Abstract
The field of preimplantation genetic testing (PGT) is evolving fast, and best practice advice is essential for regulation and standardisation of diagnostic testing. The previous ESHRE guidelines on best practice for preimplantation genetic diagnosis, published in 2005 and 2011, are considered outdated and the development of new papers outlining recommendations for good practice in PGT was necessary.
The current updated version of the recommendations for good practice is, similar to the 2011 version, split into four documents, one of which covers the organisation of a PGT centre. The other documents focus on the different technical aspects of embryo biopsy, PGT for monogenic/single-gene defects (PGT-M) and PGT for chromosomal structural rearrangements/aneuploidies (PGT-SR/PGT-A).
The current document outlines the steps prior to starting a PGT cycle, with details on patient inclusion and exclusion, and counselling and information provision. Also, recommendations are provided on the follow-up of PGT pregnancies and babies. Finally, some further recommendations are made on the practical organisation of an IVF/PGT centre, including basic requirements, transport PGT and quality management.
This document, together with the documents on embryo biopsy, PGT-M and PGT-SR/PGT-A, should assist everyone interested in PGT in developing the best laboratory and clinical practice possible.
Keywords: ESHRE, IVF, PGT centre organisation, preimplantation genetic testing, training, counselling, reporting
WHAT DOES THIS MEAN FOR PATIENTS?
The paper describes good practice recommendations for preimplantation genetic testing (or PGT). Similar documents have been published in 2011, but these needed updating to the new techniques used in IVF and genetics labs.
The recommendations should help laboratory personnel and geneticist to perform PGT according to the best laboratory and clinical practice possible. The current paper provides recommendations on the organisation of PGT and includes inclusion and exclusion of patients for PGT, counselling, consent and informed choice. The paper also describes the basic requirements of an IVF/PGT centre, including equipment, materials, and staff, reporting and follow-up of pregnancies after PGT.
These technical recommendations are not directly relevant for patients, but they should ensure that PGT patients receive the best care possible.
Disclaimer
This Good Practice Recommendations (GPR) document represents the views of ESHRE, which are the result of consensus between the relevant ESHRE stakeholders and are based on the scientific evidence available at the time of preparation.
ESHRE GPRs should be used for information and educational purposes. They should not be interpreted as setting a standard of care or be deemed inclusive of all proper methods of care, nor exclusive of other methods of care reasonably directed to obtaining the same results. They do not replace the need for application of clinical judgment to each individual presentation, nor variations based on locality and facility type.
Furthermore, ESHREs GPRs do not constitute or imply the endorsement, or favouring of any of the included technologies by ESHRE.
Introduction
The previous terms of preimplantation genetic diagnosis (PGD) and preimplantation genetic screening (PGS) have been replaced by the term preimplantation genetic testing (PGT), following a revision of terminology used in infertility care (Zegers-Hochschild et al., 2017). PGT is defined as a test performed to analyse the DNA from oocytes (polar bodies) or embryos (cleavage stage or blastocyst) for HLA typing or for determining genetic abnormalities. This includes PGT for aneuploidy (PGT-A), PGT for monogenic/single gene defects (PGT-M) and PGT for chromosomal structural rearrangements (PGT-SR) (Zegers-Hochschild et al., 2017). PGT for chromosomal numerical aberrations of high genetic risk is included within PGT-SR in the data collections of the ESHRE PGT consortium.
PGT began as an experimental procedure in the 1990s with polymerase chain reaction (PCR)-based methods used for sex selection and the detection of monogenic diseases. Interphase fluorescence in situ hybridisation (FISH) was introduced a few years later and became the standard method for sexing embryos and for detecting numerical and structural chromosomal aberrations. Genome-wide technologies began to replace the gold standard methods of FISH and PCR over the last decade, and this trend was most apparent for PGT-A. PGT-A has been carried out mainly for in vitro fertilisation (IVF) patients with original aims of increasing pregnancy rates per embryo transfer and decreasing miscarriage rates. Other outcome measures, such as increasing elective single embryo transfer and reduced time to pregnancy, have been added more recently. Cited indications for PGT-A include advanced maternal age (AMA), recurrent implantation failure (RIF), severe male factor (SMF) and couples with normal karyotypes who have experienced recurrent miscarriage (RM). The value of the procedure for all IVF patients and/or appropriate patient selection remains an ongoing discussion, but this is outside the scope of this manuscript (Harper et al., 2018).
The goal of this series of papers is to bring forward best practices to be followed in all types of PGT services, offering PGT-A as well as PGT-M and PGT-SR.
In order to take PGT to the same high-quality level as routine genetic testing, guidelines for best practice have been designed by several societies. The PGD International Society has drafted guidelines (2004, 2008) while the American Society for Reproductive Medicine reviewed PGT practice in the USA (Practice Committee of the Society for Assisted Reproductive Technology and Practice Committee of the American Society for Reproductive Medicine, 2008) and published several opinion papers (on blastocyst culture, embryo transfer and on PGT-A). The first guidelines of the ESHRE PGT Consortium were published in 2005, as one of the missions of the Consortium was to bring overall standardisation and improve quality standards (Thornhill et al., 2005). In collaboration with the Cytogenetics European Quality Assessment (CEQA) and the UK National External Quality Assessment Service (UKNEQAS), now together in Genomics Quality Assessment (GenQA), the ESHRE PGT Consortium also initiated External Quality Assessment (EQA) schemes to provide an independent evaluation of laboratories and help them in improving their techniques and reports. A review of the original guidelines yielded four sets of recommendations on different aspects of PGT: one on the organisation of PGT and three relating to the methods used: embryo biopsy, amplification-based testing and FISH-based testing (Harton et al., 2011a, Harton et al., 2011b, Harton et al., 2011c, Harton et al., 2011d). These four guidelines are now being updated and extended, taking into account the fast changes in the provision of PGT services. In these updated guidelines, the laboratory performing the diagnosis will be referred to as the PGT centre and the centre performing the IVF as the IVF centre.
General aspects of PGT, including patient selection, counselling, pregnancy and children follow-up and transport PGT, will be covered in the paper on organisation of PGT. Technical recommendations for embryo biopsy and tubing will be covered in the paper on embryo biopsy. Recommendations for genetic testing will be covered in the papers on detection of numerical and structural chromosomal aberrations and on detection of monogenic disorders. The content of the different papers is aligned with the IVF/PGT clinical procedure in Fig. 1.
Figure 1.
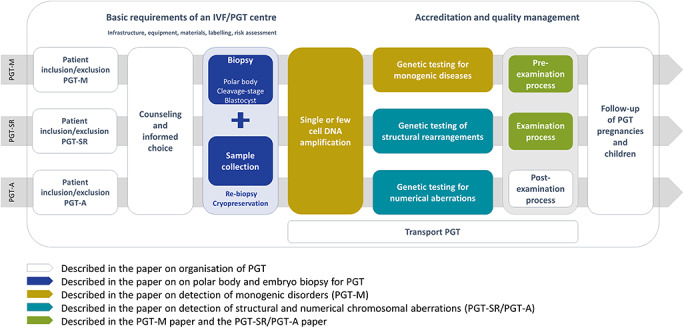
Overview of the IVF/PGT process, and how all aspects are covered by one of the four recommendations papers. IVF: in vitro fertilisation, PGT: preimplantation genetic testing.
The ESHRE PGT Consortium recognises that owing to variations in local or national regulations and specific laboratory practices, there will remain differences in the ways in which PGT is practiced (from initial referral through IVF treatment, genetic testing to follow-up of pregnancies, births and children). This does not preclude a series of consensus recommendations for best practice based on experience and available evidence. These recommendations are not intended as the only approved standard of practice, nor are they legally binding. The unique needs of individual patients may justify deviation, and the recommendations must be applied according to individual patient’s needs using professional judgement. However, recommendations and opinions may be used to frame laws and regulations, and practitioners should ensure that they comply with statutory requirements or clinical practice guidelines in their own countries. To keep the papers concise, repetitions have been excluded as much as possible and many cross-references were included. Therefore, it is recommended to not consult the papers independently but always as a set when one is seeking guidance on a PGT issue.
Materials and Methods
The current paper was developed according to the published methodology for ESHRE Recommendations for good practice papers (Vermeulen et al., 2019). The PGT-Steering Committee assessed the previous guidelines (Harton et al., 2011a) and deducted an outline for the current paper. All members of the Steering Committee, according to their expertise, wrote a section that was later discussed in depth with the entire Steering Committee until consensus was reached. As the aim was to provide technical guidance and support, it was not considered relevant to perform a formal literature search and as a result no references were added, except for references to other guidance documents. Seven online meetings were organised for discussion. The final draft of the paper was checked for consistency with the other papers of the series. The draft was then submitted for stakeholder review; it was published on the ESHRE website between 10 June and 11 July 2019, and ESHRE members were invited to send in comments. All comments were checked by the PGT-Steering Committee, discussed in an online meeting, and incorporated in the final version where relevant. A review report is published on the ESHRE website.
For easier use of the recommendations, terms in bold and italic are explained in a glossary (Supplementary Table SI) and abbreviations are listed (Supplementary Table SII).
Results/Recommendations
Patient inclusion/exclusion criteria
The decision to accept or decline patients in PGT services should be undertaken by a team of dedicated healthcare professionals (including clinical geneticists or genetic counsellors, molecular biologists/cytogeneticists, mental health professionals, clinical IVF specialists and embryologists), based on well-defined inclusion/exclusion criteria. PGT requests should be compliant with national legislation and, where needed, also be considered by local ethics boards or local/national regulatory agencies. Local regulations will vary from centre to centre as will criteria for inclusion and exclusion of patients. The following recommendations should be considered as a starting point for discussion.
General: inclusion/exclusion
It is recommended that PGT is only applied when genetic diagnosis is technically feasible, and the reliability of the diagnosis is high. Current procedures in most IVF/PGT centres allow for overall error rates (resulting in misdiagnosis) as low as 1 to 3% (De Rycke et al., 2017). Each centre should be aware of their error rates and include this information in their informed consents and reports in an open communication with the patient.
When considering PGT, safety issues, female age, impossibility to retrieve male or female gametes, body mass index (BMI) and other contraindications for IVF should be considered as possible exclusion criteria.
Furthermore, exclusion from PGT should be considered if the woman has serious signs and symptoms of an autosomal dominant or X-linked disorder (for which PGT is requested), which could introduce medical complications during ovarian stimulation, oocyte retrieval or pregnancy or medical risks at birth. PGT should be carefully considered if one of the partners has serious physical or psychological problems, either linked to the tested disease or due to other conditions.
PGT-M, mitochondrial disorders and HLA: inclusion/exclusion
PGT-M refers to testing for DNA pathogenic variant(s) causing (combinations of) monogenic disorders, X-linked, autosomal dominantly or recessively inherited, for which the disease-causing loci (nuclear or mitochondrial) has been unequivocally identified. In this respect, HLA typing of embryos is a different (no pathogenic variant detection) indication.
PGT-M
PGT-M testing can be carried out for (likely) pathogenic germline genetic variant(s) (Richards et al., 2015), shown with high likelihood to be disease causing with serious health effects that may manifest at birth, in childhood or in adulthood. Further research (e.g. functional studies, family studies) may be indicated to prove the clinical significance of genetic variants. Cases of genetic variants of unknown significance that are not predictive of a phenotype should be excluded from PGT. PGT testing is inappropriate in case of uncertain genetic diagnosis (for example genetic/molecular heterogeneity), or in case of uncertain mode of inheritance.
For autosomal recessive disorders, where a single pathogenic variant has been diagnosed in the proband and only one parent, it is acceptable to offer PGT if the pathogenic genotype is attributed to a single gene and sufficient evidence from the family pedigree allows identification of the disease-associated haplotypes. Similarly, it is acceptable to offer PGT for known X-linked recessive single gene disorders with a clear unequivocal clinical diagnosis where no pathogenic variant was found in the proband but low- and high-risk haplotypes can be identified based on the family history.
Exclusion or non-disclosure testing can be indicated for late-onset disorders, such as Huntington’s disease, to avoid pre-symptomatic testing of the partner with a family history of the disease. Exclusion testing is preferred over PGT with non-disclosure of the direct test results to the couple (Shenfield et al., 2003).
PGT for mitochondrial disorders
PGT for mitochondrial disorders caused by mitochondrial DNA (mtDNA) pathogenic variant -(s) allows the selection of embryos with an mtDNA pathogenic variant load below the threshold of clinical expression, providing an effective risk reduction strategy for heteroplasmic mtDNA pathogenic variant(s). As this threshold is often not known for rare or private pathogenic variant(s), a meta-analysis was performed for all mtDNA pathogenic variant(s), showing that embryos with a pathogenic variant load of ~18% have a likelihood of more than 95% of being unaffected, irrespective of the mtDNA pathogenic variant and can be considered for transfer. For all mtDNA pathogenic variant(s) tested so far, the pathogenic variant load in individual blastomeres is representative for the entire embryo, which was expected due to the absence of mtDNA replication in the cleavage stage. Whether the same is true for blastocysts remains to be established, as mtDNA replication has started in this stage, leading to increased variation. Therefore, it is warranted to assess the variation in pathogenic variant load within embryos.
PGT is not indicated in case of homoplasmy.
In cases where the causative pathogenic variant of the mitochondrial disease is encoded by nuclear DNA, testing is the same as for other monogenic disorders.
HLA typing
When all other clinical options have been exhausted, selection of HLA-matched embryos via PGT is acceptable for couples who already have a child affected with a malignant, acquired disorder or a genetic disorder where the affected child is likely to be cured or life expectancy is substantially prolonged by transplantation with stem cells from an HLA-matched sibling. Testing can be performed for HLA typing alone, if the recurrence risk of the disease is low, or in combination with autosomal dominant/recessive or X-linked disorders.
Attention should be given to the time required for PGT workup, cycle(s) application and for an HLA-matched sibling to be born. Therefore, cases in which the affected child has an acute medical condition prohibiting safe stem cell transplantation or an extremely low life expectancy should be carefully considered for PGT. Any request for HLA typing to create a future donor for a sibling in the absence of a specific disease should be refused.
PGT-SR: inclusion/exclusion
PGT-SR is an accepted and routine procedure in most IVF/PGT centres. It has been developed for patients who are unable to achieve a pregnancy or at high risk of pregnancy loss and of abnormal live born births, resulting from inheritance of unbalanced products of the rearrangement.
Depending on the technology used (FISH, quantitative real-time PCR (qPCR), comprehensive testing methods [array-based comparative genomic hybridisation (aCGH), single nucleotide polymorphism (SNP) array or next generation sequencing (NGS)]), different inclusion/exclusion criteria may apply. In general, PGT-SR is only recommended if the technique applied is able to detect all expected unbalanced forms of the chromosomal rearrangement. When comprehensive testing strategies are applied, it is acceptable to use information on copy number of non-indication chromosomes to refine embryo transfer strategies.
PGT-A: inclusion/exclusion
Although PGT-A remains heavily debated in clinical practice, the following indications for its use have been reported:
-
−
AMA;
-
−
RIF;
-
−
RM. It should be noted that couples with a history of RM have a high chance of successfully conceiving naturally and that PGT-A for RM without a genetic cause is not recommended in a recent evidence-based guideline (The ESHRE Guideline Group on RPL et al., 2018);
-
−
SMF.
The exact definition (e.g. age limit, number of losses) of these factors should be determined by each centre. International definitions are provided in the glossary (Supplementary Table SI).
For all, but in particular for RIF, RM and SMF couples, a previous karyotype of both partners is recommended since there is a higher chance of structural rearrangements for these indications. If an abnormal karyotype is identified, the technology for the detection of unbalanced abnormalities can differ from the regular PGT-A.
Counselling and informed choice
Relevant documents
The following documents should be available before starting PGT:
-
−
original or copy of results of genetic testing, karyotypes or other specific testing of the index patient, spouse or partner, children or other family members (when appropriate).
-
−
female reproductive history, gynaecological and fertility status;
-
−
male reproductive history, andrological history, fertility status, results of sperm analysis (especially in cases where the genetic disorder(s) for which PGT is desired has effects on sperm parameters, e.g. monogenic diseases, such as myotonic dystrophy and cystic fibrosis/congenital bilateral absence of the vas deferens and some Robertsonian translocations);
-
−
reports on health problems of female and male partners that may affect genetic diagnosis, or the outcome of IVF and pregnancy (when appropriate). Health status may need to be re-evaluated over time;
-
−
for PGT-M, PGT-SR: a genetic counselling report together with full pedigree and family data;
-
−
for HLA testing: a medical report of the affected child, current situation, prognosis, options for treatment other than PGT, suitability for stem cell transplantation, results of previous HLA typing (serologic and/or DNA markers) in affected child, parents and siblings.
As laws and regulations on PGT vary internationally, the legality of undertaking PGT in a particular country for a specific indication should be verified. If required, licenses or approval to carry out PGT should be obtained prior to the start of ovarian stimulation.
Counselling: general issues
All information, oral and written, should be in language that can be understood by a layperson as technical terminology may lead to patient misunderstanding.
Written information about treatment should be available prior to a consultation.
When PGT involves the treatment of a couple, both partners should, when possible, attend consultations.
An independent interpreter should be present when necessary, although a family member could act as translator in the absence of an alternative.
Counselling should be offered both before and after each IVF/PGT cycle.
Genetic counselling should be provided by a qualified clinical geneticist or genetic counsellor.
A specialist in reproductive medicine should provide information regarding the IVF cycle.
The counselling provided should be non-directive and include all reproductive options available to the couple, enabling them to reach their own conclusion about the suitability of treatment.
Costs and timelines should also be discussed to ensure that patients are fully informed of all aspects of IVF and PGT before treatment starts. The social and psychological impact needs to be considered, especially in couples already responsible for the care of affected children.
Additional counselling may be needed after completion of the laboratory work-up.
Individualised post-consultation letters should contain a summary of the information discussed.
The patients should sign a written informed consent for all procedures.
PGT-related counselling
PGT counselling includes counselling related to the IVF treatment on one side and genetic counselling on the other.
Related to the IVF treatment
Counselling should include discussion of:
-
−
the risk of medical complications for women during ovarian stimulation or oocyte retrieval;
-
−
the risk of spontaneous pregnancy in the waiting time or during IVF treatment, and the need for contraception;
-
−
the number of oocytes to be retrieved and the need to maximise this within the safe limits of medical practice. Different options for pooling oocytes or embryos before biopsy should be considered, when appropriate;
-
−
the expected number of embryos for biopsy, the biopsy stage, the number of cells to be biopsied and the percentage of embryos expected to survive;
-
−
the possibility that some embryos remain undiagnosed. In specific cases, re-biopsy is acceptable to achieve diagnosis. If no diagnosis is obtained, selection of these embryos for transfer is not acceptable. An exception can be made for PGT-A but requires patients’ fully informed consent;
-
−
the number of embryos to be transferred and the policy on elective single-embryo transfer in the centre. The risk of conceiving a multiple pregnancy should also be discussed;
-
−
the possibility of having no embryos for transfer if all the embryos are morphologically and/or genetically unsuitable;
-
−
the chance of pregnancy/live birth per cycle started and per embryo transfer, taking into account maternal age and indication;
-
−
the risk of miscarriage and the importance of re-analysis of placental or foetal tissue, as a tool to assess false negative rates and to advise the couple for further treatment;
-
−
cryopreservation following PGT and the predicted success of pregnancies from biopsied and cryopreserved embryos;
-
−
follow-up of pregnancies and children born from PGT;
-
−
options for embryos not transferred or frozen for future use, including donation to research, according to local regulations.
Related to the genetic analysis
Counselling should include discussion of the following:
-
−
an updated review of the genetic risk and molecular or cytogenetic confirmation of the diagnosis when appropriate, the severity and variability of the condition and presence or absence of genotype/phenotype correlation;
-
−
the principle of the test; it should be explained that depending on the indication, biological samples and genetic reports from the couple and relevant family members may be required for the laboratory work-up;
-
−
the condition(s) tested for, the testing method and the limitations of the test;
-
−
the expected time-frame for the laboratory work-up and the treatment;
-
−
the reporting of results and the centre’s policy on incidental findings;
-
−
decision-making about which embryos are acceptable for transfer/vitrification: this should be discussed with the patients before a treatment cycle begins and may need to be revisited. The fate of undiagnosed embryos and non-transferable embryos also needs to be addressed. It is acceptable to use non-transferable embryos for test optimisation;
-
−
chromosomal mosaicism as an inherent biological phenomenon in human preimplantation embryos and, when appropriate, how this may affect diagnosis and the centre’s embryo transfer policy;
-
−
the possibility of a misdiagnosis; error rates expressed as false negative or positive results should be based on ‘in-house’ work-up and follow-up analysis for specific diagnostic tests or strategies;
-
−
the option and possible recommendation for prenatal diagnosis (in case of pregnancy) for confirmation of the PGT result.
Depending on the condition, and test to be used, the following issues should also be addressed in counselling:
-
−
for structural chromosomal rearrangements, it is important to discuss that the applied technology may not allow to discriminate between normal and balanced results;
-
−
for autosomal recessive, as well as for X-linked recessive disorders, the transfer of carrier embryos should be discussed, according to the local regulations;
-
−
for X-linked diseases where specific pathogenic variant detection is not possible, the pros and cons of embryo sexing should be discussed: all male embryos, affected or unaffected, will be discarded and carrier females cannot be distinguished from unaffected female embryos;
-
−
the option of revealing the sex of the embryo should be discussed within the local legal constraints;
-
−
for monogenic disorders caused by dynamic pathogenic variants with repeat instability where testing involves repeat size determination, the couple should be fully informed on the threshold of repeat expansions below which embryos can still be transferred;
-
−for HLA typing, the theoretical number of embryos suitable for transfer should be discussed. The fate of unaffected non-HLA-matched embryos should be discussed, taking local and national regulations into consideration. Due to the complexity of the procedure it is recommended to maintain close collaboration between specialists of the IVF, PGT and transplant units, and to minimise the time of the whole procedure;
-
∘All potential limitations should be communicated to the couple, including the chance of finding a transferable embryo and hematopoietic stem cell transplantation issues (potential stem cell source, timing, expected success rate).
-
∘It is recommended to counsel prospective parents on the genetic chance of identifying a transferable embryo:
-
▪25% (1 out of 4) of biopsied embryos are genetically transferable when performing preimplantation HLA-typing only;
-
▪18.8% (3 out of 16) when concurrently excluding an autosomal recessive or X-linked recessive disease;
-
▪12.5% (1 out of 8) when concurrently excluding an autosomal dominant disease;
-
∘It is important to discuss the risk of a unique crossover in the proband, leading to very low likelihood of identifying a transferable embryo.
-
−for PGT-M or PGT-SR combined with PGT-A, the policy for embryo (ranking and) transfer should be discussed.
-
∘
Psychological support and evaluation
When available in the centre, psychological support should be offered to every couple before, during and after PGT, including unsuccessful cycles.
Psychological evaluation should be considered for the following patients:
-
−
couples for whom the geneticist, gynaecologist or other member of the IVF/PGT team has doubts regarding the welfare of existing or future children or the psychological and physical wellbeing or mental capacity of future parents;
-
−
couples in whom one of the future parents is the carrier of an autosomal dominant disorder and may have signs and/or symptoms of this disorder as determined by the appropriate specialist physician (e.g. neurodegenerative/psychiatric diseases);
-
−
couples who are undergoing PGT HLA-typing to evaluate their ‘child wish’ and the extent to which the new child is welcomed, not only as a donor but also as a full family member, appreciated for whom s/he is.
Psychological support and intervention are recommended for
-
−
couples with a history of reproductive failure;
-
−
patients with past traumatic experiences;
-
−
patients with current salient psychological distress;
-
−
couples who actively request psychological intervention.
Basic requirements of an IVF/PGT centre
A close collaboration between the IVF centre and the PGT centre is essential, particularly in complex cases.
Oocyte retrieval, fertilisation, culture, biopsy and transfer of embryos and PGT diagnosis should be undertaken in a centre with suitable laboratory infrastructure, equipment and trained staff, in accordance with the European Union Tissue and Cells directive or other local laws. Adherence to published best practice guidance on PGT is recommended.
The following recommendations apply to the preclinical work-up and testing of clinical cases.
Laboratory infrastructure, equipment and materials
Laboratory infrastructure
Oocyte and/or embryo biopsy should be performed in a specifically designated laboratory setting. Collection of the biopsied samples and initial steps of genetic testing procedures should be carried out in laboratory settings dedicated for processing single and/or few cells. Appropriate precautions should be taken both to prevent contamination of samples by physical isolation, and to detect any such contamination. Licenses for offering embryo biopsy procedures and/or genetic testing by the centre may be obtained, according to local regulations.
Equipment
-
•
All clinical equipment should meet the criteria set for the intended application, be appropriately calibrated, maintained and serviced, with all aspects supported by written standard operating procedures (SOPs). Equipment used for critical steps should have an uninterrupted power supply (UPS).
For areas within the IVF centre, whether it is a dedicated area or a room, all equipment should comply with ‘Revised Guidelines for good practice in IVF laboratories (2015)’, section 3 ‘laboratory safety’ (ESHRE Guideline Group on Good Practice in IVF Labs et al., 2016). Prior to the biopsy procedure, work surfaces, equipment and hoods should be cleaned and decontaminated with disinfectants with proven compatibility and efficacy for use in an IVF laboratory.
For areas within the PGT centre, prior to each use, work surfaces and equipment should be cleaned and decontaminated with DNA decontamination solutions or 10% bleach, or by UV-C irradiation or autoclaving (when applicable, for example tube racks). It is not recommended to use 70% ethanol solution only, as it does not decontaminate DNA.
Multichannel pipettes or automated systems may be useful in the PGT laboratory to minimise the risks of mislabelling or misallocation of samples during the post-amplification steps, but they are not recommended in the pre-amplification steps.
Materials
To prevent contamination, protective clothing for DNA amplification of a single and/or few cells should be worn, including full surgical gown (clean, not sterile and changed regularly), hair cover/hat, face mask (covering nose and mouth) and preferably shoe covers or dedicated shoes. Gloves should be worn at all times and changed frequently. These should be well-fitting (e.g. nitrile, but not vinyl examination gloves). For areas within the IVF centre, protective clothing, preferably with low particle shedding and non-powdered gloves and masks should be considered.
The pre-amplification materials and reagents should be kept away from any DNA source and preferably stored in the pre-amplification area.
Whenever possible, all solutions or reagents should be purchased ‘ready to use’ and should be of ‘molecular biology’ grade or equivalent. All reagents (purchased and in-house) should be tested and validated. All plastic-ware used, including filter tips, should be certified DNA-free and DNase-free.
Batch or lot numbers should be recorded for traceability, according to internal quality standards in the laboratory.
Whenever possible, solutions or reagents should be split into small aliquots and no aliquot should be re-used for a clinical case.
It is recommended to avoid repeated freeze-thaw cycles of all reagents.
Reagents and solutions can be DNA decontaminated by UV-C irradiation. Alternatively, reagents and solutions made in-house can be autoclaved, preferably using a PGT-dedicated autoclave.
Careful handling of all reagents employed must be ensured with regards to storage temperature and working conditions, following manufacturer’s recommendations. Vortexing and quick temperature changes should be avoided for the most sensitive components.
Specific issues for handling of reaction tubes to reduce cross-contamination:
It is recommended to avoid touching the inside or the lid of the tubes with your fingers.
It is recommended to avoid touching the outside or the cap of the tubes with the tip of the pipette. If this happens, the pipette tip should be changed immediately.
It is recommended to keep the reaction tubes open no longer than necessary.
Laboratory documentation
Well-structured (electronic and/or paper) laboratory forms should be available for recording wet-laboratory details of work-up and PGT cycle procedures.
Further specific requirements with respect to infrastructure, equipment, materials and documentation are discussed separately in the papers on embryo biopsy and PGT techniques (ESHRE PGT-M Working Group et al., 2020, ESHRE PGT-SR/PGT-A Working Group et al., 2020, ESHRE PGT Consortium and SIG-Embryology Biopsy Working Group et al., 2020).
Training and personnel
It is recommended that laboratory personnel performing clinical work should be supervised by an appropriately trained person.
Staff training and competence: embryo biopsy procedures and genetic testing should be performed by competent and adequately trained laboratory staff, according to national legislation. Joining specific training programmes (workshops, hands-on training, one-to-one training) for embryology and PGT procedures is recommended. All staff should document their competence level and continuous professional development. The number of trained laboratory staff should reflect the number of PGT cycles performed per year and also consider other duties such as administration, quality management and communication with respect to the PGT work. For centres with a low number of PGT cycles, more than one individual should be trained to avoid difficulties with absence.
It is recommended for a member of the personnel with abstinence from a specific technique to demonstrate laboratory skills before working again with clinical cases.
When the interpretation of results includes specific software, personnel may also be trained in management and interpretation of the software.
Good laboratory practice and good scientific judgement are always required.
Labelling and witnessing
It is recommended that an adequate labelling system, written or barcoded (electronic), with two unique patient identifiers plus the embryo/cell(s) number is used to match the sample’s diagnostic result with the embryo from which that sample was taken. This should ensure traceability throughout the IVF and PGT process until reporting of the final results.
The labelling system should be comprehensible and practical for both the IVF and PGT centres. Printed sticker labelling may be superior to pens, as labelling should be legible and uneditable.
Labelling and sample identification should be confirmed for critical and high-risk steps by an independent observer and signed off (Fig. 2). These critical steps are detailed in the technical papers for the various methods (ESHRE PGT-M Working Group et al., 2020, ESHRE PGT-SR/PGT-A Working Group et al., 2020, ESHRE PGT Consortium and SIG-Embryology Biopsy Working Group et al., 2020).
After biopsy, the sample may be analysed in house or sent for genetic testing in another centre (see ‘Transport PGT’).
Figure 2.
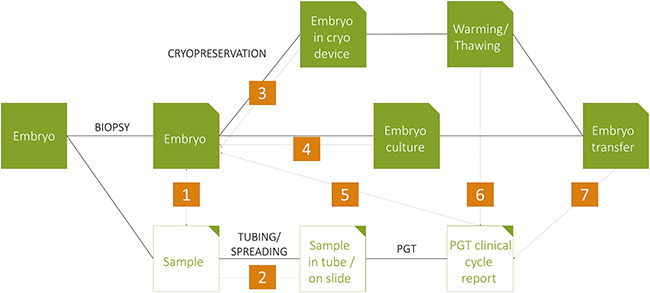
Outline of the biopsy and genetic testing procedure with indications of the seven critical steps where labelling and sample identification should be confirmed. Further details on labelling and sample identification during the PGT testing are included in the specific sections of the PGT-A/SR paper. Witnessing is recommended during the following stages: (i) immediately after biopsy to confirm the embryo and sample number match; (ii) during spreading or tubing, to confirm that the sample identification matches the labelling on the relevant slide or tube, respectively; (iii) in case of cryopreservation, immediately after biopsy before acquiring the genetic analysis results, at placing and labelling the embryo into the cryopreservation device; (iv) for further embryo culture, at placing and labelling the embryo into the culture dish; (v) when diagnostic results are issued to ensure accuracy and correlation with the correct sample identification; (vi) during the thawing/warming procedure to ensure accuracy and correlation with the correct embryo diagnostic result; and (vii) at the time of selecting the embryo(s) for embryo transfer.
Preclinical work-up report, examination and post-examination process
Preclinical work-up
The PGT work-up report should contain at least the following information (Claustres et al., 2014):
-
−administrative information:
-
∘title or name of the report;
-
∘number of the report (as used for document control, when available);
-
∘pagination including the actual and total number of pages (the patient identifier and report name/number must be present on each additional page);
-
∘full date of the report;
-
∘name and address of the physician referring the patient;
-
∘identification of the person(s) performing the diagnosis/authorising the release of the report and their signature;
-
∘identity of the IVF/PGT centre with full contact details;
-
∘
-
−patient (male and female)/sample identification:
-
∘full given name(s) and surname, or unique patient identification code;
-
∘unequivocal date of birth;
-
∘gender;
-
∘
-
−specific for the preclinical work-up report:
-
∘date of DNA sample collection;
-
∘date of DNA sample arrival in the laboratory;
-
∘samples and genetic status of relevant family members can be mentioned only with their informed consent and should be in accordance with general data protection regulations (GDPR) and/or local privacy regulations;
-
∘for PGT-SR, an overview of the most likely segregation products;
-
∘
-
−
restatement of the clinical question, i.e. the indication(s) being requested for analysis, the type of required testing, the referral reason, parental karyotypes/genomes;
-
−specification of genetic tests used:
-
∘brief information on the methods used in the analysis;
-
∘full details of the extent of the tests, including software, where appropriate;
-
∘where a commercially available kit is used, this should be clearly identified in the report, including the reference and version of the kit.
-
∘
-
−
a clear description and interpretation of results;
-
−
a clear summary of the results;
-
−error rates/limitations of the test/misdiagnosis (a general figure should be stated for the overall cycle/treatment).
- It is recommended that all reporting based on haplotyping clearly states that the accuracy of the results is based on the assumption that samples received were correctly identified, family relationships are true and the clinical diagnosis of relatives is correct.
- It is recommended that any particularity of the protocol (e.g. specifying type of biopsy, number of cells) is clearly indicated and communicated to both the patient and the IVF centre, if needed.
Examination process
The examination process and reporting of results must comply with local guidelines or law, or with the ISO 15189 standard.
Before starting a clinical PGT cycle, relevant documents should be available, labelling of samples should be checked, and genetic counselling provided to the couple.
It is recommended that the PGT laboratory has clearly documented procedures for all steps of the examination process (explicit instructions and a summary of validation results) and release of results (diagnosis, reporting, embryo transfer policy). These procedures are preferably covered in a service-level agreement between the PGT and IVF centres.
Many different methods for PGT have been published and all appropriately validated methods are acceptable for clinical cases. The method used should have been previously implemented, tested and validated in the PGT centre.
Scoring of clinical results
It is recommended that results are reviewed and signed or electronically validated by a suitably qualified person (name, qualification, date).
PGT clinical cycle report
The PGT clinical cycle report contains an interpretation of the clinical results and guidance on which embryos are genetically transferable. The same recommendations apply as specified for the preclinical work-up report (see section ‘Preclinical work-up’), together with the following items:
-
−
unique cycle/treatment code;
-
−
date of oocyte retrieval;
-
−
date of biopsy;
-
−
date of biopsy sample arrival in the laboratory;
-
−
information on the sample type (including number of samples and controls);
-
−
unique ID number for each cycle and/or biopsy sample tested;
-
−
indication for PGT.
When scoring results from polar body (PB) testing, it is recommended to report what was detected in each PB and then infer the oocyte diagnosis. It is recommended to test both PBs.
When scoring results from blastomere/trophectoderm (TE) testing, it is recommended to report what was detected in the sample and then infer the embryo diagnosis.
When results are reported from ‘pooling’ of embryos, it is advisable to refer to each oocyte and sample collection date, and clearly differentiate the embryo number between cycle/treatment.
Reporting of clinical results to the IVF centre must follow local regulations or international accreditation guidelines, including GDPR.
The embryo transfer policy should be agreed upon between stakeholders (IVF centre, genetic centre, genetic counsellors, clinicians and patients). In PGT-M and PGT-SR cases, embryos with no or inconclusive results are not recommended for transfer. Depending on local rules and following adequate counselling of the prospective parents, the carrier status of embryos (for autosomal recessive or X-linked recessive disorders) may be taken into consideration for embryo selection. In case of PGT-A in addition to PGT-M or PGT-SR, it is crucial that the centre has a clear policy on embryo (ranking and) transfer.
A written or electronic report should be securely transmitted to the IVF centre to ensure transfer and/or cryopreservation of the correct embryos. Results should not be communicated orally.
Reporting time should be kept as short as possible and when fresh transfer is intended, reporting time should be adapted to allow the IVF centre to organise the embryo transfer.
It is recommended that the report is clear, concise, accurate and easily understandable by non-geneticists.
It is recommended that the overall result and interpretation (including transfer recommendation) are presented per embryo, preferably in tabulated form. Sufficient information for genetic counselling should be included, such as the chromosome(s) involved, chromosome band(s)/nucleotides, the size of the chromosomal aberration in Mb, and the correct identification of the genetic variant. Where applicable, the latest version of the International System for Human Cytogenetic Nomenclature (ISCN)/Human Genome Variation Society (HGVS) nomenclature can be used.
In case of no diagnosis and re-biopsy to try and obtain a result, this should be included in the report.
The final clinical cycle report must be signed by appropriately qualified (authorised) personnel (name, qualification, date).
It is recommended that the clinical cycle results are discussed with the couple before embryo transfer.
It is recommended that the report is stored in the patient file in the PGT centre, according to local regulations.
It is recommended to include a disclaimer in the report to address limitations of the test and any other information that may be of significance to the addressee.
It is acceptable to indicate in the report the need for prenatal testing to confirm the result in case of pregnancy.
Further details on the specific reporting of the results and interpretation of results are outlined in the technical recommendations papers (ESHRE PGT-M Working Group et al., 2020, ESHRE PGT-SR/PGT-A Working Group et al., 2020).
Post-examination process
PGT cycle follow-up
For quality purposes, it is recommended to confirm the PGT diagnosis on a subset of embryos not transferred or cryopreserved following diagnosis, in line with local regulations. Such confirmation aims to provide internal quality assurance (QA) as well as accurate and up-to-date misdiagnosis rates for prospective PGT patients. It is recommended that this is performed on as many embryos as is practicable. It is acceptable to perform this periodically.
When a pregnancy ensues following PGT testing, it is recommended that parents are (again) made aware of the chance and risks of a misdiagnosis and be informed on the possibilities for prenatal testing. PGT and IVF centres should make special efforts to follow-up with the parents following prenatal testing or birth, especially if confirmatory testing is not possible.
Follow-up data should be used for both internal quality control (QC) and QA purposes and documented in the ESHRE PGT Consortium online database for international data collection.
It is recommended that laboratories follow local regulations or accreditation schemes on storage of clinical samples and patient records. If no local regulations or guidelines exist on storage of clinical samples and patient records, it is recommended as follows:
If embryos have been transferred and/or frozen, all relevant material (e.g. FISH slides, DNA amplification products) from the case should be retained and appropriately stored. Samples should be stored for at least 1 year. Prolonged sample storage could be considered, taking into account the availability of information on delivery and the duration of embryo cryopreservation.
If there is no genetically suitable embryo for transfer or cryopreservation, it is not necessary to keep the samples.
If there is no pregnancy after transfer of all genetically suitable embryos, samples can be discarded.
Misdiagnosis rate
It is recommended that each PGT centre performs a prospective risk analysis in order to prevent and/or eliminate possible causes of misdiagnosis.
It is recommended that misdiagnosis rates should be calculated for each type of method and for all methods from a particular centre. Misdiagnosis rates include those clinical cases in which affected pregnancies arose and cases for which re-analysis results were discordant with the biopsy result.
It is recommended that confirmatory testing should be performed at least periodically as a QA.
It is recommended that the published and in-house estimates of misdiagnosis rates should be available on request to prospective patients along with pregnancy rates and live birth rates, to allow informed consent for PGT.
Following a misdiagnosis, the IVF/PGT centre should investigate the possible causes of the misdiagnosis and make changes to protocols to eliminate the risk in the future. Many of the causes of misdiagnosis are avoidable by taking preventative actions and following the principles of quality management.
Misdiagnosis should be reported, for instance through the ESHRE PGT Consortium online database.
Baseline IVF live birth rates for PGT
Setting appropriate baseline live birth rates should be left up to the individual centres. However, it is recommended that each IVF centre should compare PGT live birth rates and matched non-PGT [routine IVF or intracytoplasmic sperm injection (ICSI)] live birth rates within that IVF centre.
Comparison of live birth rates with those reported by the ESHRE PGT Consortium or comparable peers can also be carried out to set benchmarks for continual improvement of the PGT centre.
Transport PGT
When in-house genetic analysis is not feasible, transport PGT is an option. In transport PGT, patients have the IVF treatment (oocyte retrieval, embryo culture, biopsy and transfer, pregnancy follow-up) at their local IVF centre, but genetic testing is performed at a collaborating PGT centre with significant experience in PGT.
The IVF centre and PGT centre should have in place an official agreement (Service-Level Agreement) dealing with legal, insurance and accountability issues about the transport PGT procedures.
Transportation companies entitled to transport biopsied material should certify their suitability to transport the biopsied material, provide the likelihood of a sample loss or sample delivery delay and provide actions taken against these risks.
The IVF centre and PGT centre should make arrangements to ensure that patients have had adequate PGT counselling.
The IVF centre and PGT centre should have in place a set of clinical/laboratory validated protocols, including tubing/spreading protocols, and shipment protocols specifying approximate transportation time and ensuring cell and/or DNA integrity.
In addition, practical and logistic arrangements on who will be responsible for the various stages of the PGT treatment should be clearly established.
The IVF centre and PGT centre should delineate clear and sufficient lines of communication as documented in written procedures and compliant with the GDPR during all stages of a transport PGT treatment.
Preclinical runs: before sending/receiving clinical samples from the treatment cycles, one or more ‘preclinical runs’ are recommended. This practice may detect issues related to the quality of biopsy, handling and labelling of biopsied samples, and the transport. Negative control specimens should be included in preclinical runs to assess contamination. The sensitivity and specificity of genetic testing should be evaluated and compared with in-house samples and/or samples received from other IVF centres. The reporting of the results should be agreed upon.
The IVF/PGT centres should agree on the feasibility, the number and the timing of transport PGT cycles and define a schedule.
It is recommended that all diagnostic results and reports are sent in written form (complying with the GDPR).
The IVF centre and PGT centre should agree on who is responsible for the collection of PGT data and follow-up of PGT children (www.eshre.eu/data collection).
Follow-up of PGT pregnancies and children
Prenatal diagnosis
Prenatal diagnosis should be offered to all women who become pregnant following PGT. The discussion about the tests available should be undertaken by a suitably qualified professional to ensure that all available options are presented, including prenatal invasive diagnostic tests, such as chorionic villus sampling and amniocentesis, and prenatal non-invasive diagnostic or screening tests, such as ultrasound scanning or cell-free foetal DNA testing (NIPT screening for aneuploidies or NIPD diagnosis for monogenic disorders and sexing).
As an alternative to prenatal diagnosis, patients could choose to have postnatal confirmation by cord blood sampling. However, testing of minors for non-actionable conditions should be in line with local legislation.
Follow-up of PGT pregnancies and children
There have been concerns about the health of children after assisted reproductive technologies (ART), and in particular after embryo biopsy techniques, prolonged culture to blastocyst and cryopreservation/vitrification.
So far, there is no indication that embryo biopsy causes an increased risk for adverse neonatal outcome. However, PGT includes ART for which there is evidence that ART singleton children differ from spontaneously conceived children. It is unclear whether this difference is due to the infertility status of the couple and/or the ART procedure itself.
There is uncertainty about the long-term impact of ART and/or PGT, and IVF/PGT centres should be encouraged to obtain follow-up data on babies born after treatment, preferably in collaborative prospective and retrospective studies. If this is not possible, the suggested minimum data set to collect should include:
-
−
date of birth;
-
−
singleton versus multiple pregnancy + chorionicity status;
-
−
gestational age at birth;
-
−
delivery mode;
-
−
birth weight and length;
-
−
sex;
-
−
congenital abnormalities.
Neonatal complications and APGAR score can additionally be recorded.
Accreditation and quality management
Accreditation
Accreditation, together with proficiency testing through internal (IQA) and external quality assessment (EQA), provides a means to achieve and maintain the highest quality standards. Accreditation is the formal recognition that an authoritative body gives to a laboratory/department/centre when it demonstrates competence to carry out defined tasks and involves all aspects of management, along with technical requirements.
Where possible, IVF/PGT centres should be accredited/certified, even when it is not legally required.
Because PGT is of a multidisciplinary nature, the various units involved should each be accredited/certified for their defined tasks and according to the most appropriate quality standards. For each unit, responsibilities should be clearly outlined/described and transition of responsibility from one unit to the other during the PGT process should be well defined and guaranteed.
IVF/PGT laboratories should strive for accreditation conforming with the latest version of ISO15189 or equivalent international/local standards and work with international diagnostic laboratory accreditation schemes, if available.
IVF/PGT clinical units should strive for certification conforming with the latest version of ISO9001 or equivalent international/local standards and work with medical/clinical peer review, if available.
Quality management
It is recommended that a quality management system is integrated with the IVF/PGT centre. Quality management ensures that an IVF/PGT centre and the PGT service it provides are of consistent quality. It has four main components: quality planning, QA, QC and quality improvement. To most if not all accreditation/certification schemes, QA and QC are prerequisites.
Aspects of quality management to be implemented include, among others, quality policy, quality manual, document control, compliance with SOPs, risk management, continual improvement, audits and management review. Technical requirements include personnel, laboratory conditions and environment, laboratory equipment, all stages of examination procedures, results reporting and QA.
It is recommended that PGT centres participate on a regular basis in EQA schemes; GenQA offers schemes for PGT that cover all types of analysis performed (https://www.genqa.org/).
Validation of all methods used is recommended.
Written SOPs should be available for all steps of the PGT procedure. Laboratory staff should have profound knowledge of the SOPs as these are the fundamental backbone of the service. Deviations from protocols should be recorded.
Risk assessment is part of the QC system and required for every stage of the PGT process. It should be integrated into the SOPs.
Laboratories should perform a risk assessment analysis to estimate the probability of a putative hazard and the severity of their consequences, as well as the chances for detection of error. A collaborative and multidisciplinary approach between the different operators involved in the management of a PGT cycle would lead then to the prevention of any putative procedural risk and implementation of specific corrective measures.
Supplementary Material
Acknowledgements
The authors would like to acknowledge the input of Dr Bert Smeets (Maastricht) on the section on PGT for mitochondrial diseases. The authors also thank everyone that contributed to the stakeholder review for constructive remarks that improved the quality of the paper. The list of reviewers is available in Supplementary Table SIII.
Footnotes
†ESHRE Pages content is not externally peer reviewed. The manuscript has been approved by the Executive Committee of ESHRE.
Supplementary data are available at Human Reproduction Open online.
Authors’ roles
M.D.R. chaired the working group. All authors contributed in conception and design, drafting the content and discussing it. All authors approved the final version.
Funding
European Society of Human Reproduction and Embryology.
Conflict of interest
The authors had nothing to disclose.
References
- Claustres M, Kozich V, Dequeker E, Fowler B, Hehir-Kwa JY, Miller K, Oosterwijk C, Peterlin B, Ravenswaaij-Arts C, Zimmermann U et al. . Recommendations for reporting results of diagnostic genetic testing (biochemical, cytogenetic and molecular genetic). Eur J Hum Genet 2014;22:160–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rycke M, Goossens V, Kokkali G, Meijer-Hoogeveen M, Coonen E, Moutou C. ESHRE PGD Consortium data collection XIV-XV: cycles from January 2011 to December 2012 with pregnancy follow-up to October 2013. Hum Reprod 2017;32:1974–1994. [DOI] [PubMed] [Google Scholar]
- ESHRE Guideline Group on Good Practice in IVF Labs, De los Santos MJ, Apter S, Coticchio G, Debrock S, Lundin K, Plancha CE, Prados F, Rienzi L, Verheyen G et al. . Revised guidelines for good practice in IVF laboratories (2015). Hum Reprod 2016;31:685–686. [DOI] [PubMed] [Google Scholar]
- ESHRE PGT-M Working Group, Carvalho F, Moutou C, Dimitriadou E, Dreesen J, Giménez C, Goossens V, Kakourou G, Vermeulen N, Zuccarello D et al. . ESHRE PGT Consortium good practice recommendations for the detection of monogenic disorders. Hum Reprod Open 2020. doi: 10.1093/hropen/hoaa018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ESHRE PGT-SR/PGT-A Working Group, Coonen E, Rubio C, Christopikou D, Dimitriadou E, Gontar J, Goossens V, Maurer M, Spinella F, Vermeulen N et al. . ESHRE PGT Consortium good practice recommendations for the detection of structural and numerical chromosomal aberrations. Hum Reprod Open 2020. doi: 10.1093/hropen/hoaa017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ESHRE PGT Consortium and SIG-Embryology Biopsy Working Group, Kokkali G, Coticchio G, Bronet F, Celebi C, Cimadomo D, Goossens V, Liss J, Sofia Nunes S, Sfontouris I et al. . ESHRE PGT Consortium and SIG Embryology good practice recommendations for polar body and embryo biopsy for preimplantation genetic testing. Hum Reprod Open 2020. doi: 10.1093/hropen/hoaa020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper JC, Aittomaki K, Borry P, Cornel MC, de Wert G, Dondorp W, Geraedts J, Gianaroli L, Ketterson K, Liebaers I et al. . Recent developments in genetics and medically assisted reproduction: from research to clinical applications. Eur J Hum Genet 2018;26:12–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harton G, Braude P, Lashwood A, Schmutzler A, Traeger-Synodinos J, Wilton L, Harper JC. ESHRE PGD consortium best practice guidelines for organization of a PGD centre for PGD/preimplantation genetic screening. Hum Reprod 2011a;26:14–24. [DOI] [PubMed] [Google Scholar]
- Harton GL, De Rycke M, Fiorentino F, Moutou C, SenGupta S, Traeger-Synodinos J, Harper JC. ESHRE PGD consortium best practice guidelines for amplification-based PGD. Hum Reprod 2011b;26:33–40. [DOI] [PubMed] [Google Scholar]
- Harton GL, Harper JC, Coonen E, Pehlivan T, Vesela K, Wilton L. ESHRE PGD consortium best practice guidelines for fluorescence in situ hybridization-based PGD. Hum Reprod 2011c;26:25–32. [DOI] [PubMed] [Google Scholar]
- Harton GL, Magli MC, Lundin K, Montag M, Lemmen J, Harper JC. ESHRE PGD Consortium/Embryology Special Interest Group--best practice guidelines for polar body and embryo biopsy for preimplantation genetic diagnosis/screening (PGD/PGS). Hum Reprod 2011d;26:41–46. [DOI] [PubMed] [Google Scholar]
- Practice Committee of the Society for Assisted Reproductive Technology, Practice Committee of the American Society for Reproductive Medicine. Preimplantation genetic testing: a Practice Committee opinion. Fertil Steril 2008;90:S136–S143. [DOI] [PubMed] [Google Scholar]
- Preimplantation Genetic Diagnosis International Society. Guidelines for good practice in PGD: programme requirements and laboratory quality assurance. Reprod Biomed Online 2008;16:134–147. [DOI] [PubMed] [Google Scholar]
- Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E et al. . Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015;17:405–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenfield F, Pennings G, Devroey P, Sureau C, Tarlatzis B, Cohen J, Force EET. Taskforce 5: preimplantation genetic diagnosis. Hum Reprod 2003;18:649–651. [DOI] [PubMed] [Google Scholar]
- The ESHRE Guideline Group on RPL, Bender Atik R, Christiansen OB, Elson J, Kolte AM, Lewis S, Middeldorp S, Nelen W, Peramo B, Quenby S et al. . ESHRE guideline: recurrent pregnancy loss. Hum Reprod Open 2018;2018: hoy004-hoy004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Preimplantation Genetic Diagnosis International Society (PGDIS). Guidelines for good practice in PGD. Reprod Biomed Online 2004;9:430–434. [DOI] [PubMed] [Google Scholar]
- Thornhill AR, deDie-Smulders CE, Geraedts JP, Harper JC, Harton GL, Lavery SA, Moutou C, Robinson MD, Schmutzler AG, Scriven PN et al. . ESHRE PGD Consortium ‘Best practice guidelines for clinical preimplantation genetic diagnosis (PGD) and preimplantation genetic screening (PGS)’. Hum Reprod 2005;20:35–48. [DOI] [PubMed] [Google Scholar]
- Vermeulen N, Le Clef N, Veleva Z, D'Angelo A, Tilleman K. European Recommendations for good practice in addition to an evidence-based guidelines programme: rationale and method of development. BMJ Evid Based Med 2019;24:30–34. [DOI] [PubMed] [Google Scholar]
- Zegers-Hochschild F, Adamson GD, Dyer S, Racowsky C, Mouzon J, Sokol R, Rienzi L, Sunde A, Schmidt L, Cooke ID et al. . The international glossary on infertility and fertility care. Hum Reprod 2017;32:1786–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


