Photodynamic therapy (PDT) is a rapidly evolving cancer treatment modality, wherein a light-activatable molecule (photosensitizer) is used in conjunction with light to locally produce reactive oxygen species (ROS). While PDT provides high spatiotemporal precision at the location to which the ROS-induced damage is inflicted, poor light tissue penetration (<1 cm) has been a major hurdle for PDT clinical implementation in the context of deep-seated tumors.1 A significant amount of effort has been devoted toward the development of photosensitizing materials that would be able to overcome the challenges imposed by such limited light penetration. These include the development of chemi- and bioluminescent probes as well as multi-photon and upconverting materials. On the other hand, external ionizing radiation (such as X-rays) penetrates deep into the human body and is widely used as a neoadjuvant treatment for shrinking nonresectable tumors. Given this widespread X-ray implementation in oncology, the possibility of utilizing it for deep tissue photosensitizer activation is highly enticing.2 In this issue of ACS Central Science, Deng and colleagues described novel mitochondria-targeted PLGA-based nanoconstructs (PLGA-TPP) containing ultrasmall gold nanoparticles and the clinically approved photosensitizer (verteporfin) for X-ray-induced PDT treatment of colorectal cancer (Figure 1A).3
Figure 1.
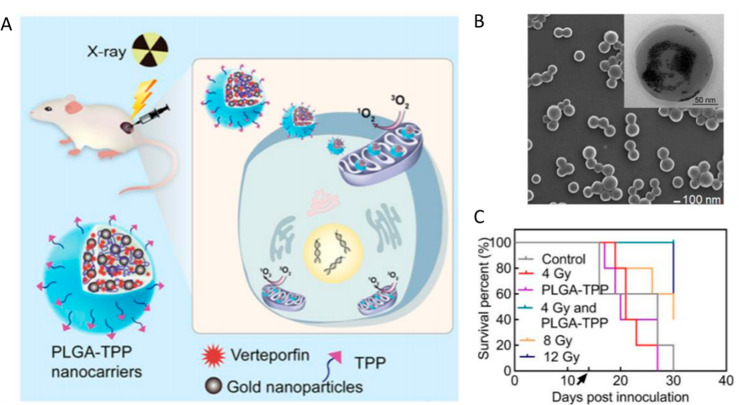
(A) Schematic illustration of X-ray-induced PDT with PLGA-TPP nanocarriers consisting of PLGA, verteporfin (VP), gold nanoparticles, and a mitochondria-targeting ligand. (B) Scanning electron microphotograph of the PLGA nanocarriers, inset is transmission electron microphotograph of the same sample at higher magnification. (C) A Kaplan–Meier curve demonstrating the extended survival in mice with colorectal tumors treated with X-ray-induced PDT and PLGA-TPP nanoconstructs (4Gy and PLGA-TPP). Black arrow indicates the time of treatment administration. Reproduced from ref (3). Copyright 2020, American Chemical Society.
The subject of X-ray-induced PDT (X-PDT) is incredibly complex and multifaceted from biological, physical, and material design standpoints. In order to enable X-ray-induced photosensitizer activation, a variety of scintillating materials—mainly rare earth metal-doped nanoparticles—have been explored in combination with photosensitizers. Nanoscintillators are thought to locally convert X-rays into visible light, serving as the in situ excitation source for colocalized photosensitizers.4 The combination of X-ray-induced ROS generation (mainly hydroxyl anions and peroxide) with photodynamic activation (often resulting in the singlet oxygen generation) initiates a variety of cytotoxic pathways, resulting in cell death at a lower total radiation dose compared with X-ray radiation alone. In the current study reported by Deng and co-authors, combining PLGA-TPP together with subtherapeutic (4 Gy) radiation inhibited tumor growth equivalent to a therapeutic (12 Gy) radiation dose (Figure 1C). Such a reduction in dose helped alleviate radiation-associated weight loss, which makes this study one of a handful that demonstrate the therapeutic potential of X-PDT in vivo.
Biologically, PDT and X-rays can trigger separate but complementary cytotoxic pathways. In practice, however, the application of X-PDT relies on a complex set of principal components, in which the photosensitizer class, subcellular localization, as well as radiation dosimetry, can result in either synergistic, additive, or antagonizing effects.5,6 It is generally accepted that mitochondrial photosensitizer localization results in more effective cell killing than lysosomal localization. In this study, a mitochondrial-targeting moiety triphenylphosphonium (TPP) was introduced to impart mitochondrial localization resulting in additional cytotoxicity compared to its nontargeted counterpart (Figure 1A). The authors validated the targeted effects of PLGA-TPP by demonstrating a decrease in the structural integrity of the mitochondrial membrane.
From a biocompatibility point of view, many X-PDT agents contain cytotoxic components that increase their long-term off-target toxicity and reduce their potential for clinical translation.7,8 Deng et al., on the other hand, utilized widely available and extensively characterized components, such as biodegradable polymer poly(lactic-co-glycolic acid), 2–5 nm gold nanoparticles, and a commonly used photosensitizer verteporfin (Figure 1B). All of these nanoparticle components are approved by the U.S. Food and Drug Administration, which opens the door for further clinical translation.
While the PLGA-TPP particles reported by Deng and colleagues have demonstrated promising therapeutic efficacy in the preclinical investigation, many questions regarding the mechanism of X-ray-induced photodynamic activation for these nanoconstructs remain open. For example, it is unclear why there was no correlation between the different gold and verteporfin molar ratios in relation to the X-ray-induced ROS generation, given that both gold nanoparticles and verteporfin are known radiosensitizers.9,10 Further studies are necessary to distinguish purely radiosensitizing effects from cathodoluminescence-induced verteporfin activation. Specifically, it is essential to establish a correlation between the nanoscintillator and photosensitizer stoichiometry, X-ray exposure, and ROS generation, as well as to characterize the role of the scintillator-photosensitizer nanostructure in the context of energy transfer. Ultimately, a set of rational guidelines will aid the field in the development of the next-generation X-PDT agents.
The use of ionizing radiation as a means for photosensitizer activation holds immense clinical promise, and the current study opens multiple avenues for the subsequent mechanistic and translational pursuits. From a clinical perspective, decreasing high radiation doses required to shrink tumors prior to surgery will provide a clear benefit to patients by alleviating radiation-induced adverse effects. To accelerate clinical translation of X-PDT nanoconstructs, there is a strong rationale for further exploration of various biocompatible materials, focusing not only on maximizing X-ray-induced photodynamic capabilities, but optimizing pharmacokinetics and biodistribution profiles.
References
- Mallidi S.; Anbil S.; Bulin A. L.; Obaid G.; Ichikawa M.; Hasan T. Beyond the Barriers of Light Penetration: Strategies, Perspectives and Possibilities for Photodynamic Therapy. Theranostics 2016, 6 (13), 2458–2487. 10.7150/thno.16183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogue B. W.; Wilson B. C. Optical and X-ray technology synergies enabling diagnostic and therapeutic applications in medicine. J. Biomed. Opt. 2018, 23 (12), 1–17. 10.1117/1.JBO.23.12.121610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKelvey K.; Guller A.; Fayzullin A.; Campbell J.; Clement S.; Habibalahi A.; Wargocka Z.; Liang L.; Shen C.; Howell V. M.; Engel A.; Goldys E.; Deng W.. Application of mitochondrially targeted nanoconstructs to neo-adjuvant X-ray-induced photodynamic therapy for rectal cancer. ACS Central Science, ACS Cent. Sci. 2020, 10.1021/acscentsci.9b01121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamkaew A.; Chen F.; Zhan Y.; Majewski R. L.; Cai W. Scintillating Nanoparticles as Energy Mediators for Enhanced Photodynamic Therapy. ACS Nano 2016, 10 (4), 3918–35. 10.1021/acsnano.6b01401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg K.; Luksiene Z.; Moan J.; Ma L. Combined treatment of ionizing radiation and photosensitization by 5-aminolevulinic acid-induced protoporphyrin IX. Radiat. Res. 1995, 142 (3), 340–6. 10.2307/3579143. [DOI] [PubMed] [Google Scholar]
- Luksiene Z.; Kalvelyte A.; Supino R. On the combination of photodynamic therapy with ionizing radiation. J. Photochem. Photobiol., B 1999, 52 (1–3), 35–42. 10.1016/S1011-1344(99)00098-6. [DOI] [PubMed] [Google Scholar]
- Tian J.; Zeng X.; Xie X.; Han S.; Liew O. W.; Chen Y. T.; Wang L.; Liu X. Intracellular Adenosine Triphosphate Deprivation through Lanthanide-Doped Nanoparticles. J. Am. Chem. Soc. 2015, 137 (20), 6550–8. 10.1021/jacs.5b00981. [DOI] [PubMed] [Google Scholar]
- Liu C.; Hou Y.; Gao M. Are rare-earth nanoparticles suitable for in vivo applications?. Adv. Mater. 2014, 26 (40), 6922–32. 10.1002/adma.201305535. [DOI] [PubMed] [Google Scholar]
- Shah S. R.; Kim J.; Schiapparelli P.; Vazquez-Ramos C. A.; Martinez-Gutierrez J. C.; Ruiz-Valls A.; Inman K.; Shamul J. G.; Green J. J.; Quinones-Hinojosa A. Verteporfin-Loaded Polymeric Microparticles for Intratumoral Treatment of Brain Cancer. Mol. Pharmaceutics 2019, 16 (4), 1433–1443. 10.1021/acs.molpharmaceut.8b00959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.; Huang F.; Ren C.; Liu J.; Yang L.; Chen S.; Chang J.; Yang C.; Wang W.; Zhang C.; Liu Q.; Liang X. J.; Liu J. Enhanced Radiosensitization by Gold Nanoparticles with Acid-Triggered Aggregation in Cancer Radiotherapy. Adv. Sci. 2019, 6 (8), 1801–806. 10.1002/advs.201970050. [DOI] [PMC free article] [PubMed] [Google Scholar]