Abstract
Heart regeneration, a relatively new field of biology, is one of the most active and controversial areas of biomedical research. The potential impact of successful human heart regeneration therapeutics cannot be overstated, given the magnitude and prognosis of heart failure. However, the regenerative process is highly complex, and premature claims of successful heart regeneration have both fueled interest and created controversy. The field as a whole is now in the process of course correction, and a clearer picture is beginning to emerge. Despite the challenges, fundamental principles in developmental biology have provided a framework for hypothesis-driven approaches toward the ultimate goal of adult heart regeneration and repair. In this review, we discuss the current state of the field and outline the potential paths forward toward regenerating the human myocardium.
Introduction
Cardiovascular diseases have long been the leading cause of death in both industrialized and developing countries. This broad term includes mortality from both vascular and myocardial disease and has been heavily driven by mortality from acute vascular events such as myocardial infarction. This epidemic of vascular death has led to important advances in both basic and clinical research, with significant results. The rates of both myocardial infarction and associated fatalities have been steadily declining (Yeh et al., 2010) thanks to advances in risk-factor management, as well as advanced therapies for coronary revascularization. Unfortunately, myocardial damage from non-lethal cardiac events has contributed to the increased prevalence of cardiomyopathy (Khera et al., 2017; Yeh et al., 2010).
Cardiomyopathy, or weakening of the heart muscle, is a devastating progressive disease with a prognosis worse than that of many malignancies (Mosterd and Hoes, 2007). Decades of advances in understanding the myocardial response to injury have led to the development of safe and effective drugs that slow the progression of cardiomyopathy and even restore function in some reversible cases where there is no significant myocyte loss (Yancy et al., 2017). These drugs mainly target sympathetic activation, afterload, and fibrosis pathways, which are responsible for the progressive nature of the disease after an initial insult. However, to date we have no answer to the disease’s central underlying basis, which is cardiomyocyte loss. The notion that it might be possible to rebuild the cardiac muscle, or to regenerate the myocardium, after injury has sparked significant interest over the past two decades, and it has created a battleground for competing theories and ideas. Although cardiac regeneration requires replenishing numerous cell types, including cardiomyocytes, vasculature, lymphatics, conduction system cells, and the interstitium, among others, this review will focus on cardiomyocyte regeneration.
Non-cardiac Models of Spontaneous Regeneration
When considering strategies to induce heart regeneration in mammals, perhaps an important question is where do we start looking for clues? Which models are likely to be most informative? The field in general has followed numerous leads, some providing important clues, although most have been dead ends.
One of the first promising possibilities came from satellite cells, the committed progenitor cells of skeletal muscles. Satellite cells are resident skeletal-muscle progenitors that are tasked with replenishing adult skeletal muscles throughout the lifespan of mammals. With the exception of some degree of impaired skeletal-muscle regeneration in aged mammals, this process is highly efficient. Apart from skeletal muscles and parts of the esophagus, the myocardium is the only other striated muscle in adult mammals. Interestingly, though satellite cells spontaneously regenerate injured muscle fibers, no such cell has been discovered in the heart. Therefore, it was logical to consider satellite cells as a potential source of exogenous cell therapy for cardiomyocyte regeneration. Some promising early animal studies paved the way for human trials, which were prematurely terminated. Unfortunately, not only did skeletal-muscle progenitors fail to significantly improve left ventricular (LV) systolic function, but they also caused ventricular arrhythmias (Menasché et al., 2008). This life-threatening side effect should be a warning that although most cell therapies are considered safe, complications are not always easily foreseen.
Another prominent misdirection came from bone marrow mononuclear cells (Orlic et al., 2001b). The bone marrow contains the only known adult stem cells, namely hematopoietic stem cells (HSCs), that are currently in clinical use. Given the relative ease of harvesting and delivering these cells, one can easily see how this is an attractive model for regeneration. In adult mammals, a single HSC is capable of repopulating the entire bone marrow, resulting in long-term engraftment (Lemischka et al., 1986). Extrapolating this concept to the heart, early animal studies suggested that bone marrow cells can regenerate the heart (Orlic et al., 2001a). However, these findings were quickly refuted by several studies demonstrating a lack of trans-differentiation potential of bone marrow cells into cardiomyocytes (Balsam et al., 2004; Murry et al., 2004). The signal of very modest improvement of LV systolic function in humans after administration of bone-marrow-derived cells (Fisher et al., 2014) remains of unclear significance (Zhang et al., 2015). As a result, a recent consensus statement by a number of prominent heart regeneration researchers concluded that the use of bone marrow cells for heart regeneration is not supported by the literature (Eschenhagen et al., 2017). Importantly, a recent study demonstrated that adult stem cells injected into the myocardium do not form new cardiomyocytes but rather activate an acute sterile immune response that underpins their modest beneficial effect (Vagnozzi et al., 2019).
The elegance of the HSC system might have also misguided the cardiac regeneration field from a conceptual standpoint. Theoretically speaking, if a model similar to the hematopoietic hierarchy exists in the heart, where an organ-specific stem cell gives rise to all lineages in that organ, then a resident cardiac stem cell might be able to regenerate all cardiac lineages, including cardiomyocytes, endothelial cells, smooth muscle cells, and fibroblasts from a single cell. However, if we consider the developmental origin of the hematopoietic and cardiac systems, the fundamental differences between the two systems become clearer. During embryonic development, the hematopoietic system consistently maintains the stem cell hierarchy, wherein a single stem cell is capable of giving rise to the majority of hematopoietic cells, as the site of definitive hematopoiesis changes from the aorta-gonad-mesonephros to the fetal liver and finally to the bone marrow (Gao et al., 2018). In contrast, no committed cardiac stem cell, one that specifically gives rise to all cardiac lineages, has been identified, although several cell types do have multipotential properties during embryogenesis. For example, Oct4+ pluripotent stem cells give rise to Nestin+ ectoderm precursors, which in turn give rise to outflow tract vascular smooth muscle cells and endocardial cushions, whereas Mesp1+ primordial cardiovascular precursor cells give rise to the majority of other cardiac lineages (Sahara et al., 2015). Of note, Mesp1+ cells are not restricted to the cardiac lineage, but are also involved in extra-embryonic and cranial-cardiac mesoderm, as well as in paraxial mesoderm to generate somites (Saga et al., 1996), and thus cannot be defined as cardiac stem cells. Isl-1-expressing cells have also been shown to have some multipotential properties during development (Moretti et al., 2006); however, they do not contribute to cardiomyogenesis in the postnatal heart. Importantly, a recent report used a dual recombinase fate-mapping system, in which non-cardiomyocytes and cardiomyocytes can be simultaneously labeled by two orthogonal recombination systems, to demonstrate that nonmyocytes contribute to cardiomyocyte formation only during early cardiac morphogenesis, and there is no evidence of a contribution to cardiomyocytes after embryonic day 10.5 (E10.5) (Li et al., 2019). Therefore, evidence that a naturally occurring cardiac-specific stem cell that gives rise to all or the majority of cells in the heart, either during development or postnatally, does not exist.
One of the noteworthy attempts at finding a bona fide cardiac stem cell focused on resident cardiac C-kit+ cells. Although initial studies suggested a multipotent ability for these cells (Beltrami et al., 2003), lineage-tracing studies later indicated that these cells have negligible cardiomyogenic potential and instead seem to be primarily endothelial cell progenitors (Elhelaly et al., 2019; Sultana et al., 2015; van Berlo et al., 2014). The question of whether or not any nonmyocyte populations (including C-kit cells) contribute to cardiomyocyte generation in the adult heart has been recently investigated using the same dual recombinase fate-mapping strategy outlined above to demonstrate conclusively that non-myocytes do not contribute to cardiomyocyte generation in the adult heart, either under homeostatic conditions or after injury (Li et al., 2018). Unfortunately, the question of the cardiomyogenic potential of C-kit+ cells has polarized the field and has been marred by scientific misconduct, lawsuits, and a staggering number of retractions (Chien et al., 2019), all of which has eroded trust in the entire cardiac regeneration field and resulted in an enormous waste of time and money.
In retrospect, it was a mistake for the field to proceed with translational studies without a solid basic understanding of the mechanisms of cardiac regeneration or rigorous fate-mapping studies. Moving forward, it will be important to capitalize on the knowledge gained thus far and to take a more objective approach to studying heart regeneration.
Spontaneous Cardiomyocyte Regeneration
Cardiomyocytes Beget Cardiomyocytes
From a theoretical point of view, naturally occurring heart regeneration can serve as an invaluable roadmap to guide the development of innovative strategies for human heart regeneration. Spontaneous cardiac regeneration does in fact occur in several non-mammalian species (Becker et al., 1974; Cano-Martínez et al., 2010; Flink, 2002; Oberpriller and Oberpriller, 1974; Poss et al., 2002), as well as both small (Porrello et al., 2011; Porrello et al., 2013) and large (Ye et al., 2018) neonatal mammals. Early studies in zebrafish demonstrated that apical resection results in spontaneous regeneration in the ensuing 60 days (Poss et al., 2002). A similar type of injury induces myocardial regeneration in amphibians such as axolotls (Cano-Martínez et al., 2010; Flink, 2002), salamanders (Becker et al., 1974), and newts (Oberpriller and Oberpriller, 1974). In embryonic and neonatal mammals, genetic ablation (Sturzu et al., 2015), apical resection (Porrello et al., 2011), and ischemic myocardial infarction (Porrello et al., 2013) result in spontaneous regeneration. In all these settings, myocardial regeneration is mediated by compensatory proliferation of preexisting cardiomyocytes, rather than a stem or progenitor cell population.
Although spontaneous heart regeneration does not seem to occur in adult mammals, it is well established that measurable cardiomyocyte turnover does occur in both rodents and humans. Elegant 14C dating studies indicate that slow but measurable cardiomyocyte turnover occurs in the postnatal human heart (Bergmann et al., 2009; Bergmann et al., 2015). Although these studies can only provide insight into DNA replication, which can occur in the absence of full abscission, combining these studies with nucleation and DNA copy number provides an additional layer of evidence to support the notion of cardiomyocyte turnover in humans.
Several lines of evidence suggest that a similar phenomenon exists in rodents, and surprisingly the rate appears to be the at about 1% per year (Kimura et al., 2015; Senyo et al., 2013). Several studies indicated that this slow turnover in rodents is mediated by cardiomyocyte proliferation, and a recent study suggests that a rare population of hypoxic cardiomyocytes mediates that slow turnover (Kimura et al., 2015).
Collectively, these studies suggest that new cardiomyocytes are derived from preexisting cardiomyocytes in lower vertebrates and in mammals. As a result, a major current focus of the field is now to understand mechanisms of cardiomyocyte proliferation in the hope of developing therapeutics that induce spontaneous cardiac regeneration in humans.
Why Can’t the Adult Mammalian Heart Regenerate?
Given the apparent ease by which lower vertebrates and neonatal mammals are able to regenerate their myocardium spontaneously, one has to wonder why this phenomenon doesn’t exist in the adult mammalian heart. There have been several theories that can, at least in part, address this question. But first, it is important to define the question a bit further. In principle, a myocardial regenerative response requires a responsive substrate (in this case cardiomyocytes) and a pro-mitotic signal (for example, injury) that induces proliferation of cardiomyocytes in the injured zone. For example, although mammalian neonatal cardiomyocytes (the substrate) are capable of proliferation, the basal rate of proliferation is low, and it is inducible by several-fold in response to injury (a mitotic signal) (Porrello et al., 2011; Porrello et al., 2013). So, which is defective in the adult heart– the substrate, the mitotic signal, or both? It is well documented that cardiac injury in adult mammals, including humans, induces a mitogenic effect on cardiomyocytes; however, this results mainly in increased polyploidization and multinucleation with minimal formation of new cardiomyocytes (Herget et al., 1997). In addition, specific mitogenic stimuli that induce profound cardiomyocyte proliferation in lower vertebrates (Gemberling et al., 2015) and neonatal mammals (Polizzotti et al., 2015) fail to induce proliferation of adult cardiomyocytes. Therefore, a major barrier against myocardial regeneration appears to be cell-cycle arrest of adult mammalian cardiomyocytes.
A more focused question then becomes: why do mammalian cardiomyocytes stop dividing?
From a growth perspective, the myocardium continues to grow during normal postnatal development and in response to various stress stimuli. However, it does so through hypertrophic rather than hyperplastic growth (Li et al., 1996), which coincides with a loss of the ability of cardiomyocytes to divide. This switch in the growth mode of the myocardium, which results in the loss of its endogenous regenerative capacity, might hold the key to understanding why the hearts of adult mammals don’t regenerate.
One theory is that mitosis of incessantly contractile cells is not feasible; otherwise, the organ would lose contractile force as cardiomyocytes undergo mitosis. However, the fetal heart is certainly contractile as it undergoes hyperplastic growth. Histological analysis of mitotic cardiomyocytes in fetal and early post-natal hearts suggests that the cell cycle of cardiomyocytes is staggered enough so that only a small percentage of cells undergo mitosis at any given time (Porrello et al., 2011; Porrello et al., 2013).
Another theory is that neonatal heart regeneration in mammals is simply a remnant of developmental programs that are lost after birth. Although it is difficult to refute this hypothesis, the adult myocardium does in fact attempt to re-activate at least some of these pathways, which have been dubbed the “fetal gene program,” upon injury. However, this does not reverse cell-cycle arrest but instead is believed to play a role in the injury-induced hypertrophic response. However, this term “fetal gene program” is somewhat ill-defined, and there are certainly a large number of other gene pathways that are either lost or activated after birth and exert potent effects on the cardiomyocyte cell cycle. Examples include pathways involving the Hippo (Heallen et al., 2011), Erbb2 (D’Uva et al., 2015), and Meis1 (Mahmoud et al., 2013) genes, to name a few.
It is also possible that the postnatal environment forces cell-cycle arrest of cardiomyocytes. Recent studies suggest that the postnatal increase in oxygenation and load induce the metabolic switch from glycolytic to beta oxidation-dependent oxidative metabolism in postnatal cardiomyocytes. This induces DNA damage, which results in activation of the DNA damage response and cell-cycle arrest (Puente et al., 2014). Both hypoxia (Nakada et al., 2017) and mechanical unloading (Canseco et al., 2015) have been shown to decrease DNA damage and reactivate the cardiomyocyte cell cycle in the adult heart.
Issues to Consider
It is worth considering how much we can actually extrapolate from studies in small model organisms such as zebrafish toward the possibility of regenerating the adult human heart. The zebrafish and neonatal hearts contain only a few hundred thousand cardiomyocytes (Naqvi et al., 2014). Following apical resection or surgical myocardial infarction, less than half of those cardiomyocytes are lost. In contrast, a typical infarct in the human heart may measure several inches in diameter and involve the loss of a billion cardiomyocytes. Efficient regeneration of the human myocardium in the face of extensive fibrotic scarring represents a major biological challenge. In addition, adult human cardiomyocytes must be seamlessly coupled to each other to ensure synchronous contractions and prevent fatal arrhythmias. Finally, widespread cardiomyocyte proliferation is disruptive to cardiac rhythmicity and might therefore have been selected against.
Where We Stand
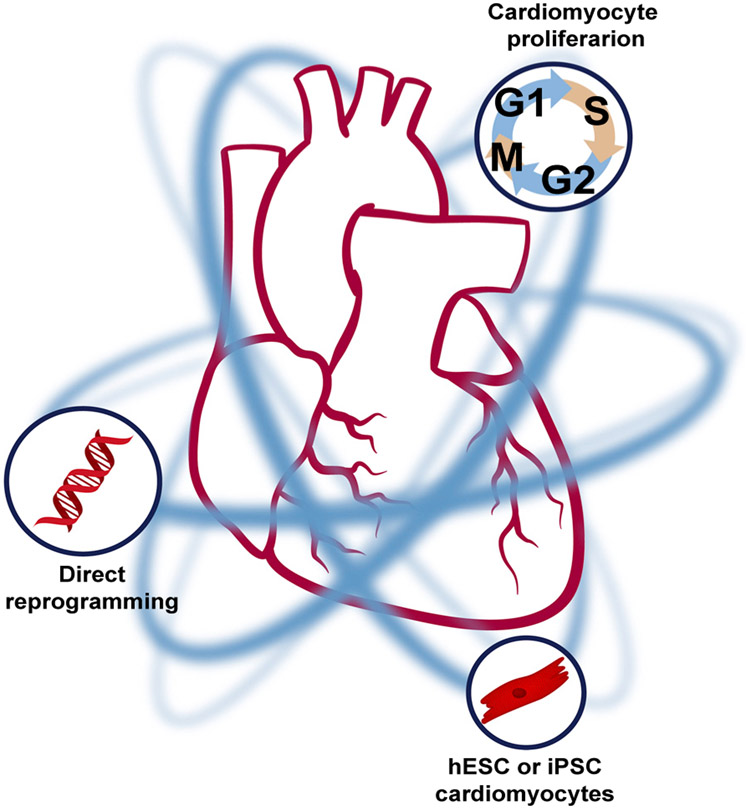
As our understanding of cardiac regeneration matures, therapeutic options will continue to emerge. In our view, the three most promising therapeutic strategies for myocardial regeneration in adult mammals are induction of endogenous cardiomyocyte mitosis, direct reprogramming of non-myocytes to a cardiac fate in vivo, and remuscularization of the myocardium via exogenously administered pluripotent-cell-derived cardiomyocytes (Figure 1).
Figure 1. Strategies for Myocardial Regeneration.
Schematic of the most promising cardiomyocyte regeneration strategies, which include induction of cardiomyocyte proliferation, the use of pluripotent-stem-cell-derived cardiomyocytes, and direct reprogramming.
Cardiomyocyte renewal by enhancing cardiomyocyte proliferation is a viable therapeutic strategy. Numerous strategies to induce adult cardiomyocyte proliferation have shown promise; these strategies include: manipulation of environmental factors (Nakada et al., 2017), nerves (Mahmoud et al., 2015; White et al., 2015), kinases (Heallen et al., 2011), transcription factors (Mahmoud et al., 2013; Malek Mohammadi et al., 2017), micro RNAs (Eulalio et al., 2012; Hodgkinson et al., 2015; Katz et al., 2016; Porrello et al., 2013), surface receptors (D’Uva et al., 2015), epicardial factors (Wei et al., 2015), and extracellular factors (Bassat et al., 2017), among others. In addition, forced expression of direct cell cycle regulators has been also shown to stimulate heart regeneration (Mohamed et al., 2018). The following section will discuss advances in this arena.
Resident Cardiomyocytes as a Source of New Cardiomyocyte Formation
Studies that examined induction of cardiomyocyte proliferation as a path toward myocardial regeneration date back several decades. One of the first reports of forced induction of cardiomyocyte proliferation came from the Field group, where they showed in a series of studies that cardiomyocyte-specific constitutively active Cyclin D2 overexpression results in persistent cardiomyocyte proliferation and myocardial regeneration following injury (Pasumarthi et al., 2005). The role of other cyclins in cardiomyocyte proliferation has also been studied. Overexpression of Cyclin A2 was shown to induce cardiomyocyte proliferation and improve LV systolic function after injury both in mice (Chaudhry et al., 2004) and in pigs (Shapiro et al., 2014). In a recent report, the Srivastava group showed that combinatorial overexpression of several cyclins and CDKs (cyclins B1 and D1, as well as CDK1 and 4) induces cardiomyocyte proliferation in the adult mouse heart (Mohamed et al., 2018). Several other cyclins appear to have little or no effect on the cardiomyocyte cell cycle. For example, within the cyclin D subfamily, cyclin D2 appears to be the main driver of cardiomyocyte proliferation, whereas cyclins D1 and D3 fail to induce significant cell cycle entry (Pasumarthi et al., 2005). In addition, cyclin G1 appears to be an important regulator of cardiomyocyte polyploidy and multinucleation. Overexpression of cyclin G1 in cardiomyocytes induces DNA synthesis but inhibits cytokinesis, resulting in polyploidy and multinucleation, whereas inhibition of cyclin G1 prevents multinucleation in response to pressure overload (Liu et al., 2010). Some of these strategies, such as cyclin A2 overexpression, have now advanced to the preclinical stage as a strategy for heart regeneration.
Cardiac innervation was also demonstrated to be critical for neonatal heart regeneration in mammals, where manipulation of autonomic nerve supply to the myocardium was shown to regulate neonatal cardiomyocyte proliferation in response to injury. Lee and colleagues demonstrated that the interruption of cholinergic signaling through vagotomy or atropine administration prevented cardiomyocyte proliferation and neonatal heart regeneration (White et al., 2015). This phenomenon appears to be regulated through muscarinic M2 receptors. Interestingly, cholinergic stimulation by carbachol extended the postnatal neonatal heart regeneration window (Mahmoud et al., 2015). Sympathetic outflow has also been shown to be critical for neonatal heart regeneration. Subepicardial sympathetic nerves that are severed during neonatal apical resection regrow during apical regeneration, and that sympathectomy abolishes the endogenous regenerative capacity of the neonatal heart (White et al., 2015). These observations are intriguing and raise some important translational questions. For example, given the proven role of sympathetic blockade in reversing cardiac remodeling in the setting of systolic heart failure, is there an autonomic outflow dose-effect where modest blockade of sympathetic outflow is critical for prevention of cardiomyocyte death and fibrosis (remodeling), whereas a more pronounced loss of sympathetic outflow inhibits cardiomyocyte proliferation? This latter scenario will most likely have little effect on the adult heart where the majority of cardiomyocytes are cell-cycle arrested. Similarly, is there a role for cholinergic stimulation in heart failure? Although early animal studies showed a potential beneficial effect (Li et al., 2004), subsequent human trials failed to demonstrate a pronounced beneficial effect when using a vagal stimulation device (Gold et al., 2016). Nevertheless, more studies are underway to further explore the role of vagal stimulation in heart failure.
A number of extracellular matrix (ECM) proteins have also been studied for their role in cardiomyocyte cell-cycle regulation and heart regeneration. The fibroblast-derived factor periostin was shown to induce adult heart regeneration through induction of cardiomyocyte proliferation (Kühn et al., 2007). However, these results were not readily reproducible by other groups (Lorts et al., 2009). It is noteworthy though to highlight an interesting discrepancy between the neonatal and adult hearts in response to periostin where neonatal heart regeneration appears to require periostin expression (Chen et al., 2017). This is thus another example of how the cell cycle state of adult cardiomyocytes prevents the response to pro-proliferative stimuli. A similar pattern is seen in the discrepancy between neonatal and adult myocytes in response to neuregulin, for example (Polizzotti et al., 2015), where loss of Erbb2 in the postnatal heart results in inhibition of the promitotic effect of neuregulin on cardiomyocytes. Intriguingly, this promitotic effect can be restored by conditional forced expression of Erbb2 in cardiomyocytes (D’Uva et al., 2015). More recently, a report from the Pilar Ruiz-Lozano group showed that epicardial-derived Fstl1, but not that generated within cardiomyocytes, induces cardiomyocyte proliferation and heart regeneration both in small and large mammals when injected into the myocardium or delivered by an epicardial patch (Wei et al., 2015). In addition, Eldad Tzahor demonstrated that the extracellular protein agrin is required for neonatal heart regeneration in mice. Intriguingly, direct administration of agrin into the adult myocardium results in reactivation of the cardiomyocyte cell cycle and heart regeneration in adult mammals, although the beneficial effects of agrin appear to be multifactorial and not singularly dependent on induction of cardiomyocyte proliferation. From a mechanistic standpoint, agrin appears to induce cardiomyocyte proliferation through a mechanism that involves YAP activation. Two additional recent studies also suggested that cardiac fibroblast senescence plays a pivotal role during neonatal heart regeneration, and in one of those reports, agrin appears to mediate that process (Feng et al., 2019; Sarig et al., 2019). Therefore, the ECM appears to play an interesting, yet poorly understood, role in myocardial regeneration, and this warrants further study. Certainly, modulating the cardiac ECM by local delivery of patches or molecules, for example at the time of cardiac surgery, would be an attractive strategy for heart regeneration.
The Hippo pathway is a highly conserved signaling cascade that plays a critical role in organ size determination. The transcriptional coactivator Yap is a key downstream effector of the Hippo signaling pathway. Phosphorylation of Yap at serine 112 (S112) results in its sequestration in the cytoplasm, whereas dephosphorylation allows for its nuclear translocation, where it induces cell-cycle progression through interaction with TEAD transcription factors. The role of this pathway in heart regeneration came to light a in 2010 study when the Martin lab published a report outlining its role in regulation of cardiomyocyte proliferation during embryonic development and early postnatal period (Heallen et al., 2011). Several subsequent studies confirmed the role of Hippo signaling in regulation of cardiomyocyte cell-cycle regulation and heart regeneration. For example, cardiomyocyte-specific overexpression of constitutively-active YAP results in a significant hyperplastic response characterized by increased cardiomyocyte proliferation, thickened myocardium, and myocardial regeneration after injury (Xin et al., 2013; Xin et al., 2011). A number of other groups have also outlined the role of Hippo signaling in cardiomyocyte cell-cycle regulation (Lin et al., 2015; von Gise et al., 2012; Xin et al., 2011). Interestingly, a recent study demonstrated that a key downstream regulator of the pro-regenerative effect of Yap in the heart is the transcription factor Pitx2, which appears to modulate redox signaling and maintain a low oxidative state (Tao et al., 2016).
This correlation between the oxidative state of cardiomyocytes and their endogenous regenerative capacity has also been previously studied. Puente et al. 2014 demonstrated that postnatal cell-cycle arrest of cardiomyocytes is mediated, at least in in part, by the metabolic switch from glycolysis to oxidative phosphorylation. The postnatal shift to mitochondrial oxidative metabolism is associated with increased mitochondrial reactive oxygen species (ROS) production and oxidative DNA damage, which in turn activates DNA damage response (DDR), including ataxia telangiectasia mutated (ATM) and downstream cell-cycle regulators such as Wee1 kinase (Puente et al., 2014). In support of this notion, two other reports supported the role of glycolytic metabolism in cell-cycle progression in cardiomyocytes. Kimura et al., (2015) used a fate-mapping strategy based on stabilization of the oxygen dependent domain (ODD) of Hif-1alpha to demonstrate that cycling cardiomyocytes in the adult heart can be identified on the basis of this hypoxic phenotype. It is important to note here that it is unclear whether these rare, cycling cardiomyocytes are truly hypoxic or whether Hif-1alpha is stabilized through non-hypoxic pathways. Intriguingly, single-cell RNA sequencing analysis suggested that prolyl hydroxylases, the oxygen-sensing negative regulators of Hif-1alpha protein stabilization, were significantly downregulated in this cardiomyocyte population (Kimura et al., 2015). More recently, Nakada and Canseco et al. (Nakada et al., 2017) demonstrated that gradual exposure to severe hypoxia blunted the oxidative metabolic phenotype of adult cardiomyocytes, decreased oxidative DNA damage, and induced cell-cycle progression. This was associated with modest functional recovery of LV systolic function when gradual hypoxia was initiated after permanent coronary ligation (Nakada et al., 2017). In support of this notion, Fan and colleagues recently demonstrated that deletion of prolyl hydroxylases 2 and 3 (PHD2 and PHD3) in endothelial cells induces cardiomyocyte proliferation and functional recovery after myocardial infarction in the adult heart, suggesting that hypoxia signaling in non-cardiomyocytes might play a critical role in cardiomyocyte cell-cycle regulation (Fan et al., 2019).
Several transcription factors have also been implicated in cardiomyocyte cell-cycle regulation and postnatal cell-cycle withdrawal of cardiomyocytes. GATA4, a member of the GATA family of transcription factors, is highly expressed in proliferative embryonic and early postnatal cardiomyocytes and is downregulated upon cell-cycle arrest. Forced expression of GATA4 prolongs the postnatal window of cardiomyocyte proliferation, whereas loss of GATA4 inhibits cardiomyocyte proliferation and neonatal heart regeneration (Malek Mohammadi et al., 2017). In addition, the homeodomain transcription factor Meis1 was recently demonstrated to regulate postnatal cardiomyocyte cell-cycle arrest (Mahmoud et al., 2013). Cardiomyocyte-specific loss of Meis1 prolonged the postnatal window of cardiomyocyte proliferation and reactivated the cardiomyocyte cell cycle in the adult heart. Mechanistically, Meis1 appears to transcriptionally activate cell-cycle inhibitors, including a number of cyclin-dependent kinase inhibitors (CDKIs). Finally, Tbx20, a member of the Tbx-1 subfamily of T-box genes that is required for embryonic cardiomyocyte proliferation (Greulich et al., 2011), was also shown to be promote heart regeneration in the adult mouse heart. In a recent report, overexpression of Tbx20 in the adult heart was found to induce cardiomyocyte proliferation and heart regeneration (Xiang et al., 2016). Tbx20, a transcriptional repressor, appears to inhibit the expression of the antiproliferative gene Btg2, which in turn induces cell-cycle arrest through repression of CyclinD1.
Lastly, microRNAs have also been tested in different contexts as potential regulators of cardiomyocyte proliferation. One of the first reports highlighted the role of the miR-15 family in the regulation of postnatal cell-cycle arrest and cardiomyocyte proliferation in the adult heart. Members of the miR-15 family are upregulated in the postnatal heart at a time point corresponding to cell-cycle arrest. In addition, inhibition of the miR-15 family prolonged the postnatal window of cardiomyocyte proliferation and improved cardiac function after infarction in young adult mice (Porrello et al., 2013). Perhaps one of the most striking demonstrations of the effect of microRNAs on cardiomyocyte cell-cycle regulation came from the Giacca group. In an elegant report, the group performed a screen on rat neonatal cardiomyocytes; the screen identified miR-199a as an important regulator of cardiomyocyte proliferation. This was confirmed using AAV-mediated overexpression in cardiomyocytes, which resulted in robust cardiomyocyte proliferation and a hyperplastic phenotype (Eulalio et al., 2012). The same AAV-mediated overexpression strategy in pigs resulted in robust cardiomyocyte proliferation resulting in functional recovery after injury. However, the intervention proved to be invariably lethal secondary to ventricular arrhythmias (Gabisonia et al., 2019). These studies highlight the potential difficulty in translating cardiomyocyte cell-cycle regulation studies to the clinic, especially because the earlier mouse studies that used miR-199a AAVs did not show evidence of arrythmias; this highlights the importance of large-animal studies before any of these therapies can be considered as a therapeutic option. It is important to note here that the aforementioned large-animal study, which used an Fstl-1 patch, did improve systolic function due to induction of cardiomyocyte proliferation in pigs and did not report a similar arrhythmogenic phenotype. It is worth noting here that the degree of induction of cardiomyocyte proliferation by miR-199a was more robust than that in response to Fstl-1, which may have played a role in ventricular arrhythmias.
The arrhythmogenic phenotype highlighted by Giacca and colleagues brings up an important translational question: is induction of cardiomyocyte proliferation safe? The simple answer is that we do not know. It is probably dependent on several factors, such as the magnitude and duration of induction of cardiomyocyte proliferation, the electrical and mechanical consequences of a particular intervention, and whether forced induction of cardiomyocyte proliferation induces cell death, to name a few. For example, a recent study suggested that dividing cardiomyocytes do not express connexin 43 at the time of cell division (Wang et al., 2017), a phenomenon that might play a role in the development of cardiac arrhythmias. However, in the pig miR-199a study (Gabisonia et al., 2019), the authors clearly showed that proliferative cardiomyocytes display a normal pattern of connexin 43 expression, and this highlights the fact that we still do not fully understand what factors mediate electrical instability secondary to forced induction of cardiomyocyte proliferation. Another consideration is the mechanical consequences of cardiomyocyte proliferation. For example, it is well established that dividing neonatal cardiomyocytes disassemble their sarcomeres at the time of mitosis with marginalization of sarcomeric proteins (possibly associating with the mitotic spindle), a phenomenon that is rarely seen in the adult heart even with forced induction of cardiomyocyte proliferation. In a recent report, induction of cardiomyocyte proliferation by forced expression of Erbb2 was found to induce sarcomere disassembly (although this manifested as the loss of sarcomeres rather than the typical marginalization of sarcomeres seen in the neonatal heart) and resulted in a significant drop in LV systolic function, which was readily reversible when proliferation stopped (D’Uva and Tzahor, 2015). Therefore, although it appears that forced induction of cardiomyocyte proliferation is a viable strategy for myocardial regeneration, potential complications must be considered when designing therapeutic strategies that might induce widespread, unchecked cardiomyocyte proliferation. In addition to the aforementioned factors, and depending on the target, caution is warranted when choosing to activate pro-mitotic pathways, which might have undesirable off-target effects, including induction of malignant transformation. In addition, in a “burned out myocardium” where there is a paucity of remaining cardiomyocytes that can divide, it is unclear whether induction of cardiomyocyte mitosis is a viable option, especially in light of the scalability issue of regenerating a human myocardium that has several billion cardiomyocytes. If these precautions can be adequately addressed, then in our view, induction of resident cardiomyocyte proliferation is a leading therapeutic goal.
Creation of New Cardiomyocytes
Another important strategy that has recently emerged is remuscularization of the myocardium via exogenous cardiomyocytes generated from either human embryonic stem cells (hESCs) (Chong et al., 2014) or induced pluripotent stem cells (iPSCs) (Shiba et al., 2016). Although the current review is not focused on this topic, it is our view that this represents one of the leading strategies for myocardial regeneration. Recent studies indicate that pluripotent-cell-derived cardiomyocytes can engraft into the myocardium of small and large animals, including primates. This is associated with electromechanical coupling with the native myocardium and improvement of LV systolic function. However, some concerns remain regarding the immaturity of the engrafted cardiomyocytes, the propensity for ventricular arrhythmias (Chong et al., 2014), and the need for immunosuppression where non-autologous cells are used (Menasché et al., 2018). In cases of ventricular aneurysm where the myocardium is replaced by a thin fibrotic scar, prefabricated grafts, which include several layers of cardiomyocytes with vascular and interstitial cells, might be a viable strategy.
Beyond strategies for promoting the proliferation of preexisting cardiomyocytes and the administration of hESC- or iPSC-derived myocytes, reprogramming of cardiac fibroblasts to cardiomyocytes with defined transcription factors, microRNAs, and small molecules represents an attractive alternative for repair of the adult heart. Initial demonstration of this approach was provided by the observation that three cardiogenic transcription factors, GATA4, Mef2c, and Tbx5 (abbreviated GMT), were capable of activating a subset of cardiac genes in cultured mouse fibroblasts (Ieda et al., 2010). A rare subset of these reprogrammed cells also displayed spontaneous contractility and sarcomere formation. However, the efficiency of this method was low, and only a small percentage of reprogrammed cells acquired the more mature phenotype. Subsequent studies from numerous labs revealed additional transcription factors, signaling molecules, micro RNAs, and chemicals that could further augment the reprogramming process, albeit still without complete efficiency (Jayawardena et al., 2012; Qian et al., 2012; Song et al., 2012; Zhao et al., 2015). Moreover, the reprogramming cocktail required for reprogramming human fibroblasts is different from that for mice (Nam et al., 2013), and human cells are clearly more resistant to the process, posing potential challenges for possible clinical translation.
Viral delivery of combinations of cardiogenic transcription factors in mice post-MI has shown significant generation of new cardiomyocytes with improved cardiac systolic function (Qian et al., 2012; Song et al., 2012). This approach has the advantage of targeting existing resident fibroblasts, which could have the dual effect of inhibiting fibrosis as well as generating new functional cardiomyocytes. Despite the relative inefficiency of reprogramming in vitro, studies thus far suggest that the process may be more efficient in vivo for reasons yet to be understood (Srivastava and DeWitt, 2016). Perhaps the persistent contractility of the heart or local cell-cell interactions and other signaling events that are lacking in culture are able to promote the phenotypic transformation in vivo.
Nevertheless, several notable challenges remain before the potential of fibroblast-to-cardiomyocyte reprogramming can be fully realized as a regenerative therapy. For example, the age of fibroblasts might be an important factor in the efficiency of reprogramming (Mahmoudi et al., 2019). Beyond the efficiency of the process, it will be important that reprogrammed cardiomyocytes adopt an adult phenotype and seamlessly couple with each other and with residual cardiomyocytes surrounding the infarct zone, so as to ensure rhythmic contractility and avoid arrhythmias. The longevity of reprogrammed cardiomyocytes in vivo also remains to be demonstrated. Whether they will last life-long remains unknown. Revascularization of the neomyocardium will also be essential. Finally, delivery remains a challenge. To date, local delivery of viral expression cassettes has been used for reprogramming studies in mice and pigs, but this requires open-chest surgery. It will be important to adapt and optimize these delivery methods to less invasive, catheter-based approaches. Safety and efficacy studies of optimized reprogramming cocktails in large-animal models of heart disease represent important additional pre-clinical goals. Expanding the potential of reprogramming beyond post-MI cardiac remodeling to other forms of heart disease could also have an important impact on cardiovascular medicine.
Prospects for the Future
Regenerating the human myocardium remains a laudable goal, and despite misfires and controversies, the field of myocardial regeneration has, by all accounts, made significant strides in understanding key mechanisms and pathways. A clear path to myocardial regeneration is now starting to emerge. As outlined earlier, we envision three main strategies that have the highest likelihood of yielding clinically relevant therapeutics; these include induction of cardiomyocyte proliferation, the use of pluripotent-stem-cell-derived cardiomyocytes, and direct reprogramming. Although large-animal and primate studies that examined the role of all three strategies in myocardial regeneration have been conducted, little progress has been made in developing pharmacological therapeutics for induction of cardiomyocyte proliferation. Although concerns for neoplastic transformation and off-target effects are valid, these concerns should not dissuade us from attempting to develop pro-regenerative drugs, given the transient nature of the potential intervention and the possibility of identifying non-tumorigenic targets. Importantly, although myocardial regeneration is the goal in conditions where the underlying cause of cardiomyopathy is cardiomyocyte loss, there are numerous other scenarios where the disease complexity goes beyond replenishing lost cardiomyocytes. For example, neovascularization might be needed before attempting myocardial regeneration in some cases of ischemic cardiomyopathy. In addition, in some scenarios of dilated cardiomyopathy (DCM), where the underlying etiology is genetic aberrations resulting in poor contractile force even in the absence of cell death, or in conditions of infiltrative cardiomyopathy, generating new cardiomyocytes is likely to have little to no role in functional recovery. Nevertheless, in the vast majority of heart failure scenarios associated with systolic dysfunction, the underlying mechanism is cardiomyocyte loss, for which the ultimate therapeutic goal is new cardiomyocyte generation. In summary, continuing to focus on fundamental biology while concomitantly developing innovative therapeutic strategies that are rooted in solid basic science will remain the best strategy for finding a cure for heart failure.
ACKNOWLEDGMENTS
H. A.S. was supported by grants from the National Institutes of Health (NIH) (R01HL13177803, 5R01H2131778, and R01HL147276), the American Heart Association (16EIA27740034), and the Fondation Leducq. E.N.O. was supported by grants from the NIH (AR-067294, HL-130253, and DK-099653) and the Robert A. Welch Foundation (grant 1-0025).
REFERENCES
- Balsam LB, Wagers AJ, Christensen JL, Kofidis T, Weissman IL, and Robbins RC (2004). Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature 428, 668–673. [DOI] [PubMed] [Google Scholar]
- Bassat E, Mutlak YE, Genzelinakh A, Shadrin IY, Baruch Umansky K, Yifa O, Kain D, Rajchman D, Leach J, Riabov Bassat D, et al. (2017). The extracellular matrix protein agrin promotes heart regeneration in mice. Nature 547, 179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker RO, Chapin S, and Sherry R (1974). Regeneration of the ventricular myocardium in amphibians. Nature 248, 145–147. [DOI] [PubMed] [Google Scholar]
- Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, et al. (2003). Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell 114, 763–776. [DOI] [PubMed] [Google Scholar]
- Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabé-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, et al. (2009). Evidence for cardiomyocyte renewal in humans. Science 324, 98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann O, Zdunek S, Felker A, Salehpour M, Alkass K, Bernard S, Sjostrom SL, Szewczykowska M, Jackowska T, Dos Remedios C, et al. (2015). Dynamics of cell generation and turnover in the human heart. Cell 161, 1566–1575. [DOI] [PubMed] [Google Scholar]
- Cano-Martínez A, Vargas-González A, Guarner-Lans V, Prado-Zayago E, León-Oleda M, and Nieto-Lima B (2010). Functional and structural regeneration in the axolotl heart (Ambystoma mexicanum) after partial ventricular amputation. Arch. Cardiol. Mex 80, 79–86. [PubMed] [Google Scholar]
- Canseco DC, Kimura W, Garg S, Mukherjee S, Bhattacharya S, Abdisalaam S, Das S, Asaithamby A, Mammen PP, and Sadek HA (2015). Human ventricular unloading induces cardiomyocyte proliferation. J. Am. Coll. Cardiol 65, 892–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry HW, Dashoush NH, Tang H, Zhang L, Wang X, Wu EX, and Wolgemuth DJ (2004). Cyclin A2 mediates cardiomyocyte mitosis in the postmitotic myocardium. J. Biol. Chem 279, 35858–35866. [DOI] [PubMed] [Google Scholar]
- Chen Z, Xie J, Hao H, Lin H, Wang L, Zhang Y, Chen L, Cao S, Huang X, Liao W, et al. (2017). Ablation of periostin inhibits post-infarction myocardial regeneration in neonatal mice mediated by the phosphatidylinositol 3 kinase/glycogen synthase kinase 3β/cyclin D1 signalling pathway. Cardiovasc. Res 113, 620–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien KR, Frisén J, Fritsche-Danielson R, Melton DA, Murry CE, and Weissman IL (2019). Regenerating the field of cardiovascular cell therapy. Nat. Biotechnol 37, 232–237. [DOI] [PubMed] [Google Scholar]
- Chong JJ, Yang X, Don CW, Minami E, Liu YW, Weyers JJ, Mahoney WM, Van Biber B, Cook SM, Palpant NJ, et al. (2014). Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature 510, 273–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Uva G, Aharonov A, Lauriola M, Kain D, Yahalom-Ronen Y, Carvalho S, Weisinger K, Bassat E, Rajchman D, Yifa O, et al. (2015). ERBB2 triggers mammalian heart regeneration by promoting cardiomyocyte dedifferentiation and proliferation. Nat. Cell Biol 17, 627–638. [DOI] [PubMed] [Google Scholar]
- D’Uva G, and Tzahor E (2015). The key roles of ERBB2 in cardiac regenerationation. Cell Cycle 14, 2383–2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elhelaly WM, Cardoso AC, Pereira AHM, Elnawasany A, Ebrahimi S, Nakada Y, and Sadek HA (2019). C-kit cells do not significantly contribute to cardiomyogenesis during neonatal heart regeneration. Circulation 139, 559–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eschenhagen T, Bolli R, Braun T, Field LJ, Fleischmann BK, Frisén J, Giacca M, Hare JM, Houser S, Lee RT, et al. (2017). Cardiomyocyte regeneration: A consensus statement. Circulation 136, 680–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eulalio A, Mano M, Dal Ferro M, Zentilin L, Sinagra G, Zacchigna S, and Giacca M (2012). Functional screening identifies miRNAs inducing cardiac regeneration. Nature 492, 376–381. [DOI] [PubMed] [Google Scholar]
- Fan Q, Mao H, Angelini A, Coarfa C, Robertson MJ, Lagor WR, Wehrens XHT, Martin JF, Pi X, and Xie L (2019). Depletion of endothelial prolyl hydroxylase domain protein 2 and 3 promotes cardiomyocyte proliferation and prevents ventricular failure induced by myocardial infarction. Circulation 140, 440–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng T, Meng J, Kou S, Jiang Z, Huang X, Lu Z, Zhao H, Lau LF, Zhou B, and Zhang H (2019). CCN1-induced cellular senescence promotes heart regeneration. Circulation 139, 2495–2498. [DOI] [PubMed] [Google Scholar]
- Fisher SA, Brunskill SJ, Doree C, Mathur A, Taggart DP, and Martin-Rendon E (2014). Stem cell therapy for chronic ischaemic heart disease and congestive heart failure. Cochrane Database Syst. Rev. (4), CD007888. [DOI] [PubMed] [Google Scholar]
- Flink IL (2002). Cell cycle reentry of ventricular and atrial cardiomyocytes and cells within the epicardium following amputation of the ventricular apex in the axolotl, Amblystoma mexicanum: Confocal microscopic immunofluorescent image analysis of bromodeoxyuridine-labeled nuclei. Anat. Embryol. (Berl.) 205, 235–244. [DOI] [PubMed] [Google Scholar]
- Gabisonia K, Prosdocimo G, Aquaro GD, Carlucci L, Zentilin L, Secco I, Ali H, Braga L, Gorgodze N, Bernini F, et al. (2019). MicroRNA therapy stimulates uncontrolled cardiac repair after myocardial infarction in pigs. Nature 569, 418–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Xu C, Asada N, and Frenette PS (2018). The hematopoietic stem cell niche: From embryo to adult. Development 145, dev139691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemberling M, Karra R, Dickson AL, and Poss KD (2015). Nrg1 is an injury-induced cardiomyocyte mitogen for the endogenous heart regeneration program in zebrafish. eLife 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold MR, Van Veldhuisen DJ, Hauptman PJ, Borggrefe M, Kubo SH, Lieberman RA, Milasinovic G, Berman BJ, Djordjevic S, Neelagaru S, et al. (2016). Vagus nerve stimulation for the treatment of heart failure: The IN-OVATE-HF trial. J. Am. Coll. Cardiol 68, 149–158. [DOI] [PubMed] [Google Scholar]
- Greulich F, Rudat C, and Kispert A (2011). Mechanisms of T-box gene function in the developing heart. Cardiovasc. Res 91, 212–222. [DOI] [PubMed] [Google Scholar]
- Heallen T, Zhang M, Wang J, Bonilla-Claudio M, Klysik E, Johnson RL, and Martin JF (2011). Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size. Science 332, 458–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herget GW, Neuburger M, Plagwitz R, and Adler CP (1997). DNA content, ploidy level and number of nuclei in the human heart after myocardial infarction. Cardiovasc. Res 36, 45–51. [DOI] [PubMed] [Google Scholar]
- Hodgkinson CP, Kang MH, Dal-Pra S, Mirotsou M, and Dzau VJ (2015). MicroRNAs and cardiac regeneration. Circ. Res 116, 1700–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ieda M, Fu JD, Delgado-Olguin P, Vedantham V, Hayashi Y, Bruneau BG, and Srivastava D (2010). Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell 142, 375–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayawardena TM, Egemnazarov B, Finch EA, Zhang L, Payne JA, Pandya K, Zhang Z, Rosenberg P, Mirotsou M, and Dzau VJ (2012). MicroRNA-mediated in vitro and in vivo direct reprogramming of cardiac fibroblasts to cardiomyocytes. Circ. Res 110, 1465–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz MG, Fargnoli AS, Kendle AP, Hajjar RJ, and Bridges CR (2016). The role of microRNAs in cardiac development and regenerative capacity. Am. J. Physiol. Heart Circ. Physiol 310, H528–H541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khera R, Pandey A, Ayers CR, Agusala V, Pruitt SL, Halm EA, Drazner MH, Das SR, de Lemos JA, and Berry JD (2017). Contemporary epidemiology of heart failure in fee-for-service medicare beneficiaries across healthcare settings. Circ Heart Fail 10, e004402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura W, Xiao F, Canseco DC, Muralidhar S, Thet S, Zhang HM, Abderrahman Y, Chen R, Garcia JA, Shelton JM, et al. (2015). Hypoxia fate mapping identifies cycling cardiomyocytes in the adult heart. Nature 523, 226–230. [DOI] [PubMed] [Google Scholar]
- Kühn B, del Monte F, Hajjar RJ, Chang YS, Lebeche D, Arab S, and Keating MT (2007). Periostin induces proliferation of differentiated cardiomyocytes and promotes cardiac repair. Nat. Med 13, 962–969. [DOI] [PubMed] [Google Scholar]
- Lemischka IR, Raulet DH, and Mulligan RC (1986). Developmental potential and dynamic behavior of hematopoietic stem cells. Cell 45, 917–927. [DOI] [PubMed] [Google Scholar]
- Li F, Wang X, Capasso JM, and Gerdes AM (1996). Rapid transition of cardiac myocytes from hyperplasia to hypertrophy during postnatal development. J. Mol. Cell. Cardiol 28, 1737–1746. [DOI] [PubMed] [Google Scholar]
- Li M, Zheng C, Sato T, Kawada T, Sugimachi M, and Sunagawa K (2004). Vagal nerve stimulation markedly improves long-term survival after chronic heart failure in rats. Circulation 109, 120–124. [DOI] [PubMed] [Google Scholar]
- Li Y, He L, Huang X, Bhaloo SI, Zhao H, Zhang S, Pu W, Tian X, Li Y, Liu Q, et al. (2018). Genetic lineage tracing of nonmyocyte population by dual recombinases. Circulation 138, 793–805. [DOI] [PubMed] [Google Scholar]
- Li Y, Lv Z, He L, Huang X, Zhang S, Zhao H, Pu W, Li Y, Yu W, Zhang L, et al. (2019). Genetic tracing identifies early segregation of the cardiomyocyte and nonmyocyte lineages. Circ. Res 125, 343–355. [DOI] [PubMed] [Google Scholar]
- Lin Z, Zhou P, von Gise A, Gu F, Ma Q, Chen J, Guo H, van Gorp PR, Wang DZ, and Pu WT (2015). Pi3kcb links Hippo-YAP and PI3K-AKT signaling pathways to promote cardiomyocyte proliferation and survival. Circ. Res 116, 35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Yue S, Chen X, Kubin T, and Braun T (2010). Regulation of cardiomyocyte polyploidy and multinucleation by CyclinG1. Circ. Res 106, 1498–1506. [DOI] [PubMed] [Google Scholar]
- Lorts A, Schwanekamp JA, Elrod JW, Sargent MA, and Molkentin JD (2009). Genetic manipulation of periostin expression in the heart does not affect myocyte content, cell cycle activity, or cardiac repair. Circ. Res 104, e1–e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud AI, Kocabas F, Muralidhar SA, Kimura W, Koura AS, Thet S, Porrello ER, and Sadek HA (2013). Meis1 regulates postnatal cardiomyocyte cell cycle arrest. Nature 497, 249–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud AI, O’Meara CC, Gemberling M, Zhao L, Bryant DM, Zheng R, Gannon JB, Cai L, Choi WY, Egnaczyk GF, et al. (2015). Nerves regulate cardiomyocyte proliferation and heart regeneration. Dev. Cell 34, 387–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoudi S, Mancini E, Xu L, Moore A, Jahanbani F, Hebestreit K, Srinivasan R, Li X, Devarajan K, Prélot L, et al. (2019). Heterogeneity in old fibroblasts is linked to variability in reprogramming and wound healing. Nature 574, 553–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malek Mohammadi M, Kattih B, Grund A, Froese N, Korf-Klingebiel M, Gigina A, Schrameck U, Rudat C, Liang Q, Kispert A, et al. (2017). The transcription factor GATA4 promotes myocardial regeneration in neonatal mice. EMBO Mol. Med 9, 265–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menasché P, Alfieri O, Janssens S, McKenna W, Reichenspurner H, Trinquart L, Vilquin JT, Marolleau JP, Seymour B, Larghero J, et al. (2008). The Myoblast Autologous Grafting in Ischemic Cardiomyopathy (MAGIC) trial: First randomized placebo-controlled study of myoblast transplantation. Circulation 117, 1189–1200. [DOI] [PubMed] [Google Scholar]
- Menasché P, Vanneaux V, Hagège A, Bel A, Cholley B, Parouchev A, Cacciapuoti I, Al-Daccak R, Benhamouda N, Blons H, et al. (2018). Transplantation of human embryonic stem cell-derived cardiovascular progenitors for severe ischemic left ventricular dysfunction. J. Am. Coll. Cardiol 71, 429–438. [DOI] [PubMed] [Google Scholar]
- Mohamed TMA, Ang YS, Radzinsky E, Zhou P, Huang Y, Elfenbein A, Foley A, Magnitsky S, and Srivastava D (2018). Regulation of cell cycle to stimulate adult cardiomyocyte proliferation and cardiac regeneration. Cell 173, 104–116.e112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretti A, Caron L, Nakano A, Lam JT, Bernshausen A, Chen Y, Qyang Y, Bu L, Sasaki M, Martin-Puig S, et al. (2006). Multipotent embryonic isl1 + progenitor cells lead to cardiac, smooth muscle, and endothelial cell diversification. Cell 127, 1151–1165. [DOI] [PubMed] [Google Scholar]
- Mosterd A, and Hoes AW (2007). Clinical epidemiology of heart failure. Heart 93, 1137–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murry CE, Soonpaa MH, Reinecke H, Nakajima H, Nakajima HO, Rubart M, Pasumarthi KB, Virag JI, Bartelmez SH, Poppa V, et al. (2004). Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature 428, 664–668. [DOI] [PubMed] [Google Scholar]
- Nakada Y, Canseco DC, Thet S, Abdisalaam S, Asaithamby A, Santos CX, Shah AM, Zhang H, Faber JE, Kinter MT, et al. (2017). Hypoxia induces heart regeneration in adult mice. Nature 541, 222–227. [DOI] [PubMed] [Google Scholar]
- Nam YJ, Song K, Luo X, Daniel E, Lambeth K, West K, Hill JA, Di-Maio JM, Baker LA, Bassel-Duby R, and Olson EN (2013). Reprogramming of human fibroblasts toward a cardiac fate. Proc. Natl. Acad. Sci. USA 110, 5588–5593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi N, Li M, Calvert JW, Tejada T, Lambert JP, Wu J, Kesteven SH, Holman SR, Matsuda T, Lovelock JD, et al. (2014). A proliferative burst during preadolescence establishes the final cardiomyocyte number. Cell 157, 795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberpriller JO, and Oberpriller JC (1974). Response of the adult newt ventricle to injury. J. Exp. Zool 187, 249–253. [DOI] [PubMed] [Google Scholar]
- Orlic D, Kajstura J, Chimenti S, Bodine DM, Leri A, and Anversa P (2001a). Transplanted adult bone marrow cells repair myocardial infarcts in mice. Ann. N Y Acad. Sci 938, 221–229, discussion 229–230. [DOI] [PubMed] [Google Scholar]
- Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, et al. (2001b). Bone marrow cells regenerate infarcted myocardium. Nature 470, 701–705. [DOI] [PubMed] [Google Scholar]
- Pasumarthi KB, Nakajima H, Nakajima HO, Soonpaa MH, and Field LJ (2005a). Targeted expression of cyclin D2 results in cardiomyocyte DNA synthesis and infarct regression in transgenic mice. Circ. Res 96, 110–118. [DOI] [PubMed] [Google Scholar]
- Polizzotti BD, Ganapathy B, Walsh S, Choudhury S, Ammanamanchi N, Bennett DG, dos Remedios CG, Haubner BJ, Penninger JM, and Kühn B (2015). Neuregulin stimulation of cardiomyocyte regeneration in mice and human myocardium reveals a therapeutic window. Sci. Transl. Med 7, 281ra45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, Olson EN, and Sadek HA (2011). Transient regenerative potential of the neonatal mouse heart. Science 331, 1078–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrello ER, Mahmoud AI, Simpson E, Johnson BA, Grinsfelder D, Canseco D, Mammen PP, Rothermel BA, Olson EN, and Sadek HA (2013). Regulation of neonatal and adult mammalian heart regeneration by the miR-15 family. Proc. Natl. Acad. Sci. USA 110, 187–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poss KD, Wilson LG, and Keating MT (2002). Heart regeneration in zebrafish. Science 298, 2188–2190. [DOI] [PubMed] [Google Scholar]
- Puente BN, Kimura W, Muralidhar SA, Moon J, Amatruda JF, Phelps KL, Grinsfelder D, Rothermel BA, Chen R, Garcia JA, et al. (2014). The oxygen-rich postnatal environment induces cardiomyocyte cell-cycle arrest through DNA damage response. Cell 157, 565–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian L, Huang Y, Spencer CI, Foley A, Vedantham V, Liu L, Con-way SJ, Fu JD, and Srivastava D (2012). In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature 485, 593–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saga Y, Hata N, Kobayashi S, Magnuson T, Seldin MF, and Taketo MM (1996). MesP1: A novel basic helix-loop-helix protein expressed in the nascent mesodermal cells during mouse gastrulation. Development 122, 2769–2778. [DOI] [PubMed] [Google Scholar]
- Sahara M, Santoro F, and Chien KR (2015). Programming and reprogramming a human heart cell. EMBO J. 34, 710–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarig R, Rimmer R, Bassat E, Zhang L, Umansky KB, Lendengolts D, Perlmoter G, Yaniv K, and Tzahor E (2019). Transient p53-mediated regenerative senescence in the injured heart. Circulation 139, 2491–2494. [DOI] [PubMed] [Google Scholar]
- Senyo SE, Steinhauser ML, Pizzimenti CL, Yang VK, Cai L, Wang M, Wu T-D, Guerquin-Kern J-L, Lechene CP, and Lee RT (2013). Mammalian heart renewal by pre-existing cardiomyocytes. Nature 493, 433–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro SD, Ranjan AK, Kawase Y, Cheng RK, Kara RJ, Bhattacharya R, Guzman-Martinez G, Sanz J, Garcia MJ, and Chaudhry HW (2014). Cyclin A2 induces cardiac regeneration after myocardial infarction through cytokinesis of adult cardiomyocytes. Sci. Transl. Med 6, 224ra27. [DOI] [PubMed] [Google Scholar]
- Shiba Y, Gomibuchi T, Seto T, Wada Y, Ichimura H, Tanaka Y, Ogasawara T, Okada K, Shiba N, Sakamoto K, et al. (2016). Allogeneic transplantation of iPS cell-derived cardiomyocytes regenerates primate hearts. Nature 538, 388–391. [DOI] [PubMed] [Google Scholar]
- Song K, Nam Y-J, Luo X, Qi X, Tan W, Huang GN, Acharya A, Smith CL, Tallquist MD, Neilson EG, et al. (2012). Heart repair by reprogramming non-myocytes with cardiac transcription factors. Nature 485, 599–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava D, and DeWitt N (2016). In vivo cellular reprogramming: The next generation. Cell 166, 1386–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturzu AC, Rajarajan K, Passer D, Plonowska K, Riley A, Tan TC, Sharma A, Xu AF, Engels MC, Feistritzer R, et al. (2015). Fetal mammalian heart generates a robust compensatory response to cell loss. Circulation 132,109–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultana N, Zhang L, Yan J, Chen J, Cai W, Razzaque S, Jeong D, Sheng W, Bu L, Xu M, et al. (2015). Resident c-kit(+) cells in the heart are not cardiac stem cells. Nat. Commun 6, 8701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao G, Kahr PC, Morikawa Y, Zhang M, Rahmani M, Heallen TR, Li L, Sun Z, Olson EN, Amendt BA, and Martin JF (2016). Pitx2 promotes heart repair by activating the antioxidant response after cardiac injury. Nature 534, 119–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vagnozzi RJ, Maillet M, Sargent MA, Khalil H, Johansen AK, Schwanekamp JA, York AJ, Huang V, Nahrendorf M, Sadayappan S, and Molkentin JD (2019). An acute immune response underlies the benefit of cardiac stem-cell therapy. Nature. 10.1038/s41586-019-1802-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Berlo JH, Kanisicak O, Maillet M, Vagnozzi RJ, Karch J, Lin SC, Middleton RC, Marbán E, and Molkentin JD (2014). c-kit+ cells minimally contribute cardiomyocytes to the heart. Nature 509, 337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Gise A, Lin Z, Schlegelmilch K, Honor LB, Pan GM, Buck JN, Ma Q, Ishiwata T, Zhou B, Camargo FD, and Pu WT (2012). YAP1, the nuclear target of Hippo signaling, stimulates heart growth through cardiomyocyte proliferation but not hypertrophy. Proc. Natl. Acad. Sci. USA 109, 2394–2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WE, Li L, Xia X, Fu W, Liao Q, Lan C, Yang D, Chen H, Yue R, Zeng C, et al. (2017). Dedifferentiation, proliferation, and redifferentiation of adult mammalian cardiomyocytes after ischemic injury. Circulation 136, 834–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei K, Serpooshan V, Hurtado C, Diez-Curñado M, Zhao M, Maruyama S, Zhu W, Fajardo G, Noseda M, Nakamura K, et al. (2015). Epicardial FSTL1 reconstitution regenerates the adult mammalian heart. Nature 525, 479–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White IA, Gordon J, Balkan W, and Hare JM (2015). Sympathetic reinnervation is required for mammalian cardiac regeneration. Circ. Res. 117, 990–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang FL, Guo M, and Yutzey KE (2016). Overexpression of Tbx20 in adult cardiomyocytes promotes proliferation and improves cardiac function after myocardial infarction. Circulation 133, 1081–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin M, Kim Y, Sutherland LB, Qi X, McAnally J, Schwartz RJ, Richardson JA, Bassel-Duby R, and Olson EN (2011). Regulation of insulin-like growth factor signaling by Yap governs cardiomyocyte proliferation and embryonic heart size. Sci. Signal. 4, ra70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin M, Kim Y, Sutherland LB, Murakami M, Qi X, McAnally J, Porrello ER, Mahmoud AI, Tan W, Shelton JM, et al. (2013). Hippo pathway effector Yap promotes cardiac regeneration. Proc. Natl. Acad. Sci. USA 110, 13839–13844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr., Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, et al. (2017). 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J. Card. Fail 23, 628–651. [DOI] [PubMed] [Google Scholar]
- Ye L, D’Agostino G, Loo SJ, Wang CX, Su LP, Tan SH, Tee GZ, Pua CJ, Pena EM, Cheng RB, et al. (2018). Early regenerative capacity in the porcine heart. Circulation 138, 2798–2808. [DOI] [PubMed] [Google Scholar]
- Yeh RW, Sidney S, Chandra M, Sorel M, Selby JV, and Go AS (2010). Population trends in the incidence and outcomes of acute myocardial infarction. N. Engl. J. Med 362, 2155–2165. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Mignone J, and MacLellan WR (2015). Cardiac regeneration and stem cells. Physiol. Rev 95, 1189–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Londono P, Cao Y, Sharpe EJ, Proenza C, O’Rourke R, Jones KL, Jeong MY, Walker LA, Buttrick PM, et al. (2015). High-efficiency reprogramming of fibroblasts into cardiomyocytes requires suppression of pro-fibrotic signalling. Nat. Commun 6, 8243. [DOI] [PMC free article] [PubMed] [Google Scholar]