Abstract
Shedding of cell surface antigens is an important biological process that is used by cells to modulate responses to signals in the extracellular environment. Because antibody-based therapies of cancer target cell surface antigens, it is important to understand more about the shedding process and how it affects tumor responses to this type of therapy. Up to now most attention has been focused on measuring the concentration of shed antigens in the blood and using these to determine the presence of a tumor and as a measure of response. The recent finding that the concentration of the tumor antigen mesothelin is extremely high within the interstitial space of tumors, where it can block antibody action, and that the concentration of shed mesothelin within the tumor is lowered by chemotherapy has important implications for the successful treatment of solid tumors by immunoconjugates and whole antibodies.
Background
Antibody-based therapies now play a major role in the treatment of cancer (1). Because there are barriers that limit the penetration of these large proteins into solid tumors, antibodies are usually given in very large amounts to try and reach all cells in the interior of the tumor (2, 3), but in some cases, large amounts of antibody cannot be given because of undesirable side effects. Antibodies or antibody fragments are also used to deliver radioisotopes, cytotoxic drugs, and protein toxins to tumors. These immunoconjugates cannot be given in as large amounts as naked antibodies because of the nonspecific side effects of the radioisotope, drug, or toxin on normal tissues. The inability to give large amounts of immunoconjugates has limited their efficacy against solid tumors (4, 5). This review discusses the role of antigen shedding in diminishing the responses to antibodies and immunoconjugates and approaches to lower the concentration of shed antigen in the tumor.
Shedding of Cell Surface Antigens
Shedding of cell surface proteins, also known as ectodomain shedding, is a process used by cells to modulate the function of surface proteins. Shedding is usually due to limited proteolysis; although phosphatidylinositol (PI)-linked proteins may also be released by activation of phospholipases. Virtually all structural and functional categories of membrane proteins have been found to be shed from cells (6, 7). These include the following:
Growth factors and cytokines, which are made as precursor proteins [epidermal growth factor (EGF), transforming growth factor α, HB-EGF, tumor necrosis factor (TNF)-α, Fas ligand, etc.); many of these are involved in physiologic and pathologic processes including carcinogenesis, inflammation, cell degeneration, and apoptosis.
Receptors for growth factors and cytokines (EGF-R, ErbB2, HER-4, ILl-R, IL2-R, platelet-derived growth factor-R, CD30, etc).
Proteins involved in adhesion and cell-cell interactions (integrins, cadherins, syndecans, mesothelin).
Receptors for essential nutrients (transferrin receptor, folate receptor).
A variety of other cell surface proteins, whose functions are not yet established. In many cases, these trans-membrane proteins are processed in one or several proteolytic steps to produce the biologically active form of the protein.
Identification of specific proteases responsible for antigen shedding is a challenge because of the multitude of candidate proteases, the lack of a consensus motif for cleavage, the lack of specific protease inhibitors, and the complex regulation of protease activity. The first protease shown to carry out a specific cleavage is tumor necrosis factor α converting enzyme (TACE) or ADAM 17, which releases active TNF-α from the cell membrane (8, 9). TACE belongs to the disintegrin and metalloproteinase (ADAM) family, which shares a metalloproteinase domain with matrix metalloproteinases. It is now recognized that ADAMs are major players in the shedding process (10, 11). ADAM17/TACE and ADAM10 participate in the shedding of many cytokines and receptors (12). These include many members of the TNFR super family (TNFR1, TNFR2, CD30, and CD40), the extracellular domains of growth factors receptors (erbB2 and erbB4), cytokine receptors (IL-1RII, IL-6R α chain, IL-15R α chain, and c-kit), growth factors and cytokines (EGF, HB-EGF, and transforming growth factor α), adhesion molecules (L-selectin, VCAM-1, and CX3CL1), as well as other important molecules (Notch, macrophage colony-stimulating factor, neurotrophin receptor, CD44, and RANK ligand). ADAM17 also regulates growth hormone signaling by releasing the extracellular domain of growth hormone receptors (13). The matrix metalloproteinase family of proteases is also involved in the shedding process. Overall the shedding process is complex with different sheddases recognizing the same substrate protein and different substrates being hydrolyzed by the same sheddase.
Because of the importance of sheddases in various disease processes, many inhibitors have been developed and tested in the clinic. Both macromolecular inhibitors [endogenous tissue inhibitor of metalloproteinases (14) and monoclonal antibodies (15)] and small molecules have been considered as potential therapies. But most of the inhibitors that have been developed are small molecules with either high or low selectivity. Unfortunately, the results of clinical trials have been disappointing possibly because of the lack of specificity of the inhibitors evaluated. TACE has been shown to play an important role in inflammatory processes and cancer development, and 3 TACE inhibitors [Ro 32–7315 from Roche (16), TMI-1 from Wyeth (17), and GW3333 from GSK (18)] were evaluated in clinical trials and showed disappointing results despite efficacy in animal models. It is still possible that the development of more selective inhibitors of other proteases may show some clinical activity.
The importance of the shedding process in biological function implies that the proteolytic activity must be under strict regulation, and it has been classified into two processes, constitutive shedding and regulated shedding. Constitutive matrix metalloproteinase 7 activity is responsible for the basal level of proTNF-α shedding in macrophages, and TACE cleaves proTNF-α in response to an activator (19). It has been found that shedding is frequently regulated by phorbol esters (PMA) through PKC, although PKC-independent mechanisms also exist. In addition, mitogen-activated protein kinase has a role in the shedding of many important proteins, such as HB-EGF, transforming growth factor α, TNF-α, c-Met receptor, etc., and this pathway is triggered by growth factors and cytokines. The level of intracellular calcium is also thought to have an important role, as shown in the shedding of L-selectin (20).
The levels of sheddases (metalloproteinases) can be regulated at three levels, transcription, proenzyme activation, and inhibition of activity. The expression level and pattern of metalloproteinase expression are modulated by cytokines and growth factors, such as TNF-α, IL-1, and transforming growth factor β. This is carried out through signaling pathways, such as p38 mitogen-activated protein kinase, affecting transcription factor API and many others (21). Single-nucleotide polymorphisms (22) and DNA methylation (23) also play a role in the regulation. Most metalloproteinases are synthesized as zymogens, so zymogen activation is also an important regulatory step. The prodomain that keeps sheddases inactive can be removed by proprotein convertases (furin, PC7, PC6, and PACE) or other metalloproteinases (6). Natural metalloproteinase activity inhibitors also exist, including general inhibitors, such as α2-macroglobulin, and more specific ones such as tissue inhibitor of metalloproteinases (24). These have an important role in modulating proteolytic activity at the protein level.
Clinical Translational Advances
Clinical studies.
A large number of monoclonal antibodies that react with antigens on the surface of cancer cells have been investigated in clinical trials, but to date, only the Food and Drug Administration has approved five and two of the five target the EGF receptor (1). In addition, two radiolabeled antibodies targeting CD20 are approved for non - Hodgkin’s lymphoma, an immunoconjugate of an anti-CD33 antibody with calicheamicin (gemtuzumab ozogamicin/Mylotarg) is approved for recurrent acute myelogenous leukemia, and an IL2-diphtheria toxin fusion protein (Ontak) is approved for cutaneous T-cell lymphoma. The targets of all these therapeutic proteins are shed from the cancer cells and are present in the blood usually in the pg/mL or ng/mL range (25–28). Because large amounts of monoclonal antibodies are given to treat cancer, the blood levels of the antibodies are often over 100 μg/mL so that neutralization of the antibody by soluble antigen is not a significant factor. These concentrations are believed to be high enough to enable the antibody to reach all the cells within solid tumor masses.
In contrast, the amounts of immunoconjugates, which can be safely given to patients, are much lower because they exert toxic effects on normal cells, such as bone marrow suppression with antibodies carrying radioisotopes (29, 30), liver thrombosis with Mylotarg (31), and liver toxicity with Ontak (32). In addition, Campath itself causes bone marrow suppression so that the maximum tolerated dose of Campath is much less than that of other antibodies now in clinical use (33). Because of these low doses, soluble antigen levels can be high enough to interfere with the action of immunoconjugates. In addition, the rapid binding of immunoconjugates to cells in the blood can greatly reduce the amount of immunoconjugate reaching cells in lymph nodes or packed bone marrows (34, 35).
Solid tumors.
As summarized above, the entry of antibodies and antibody-derived therapeutics into tumor masses and also, in some cases, into tumor-packed bone marrow, is limited by a site barrier due to the close packing of tumor cells, high interstitial pressure within tumors, and a lack of functional lymphatics (2, 36). These serve as a barrier to the entry of antibodies and immunoconjugates into the interior of solid tumors. Despite this barrier, radioimmunoconjugates have been found to be active in lymphomas most likely because the cells are very radiation sensitive, and because the isotopes used (Y-90/I-131) are β emitters that can kill nearby cells that have not bound the immunoconjugate by crossfire (37). In marked contrast, carcinomas are very radiation resistant, and it has not been possible to achieve sufficient levels of radioactivity in the tumors to produce clinical benefit.
Combinations of antibodies and chemotherapy.
In an effort to improve responses, antibodies have been combined with various types of chemotherapy or radiation therapy (38). In some cases, the combination has produced a useful increase in patient response or survival, but in many cases, it has not. The mechanism by which combining different agents results in improved activity have not yet been clarified (39, 40).
Shed antigen in tumors.
Our laboratory has been involved in the development of recombinant immunotoxins, a type of immunoconjugate in which a tumor-specific Fv is fused to a bacterial toxin. The Fv binds to the cells and the immunotoxin is internalized by endocytosis enabling the toxin to reach the cytosol and kill the target cell (41). Using an immunotoxin (BL22) targeting CD22-expressing cells, many complete remissions were obtained in drug-resistant hairy cell leukemia (34). In this disease, the leukemic cells are in the blood, spleen, and bone marrow and quite accessible to the immunotoxin. There has been much less success in targeting solid tumors (42, 43). For example, in a recent trial targeting mesothelin expressing cancers with immunotoxin SS1P, only minor responses were observed (44, 45), yet both of these immunotoxins, BL22 and SS1P, have similar cytotoxic activities on cancer cells isolated from patients (IC50, 1–10 ng/mL). It is very likely that poor tumor entry is a major factor limiting the activity of SS1P, and several strategies are being pursued to overcome the entry barrier and improve immunoconjugate entry.
One approach is to combine immunotoxins with chemotherapy. When immunotoxin SS1P was combined with Taxol in mice with human tumor xenografts, remarkable synergy was observed with many complete tumor regressions. In contrast, when Taxol with SS1P in cell culture, only minor additive effects were observed (46). These findings indicated that the synergistic response was due to some special property of the solid tumor. In the initial synergy experiments, Taxol was combined with SS1P, but to be certain that synergy was a common event and not just observed with one immunotoxin and one tumor type, studies were carried out with three different immunotoxins reacting with three different target antigens on three different types of tumors using six different chemotherapeutic agents (Table 1). In all cases, synergy was observed indicating the response is quite general. Only when the tumor was drug resistant was synergy not observed (47). Because it is known that entry of antibodies and immunoconjugates into solid tumors is poor (2), the effect of chemotherapy on immunotoxin uptake by the tumors was measured, and no increase in total immunotoxin uptake was detected, indicating another mechanism was needed to explain synergy (46).
Table 1.
Synergy was observed with three different tumors, three immunotoxins, and six chemotherapies
| Tumor | Target | Immunotoxin | Chemotherapy |
|---|---|---|---|
| A431/K5 | Mesothelin | SS1P | Taxol |
| A431/K5 | Mesothelin | SS1P | CDDP |
| A431/K5 | Mesothelin | SS1P | Cytoxan |
| A431/K5 | Mesothelin | SS1P | Gemcitabine |
| KB (HeLa) | Mesothelin | SS1P | Taxol |
| KB (HeLa) | TFR | HB21(Fv)PE40 | Taxol |
| CA46 | CD22 | HA22 | Taxol |
| CA46 | CD22 | HA22 | Adriamycin |
It has been found that mesothelin, like many tumor antigens, is shed into the blood and that mesothelin levels in the blood are elevated in many patients with mesothelioma and ovarian cancer (48, 49). Because the tumor is the source of mesothelin, we hypothesized that the levels of shed mesothelin within the tumor were much higher than in the blood and could act as a decoy and block immunotoxin action more effectively than antigen in the blood. Using a method developed by Wiig et al. (50) to measure albumin present in the extracellular fluid (ECF) of tumors, the levels of shed mesothelin in the ECF of tumors was measured and found to be extremely high, up to 100 nmol/L, greatly exceeding the amount of immunotoxin in the tumor (up to 10 nmol/L). In addition, soluble mesothelin was being produced and released into the blood very rapidly (47). Recently, investigations in our laboratory showed high levels of two other shed antigens in tumors: CD22 in the ECF of CA46 lymphomas and transferrin receptor in KB tumors.1 These results indicate that soluble antigen in the tumor ECF can present an additional barrier to the entry and activity of immunoconjugates.
Control of antigen in ECF.
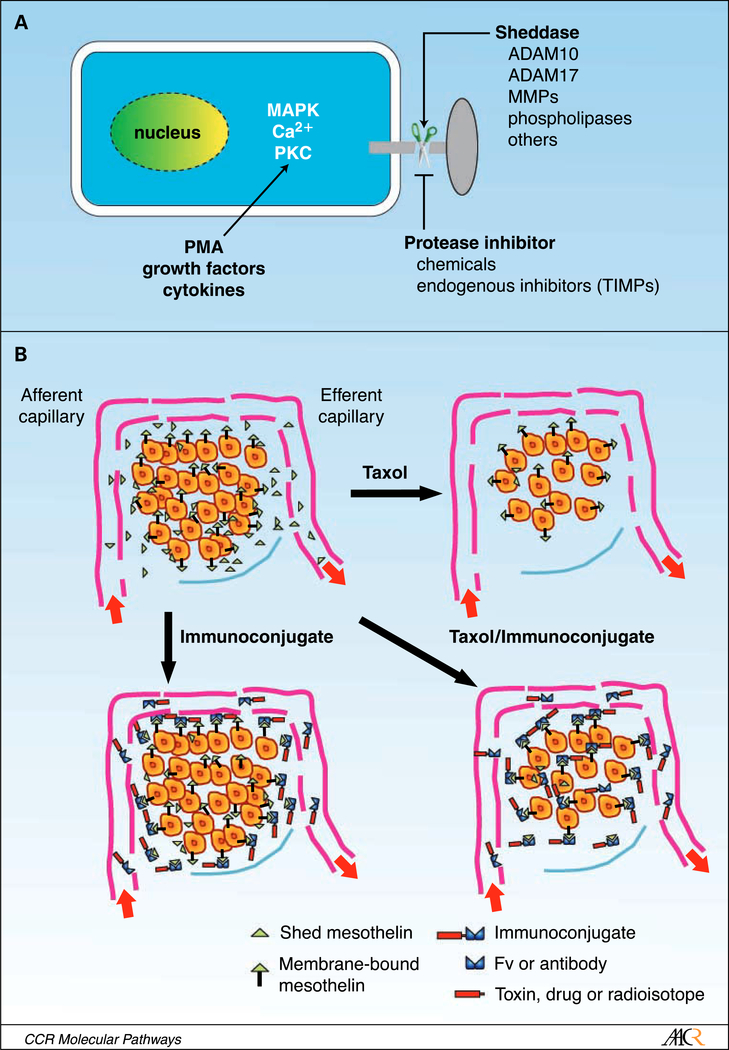
The levels of mesothelin within the ECF of KB tumors were measured before and after Taxol treatment, and were found to decrease dramatically over the 2- to 5-day period after treatment as tumor cells underwent apoptosis (47). Presumably, this decrease in shed antigen is due to the slowing of synthesis by dying and dead tumor cells and the increased transfer of antigen from the ECF into the blood. These changes combine to allow the immunotoxin to reach more cells within the tumor accounting for the synergistic interaction of immunetoxins with chemotherapy. Another way to decrease the release of shed antigen is to use specific protease inhibitors (51, 52). Figure 1 illustrates some of the important steps involved in the pathway of antigen shedding and how shed antigen and the tight packing of tumor cells can act as a barrier to the entry of antibodies and immunoconjugates into solid tumors.
Fig. 1.
A. cancer cells contain many different proteases capable of releasing cell surface antigens as well as phospholipases that can release PI-linked proteins, although such proteins may also be released by proteases. Activation of protein kinase C, various signaling pathways, and the level of intracellular calcium can modulate the activity of these proteases. Shedding can be promoted by treatment of cells with tissue plasminogen activator and inhibited by specific protease inhibitors. B. illustrates a representation of a microscopic section of a cancer showing a nest of cancer cells surrounded by a capillary supplying nutrients and capable of delivering an antibody or immunoconjugate (top left). Antigen is present on the surface of the cancer cells, and shed antigen is present at high concentrations in the ECF and much lower concentrations in the afferent capillary reflecting the average concentration in the blood. Top right, how chemotherapy decreases the number of tumor cells, disrupts their organization, and lowers shed antigen levels. Bottom left, the barriers to immunoconjugate entry, which are tight cell packing producing a so-called site barrier and shed antigen, which acts as a decoy binding immunoconjugate. Bottom right, immunoconjugate can now access more or ail tumor cells when the site barrier and shed antigen is removed.
Summary
Antigen shedding is a common biological event and tumor cells shed many antigens into the blood. Mesothelin levels are elevated in the blood of humans and mice with mesothelin expressing tumors. The levels of shed mesothelin in the tumor exceed that in the blood by >20-fold and can act as a decoy to prevent immunoconjugates and antibodies from reaching cells in the interior of solid tumors. Chemotherapy causes a dramatic decrease in mesothelin levels and allows more effective immunotoxin therapy. We propose that shed antigen within tumors constitutes an unrecognized and important barrier to antibody-based therapies. Reduction of shed antigen by chemotherapy or possibly by protease inhibitors should enhance the efficacy of immunoconjugate therapies.
Acknowledgments
Grant support: Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
Y.Zhang, R.J. Kreitman, and I. Pastan, unpublished data.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Oldham RK, Dillman RO. Monoclonal antibodies in cancer therapy: 25 years of progress. J Clin Oncol 2008;26:1774–7. [DOI] [PubMed] [Google Scholar]
- 2.Jain RK. Transport of molecules, particles, and cells in solid tumors. Annu Rev Biomed Eng 1999;1: 241–63. [DOI] [PubMed] [Google Scholar]
- 3.Al Minchinton, Tannock IF. Drug penetration in solid tumours. Nat Rev Cancer 2006;6:583–92. [DOI] [PubMed] [Google Scholar]
- 4.Pastan I, Hassan R, FitzGerald DJ, Kreitman RJ. lmmunotoxin treatment of cancer. Annu Rev Med 2007;58:221–37. [DOI] [PubMed] [Google Scholar]
- 5.Brumlik MJ, Daniel BJ, Waehler R, Curiel DT, Giles FJ, Curiel TJ. Trends in immunoconjugate and ligand-receptor based targeting development for cancer therapy. Expert Opin Drug Deliv 2008;5: 87–103. [DOI] [PubMed] [Google Scholar]
- 6.Arribas J, Borroto A. Protein ectodomain shedding. Chem Rev 2002;102:4627–38. [DOI] [PubMed] [Google Scholar]
- 7.Dello Sbarba P, Rovida E. Transmodulation of cell surface regulatory molecules via ectodomain shedding. Biol Chem 2002;383:69–83. [DOI] [PubMed] [Google Scholar]
- 8.Black RA, Rauch CT, Kozlosky CJ, et al. A metalloproteinase disintegrin that releases tumour-necrosis factor-α from cells. Nature 1997;385:729–33. [DOI] [PubMed] [Google Scholar]
- 9.Moss ML, Jin SL, Milla ME, et al. Cloning of a disintegrin metalloproteinase that processes precursor tumour-necrosis factor-α. Nature 1997;385:733–6. [DOI] [PubMed] [Google Scholar]
- 10.Blobel CP. ADAMs: key components in EGFR signalling and development. Nat Rev Mol Cell Biol 2005;6:32–43. [DOI] [PubMed] [Google Scholar]
- 11.Huovila AP, Turner AJ, Pelto-Huikko M, Karkkainen I, Ortiz RM. Shedding light on ADAM metalloproteinases. Trends Biochem Sci 2005;30:413–22. [DOI] [PubMed] [Google Scholar]
- 12.Kenny PA. TACE: a new target in epidermal growth factor receptor dependent tumors. Differentiation 2007;75:800–8. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y, Jiang J, Black RA, Baumann G, Frank SJ. Tumor necrosis factor-a converting enzyme (TACE) is a growth hormone binding protein (GHBP) sheddase: the metalloprotease TACE/AD-Am-17 is critical for (PMA-induced) GH receptor proteolysis and GHBP generation. Endocrinology 2000;141:4342–8. [DOI] [PubMed] [Google Scholar]
- 14.Chirco R, Liu XW, Jung KK, Kim HR. Novel functions of T MPs in cell signaling. Cancer Metastasis Rev 2006;25:99–113. [DOI] [PubMed] [Google Scholar]
- 15.Hu J, Van den Steen PE, Houde M, llenchukTT, Opdenakker G. Inhibitors of gelatinase B/matrix metalloproteinase-9 activity comparison of a peptidomimetic and polyhistidine with single-chain derivatives of a neutralizing monoclonal antibody. Biochem Pharmacol 2004;67:1001–9. [DOI] [PubMed] [Google Scholar]
- 16.Beck G, Bottomley G, Bradshaw D, et al. (E)-2(R)-[1 (S)-(Hydroxycarbamoyl)-4-phenyl-3-butenyl]-2′-isobutyl-2′-(methanesulfonyl)-4-methyl-valerohydrazide (Ro 32–7315), a selective and orally active inhibitor of tumor necrosis factor-α convertase. J Pharmacol Exp Ther 2002;302:390–6. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y, Hegen M, Xu J, et al. Characterization of (2R, 3S)-2-([4-(2-butynyloxy)phenyl]sulfonyl]a-mino)-N,3-dihydroxybutanamide, a potent and selective inhibitor of TNF-α converting enzyme. Int lmmunopharmacol 2004;4:1845–57. [DOI] [PubMed] [Google Scholar]
- 18.Conway JG, Andrews RC, Beaudet B, et al. Inhibition of tumor necrosis factor-α (TNF-α) production and arthritis in the rat by GW3333, a dual inhibitor of TNF-α-converting enzyme and matrix metalloproteinases. J Pharmacol Exp Ther 2001; 298:900–8. [PubMed] [Google Scholar]
- 19.Haro H, Crawford HC, Fingleton B, Shinomiya K, Spengler DM, Matrisian LM. Matrix metalloproteinase-7-dependent release of tumor necrosis factor-a in a model of herniated disc resorption. J Clin Invest 2000;105:143–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diaz-Rodriguez E, Esparis-Ogando A, Montero JC, Yuste L, Pandiella A. Stimulation of cleavage of membrane proteins by calmodulin inhibitors. Biochem J 2000;2:359–67. [PMC free article] [PubMed] [Google Scholar]
- 21.Overall CM, Lopez-Otin C. Strategies for MMP inhibition in cancer: innovations for the post-trial era. Nat Rev Cancer 2002;2:657–72. [DOI] [PubMed] [Google Scholar]
- 22.Biondi ML,Turri O, Leviti S, et al. MMP1 and MMP3 polymorphisms in promoter regions and cancer. Clin Chem 2000;46:2023–4. [PubMed] [Google Scholar]
- 23.Shukeir N, Pakneshan P, Chen G, Szyf M, Rabbani SA. Alteration of the methylation status of tumor-promoting genes decreases prostate cancer cell invasiveness and tumorigenesis in vitro and in vivo. Cancer Res 2006;66:9202–10. [DOI] [PubMed] [Google Scholar]
- 24.Brew K, Dinakarpandian D, Nagase H. Tissue inhibitors of metalloproteinases: evolution, structure and function. Biochim Biophys Acta 2000;1477: 267–83. [DOI] [PubMed] [Google Scholar]
- 25.Giles FJ, Vose JM, Do KA, et al. Circulating CD20 and CD52 in patients with non-Hodgkin’s lymphoma or Hodgkin’s disease. Br J Haematol 2003;123: 850–7. [DOI] [PubMed] [Google Scholar]
- 26.Manshouri T, Do KA, Wang X, et al. Circulating CD20 is detectable in the plasma of patients with chronic lymphocytic leukemia and is of prognostic significance. Blood 2003;101:2507–13. [DOI] [PubMed] [Google Scholar]
- 27.Murakami S. Soluble interleukin-2 receptor in cancer. Front Biosci 2004;9:3085–90. [DOI] [PubMed] [Google Scholar]
- 28.Biedermann B, Gil D, Bowen DT, Crocker PR. Analysis of the CD33-related siglec family reveals that Siglec-9 is an endocytic receptor expressed on sub-sets of acute myeloid leukemia cells and absent from normal hematopoietic progenitors. Leuk Res 2007; 31:211–20. [DOI] [PubMed] [Google Scholar]
- 29.Witzig TE, White CA, Gordon LI, et al. Safety of yttrium-90 ibritumomab tiuxetan radioimmunotherapy for relapsed low-grade, follicular, or transformed non-Hodgkin’s lymphoma. J Clin Oncol 2003;21:1263–70. [DOI] [PubMed] [Google Scholar]
- 30.Kaminski MS, Tuck M, Estes J, et al. 131I-tositumomab therapy as initial treatment for follicular lymphoma. N Engl J Med 2005;352:441–9. [DOI] [PubMed] [Google Scholar]
- 31.Larson RA, Sievers EL, Stadtmauer EA, et al. Final report of the efficacy and safety of gemtuzumab ozogamicin (Mylotarg) in patients with CD33-positive acute myeloid leukemia in first recurrence. Cancer 2005;104:1442–52. [DOI] [PubMed] [Google Scholar]
- 32.Olsen E, Duvic M, Frankel A, et al. Pivotal phase Ill trial of two dose levels of denileukin diftitox for the treatment of cutaneousT-cell lymphoma. J Clin Oncol 2001;19:376–88. [DOI] [PubMed] [Google Scholar]
- 33.Fraser G, Smith CA, Imrie K, Meyer R. Alemtuzumab in chronic lymphocytic leukemia. Curr Oncol 2007;14:96–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kreitman RJ, Wilson WH, Bergeron K, et al. Efficacy of the anti-CD22 recombinant immunotoxin BL22 in chemotherapy-resistant hairy-cell leukemia. N Engl J Med 2001;345:241–7. [DOI] [PubMed] [Google Scholar]
- 35.van derVelden VH, Boeckx N, Jedema I, et al. High CD33-antigen loads in peripheral blood limit the efficacy of gemtuzumab ozogamicin (Mylotarg) treatment in acute myeloid leukemia patients. Leukemia 2004;18:983–8. [DOI] [PubMed] [Google Scholar]
- 36.Juweid M, Neumann R, Paik C, et al. Micropharmacology of monoclonal antibodies in solid tumors: direct experimental evidence for a binding site barrier. Cancer Res 1992;52:5144–53. [PubMed] [Google Scholar]
- 37.Waldmann T. ABCs of radioisotopes used for radioimmunotherapy: α- and (β-emitters. Leuk Lymphoma (Suppl) 2003;3:S107–13. [DOI] [PubMed] [Google Scholar]
- 38.Dancey JE, Chen HX. Strategies for optimizing combinations of molecularly targeted anticancer agents. Nat Rev Drug Discov 2006;5:649–59. [DOI] [PubMed] [Google Scholar]
- 39.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001; 344:783–92. [DOI] [PubMed] [Google Scholar]
- 40.Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med 2002;346:235–42. [DOI] [PubMed] [Google Scholar]
- 41.Pastan I, Hassan R, Fitzgerald DJ, Kreitman RJ. lmmunotoxin therapy of cancer. Nat Rev Cancer 2006;6:559–65. [DOI] [PubMed] [Google Scholar]
- 42.Pai LH, Wittes R, Setser A, Willingham MC, Pastan I. Treatment of advanced solid tumors with immunotoxin LMB-1: an antibody linked to Pseudomonas exotoxin. Nat Med 1996;2:350–3. [DOI] [PubMed] [Google Scholar]
- 43.Fidias P, Grossbard M, LynchTJ, Jr. A phase II study of the immunotoxin N901-blocked ricin in small-cell lung cancer. Clin Lung Cancer 2002;3:219–22. [DOI] [PubMed] [Google Scholar]
- 44.K reitman R, Squires D, O’Hagan D, et al. SS1 (dsFv)PE38 anti-mesothelin immunotoxin in advanced malignancies: phase I study of continuous infusion [abstract]. Proc Am Soc Clin Oncol 2002; 21:22b. [Google Scholar]
- 45.Hassan R, Bullock S, Premkumar A, et al. Phase I study of SS1P, a recombinant anti-mesothelin immunotoxin given as a bolus I.V. infusion to patients with mesothelin-expressing mesothelioma, ovarian, and pancreatic cancers. Clin Cancer Res 2007;13: 5144–9. [DOI] [PubMed] [Google Scholar]
- 46.Zhang Y, Xiang L, Hassan R, et al. Synergistic antitumor activity of taxol and immunotoxin SS1P in tumor-bearing mice. Clin Cancer Res 2006;12: 4695–701. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Y, Xiang L, Hassan R, Pastan I. Immunotoxin and Taxol synergy results from a decrease in shed mesothelin levels in the extracellular space of tumors. Proc Natl Acad Sci USA 2007;104: 17099–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Robinson BW, Creaney J, Lake R, et al. Mesothelin-family proteins and diagnosis of mesothelioma. Lancet 2003;362:1612–6. [DOI] [PubMed] [Google Scholar]
- 49.Hassan R, Ho M. Mesothelin targeted cancer immunotherapy. Eur J Cancer 2008;44:46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wiig H, Aukland K, Tenstad O. Isolation of interstitial fluid from rat mammary tumors by a centrifugation method. Am J Physiol 2003;284: H416–24. [DOI] [PubMed] [Google Scholar]
- 51.Liu X, Fridman JS, Wang Q, et al. Selective inhibition of ADAM metalloproteases blocks HER-2 extracellular domain (ECD) cleavage and potentiates the anti-tumor effects of trastuzumab. Cancer Biol Ther 2006;5: 648–56. [DOI] [PubMed] [Google Scholar]
- 52.Fridman JS, Caulder E, Hansbury M, et al. Selective inhibition of ADAM metalloproteases as a novel approach for modulating ErbB pathways in cancer. Clin Cancer Res 2007;13:1892–902. [DOI] [PubMed] [Google Scholar]