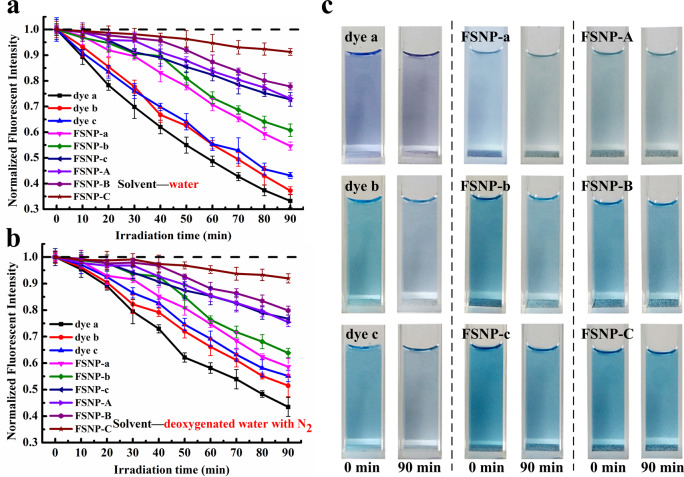
After a good chemical durability and physiological stability of FSNPs was confirmed, greater attention should be paid to the photostability. This is because of the fact that photobleaching of cyanine dyes is the biggest issue that often causes distortion of the fluorescence signal.54,55 Therefore, free dyes and FSNPs dispersed in aqueous solutions or deoxygenated aqueous solutions were irradiated with a W-halogen lamp (500 W) for 90 min to evaluate their photostability. It could be observed from Figure 4a that a long-term irradiation could rapidly reduce the fluorescence intensity of free dyes and FSNP-a–c, leading to significant time-dependent photofading. Especially, the fluorescence signal of both free dye a and dye b was less than 40% after 90 min of exposure. This was because reactive oxygen species (ROS) was produced, resulting in the photooxidative degradation of cyanine in situ.56 However, the photostability of FSNPs had been greatly improved when cyanine dyes were covalently encapsulated in the silica matrix, which retained 54.6% (FSNP-a), 60.8% (FSNP-b), and 72.8% (FSNP-c) of the initial fluorescent value. Obviously, the photostability of FSNP-c was better than FSNP-a and FSNP-b. This was because three covalent anchoring sites combining three rigid hindrance groups made the doped cyanine dyes less susceptible to be attacked by ROS through limiting the free internal rotation of cyanine in the excited state and protecting the conjugate structure. Remarkably, the phototstability of FSNP-A–C showed more obvious enhancement after doping with OTES (Figure 4a,c), which retained 73.1% (FSNP-A) and 77.8% (FSNP-B) of he initial fluorescent value. Especially, FSNP-C with the best optimal anti-photobleaching performance still retained 91.3% of the initial fluorescence intensity in water after 90 min irradiation, showing further anti-photobleaching capability for continuous long-term fluorescence imaging.
Figure 4.
Photostability evaluation of free dye a–c, FSNP-a–c, and FSNP-A–C in water (a) and deoxygenated water with N2 (b); (c) photobleaching behavior of nanoparticle aqueous solution after irradiation for 90 min. Free dyes and FSNPs are dissolved in different solvents, to ensure each group of free dyes and FSNPs had almost the same fluorescence intensity by adjusting concentration. λex free dye a, b, c = 585, 609, and 619 nm, respectively. λex FSNP-a and A, b and B, c and C = 604, 623, and 630 nm, respectively. Error bars represent ± σ for triplicate measurements.
Thus, we speculated that a relatively hydrophobic environment provided by the local hydrophobic cage could improve the photostability of dyes. So an experiment that simulated a hydrophobic environment was designed to investigate the effect of ACQ on the photostability. As shown in Figure S7a, the fluorescence of dye c in methanol was higher than that of in water, indicating a higher aggregation of dye c in water. Then, the fluorescence of dye c in water was enhanced with the addition of surfactant (sodium dodecyl benzenesulfonate). This was because the surfactant provided a hydrophobic environment for the dyes by forming micelles in the aqueous solution, leading to fluorescence enhancement.57 Subsequently, the photostability before and after micellar formation was measured. We could see from Figure S7b that the photostability of dye c that formed micelles was improved obviously after 60 min of irradiation. Therefore, the photostability of dye could be improved by inhibiting ACQ. This result agreed with the work in the literature.57,58 Meanwhile, this result also proved that FSNP-A–C with a local hydrophobic cage could effectively inhibit ACQ and further improve the photostability.
It is well-known that 1O2 was recognized as a major ROS in the photooxidation process of cyanine dyes. Nevertheless, on the basis of the electronic transfer mechanism, the interaction of the excited dye with dioxygen can form O2– through an electron transfer process. Because of the instability of O2– in water, the dismutation of O2– with H2O produced H2O2, O2, and •OH.59 These highly oxidizing species would accelerate rapid oxidative degradation of cyanine dyes. Therefore, when the photostability was measured in deoxygenated water, we found that the resistance to photodegradation of free dyes and FSNP-a–c had generally improved (Figure 4b). However, the photostability of FSNP-A–C did not change significantly. Once again, this result indicated that the local nonpolar environment provided by the hydrophobic cage could repel water molecules to prevent them from colliding with Cy7 dyes, which could reduce the generation of additional ROS (H2O2, HO2•, or •OH) and further reduce the photooxidation process. As is clear from the above results, the relatively hydrophobic property of OTES-precursor alkyl chains greatly enhanced the final photophysical performance of FSNP-A–C.
Singlet Oxygen Production of FSNPs in Aqueous Solution
The capability to produce 1O2 is one of the essential properties of cyanine dyes as photosensitizers. Therefore, the relative singlet oxygen quantum yield of the prepared FSNPs was evaluated indirectly by using a commercially available 1O2 indicator 1,3-diphenylisobenzofuran (DPBF) as a chemical trap.60 Distinctly, FSNP-A–C had a higher relative singlet oxygen quantum yield (Table S3) compared to the corresponding FSNP-a–c and free dye a–c (ΦMB = 0.52 as a reference),61,62 while ICG or IR-787 had a value of only 0.008 or 0.007.63 According to comparative tests for FSNP-C (Figure S8), the absorbance of DPBF decreased remarkably in the presence of FSNP-C under irradiation, giving the highest relative singlet oxygen quantum yield of ΦΔ = 0.085. This was 12.1- and 8.5-fold of free dye c and FSNP-c, respectively. When FSNP-a, FSNP-b, and FSNP-c are irradiated with light source and self-sensitized to produce 1O2, water molecules as the strong quencher of 1O2 will directly quench 1O2 to inhibit its escape outside the nanoparticles. However, when the silane coupling agent (OTES) is doped, the long hydrophobic chains of OTES may provide a local hydrophobic cage for the Cy7 dyes inside FSNP-A–C and inhibit the proximity of water molecules. Thus, these hydrophobic modification nanoparticles FSNPs-A–C can increase the amount of 1O2 and reduce the inactivation of 1O2 by water in situ.
Fluorescence Intensity and Photostability Evaluation of FSNPs in HeLa Cells
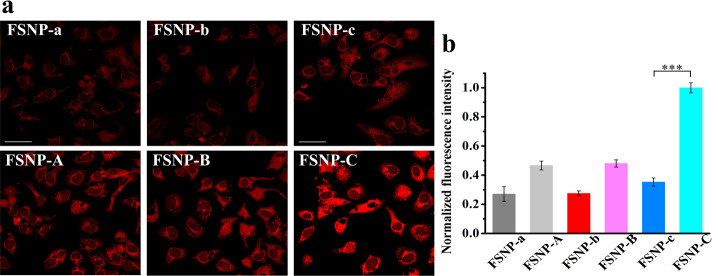
Though we have obtained the exciting results in the testing of FSNP-A–C in aqueous solution, various uncertain factors in the intracellular microenvironment, including various ions, proteins, etc., may result in unstable fluorescence signals and poor fluorescence images. Therefore, we systematically measured the photostability and fluorescence intensity of FSNP-A–C in HeLa cells via confocal laser scanning microscopy (CLSM). On one hand, as seen in Figure 5a, both FSNP-a–c and FSNP-A–C could be effective internalization by HeLa cells because of their appropriate nanometer size distribution.64−66 On the other hand, it can be clearly seen that FSNP-A–C doped with OTES generally exhibited a higher fluorescence intensity than FSNP-a–c (Figure 5b). At the same time, it can be seen that the increase of covalent anchoring sites also improved the fluorescence intensity of FSNP-C and FSNP-c compared to FSNP-B and FSNP-b, respectively. Thus, the nanoparticles FSNP-A–C obtained enhanced brightness in living cells via inhibiting the ACQ effect. Especially, FSNP-C exhibited the brightest intracellular red fluorescence among the prepared FSNPs.
Figure 5.
(a) Fluorescence images of HeLa cells incubated with FSNP-a–c and FSNP-A–C at 2 μg mL–1. Irradiation sources: a standard 635 nm laser of OLYMPUS (laser transmissivity: 10%, λem = 655–755 nm). Scale bars = 40 μm; (b) plots of normalized intracellular fluorescence intensity from (a). The statistical significance level is ***P < 0.001.
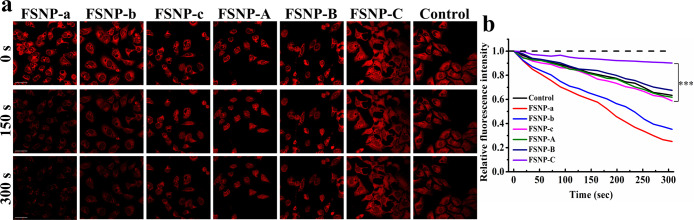
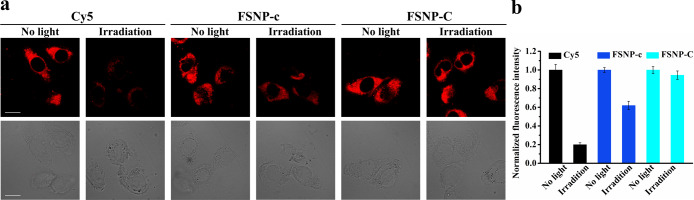
Next, the intracellular photostability of FSNPs was evaluated via continuous laser scanning of CLSM. As can be seen from Figure 6a, FSNP-A–C had an intracellular enhanced photostability compared to corresponding FSNP-a–c. Most importantly, the intensity of FSNP-C fluorescence signal remained virtually unchanged (fluorescent intensity remained at 90.1% of the initial value, Figure 6b) after 300 s of laser scanning, whereas an obvious decrease in signal intensity was detected for cells stained with FSNP-A and FSNP-B (fluorescent intensity of FSNP-A and FSNP-B only remained at 61.8% and 67.6% of the initial value, respectively). By contrast, the fluorescent signal of FSNP-a–c was significantly affected by the complicated dynamic water-based microenvironment in living cells, exhibiting significant photobleaching (fluorescent intensity of FSNP-a–c only remained at 25.1%, 35.2%, and 58.8% of the initial value). These results indicated again that the doping of the long hydrophobic chains played an important role in simultaneously enhancing the photostability and fluorescence intensity of encapsulated cyanine dyes by providing a local hydrophobic cage. In order to verify the potential of FSNPs for long-term imaging, a commercially available Cyanine5 carboxylic acid (Cy5) dye was selected as a control for intracellular photostability testing. As we all know, Cy5 dye has a shorter π-conjugation system than Cy7 dye (Figure S9), so its photostability is generally higher than that of traditional Cy7 dye. After 300 s of laser irradiation under the same conditions, the fluorescent intensity of Cy5 remained at only 63.4% of the initial value, which is much lower than that of FSNP-C. So, taking all these factors into consideration, it is demonstrated that FSNP-C was the most photostable nanoparticles that was reasonably designed. Therefore, the excellent photostability and high fluorescence intensity should impart FSNP-C with the potential for long-term or multiple fluorescence imaging in living cells.
Figure 6.
(a) Comparison of the photostabilities of FSNP-a–c and FSNP-A–C in HeLa cells under the mode of time series scanning. A commercially available Cyanine5 carboxylic acid (Cy5) was selected as the control group. Irradiation sources: a standard 635 nm laser of OLYMPUS (laser transmissivity: 10%) was used and image detection range was from 655 to 755 nm. The initial fluorescence intensity (0 s) was adjusted to almost the same level. Scale bars = 40 μm; (b) plots of relative intracellular fluorescence intensity from recorded fluorescence images (a) as a function of the time show different levels of photobleaching. The statistical significance level is ***P < 0.001.
PDT Effect of FSNPs on HeLa Cells
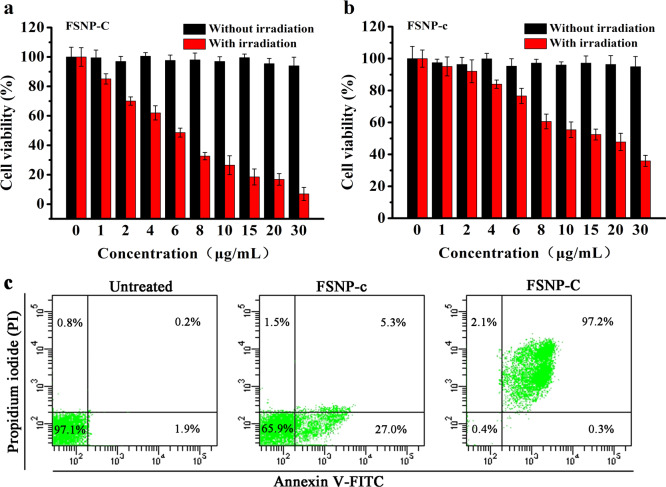
Since FSNP-C exhibited excellent photophysical properties in living cells, the PDT effect of FSNP-C on HeLa cells was further investigated using an MTT assay. As shown in Figure 7a,b, FSNP-C showed a stronger dose-dependent phototoxicity (IC50 = 5.02 μg mL–1) than FSNP-c (IC50 = 14.61 μg mL–1). Also, a commercial ROS probe 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) was selected to indicate the intracellular ROS level (Figure S10). After irradiation, the significantly green enhanced fluorescence was found in HeLa cells stained with FSNP-C. Meanwhile, both FSNP-C and FSNP-c had a low dark cytotoxicity due to a good biocompatibility of silica nanoparticles (Figure S11). Therefore, the phototoxicity index (PI) value (the ratio of IC50 of dark cytotoxicity to IC50 of phototoxicity) calculated for FSNP-C was 138.72, while that of FSNP-c was only 46.83. Besides, the HeLa cell death pathway during PDT was also determined by flow cytometry analysis using double-labeled annexin V-FITC and propidium iodide (PI). There was an obvious difference in the percentage of late apoptotic cells between the two groups that is shown in Figure 7c (97.2% for FSNP-C, only 5.3% for FSNP-c), which was because cells treated with FSNP-C could produce more cytotoxic 1O2 than those cells treated with FSNP-c. Also, the necrotic cells had a low percentage in the two groups, indicating that the death pathway of HeLa cells treated with FSNPs during PDT was apoptosis rather than necrosis. These results suggested that FSNP-C could be a better nanophotosensitizer for PDT.
Figure 7.
PDT effect of FSNP-C (a) and FSNP-c (b) on HeLa cells. Data are presented as the mean value ± SD (n = 6); (c) apoptosis in HeLa cells detected by fluorescence activated cell sorting (FACS). HeLa cells treated with FSNPs (5 μg mL–1) are irradiated by a LED array (λex = 660 nm, 50 mW/cm2) for 15 min. Percentages of live (PI-/Annexin V-), early apoptotic (PI-/Annexin V+), late apoptotic (PI+/Annexin V+), and necrotic (PI+/Annexin V-) cells are indicated in each quadrant.
PDT Efficacy of FSNPs on 3D Spheroidal Models of HeLa cells
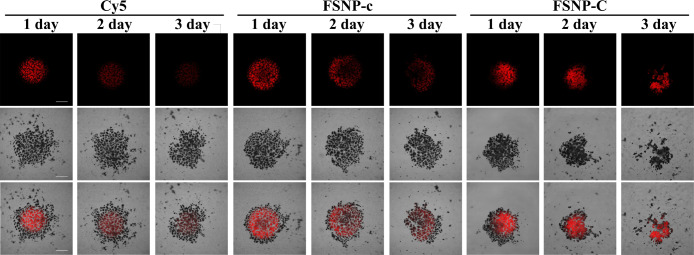
Tumor spheroids established in vitro are considered to be a better model to simulate the situation in vivo than tumor cells in the monolayer.67,68 We evaluated the PDT efficacy of FSNP-C and FSNP-c against 3D spheroidal models of HeLa cells by using Cy5 as a control. As shown by the bright red emission of FSNPs observed under CLSM (Figure 8), FSNPs could effectively enter the tumor spheroids. More importantly, a significant decrease in tumor spheroids’ diameter for the FSNP-C group could be observed after 2 days of PDT, along with the disintegration of the tumor spheroids. However, the tumor spheroids diameter did not decrease obviously for the groups of FSNP-c and Cy5. Meanwhile, we could also observe that the fluorescence of FSNP-C was barely photobleached during PDT. Therefore, FSNP-C with a good photostability and high PDT efficacy was extremely suitable for a long-term fluorescent imaging-guided PDT.
Figure 8.
Photodynamic activity of FSNP-c and FSNP-C against 3D spheroidal models of HeLa cells. 3D tumor spheroids are stained with FSNPs (5 μg mL–1, 4 h of incubation) after 3 days of incubation. PDT experiment of 3D tumor spheroids lasts for 3 days and is irradiated for 15 min day–1 with a LED array (λex = 660 nm, 50 mW/cm2). Scale bars = 200 μm.
Evaluation of Intracellular Anti-ROS Ability of FSNPs during PDT
In the PDT process, ROS produced by self-sensitization of the fluorophores not only cause cytotoxicity, but also potentially cause the fluorophores to undergo photooxidation and lose photosensitization. Meanwhile, the fluorophores may be decomposed by highly reactive species or nucleophiles in biological system, which faces with a false signal problem.69,70 Therefore, an intracellular experiment was designed to further assess he “anti-ROS” ability of FSNPs by monitoring the change in fluorescence intensity of FSNPs via CLSM (Figure 9). To accelerate the progress of the anti-ROS experiment, protoporphyrin IX (PpIX) widely used in PDT was selected as the source of ROS production because it could produce a large amount of 1O2.71−73 After irradiation with a red LED array, we found that FSNP-C still retained more than 90% of the initial fluorescence intensity (Figure 9b), while FSNP-c and Cy5 both had a significant loss of fluorescence intensity due to severe photooxidation (fluorescent intensity of FSNP-c and Cy5 only remained at 62% and 20% of the initial value, respectively). The results showed that FSNP-C could significantly reduce the occurrence rate of the photodegradation reaction during the PDT process, which was difficult to achieve in free cyanine dyes.
Figure 9.
(a) Intracellular anti-ROS experiment of FSNPs. HeLa cells are stained with PpIX (10 μM) and FSNPs (5 μg mL–1). HeLa cells are irradiated for 5 min with a LED array (λex = 660 nm, 50 mW/cm2). The initial fluorescence intensity (before irradiation) is adjusted to almost the same level. Irradiation sources: a standard 635 nm laser of OLYMPUS (laser transmissivity: 10%) is used, and the image detection range is from 655 to 755 nm. Scale bars = 20 μm; (b) plots of relative intracellular fluorescence intensity from recorded fluorescence images (a) as a function of the time show different levels of photobleaching after PDT.
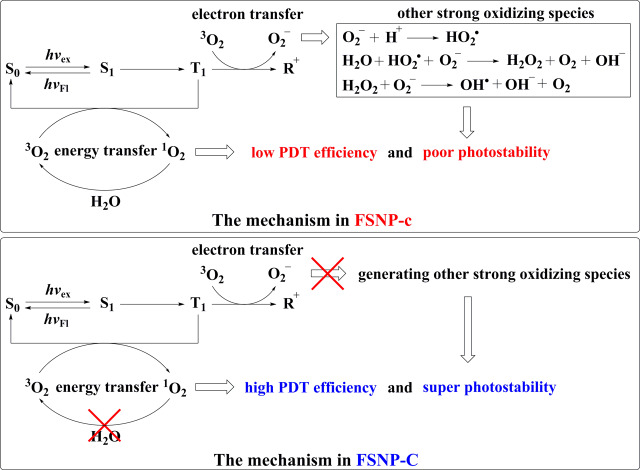
Mechanism of FSNP-C with Super Photostability and High PDT Efficiency in Aqueous Media
The experiments of PDT efficacy and intracellular anti-ROS in living cells had shown that FSNP-C had super photostability and a high PDT effect than that of FSNP-c. Obviously, the hydrophobic cage inside FSNP-C played an extremely important role. As is well-known, the interaction of an excited dye molecule and O2 could form 1O2 and O2– through two possible mechanisms (energy transfer and electron transfer), respectively.59 Therefore, the mechanism of FSNP-C with super photostability and high PDT efficiency in aqueous media is shown in Figure 10. The hydrophobic environment provided by OTES could avoid the penetration of water molecules into FSNP-C and thus inhibit the reaction of water molecules with O2– to generate other strong oxidizing species (•OH and H2O2). As a result, the electron transfer path was suppressed, avoiding further photooxidation by other strong oxidizing species. Meanwhile, 1O2 could not be quenched by water molecules, leading to a higher PDT efficiency. For FSNP-c, however, the electron transfer mechanism was enhanced and further accelerated the photooxidation of dyes due to the inability to effectively inhibit the inward diffusion of water molecules. This was because without a local hydrophobic cage inside FSNP-c, the water-soluble ROS such as H2O2 could be easily diffused inside nanoparticles as water molecules penetrated,74 leading to the occurrence of photooxidation. Besides, FSNP-c obtained a lower PDT efficiency than FSNP-C due to the quenching of 1O2 by water molecules.
Figure 10.
Mechanism of FSNP-C with super photostability and high PDT efficiency in aqueous media. S0: cyanine dyes in the ground state; S1: cyanine dyes in the singlet excited state; T1: cyanine dyes in the triplet excited state; R+: cyanine dyes in the radical cationic state.
Conclusions
In summary, we proposed a collaborative hydrophobic cage and molecular engineering strategy to achieve super bright and super photostable NIR fluorescent silica nanoparticles in aqueous media. This collaborative strategy enabled the prepared FSNP-C to exhibit the longest fluorescence lifetime, the highest fluorescence intensity, and the best photostability in aqueous solution. The excellent optical physical properties of FSNP-C were attributed to the local hydrophobic cage to inhibit the ACQ effect and inward diffusion of water molecules, the larger steric hindrance, and the increasing covalently anchored sites. Therefore, we successfully achieved long-term fluorescence imaging of HeLa cells without serious photobleaching in the physiological aqueous media even after durative irradiation for 300 s. Also, in the application of PDT, FSNP-C showed highly efficient production of 1O2 to kill HeLa cells and tumor spheroids of HeLa cells. In brief, we provided a feasible strategy for organic fluorophores which are susceptible to the ACQ effect and photobleaching to improve their photophysical performances for long-term bright fluorescence imaging and a stable PDT process.
Acknowledgments
This work was supported financially by the National Natural Science Foundation of China (21877011, 21576038, 21421005) and the Talent Fund of Shandong Collaborative Innovation Center of Eco-Chemical Engineering (XTCXYX03).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acscentsci.0c00071.
Reagents, instruments, and methods; TEM images; fluorescence lifetime decays of FSNPs; FTIR of FSNP-A–C; MS and 1H NMR of free dye-a–c; cell culture and staining (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Mitra K.; Lyons C. E.; Hartman M. C. T. A Platinum(II) Complex of Heptamethine Cyanine for Photoenhanced Cytotoxicity and Cellular Imaging in Near-IR Light. Angew. Chem., Int. Ed. 2018, 57, 10263–10267. 10.1002/anie.201806911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas A. P.; Palanikumar L.; Jeena M. T.; Kim K.; Ryu J. Cancer-mitochondria-targeted photodynamic therapy with supramolecular assembly of HA and a water soluble NIR cyanine dye. Chemical Science 2017, 8, 8351–8356. 10.1039/C7SC03169F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X.; Yang G.; Zhang L.; Liu Z.; Cheng Z.; Zhu X. Photosensitizer cross-linked nano-micelle platform for multimodal imaging guided synergistic photothermal/photodynamic therapy. Nanoscale 2016, 8, 15323–15339. 10.1039/C6NR04835H. [DOI] [PubMed] [Google Scholar]
- Zhang K.; Zhang J.; Xi Z.; Li L.; Gu X.; Zhang Q.; Yi L. A new H2S-specific near-infrared fluorescence-enhanced probe that can visualize the H2S level in colorectal cancer cells in mice. Chemical Science 2017, 8, 2776–2781. 10.1039/C6SC05646F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K.; Zhang Y.; Wang J.; Yuan A.; Sun M.; Wu J.; Hu Y., Self-assembled IR780-loaded transferrin nanoparticles as an imaging, targeting and PDT/PTT agent for cancer therapy. Sci. Rep. 2016, 6. [DOI] [PMC free article] [PubMed]
- Porcu E. P.; Salis A.; Gavini E.; Rassu G.; Maestri M.; Giunchedi P. Indocyanine green delivery systems for tumour detection and treatments. Biotechnol. Adv. 2016, 34, 768–789. 10.1016/j.biotechadv.2016.04.001. [DOI] [PubMed] [Google Scholar]
- Noh I.; Lee D.; Kim H.; Jeong C.; Lee Y.; Ahn J.; Hyun H.; Park J.; Kim Y. Enhanced Photodynamic Cancer Treatment by Mitochondria-Targeting and Brominated Near-Infrared Fluorophores. Advanced Science 2018, 5, 1700481. 10.1002/advs.201700481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J.; Weinfurter S.; Daniele C.; Perciaccante R.; Federica R.; Della Ciana L.; Pill J.; Gretz N. Zwitterionic near infrared fluorescent agents for noninvasive real-time transcutaneous assessment of kidney function. Chemical Science 2017, 8, 2652–2660. 10.1039/C6SC05059J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao M.; Yu F.; Lv C.; Choo J.; Chen L. Fluorescent chemical probes for accurate tumor diagnosis and targeting therapy. Chem. Soc. Rev. 2017, 46, 2237–2271. 10.1039/C6CS00908E. [DOI] [PubMed] [Google Scholar]
- Sun W.; Guo S.; Hu C.; Fan J.; Peng X. Recent Development of Chemosensors Based on Cyanine Platforms. Chem. Rev. 2016, 116, 7768–7817. 10.1021/acs.chemrev.6b00001. [DOI] [PubMed] [Google Scholar]
- Wang S.; Fan Y.; Li D.; Sun C.; Lei Z.; Lu L.; Wang T.; Zhang F., Anti-quenching NIR-II molecular fluorophores for in vivo high-contrast imaging and pH sensing. Nat. Commun. 2019, 10. 10.1038/s41467-019-09043-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dearman H. H.; Birks J. B. Photophysics of Aromatic Molecules. Am. Scientist 1971, 59, 747. [Google Scholar]
- Berberan-Santos M. N.; Valeur B., Molecular Fluorescence: Principles and Application. 2001. [Google Scholar]
- Ding D.; Li K.; Liu B.; Tang B. Z. Bioprobes Based on AIE Fluorogens. Acc. Chem. Res. 2013, 46, 2441–2453. 10.1021/ar3003464. [DOI] [PubMed] [Google Scholar]
- Wang S.; Fan Y.; Li D.; Sun C.; Lei Z.; Lu L.; Wang T.; Zhang F., Anti-quenching NIR-II molecular fluorophores for in vivo high-contrast imaging and pH sensing. Nat. Commun. 2019, 10. 10.1038/s41467-019-09043-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Méallet-Renault R.; Hérault A.; Vachon J.; Pansu R. B.; Amigoni-Gerbier S.; Larpent C. Fluorescent nanoparticles as selective Cu(II) sensors. Photochem. Photobiol. Sci. 2006, 5, 300–310. 10.1039/B513215K. [DOI] [PubMed] [Google Scholar]
- Trofymchuk K.; Reisch A.; Shulov I.; Mely Y.; Klymchenko A. S. Tuning the color and photostability of perylene diimides inside polymer nanoparticles: towards biodegradable substitutes of quantum dots. Nanoscale 2014, 6, 12934–12942. 10.1039/C4NR03718A. [DOI] [PubMed] [Google Scholar]
- Huang S.; Wang K.; Wang S.; Wang Y.; Wang M. Highly Fluorescent Polycaprolactones with Tunable Light Emission Wavelengths across Visible to NIR Spectral Window. Adv. Mater. Interfaces 2016, 3, 1600259. 10.1002/admi.201600259. [DOI] [Google Scholar]
- Andreiuk B.; Reisch A.; Bernhardt E.; Klymchenko A. S. Fighting Aggregation-Caused Quenching and Leakage of Dyes in Fluorescent Polymer Nanoparticles: Universal Role of Counterion. Chem. - Asian J. 2019, 14, 836–846. 10.1002/asia.201801592. [DOI] [PubMed] [Google Scholar]
- Gorka A. P.; Nani R. R.; Schnermann M. J. Cyanine polyene reactivity: scope and biomedical applications. Org. Biomol. Chem. 2015, 13, 7584–7598. 10.1039/C5OB00788G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nani R. R.; Kelley J. A.; Ivanic J.; Schnermann M. J. Reactive species involved in the regioselective photooxidation of heptamethine cyanines. Chemical Science 2015, 6, 6556–6563. 10.1039/C5SC02396C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Q.; Lavis L. D. Development of photostable fluorophores for molecular imaging. Curr. Opin. Chem. Biol. 2017, 39, 32–38. 10.1016/j.cbpa.2017.04.017. [DOI] [PubMed] [Google Scholar]
- Engel E.; Schraml R.; Maisch T.; Kobuch K.; König B.; Szeimies R.; Hillenkamp J.; Bäumler W.; Vasold R. Light-induced decomposition of indocyanine green. Invest. Ophthalmol. Visual Sci. 2008, 49, 1777–1783. 10.1167/iovs.07-0911. [DOI] [PubMed] [Google Scholar]
- Gorka A. P.; Nani R. R.; Zhu J.; Mackem S.; Schnermann M. J. A Near-IR Uncaging Strategy Based on Cyanine Photochemistry. J. Am. Chem. Soc. 2014, 136, 14153–14159. 10.1021/ja5065203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renikuntla B. R.; Rose H. C.; Eldo J.; Waggoner A. S.; Armitage B. A. Improved Photostability and Fluorescence Properties through Polyfluorination of a Cyanine Dye. Org. Lett. 2004, 6, 909–912. 10.1021/ol036081w. [DOI] [PubMed] [Google Scholar]
- Toutchkine A.; Nguyen D. V.; Hahn K. M. Merocyanine dyes with improved photostability. Org. Lett. 2007, 9, 2775–2777. 10.1021/ol070780h. [DOI] [PubMed] [Google Scholar]
- Redy-Keisar O.; Huth K.; Vogel U.; Lepenies B.; Seeberger P. H.; Haag R.; Shabat D. Enhancement of fluorescent properties of near-infrared dyes using clickable oligoglycerol dendrons. Org. Biomol. Chem. 2015, 13, 4727–4732. 10.1039/C5OB00299K. [DOI] [PubMed] [Google Scholar]
- Song F.; Peng X.; Lu E.; Zhang R.; Chen X.; Song B. Syntheses, spectral properties and photostabilities of novel water-soluble near-infrared cyanine dyes. J. Photochem. Photobiol., A 2004, 168, 53–57. 10.1016/j.jphotochem.2004.05.012. [DOI] [Google Scholar]
- Zheng Q.; Jockusch S.; Zhou Z.; Blanchard S. C. The Contribution of Reactive Oxygen Species to the Photobleaching of Organic Fluorophores. Photochem. Photobiol. 2014, 90, 448–454. 10.1111/php.12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman R. B.; Terry D. S.; Zhou Z.; Zheng Q.; Geggier P.; Kolster R. A.; Zhao Y.; Javitch J. A.; Warren J. D.; Blanchard S. C. Cyanine fluorophore derivatives with enhanced photostability. Nat. Methods 2012, 9, 68–71. 10.1038/nmeth.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H.; Li Y.; Tang B. Z.; Yoon J. Assembly strategies of organic-based imaging agents for fluorescence and photoacoustic bioimaging applications. Chem. Soc. Rev. 2020, 49, 21–31. 10.1039/C9CS00326F. [DOI] [PubMed] [Google Scholar]
- Li X.; Yu S.; Lee D.; Kim G.; Lee B.; Cho Y.; Zheng B. Y.; Ke M. R.; Huang J. D.; Nam K. T.; et al. Facile Supramolecular Approach to Nucleic-Acid-Driven Activatable Nanotheranostics That Overcome Drawbacks of Photodynamic Therapy. ACS Nano 2018, 12, 681–688. 10.1021/acsnano.7b07809. [DOI] [PubMed] [Google Scholar]
- Li X.; Yu S.; Lee Y.; Guo T.; Kwon N.; Lee D.; Yeom S. C.; Cho Y.; Kim G.; Huang J.; et al. In Vivo Albumin Traps Photosensitizer Monomers from Self-Assembled Phthalocyanine Nanovesicles: A Facile and Switchable Theranostic Approach. J. Am. Chem. Soc. 2019, 141, 1366–1372. 10.1021/jacs.8b12167. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Bhattarai P.; Dai Z.; Chen X. Photothermal therapy and photoacoustic imaging via nanotheranostics in fighting cancer. Chem. Soc. Rev. 2019, 48, 2053–2108. 10.1039/C8CS00618K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P.; Wang J.; Chen H.; Zhao L.; Chen B.; Chu C.; Liu H.; Qin Z.; Liu J.; Tan Y.; et al. Tumor Microenvironment-Responsive Ultrasmall Nanodrug Generators with Enhanced Tumor Delivery and Penetration. J. Am. Chem. Soc. 2018, 140, 14980–14989. 10.1021/jacs.8b09396. [DOI] [PubMed] [Google Scholar]
- Li S.; Zou Q.; Li Y.; Yuan C.; Xing R.; Yan X. Smart Peptide-Based Supramolecular Photodynamic Metallo-Nanodrugs Designed by Multicomponent Coordination Self-Assembly. J. Am. Chem. Soc. 2018, 140, 10794–10802. 10.1021/jacs.8b04912. [DOI] [PubMed] [Google Scholar]
- Li L.; Wang W.; Tang J.; Wang Y.; Liu J.; Huang L.; Wang Y.; Guo F.; Wang J.; Shen W.; Belfiore L. A., Classification, Synthesis, and Application of Luminescent Silica Nanoparticles: a Review. Nanoscale Res. Lett. 2019, 14. 10.1186/s11671-019-3006-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L.; Song J.; Yang H.; Chen X. Yolk-Shell Nanostructures: Design, Synthesis, and Biomedical Applications. Adv. Mater. 2018, 30, 1704639. 10.1002/adma.201704639. [DOI] [PubMed] [Google Scholar]
- Nakamura M.; Shono M.; Ishimura K. Synthesis, Characterization, and Biological Applications of Multifluorescent Silica Nanoparticles. Anal. Chem. 2007, 79, 6507–6514. 10.1021/ac070394d. [DOI] [PubMed] [Google Scholar]
- Rossi L. M.; Shi L.; Quina F. H.; Rosenzweig Z. Stöber Synthesis of Monodispersed Luminescent Silica Nanoparticles for Bioanalytical Assays. Langmuir 2005, 21, 4277–4280. 10.1021/la0504098. [DOI] [PubMed] [Google Scholar]
- Lu X.; Hou Y.; Zha J.; Xin Z. Facile Synthesis of Rhodamine B-Doped Poly(3-mercaptopropylsilsesquioxane) Fluorescent Microspheres with Controllable Size. Ind. Eng. Chem. Res. 2013, 52, 5880–5886. 10.1021/ie302556t. [DOI] [Google Scholar]
- Santra S.; Zhang P.; Wang K.; Tapec R.; Tan W. Conjugation of biomolecules with luminophore-doped silica nanoparticles for photostable biomarkers. Anal. Chem. 2001, 73, 4988–4993. 10.1021/ac010406+. [DOI] [PubMed] [Google Scholar]
- Kabanov V.; Press D. J.; Huynh R. P. S.; Shimizu G. K. H.; Heyne B. Assessment of encapsulated dyes’ distribution in silica nanoparticles and their ability to release useful singlet oxygen. Chem. Commun. 2018, 54, 6320–6323. 10.1039/C8CC03413C. [DOI] [PubMed] [Google Scholar]
- Zhelev Z.; Ohba H.; Bakalova R. Single Quantum Dot-Micelles Coated with Silica Shell as Potentially Non-Cytotoxic Fluorescent Cell Tracers. J. Am. Chem. Soc. 2006, 128, 6324–6325. 10.1021/ja061137d. [DOI] [PubMed] [Google Scholar]
- Sun Y.; Guo Z. Recent advances of bioinspired functional materials with specific wettability: from nature and beyond nature. Nanoscale Horizons 2019, 4, 52–76. 10.1039/C8NH00223A. [DOI] [PubMed] [Google Scholar]
- Huang H.; Liu H.; Hsu P.; Chiang C.; Tsai C.; Chi H.; Chen S.; Chen Y. A Multitheragnostic Nanobubble System to Induce Blood-Brain Barrier Disruption with Magnetically Guided Focused Ultrasound. Adv. Mater. 2015, 27, 655–661. 10.1002/adma.201403889. [DOI] [PubMed] [Google Scholar]
- Zheng M.; Lu Y.; Dong L.; Guo P.; Deng Q.; Li W.; Feng Y.; Huang F. Immobilization of Candida rugosa lipase on hydrophobic/strong cation-exchange functional silica particles for biocatalytic synthesis of phytosterol esters. Bioresour. Technol. 2012, 115, 141–146. 10.1016/j.biortech.2011.11.128. [DOI] [PubMed] [Google Scholar]
- Jiao L.; Song F.; Zhang B.; Ning H.; Cui J.; Peng X. Improving the brightness and photostability of NIR fluorescent silica nanoparticles through rational fine-tuning of the covalent encapsulation methods. J. Mater. Chem. B 2017, 5, 5278–5283. 10.1039/C7TB00856B. [DOI] [PubMed] [Google Scholar]
- Peng X.; Song F.; Lu E.; Wang Y.; Zhou W.; Fan J.; Gao Y. Heptamethine Cyanine Dyes with a Large Stokes Shift and Strong Fluorescence: A Paradigm for Excited-State Intramolecular Charge Transfer. J. Am. Chem. Soc. 2005, 127, 4170–4171. 10.1021/ja043413z. [DOI] [PubMed] [Google Scholar]
- Jiao L.; Song F.; Zhang B.; Ning H.; Cui J.; Peng X. Improving the brightness and photostability of NIR fluorescent silica nanoparticles through rational fine-tuning of the covalent encapsulation methods. J. Mater. Chem. B 2017, 5, 5278–5283. 10.1039/C7TB00856B. [DOI] [PubMed] [Google Scholar]
- Steinmark I. E.; James A. L.; Chung P.; Morton P. E.; Parsons M.; Dreiss C. A.; Lorenz C. D.; Yahioglu G.; Suhling K. Targeted fluorescence lifetime probes reveal responsive organelle viscosity and membrane fluidity. PLoS One 2019, 14, e0211165 10.1371/journal.pone.0211165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabanov V.; Press D. J.; Huynh R. P. S.; Shimizu G. K. H.; Heyne B. Assessment of encapsulated dyes’ distribution in silica nanoparticles and their ability to release useful singlet oxygen. Chem. Commun. 2018, 54, 6320–6323. 10.1039/C8CC03413C. [DOI] [PubMed] [Google Scholar]
- Shiju N. R.; Alberts A. H.; Khalid S.; Brown D. R.; Rothenberg G. Mesoporous Silica with Site-Isolated Amine and Phosphotungstic Acid Groups: A Solid Catalyst with Tunable Antagonistic Functions for One-Pot Tandem Reactions. Angew. Chem., Int. Ed. 2011, 50, 9615–9619. 10.1002/anie.201101449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorka A. P.; Nani R. R.; Schnermann M. J. Cyanine polyene reactivity: scope and biomedical applications. Org. Biomol. Chem. 2015, 13, 7584–7598. 10.1039/C5OB00788G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Q.; Lavis L. D. Development of photostable fluorophores for molecular imaging. Curr. Opin. Chem. Biol. 2017, 39, 32–38. 10.1016/j.cbpa.2017.04.017. [DOI] [PubMed] [Google Scholar]
- Nani R. R.; Kelley J. A.; Ivanic J.; Schnermann M. J. Reactive species involved in the regioselective photooxidation of heptamethine cyanines. Chemical Science 2015, 6, 6556–6563. 10.1039/C5SC02396C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou W.; Wang C.; Wang G.; Ma Q.; Su X. Enhance Effect of Surfactants on the Photoluminescence and Photostability of Water-Soluble Poly(phenylene ethynylene). J. Phys. Chem. B 2008, 112, 12681–12685. 10.1021/jp805345d. [DOI] [PubMed] [Google Scholar]
- Trofymchuk K.; Reisch A.; Shulov I.; Mely Y.; Klymchenko A. S. Tuning the color and photostability of perylene diimides inside polymer nanoparticles: towards biodegradable substitutes of quantum dots. Nanoscale 2014, 6, 12934–12942. 10.1039/C4NR03718A. [DOI] [PubMed] [Google Scholar]
- Chen C.; Zhou B.; Lu D.; Xu G. Electron transfer events in solutions of cyanine dyes. J. Photochem. Photobiol., A 1995, 89, 25–29. 10.1016/1010-6030(94)04024-V. [DOI] [Google Scholar]
- Zou J.; Yin Z.; Wang P.; Chen D.; Shao J.; Zhang Q.; Sun L.; Huang W.; Dong X. Photosensitizer synergistic effects: D-A-D structured organic molecule with enhanced fluorescence and singlet oxygen quantum yield for photodynamic therapy. Chemical Science 2018, 9, 2188–2194. 10.1039/C7SC04694D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva É. R.; Pavanelli A. L. S.; Mostaço L. B.; Schaberle F. A.; Galembeck S. E.; Gonçalves P. J.; Costa E Silva R.; Ferreira L. P.; Nekipelova T. D.; Kostyukov A. A.; Radchenko A. S.; Shtil A. A.; Kuzmin V. A.; Borissevitch I. E. Phototransformation of cyanine dye with two chromophores. Effects of oxygen and dye concentration. J. Photochem. Photobiol., A 2017, 349, 42–48. 10.1016/j.jphotochem.2017.08.063. [DOI] [Google Scholar]
- Bonacin J. A.; Engelmann F. M.; Severino D.; Toma H. E.; Baptista M. S. Singlet oxygen quantum yields (ΦΔ) in water using beetroot extract and an array of LEDs. J. Braz. Chem. Soc. 2009, 20, 31–36. 10.1590/S0103-50532009000100006. [DOI] [Google Scholar]
- Shi C.; Wu J. B.; Pan D. Review on near-infrared heptamethine cyanine dyes as theranostic agents for tumor imaging, targeting, and photodynamic therapy. J. Biomed. Opt. 2016, 21, 050901. 10.1117/1.JBO.21.5.050901. [DOI] [PubMed] [Google Scholar]
- Xu C.; Wang Y.; Yu H.; Tian H.; Chen X. Multifunctional Theranostic Nanoparticles Derived from Fruit-Extracted Anthocyanins with Dynamic Disassembly and Elimination Abilities. ACS Nano 2018, 12, 8255–8265. 10.1021/acsnano.8b03525. [DOI] [PubMed] [Google Scholar]
- Fang J.; Nakamura H.; Maeda H. The EPR effect: Unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv. Drug Delivery Rev. 2011, 63, 136–151. 10.1016/j.addr.2010.04.009. [DOI] [PubMed] [Google Scholar]
- Danhier F. To exploit the tumor microenvironment: Since the EPR effect fails in the clinic, what is the future of nanomedicine?. J. Controlled Release 2016, 244, 108–121. 10.1016/j.jconrel.2016.11.015. [DOI] [PubMed] [Google Scholar]
- Mueller-Klieser W. Three-dimensional cell cultures: from molecular mechanisms to clinical applications. American journal of physiology 1997, 273, C1109. 10.1152/ajpcell.1997.273.4.C1109. [DOI] [PubMed] [Google Scholar]
- Andersson T. B. Evolution of Novel 3D Culture Systems for Studies of Human Liver Function and Assessments of the Hepatotoxicity of Drugs and Drug Candidates. Basic Clin. Pharmacol. Toxicol. 2017, 121, 234–238. 10.1111/bcpt.12804. [DOI] [PubMed] [Google Scholar]
- Cheng D.; Peng J.; Lv Y.; Su D.; Liu D.; Chen M.; Yuan L.; Zhang X. De Novo Design of Chemical Stability Near-Infrared Molecular Probes for High-Fidelity Hepatotoxicity Evaluation In Vivo. J. Am. Chem. Soc. 2019, 141, 6352–6361. 10.1021/jacs.9b01374. [DOI] [PubMed] [Google Scholar]
- Peng J.; Samanta A.; Zeng X.; Han S.; Wang L.; Su D.; Loong D. T. B.; Kang N.; Park S.; All A. H.; Jiang W.; Yuan L.; Liu X.; Chang Y. Real-Time In Vivo Hepatotoxicity Monitoring through Chromophore-Conjugated Photon-Upconverting Nanoprobes. Angew. Chem., Int. Ed. 2017, 56, 4165–4169. 10.1002/anie.201612020. [DOI] [PubMed] [Google Scholar]
- Abrahamse H.; Hamblin M. R. New photosensitizers for photodynamic therapy. Biochem. J. 2016, 473, 347–364. 10.1042/BJ20150942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi L. M.; Silva P. R.; Vono L. L. R.; Fernandes A. U.; Tada D. B.; Baptista M. S. Protoporphyrin IX nanoparticle carrier: preparation, optical properties, and singlet oxygen generation. Langmuir 2008, 24, 12534–12538. 10.1021/la800840k. [DOI] [PubMed] [Google Scholar]
- Jiao L.; Zhang X.; Cui J.; Peng X.; Song F. Three-in-One Functional Silica Nanocarrier with Singlet Oxygen Generation, Storage/Release, and Self-Monitoring for Enhanced Fractional Photodynamic Therapy. ACS Appl. Mater. Interfaces 2019, 11, 25750–25757. 10.1021/acsami.9b08371. [DOI] [PubMed] [Google Scholar]
- Jin Z.; Wang L.; Zuidema E.; Mondal K.; Zhang M.; Zhang J.; Wang C.; Meng X.; Yang H.; Mesters C.; et al. Hydrophobic zeolite modification for in situ peroxide formation in methane oxidation to methanol. Science 2020, 367, 193. 10.1126/science.aaw1108. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.