Abstract
Purpose:
To identify whether financial incentives promote improved disease management in Medicaid recipients diagnosed with hypertension or diabetes, respectively.
Design:
Four-group, multicenter, randomized clinical trials.
Setting and Participants:
Between 2013 and 2016, New York State Medicaid managed care members diagnosed with hypertension (N = 920) or with diabetes (N = 959).
Intervention:
Participants in each six-month trial were randomly assigned to one of four arms: 1) process incentives – earned by attending primary care visits and/or receiving prescription medication refills; 2) outcome incentives – earned by reducing systolic blood pressure (hypertension) or HbA1c (diabetes) levels; 3) combined process and outcome incentives; 4) control (no incentives).
Measures:
Systolic blood pressure (hypertension) and HbA1c (diabetes) levels; primary care visits; medication prescription refills.
Analysis and Results:
At six months, there were no statistically significant differences between intervention arms and the control arm in the change in systolic blood pressure, p = .531. Similarly, there were no significant differences in blood glucose control (HbA1c) between the intervention arms and control after six months, p = .939. The majority of participants had acceptable systolic blood pressure (<140 mmHg) or blood glucose (< 8.0%) levels at baseline and throughout the study.
Conclusion:
Financial incentives—regardless of whether they were delivered based on disease-relevant outcomes, process activities, or a combination of the two—have a negligible impact on health outcomes for Medicaid recipients diagnosed with either hypertension or diabetes in two studies in which, among other design and operational limitations, the majority of recipients had relatively well-controlled diseases at the time of enrollment.
Purpose
Roughly half of all American adults have at least one of 10 major chronic conditions (hypertension, coronary heart disease, stroke, diabetes, cancer, arthritis, hepatitis, weak or failing kidneys, asthma, or COPD), and approximately one in four Americans have multiple chronic conditions.1 Costs related to chronic disease accounted for 86% of all health care spending in 2010.2
Proper adherence to a treatment regimen could reduce the likelihood of adverse consequences and the cost of these preventable diseases,3 but treatment adherence has been identified as a major challenge among those with chronic diseases.4 Medicaid beneficiaries have higher rates of chronic diseases than those with private or employer based insurance.5 In this project, we test alternative strategies to improve control for two common chronic diseases in which treatment adherence is important: hypertension6 and diabetes.7 Medication adherence helps hypertensive patients to control their blood pressure, which in turn reduces the risk of stroke and heart attack. With adequate treatment compliance, the vast majority (approximately 92%) of patients would be able to control their blood pressure.3 For diabetics, improved adherence to drug therapy is consistently linked to better glycemic control and fewer hospitalizations.7 Interventions such as the diabetes self-management education program, which aims to improve diabetes monitoring and treatment adherence, have successfully reduced diabetes complications by reducing cholesterol and HbA1c levels.8
Financial incentives can increase patient motivation and help to overcome patient barriers to adherence,9 yet the relative effectiveness of incentivizing based on process (e.g., medication adherence) vs. outcomes (e.g., improvement in blood pressure) among patients with chronic diseases is unknown. To address this question, we conducted two randomized trials that tested incentives for processes, outcomes, or a combination of the two among Medicaid recipients. The projects were funded by the Centers for Medicare and Medicaid Services (CMS) Medicaid Incentives for Prevention of Chronic Disease (MIPCD) initiative and implemented by the New York Department of Health and Medicaid managed care organizations (MCOs); in New York, MCOs serve the majority of the state’s 6 million Medicaid recipients. The design of these incentive interventions as randomized clinical trials is consistent with the rigorous evaluations used in other New York-based studies10 and in nine other states to test the impact of incentives for outcomes such as tobacco cessation, diabetes prevention, and weight loss.11
Both programs included primary care visits and prescription medication, both of which were covered Medicaid benefits. Some participating enrollees in each study were randomly assigned to become eligible to receive incentives through a tiered incentive program. We first describe the methods used in both studies, and then discuss the results for each study in turn. Study 1 recruited New York Medicaid managed care enrollees who had been diagnosed with hypertension, and Study 2 recruited enrollees with diabetes.
Methods
Design
For each group, we conducted a four-arm randomized controlled trial (RCT) comparing three incentive-based programs aimed at improving chronic condition management. These programs consisted of information regarding how to lower one’s blood pressure or blood glucose levels, respectively, as well as incentives for achieving reduced blood pressure/glucose levels or for attending doctor visits and refilling medication prescriptions. Each of these services was covered under the participant’s benefits at no cost to participants.
Sample
Potential participants were deemed eligible if they were currently enrolled in Medicaid, were between 18–64 years of age, and had been diagnosed with hypertension for the hypertension management program or with Type 2 diabetes for the diabetes management program. Initial recruitment procedures targeted only those with poorly controlled hypertension (systolic blood pressure > 140 mmHg) or poorly controlled diabetes (HbA1c > 8%), but logistical challenges in identifying participants based on these criteria led to a change in recruitment procedures to allow all diagnosed Medicaid patients of the proper age to enroll. The hypertension study enrolled 920 participants, and the diabetes study enrolled 959 participants. The baseline demographic characteristics are provided in Tables 1 and 2, and the flow of participants throughout each study is in the supplemental appendix materials.
Table 1.
Demographics of participants in hypertension management program.
| Total (N = 920) |
Control (n = 220) |
Process Incentives (n = 245) |
Outcome Incentives (n = 223) |
Combined Incentives (n = 232) |
Omnibus p-value |
|
|---|---|---|---|---|---|---|
| Age, Mean (SD) | 54.0 (8.6) | 54.5 (8.5) | 54.0 (8.8) | 53.7 (8.5) | 54.1 (8.5) | 0.801 |
| Female gender | 539 (59%) | 111 (50%) | 147 (60%) | 143 (64%) | 138 (59%) | 0.028 |
| Race | 0.565 | |||||
| White non-Hispanic | 217 (24%) | 54 (25%) | 62 (25%) | 47 (21%) | 54 (23%) | |
| Black non-Hispanic | 258 (28%) | 51 (23%) | 77 (31%) | 63 (28%) | 67 (29%) | |
| Hispanic | 279 (30%) | 69 (31%) | 68 (28%) | 76 (34%) | 66 (29%) | |
| Other | 69 (8%) | 20 (9%) | 14 (6%) | 16 (7%) | 19 (8%) | |
| Unknowna | 97 (10%) | 26 (12%) | 24 (10%) | 21 (10%) | 26 (11%) | |
| Baseline systolic blood pressure, Mean (SD) | 131.27 (15.87) | 131.96 (16.85) | 130.91 (14.59) | 132.56 (17.41) | 129.75 (14.59) | 0.250 |
| Baseline diastolic blood pressure, Mean (SD) | 79.78 (10.24) | 80.18 (10.51) | 79.93 (10.21) | 80.02 (9.62) | 78.99 (10.59) | 0.593 |
Participants who did not complete the race questions during enrollment in Medicaid Managed Care.
Source: Authors’ analyses.
Table 2.
Demographics of participants in diabetes management program.
| Total (N = 959) |
Control (n = 232) |
Process Incentives (n = 254) |
Outcome Incentives (n = 243) |
Combined Incentives (n = 230) |
Omnibus p-value |
|
|---|---|---|---|---|---|---|
| Age, Mean (SD) | 53.0 (9.0) | 53.4 (9.3) | 52.5 (9.2) | 52.8 (8.7) | 53.4 (8.9) | 0.599 |
| Female gender | 586 (61%) | 144 (62%) | 150 (59%) | 151 (62%) | 141 (61%) | 0.884 |
| Race | 0.015 | |||||
| White non-Hispanic | 171 (18%) | 42 (18%) | 33 (13%) | 60 (25%) | 36 (16%) | |
| Black non-Hispanic | 246 (26%) | 71 (31%) | 69 (27%) | 57 (23%) | 49 (21%) | |
| Hispanic | 337 (35%) | 68 (29%) | 95 (37%) | 83 (34%) | 91 (40%) | |
| Other | 99 (10%) | 21 (9%) | 32 (13%) | 22 (9%) | 24 (10%) | |
| Unknowna | 106 (11%) | 30 (13%) | 25 (10%) | 21 (9%) | 30 (13%) | |
| Baseline HbA1c, Mean (SD) | 7.81 (1.87) | 7.89 (1.89) | 7.77 (1.81) | 7.71 (1.87) | 7.87 (1.90) | 0.688 |
Participants who did not complete the race questions during enrollment in Medicaid Managed Care.
Source: Authors’ analyses.
Measures
For those in the hypertension management program, the primary outcome was change in systolic blood pressure over six months. The program-wide goal for participants was to reduce blood pressure measures to 10 mmHg below one’s last measurement or below the JNC-8 targets (systolic blood pressure <140 mmHg); these were the targets specified as incentivized outcomes for those in the outcomes or combined incentives arms. As a secondary outcome, we also measured diastolic blood pressure.
In the diabetes management program, the primary outcome was change in blood glucose (HbA1c) over six months. The goal for participants was to lower one’s HbA1c by at least 0.6% relative to one’s baseline HbA1c, or to reach an HbA1c of <8.0%; participants who achieved these outcomes in the outcome or combined incentive arms received incentive payments accordingly.
In both studies, we measured the number of primary care visits and the number of disease-related prescriptions filled by participants, as these constituted our measure of engagement in process activities. Although participants may have engaged in more than five total process activities (primary care visits + disease related prescription fills) during the six-month period, the data obtained from Medicaid managed care organizations (MCOs) records only included five activities engaged in by participants for this study.
Intervention
Participants were randomized to one of four conditions: process-based incentives, outcome-based incentives, combined incentives where money could be earned by completing process- and/or outcome-based objectives, and usual care. Participants assigned to process-based incentives could earn up to five $50 payments ($250 maximum) for attending primary care and/or endocrinologist appointments (up to 2 for hypertension management; no limit for diabetes management) and for each disease-related medication refill they completed. These payments were capped after five process events. Participants in the outcome-based incentive arm could receive $100 at 3 months after study enrollment and $150 at 6 months after study enrollment (up to $250 total) for achieving or maintaining targeted reductions in systolic blood pressure (reduced by 10 mmHg or to below JNC-8 targets12) or blood glucose levels (lowered HbA1c by 0.6% or to below the HEDIS target of 8.0%13). Participants randomized to the combined incentive arm could receive half-size payments for each of the milestones described in the process and the outcome arms (up to $250 total). The size of incentives was determined by CMS, and usual care participants did not receive any incentives for either process activities or outcome measures. All participants received typical primary and preventive care from doctors throughout the study and received a participation incentive of $50 for enrollment.
Analysis
Our studies were designed to have at least 80% power to identify differences of 4 mmHg in systolic blood pressure for those with hypertension, and 0.5% in blood glucose levels for those with diabetes, after adjustment for multiple comparisons among the four arms in each study, respectively. The longitudinal design for both studies resulted in substantial numbers of missing outcome measurements at both 3 month (38% in hypertension study and 40% in diabetes study) and 6 months (43% in hypertension study and 39% in diabetes study). We assumed that missing outcome measures were missing-at-random, so we conducted participant-level mixed effect modeling with random intercepts to estimate the effect of experimental arm on change in primary outcomes of blood pressure or blood glucose, relative to the control arm, controlling for observed participant characteristics including age, gender, race and ethnicity, and baseline systolic blood pressure or HbA1c readings.
We also fit participant-level generalized estimation equation models to estimate the odds of completing required processes including primary care visits and filling medication, and performed Kruskal-Wallis tests for differences in the number of process measures completed by participants. Some participants lost Medicaid eligibility during the study, in which case their outcomes and process measures were censored after the date of eligibility loss. When a significant effect of experimental arm was observed, we conducted pairwise comparisons between pairs of intervention arms using the sequential Holm-Bonferroni method (α = .05) to account for multiple tests.
All analyses were performed using SAS version 9.4 (SAS Institute). Participant level mixed effect models were specified in SAS 9.4 using the PROC GLIMMIX procedure with random intercepts. Each Kruskal-Wallis test was performed using the NPAR1WAY procedure. Participant-level generalized estimation equation models were conducted using the GENMOD procedure with repeated statements to account for repeated observations of study subjects.
Since participation in Medicaid program was terminated during the study for a small number of study subjects (n = 39 for the hypertension study; n = 33 for the diabetes study) due to loss of Medicaid eligibility, we censored their data after each individual’s respective plan termination dates.
Hypertension Study Results
Systolic blood pressure.
Average systolic blood pressure was 131 mmHg at baseline and thus relatively well-controlled across all four arms (see Figure 1). Through participants’ first three months in the study, there was a marginally significant difference among intervention arms in the change in systolic blood pressure, p = .053. The provision of process incentives had no statistically meaningful impact on blood pressure (0.53 mmHg decrease relative to control, 95% CI [−3.99, 2.92], p = .762). Similarly, the provision of outcome incentives had no statistically significant impact (0.28 mmHg increase relative to control, 95% CI [−3.19, 3.76], p = .873). However, for those offered combined incentives, there was a 3.79 mmHg increase relative to the control arm, 95% CI [0.30, 7.27], p = .033.
Figure 1: Systolic blood pressure over time, by arm (N = 920) (Hypertension Study).
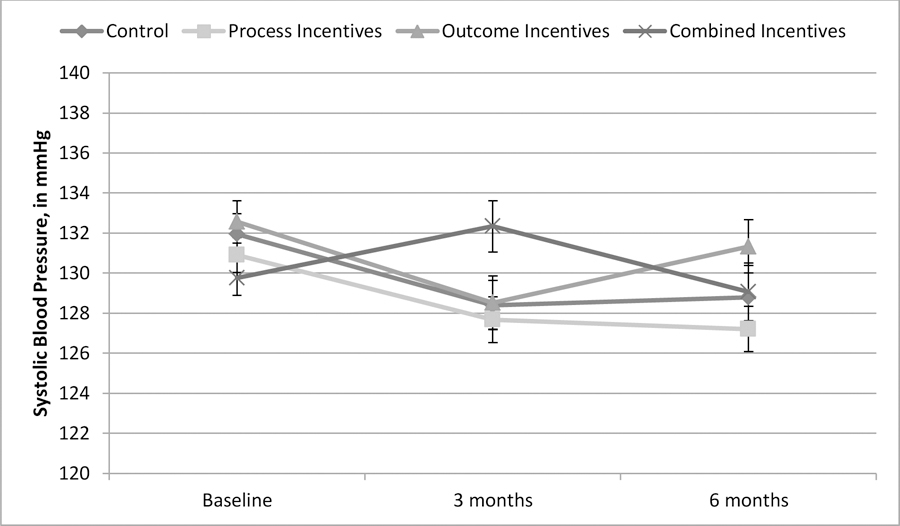
Note: Error bars +/− 1 standard error.
Source: Authors’ analyses.
After six months, the differences among intervention arms in the change in systolic blood pressure were not statistically significant, p = .531. The provision of process incentives (2.12 mmHg decrease relative to control, 95% CI [−5.78, 1.54], p = .256), outcome incentives (1.35 mmHg increase relative to control, 95% CI [−2.48, 5.19], p = .488), and combined incentives (0.26 mmHg increase relative to control, 95% CI [−3.61, 4.13], p = .896) all failed to have statistically significant impacts on blood pressure (see Figure 1).
There was no significant difference in likelihood of reaching the six-month goal of reducing systolic blood pressure by 10 mmHg (control: 47%; process incentives: 55%; outcome incentives 47%; combined incentives: 45%), p = 0.185.
Because only 24% of participants had elevated systolic blood pressure (>140 mmHg) at baseline, we re-ran the analyses for change in systolic pressure with only those participants with baseline systolic blood pressure greater than 140 mmHg and found no significant differences among arms at 3 months, p = 0.884, or at 6 months, p = 0.827. Importantly, though, the six-month average systolic blood pressure reduction among these participants was substantial in all four conditions (control: −12.10, process incentives: −17.48, outcome incentives: −15.22, combined incentives: −14.83).
Diastolic blood pressure.
Through three months, there was a marginally significant difference among intervention arms in the change in diastolic blood pressure, p = 0.065. Those provided with combined incentives had a 2.36 mmHg increase in diastolic blood pressure relative to those in the control arm, 95% CI [0.31, 4.42], p = .025. Those provided with process-based incentives reduced diastolic blood pressure by 0.03 mmHg relative to control, 95% CI [−2.36, 2.00], p = .977, and those offered outcome-based incentives had an increase of 1.14 mmHg relative to control, 95% CI [−0.90, 3.18], p = .273.
After six months, however, there were no significant differences among intervention arms in the changes in diastolic blood pressure, p = 0.820. Those offered combined incentives reduced diastolic blood pressure by 0.23 mmHg relative to control, 95% CI [−2.57, 2.10], p = .844; those in the process-based incentives arm had an increase of 0.53 mmHg relative to control, 95% CI [−1.68, 2.74], p = .637; and those offered outcome-based incentives had an increase of 1.77 mmHg relative to control, 95% CI [−0.55, 4.08], p = .135.
We also re-ran analyses with only participants whose baseline diastolic blood pressure was above 90 mmHg, and found no significant differences among arms at 3 months, p = 0.348, or at 6 months, p = 0.432.
Primary care visits.
A Kruskal-Wallis test revealed that there were no significant differences among the arms in the total number of recorded visits to a primary care physician, H(3) = 2.33, p = .507. On average, participants in the control arm made 0.6 primary care visits over the six months of the study, and participants in the process, outcome, and combined incentives conditions made 0.6, 0.6, and 0.7 visits respectively.
Medication prescriptions filled.
There were no significant differences among the arms in the recorded number of 30-day medication prescriptions that were refilled, H(3) = 2.48, p = .479. On average, control arm participants received 3.5 refills, process incentive participants received 3.7 refills, outcome incentive participants received 3.8 refills, and combined incentive participants received 3.7 refills during the six-month period.
Diabetes Study Results
Blood glucose levels.
Average HbA1c levels were 7.8% at baseline and the majority of participants (64%) had initial HbA1c levels below 8%, suggesting that a substantial number of participants had relatively well-controlled blood glucose when the program began. Through three months, there was no significant difference among intervention arms in the change in HbA1c, p = .788. Relative to control, those in the process incentives condition experienced an HbA1c reduction of 0.18%, 95% CI [−0.51, 0.15], p = .283; those in the outcome incentives condition experienced an HbA1c reduction of 0.31%, 95% CI [−0.65, 0.03], p = .075; those in the combined incentives condition experienced a reduction of 0.11%, 95% CI [−0.45, 0.23], p = .524.
Similarly, there was not any significant difference in blood glucose control between the intervention arms and control after six months, p = .939 (see Figure 2). Relative to control, those offered process-based incentives reduced their HbA1c by 0.09%, 95% CI [−0.42, 0.24], p = .606; those offered outcome-based incentives reduced HbA1c by 0.06%, 95% CI [−0.40, 0.28], p = .718; those offered combined incentives increased HbA1c by 0.02%, 95% CI [−0.33, 0.36], p = .930.
Figure 2: Blood glucose level over time, by arm (N = 959) (Diabetes Study).
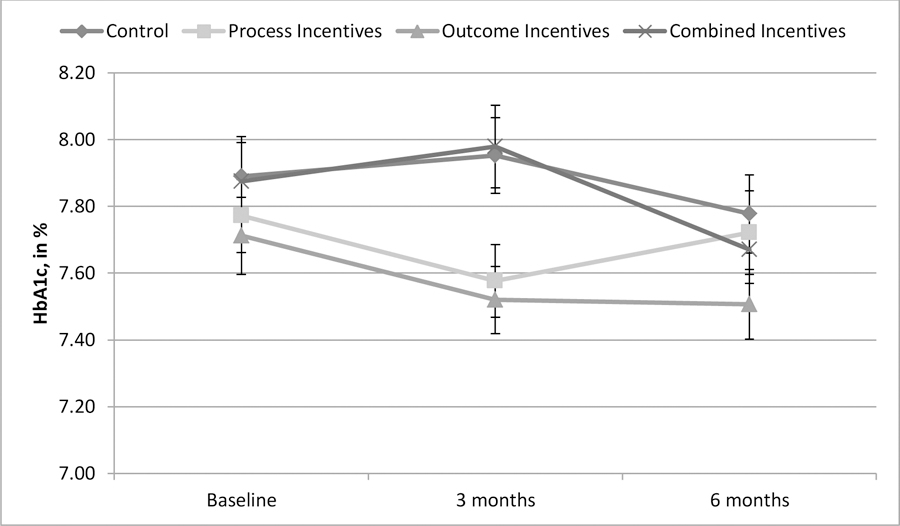
Note: Error bars +/− 1 standard error.
Source: Authors’ analyses.
There were no significant differences among arms in the rate at which participants reached the program’s target goal (reducing HbA1c by 0.6% relative to baseline or achieving an HbA1c < 8%) at six months (control = 45.3%, process = 47.6%, outcome = 46.2%, combined = 45.2%), p = 0.801.
Because not all participants had high blood glucose levels (HbA1c >8%) at baseline, we re-ran the analyses with only those participants with baseline HbA1c above 8, and again found no significant differences among arms at 3 months, p = .549, or at 6 months, p = .609. Similarly, the rate at which these participants achieved goals did not differ across arms over the six-month period, p = 0.467.
Primary care visits.
A Kruskal-Wallis test revealed that there were no significant differences in the total number of recorded visits to a primary care physician, H(3) = 0.64, p = .886. On average, participants in the control arm made 1.0 primary care appointments over the six months of the study, and those in the process, outcome, and combined incentives conditions made 1.0, 1.0, and 0.9 visits respectively.
Medication prescriptions filled.
Over the six months of the intervention period, a Kruskal-Wallis test revealed only a marginally significant difference among the arms in the recorded number of medication prescriptions that were refilled, H(3) = 6.70, p = .082. On average, control arm participants received 3.3 refills, process incentive participants received 3.3 refills, outcome incentive participants received 3.3 refills, and combined incentive participants received 3.6 refills.
Discussion
In two studies featuring different populations of Medicaid managed care enrollees with one of two common chronic diseases, we tested the impact of adding a financial incentive to standard benefits. We observed no significant improvements in health-related outcomes (blood pressure or blood glucose control) or increases in health-oriented process behaviors (physician visits or medication refills) by providing incentives worth up to $250 over 6 months, regardless of whether the incentives were targeted at improving outcomes, process measures, or a combination of the two. These results—in combination with a previous study that similarly found no effect of nearly identical incentives for weight loss and class attendance in a Medicaid diabetes prevention program10—imply that financial incentives of this magnitude implemented in the manner in which these were implemented have a negligible impact on Medicaid recipients’ disease-related behavior.
For hypertensive participants, the average 6-month reduction in systolic blood pressure did not exceed 3 mmHg in any of the four arms; the targeted reduction of 10mmHg was achieved by fewer than 50% of participants across all arms. However, we conducted supplemental analyses (reported in the technical appendix) among the subset of participants most in need of a sizable blood pressure reduction—participants with baseline systolic blood pressure above 140 mmHg—and found that these participants reduced their blood pressure by an average of more than 12 mmHg in each arm (including control), even though there were no significant differences between arms. The fact that only a minority of participants’ blood pressures were poorly-controlled at the time of enrollment likely contributed to a lack of an overall intervention effect.
Among diabetics, the average blood glucose change over the six month period across each of the four arms was not significantly different from zero, indicating that the program did little to improve these participants’ diabetes management (see technical appendix for additional analyses).
The two studies have several important limitations. First, due to challenges in identifying potential participants with poorly-controlled hypertension or diabetes, participants recruited for either study were eligible if they had been diagnosed with the disease in question. However, many of the participants opting to enroll in the studies had relatively well-controlled disease profiles (i.e., three-quarters of participants with hypertension had systolic blood pressure < 140 mmHg on enrollment and two-thirds of participants with diabetes had HbA1c < 8.0% on enrollment). The primary outcome measure of change in blood pressure or change in blood glucose levels was correspondingly difficult to improve in these participants. Missing data at six months may have further reduced the statistical power to detect significant differences. Additionally, depending on the date of their last visit, those participants in good control may not have needed to visit their doctor during the six-month study window. Future studies should restrict recruitment to those with poorly-controlled diseases for whom improvement in both outcomes and processes is more needed.
Importantly, delays between activity completion and incentive provision may have limited the effectiveness of the incentives. There is strong evidence that incentives work better when there is tight coupling of the timing of the reward with the behavior being incented.14,15 For example, delivering process-based incentive payments at the same time that participants visited the doctor or refilled their medication likely would have strengthened the connection between behavior and reward and likely would have been more effective. An even stronger coupling would be to tie financial incentives to each time a medication is actually taken, not just when the prescription was refilled, perhaps through a direct deposit into a virtual account tied to the opening of a pill bottle. However, provision of this feedback close in time to the activity being incented was not possible, with the downside that program participants on average did not receive the incentives they had earned early in the program until they were nearly done with the entire six-month program. Future research could examine the impact of different incentive timing, as well as the possibility that larger incentives might be needed to better motivate behavior and change health outcomes.
Finally, limitations in how process activities were measured and recorded may have underestimated the number of primary care visits and medication prescription fills that participants completed. Specifically, we were unable to identify in the data whether participants completed more than five process activities (primary care visits and medication prescription fills combined). Since blood pressure and blood sugar control did not improve across any arm, this is less important, but it would be interesting to know whether capping incentives at five process events for those in the relevant incentive arms increased the number of participants who completed exactly five process activities. Due to the censored nature of the current data (see technical appendix for full details), this cannot be assessed.
Supplementary Material
Appendix Exhibit 1: Figure; CONSORT Diagram for hypertension management Study; Source: Authors’ analyses.
Appendix Exhibit 2: Figure; CONSORT Diagram for diabetes management Study; Source: Authors’ analyses.
So What? Implications for Health Promotion Practitioners and Researchers.
Neither process incentives, outcomes incentives, nor a combination of the two incentives improved hypertension or diabetes management in a population of New York Medicaid managed care patients who, for the most part, had acceptable control of their disease. Future efforts to study the effects of financial incentives should focus on enrollees with poor disease control with payment of incentives done much closer in time to the behaviors being incented.
References
- 1.Ward BW, Schiller JS, Goodman RA. Multiple chronic conditions among US adults: A 2012 update. Prev Chronic Dis 2014;11:130389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gerteis J, Izrael D, Deitz D, LeRoy L, Ricciardi R, Miller T, Basu J. Multiple Chronic Conditions Chartbook.[PDF - 10.62 MB] AHRQ Publications No, Q14–0038. Rockville, MD: Agency for Healthcare Research and Quality; 2014. [Google Scholar]
- 3.Elzubier AG, Husain AA, Suleiman IA, Hamid ZA. Drug compliance among hypertensive patients in Kassala, eastern Sudan. East Mediter Health J 2000;6:100–105. [PubMed] [Google Scholar]
- 4.DiMatteo MR, Giordani PJ, Lepper HS, Croghan TW. Patient adherence and medical treatment outcomes: A meta-analysis. Med Care 2002;40(9):794–811. [DOI] [PubMed] [Google Scholar]
- 5.Croke AS. The faces of Medicaid: The complexities of caring for people with chronic illnesses and disabilities. Hamilton, NJ: Center for Health Care Strategies, Inc. 2000. [Google Scholar]
- 6.Fung V, Huang J, Brand R, Newhouse JP, Hsu J. Hypertension treatment in a Medicare population: Adherence and systolic blood pressure control. Clin Therapeutics. 2007;29(5):972–984. [DOI] [PubMed] [Google Scholar]
- 7.Asche C, LaFleur J, Conner C. A review of diabetes treatment adherence and the association with clinical and economic outcomes. Clin Therapeutics. 2011;33(1):74–109. [DOI] [PubMed] [Google Scholar]
- 8.UnitedHealth Center for Health Reform and Modernization. The United States of Diabetes: Challenges and opportunities in the decade ahead. Working Paper 5 November 2010. [Google Scholar]
- 9.Seal KH, Kral AH, Lorvick J, McNees A, Gee L, Edlin BR. A randomized controlled trial of monetary incentives vs. outreach to enhance adherence to the hepatitis B vaccine series among injection drug users. Drug and Alcohol Dependence. 2003;71:127–131. [DOI] [PubMed] [Google Scholar]
- 10.VanEpps EM, Troxel AB, Villamil E, Saulsgiver KA, Zhu J, Anarella J, Chin J-Y, Matson J, Roohan P, Gesten F, Volpp KG. Effect of process- and outcome-based financial incentives on weight loss among pre-diabetic New York Medicaid patients: A randomized clinical trial. Amer J Health Prom In press. [DOI] [PubMed]
- 11.Blumenthal KJ, Saulsgiver KA, Norton L, Troxel AB, Anarella JP, Gesten FC, Chernew ME, Volpp KG. Medicaid incentive programs to encourage healthy behavior show mixed results to date and should be studied and improved. Health Aff (Millwood). 2013;32(3):497–507. [DOI] [PubMed] [Google Scholar]
- 12.James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O, Smith SC. 2014 evidence-based guideline for the management of high blood pressure in adults: Report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014;311(5):507–520. [DOI] [PubMed] [Google Scholar]
- 13.National Committee for Quality Assurance. Comprehensive Diabetes Care. 2016. http://www.ncqa.org/report-cards/health-plans/state-of-health-care-quality/2016-table-of-contents/diabetes-care. Last accessed July 13, 2017.
- 14.Lussier JP, Heil SH, Mongeon JA, Badger GJ, Higgins ST. A meta-analysis of voucher-based reinforcement therapy for substance use disorders. Addiction. 2006;101:192–203. [DOI] [PubMed] [Google Scholar]
- 15.Volpp KG, Pauly MV, Loewenstein G, Bangsberg D. P4P4P: An agenda for research on pay-for-performance for patients. Health Aff (Millwood). 2009;28(1):206–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix Exhibit 1: Figure; CONSORT Diagram for hypertension management Study; Source: Authors’ analyses.
Appendix Exhibit 2: Figure; CONSORT Diagram for diabetes management Study; Source: Authors’ analyses.


