Abstract
Background
Peritoneal fibrosis (PF) is a frequent complication caused by peritoneal dialysis (PD). Peritoneal mesothelial cells (PMCs), the first barrier of the peritoneum, play an important role in maintaining structure and function in the peritoneum during PD. Mesothelial-mesenchymal transition (MMT) and oxidative stress of PMCs are two key processes of PF.
Purpose
To elucidate the efficacy and possible mechanism of asiaticoside inhibition of MMT and ROS generation in TGF-β1-induced PF in human peritoneal mesothelial cells (HPMCs).
Methods
MMT and ROS generation of HPMCs were induced by TGF-β1. To explain the anti-MMT and antioxidant role of asiaticoside, varied doses of asiaticoside, oxygen radical scavenger (NAC), TGF-β receptor kinase inhibitor (LY2109761) and Nrf2 inhibitor (ML385) were used separately. Immunoblots were used to detect the expression of signaling associated proteins. DCFH-DA was used to detect the generation of ROS. Transwell migration assay and wound healing assay were used to verify the capacity of asiaticoside to inhibit MMT. Immunofluorescence assay was performed to observe the subcellular translocation of Nrf2 and expression of HO-1.
Results
Asiaticoside inhibited TGF-β1-induced MMT and suppressed Smad signaling in a dose-dependent manner. Migration and invasion activities of HPMCs were decreased by asiaticoside. Asiaticoside decreased TGF-β1-induced ROS, especially in a high dose (150 μM) for 6 h. Furthermore, ML385 partly abolished the inhibitory effect of asiaticoside on MMT, ROS and p-Smad2/3.
Conclusions
Asiaticoside inhibited the TGF-β1-induced MMT and ROS via Nrf2 activation, thus protecting the peritoneal membrane and preventing PF.
Keywords: Human peritoneal mesothelial cells (HPMCs), Mesothelial-mesenchymal transition (MMT), Reactive oxygen species (ROS), TGF-β/Smad, Nrf-2/HO-1
Introduction
Peritoneal fibrosis (PD) is one of the effective treatments for patients with end-stage renal disease (ESRD), whose efficacy depends on the structure and function of the peritoneum. Compared with hemodialysis (HD), PD has the advantage of retention of residual renal function (RRF), leading to a higher quality of life [1]. HD and PD treatment have the same mortality and their impact on survival does not seem to change over time [2]. The PMC monolayer is the first barrier against external injury factors, maintaining the peritoneum integrity, functional stability and damage repair [3]. Under physiological conditions, PMCs secrete various cytokines to perform immune surveillance and regulate inflammation and tissue repair, maintaining the structure and function of the peritoneum. Unfortunately, accumulated evidence has highlighted that long-term exposure to conventional PD solutions (PDS) may damage morphology and function of PMCs, leading to progressive PF and dialysis failure [4].
MMT has been widely considered as an early and crucial process in PF [5]. Despite recent controversies about the source of myofibroblasts [6, 7], MMT is still a potential therapeutic target for the prevention of PF [8]. Oxidative stress is a disordered state between oxidative molecules and insufficient antioxidant defense, causing tissue injury and systemic damage. Currently, a growing number of studies have found that PDS-induced ROS may alter peritoneal structure and function during long-term PD, whereas antioxidants may prevent such changes [9]. It has been reported that several signaling pathways, including oxidative stress, TGF-β/Smad, non-Smad and noncoding RNAs, participate in regulating the process of MMT [10]. Myofibroblasts are known to provide an unfavorable environment for generation of pro-fibrotic cytokines and ROS. These results suggest that MMT and ROS generation are important causes of PF in PD. Patients with these complications ultimately quit PD after several years of therapy.
To clarify the role of asiaticoside in inhibiting TGF-β1-induced MMT and ROS generation, we focused on the pro-fibrotic signaling pathway TGF-β/Smad and the antioxidant signaling pathway Nrf2/HO-1 to illustrate a potential method for inhibiting PF. TGF-β1 promotes fibrosis mainly by activating TGF-β type I and type II receptors, and then activating Smad protein to mediate MMT [11]. The transcription factor nuclear factor erythroid-2-related factor 2 (Nrf2) could regulate the induction of genes encoding antioxidant proteins and phase2 detoxifying enzymes by activating antioxidant response elements (AREs). In addition, heme oxygenase 1 (HO-1) responds to oxidative stress and is up-regulated by Nrf2, exerting antioxidant properties [12]. Several recent studies have shown that Nrf2 alleviates kidney damage by inducing antioxidant enzymes in vivo and in vitro [13]. Despite a deep understanding of the mechanism of PF, there is no effective treatment for this. Therefore, effective antifibrotic therapies remain to be explored. To date, several studies have focused on herbal medicine as alternative treatments.
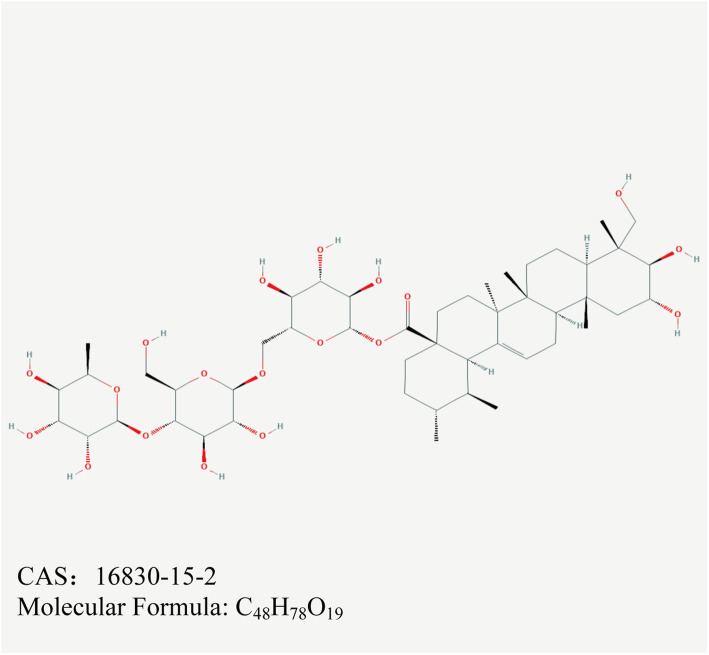
Centella asiatica (L.) Urban (Apiaceae) has been used in traditional Chinese medicine in treating various diseases for over 2000 years. Asiaticoside (shown in Fig.1) is the main component of triterpenoid saponins extracted from Centella asiatica with a clear formula. Emerging evidence has indicated that asiaticoside shows antioxidant, anti-inflammatory, anti-fibrotic and other pharmacological effects [14–16]. In the PD field, the protective effects of asiaticoside against MMT and PD-related ROS remain unknown. In this study, we used TGF-β1-induced HPMCs to investigate the role of asiaticoside in MMT and ROS generation and to elucidate its underlying mechanisms.
Fig. 1.
Chemical structure of asiaticoside. (Abbreviated AS in the figures)
Materials and methods
Cell lines and culture conditions
HMrSV5 cells (Lian Mai Bioengineering Co., Ltd., Shanghai, China) are immortal cell lines and are equivalent to HPMCs isolated from human peritoneum. HPMCs were cultured in 1640 basic medium (RPMI 1640; Gibco, USA) supplemented with 1% penicillin-streptomycin (Invitrogen; Carlsbad, CA, USA) and 10% fetal calf serum (FCS; Invitrogen) in a humidified incubator with 5% CO2 at 37 °C. All experiments were carried out after cells were seeded in culture plates containing 1% FCS for 24 h. 10 ng/cm3 TGF-β1 (R&D; Minneapolis, MN, USA) was used to induce MMT and ROS in HPMCs. Asiaticoside (C48H78O19; CAS: 16830–15-2; HPLC ≥98%; Yuanye Biotechnology Co., Ltd. Shanghai, China) was dissolved in DMSO for a stock concentration of 1.5 × 105 μM. The final concentration of DMSO in the medium was lower than 0.1% to avoid affecting the cell viability.
Cell viability assay
Cell Counting Kit-8 (CCK-8; Dojindo, Kumamoto, Japan) was used to measure cell viability. Cells were seeded at a density of 2 × 103 cells per well in 96-well plates and subjected to various interventions. Then CCK-8 solution (10 mm3) was added to each well, incubating for another 1 h at 37 °C. Optical density was measured at 450 nm (Bio-Rad 550, USA).
Immunoblotting assay
Cells were lysed in ice-cold RIPA lysis buffer (Thermo Fisher Scientific, Waltham, MA, USA) containing 0.1 mM PMSF. The lysates were centrifuged, and the supernatants were collected for immunoblotting. NE-PER nuclear and cytoplasmic extraction reagents (Thermo) were used to obtain nuclear and cytoplasmic proteins, respectively. The protein concentration was measured using the BCA Protein Assay Kit (Thermo). Extracted protein lysates were separated at a quality of 20 μg/lane using SDS-PAGE and electro transferred onto PVDF membranes. After blocking with 5% BSA in TBST, the membranes were incubated with primary antibody at 4 °C overnight, followed by incubation with HRP-conjugated anti-mouse/rabbit IgG secondary antibodies for 1 h. Finally, the bands were soaked with immobilon ECL ultra western HRP substrate (Millipore, Bedford, USA) and visualized using a chemidoc imaging system. The following antibodies were used: E-Cadherin (3195), Vimentin (5741), α-SMA (19245), p-Smad2/3 (8828), Smad2/3 (8685), β-actin (4970), H3 (4499) and secondary HRP-conjugated anti-rabbit (7074) antibodies were obtained from Cell Signaling Technology (Boston, MA, USA). HO-1 (sc136960) and secondary HRP-conjugated anti-mouse (sc516102) antibodies were obtained from Santa Cruz Biotechnology. Nrf2 (ab62352) was obtained from Abcam (Cambridge, UK). TGF-β1 receptor blocker LY2109761 was obtained from APExBIO Technology (Houston, USA). Nrf2 inhibitor ML385 was obtained from Selleck Chemicals (Houston, TX, USA).
Transwell migration assay
The transwell chamber was placed in a 24-well plate, and HPMC cells at the logarithmic phase (treated with 150 μM asiaticoside with or without 10 ng/cm3 TGF-β1 for 24 h) were selected, which were then digested and centrifuged, and the cells were diluted with serum-free RPMI 1640 medium to 2.5 × 105/cm3. 200 mm3 of cell suspension was added to each upper transwell chamber (pore size = 8 μm), and 800 mm3 of 1640 basic medium was added to each well of the lower chamber. After 24 h of culture, the chamber was removed, and the upper layer was wiped off with a cotton swab. Migrated cells were fixed with 4% paraformaldehyde for 15 min and stained with 0.1% crystal violet for 15 min. Five microscopic fields were selected randomly to take pictures and calculate the number of migrated cells.
Wound healing assay
The wound healing assay was used to detect cell migratory ability. HPMCs (1.0 × 105/cm3) were seeded in 24-well plates. After cells grew to 90–100% confluence, a wound line was produced with a sterile pipette tip based on a ruler. Cellular debris was removed by washing with PBS, and cells were allowed to migrate for 24 h after intervention. Images were taken at 0 and 24 h after wounding under an Olympus BX45 inverted microscope. The relative distance traveled by the leading edge from 0 to 24 h was detected using ImageJ software (n = 5).
Measurement of ROS levels
The generation of ROS was detected using 2′,7′-dichloroflfluorescin diacetate (DCFH-DA; Sigma, Saint Louis, MO, USA). After treatment, cells were incubated with 30 μM DCFH-DA in a dark environment at 37 °C for 30 min. After three washes with PBS, each well was fixed with 1 cm3 formaldehyde for 30 min at room temperature. After three washes with PBS, the cells were analyzed using a fluorescence microscope (Carl Zeiss, Oberkochen, Germany). Cells with green fluorescence were considered as ROS-positive ones (at least 50 cells from a single capture field).
Immunofluorescence assay
Cells (5 × 104/well) were grown to adherence on 8-well glass Nunc Lab-Tek chamber slides and were treated with TGF-β1 with or without asiaticoside. After treatment for 24 h, the slides were washed to fix, permeate, and block. Next, cells were incubated with primary antibody at 4 °C overnight, and then incubated with fluorescent secondary antibody for 1 h at room temperature, and stained with DAPI (Beyotime Biotechnology Co., Ltd. Shanghai, China) for 15 min. Images were acquired with a Zeiss AX10 fluorescence microscope.
Statistical analysis
We performed at least 3 individual experiments for each object on different days. All data were expressed as the mean ± standard error of the mean (SEM) using the SPSS 19.0 statistical software. For those data with few samples, we performed logarithmic transformation to make it conform closely to a normal distribution. Comparisons of two populations were performed by Student′s t-test. For multiple comparisons, one-way analysis of variance (ANOVA) followed by Dunnett′s test was employed. Values of P less than 0.05 were considered statistically significant.
Results
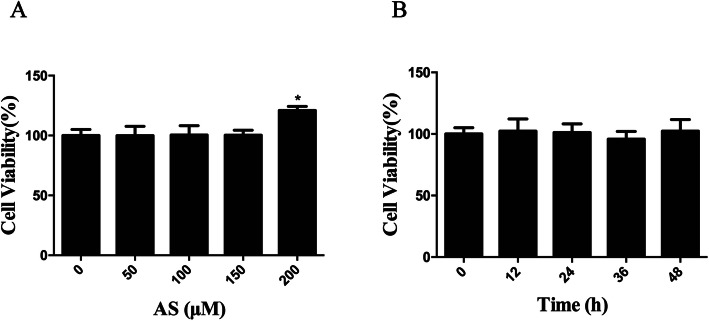
Asiaticoside showed no effect on cell growth and apoptosis under 150 μM
According to the results from the CCK-8 assay, neither enhanced cell proliferation nor apoptosis was found when the concentration was less than 150 μM or 150 μM for various times, but cell proliferation was enhanced when the concentration of asiaticoside was above 200 μM (Fig. 2a-b). Accordingly, 150 μM of asiaticoside was the dose chosen for the subsequent experiments.
Fig. 2.
Effect of asiaticoside on HPMCs viability. Notes: Cell viability was evaluated using CCK-8 assay. Data were expressed as the percentages of living cells versus the control. (A) HPMCs were treated with various dose of asiaticoside (0, 50, 100, 150 and 200 μM) for 24 h or (B) with 150 μM of asiaticoside for various times (0, 12, 24, 36 and 48 h). Abbreviations: HPMCs, human peritoneal mesothelial cells; CCK-8, cell counting kit-8
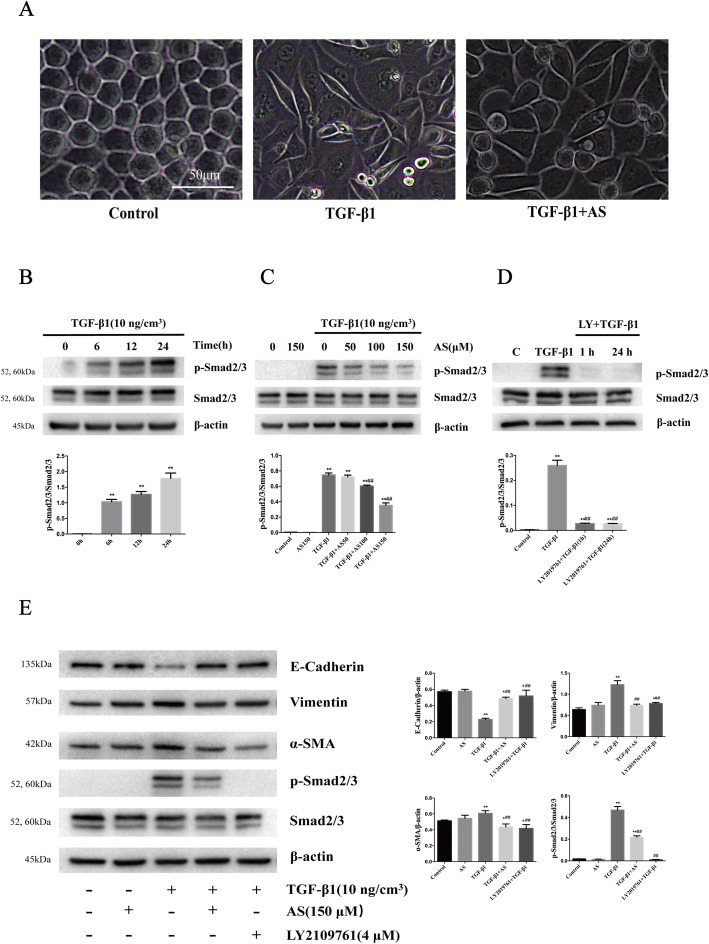
Asiaticoside attenuated TGF-β1-induced MMT by inhibiting TGF-β/Smad signaling pathway
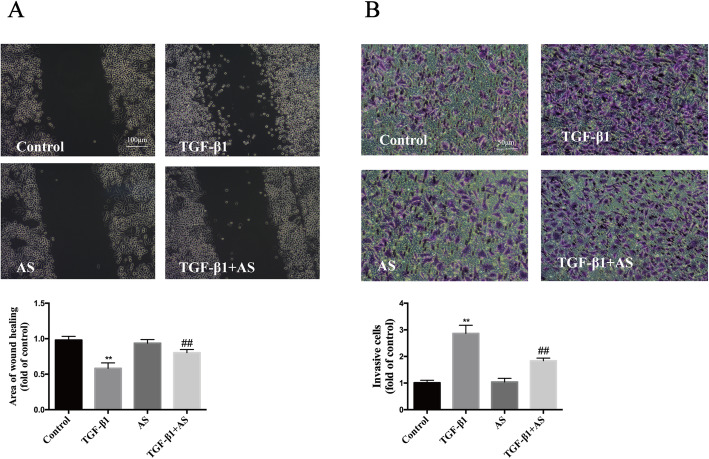
After treatment with 10 ng/cm3 TGF-β1 for 24 h, HPMCs appeared elongated and branched, with a loss of paving stone-like appearance, whereas asiaticoside treatment reduced these changes from a cuboidal shape to elongated spindle-shaped cells (Fig. 3a). Subsequently, we observed the effect of 10 ng/cm3 TGF-β1 on time gradients. The expression of p-Smad2/3 was strongly increased at 24 h after treatment (Fig. 3b). Asiaticoside attenuated the p-Smad2/3 expression in TGF-β1-stimulated HPMCs in a dose-dependent manner (Fig. 3c). Immunoblot analysis demonstrated that the expression of the mesothelial cell marker (E-Cadherin) decreased and the expressions of mesenchymal cell markers (Vimentin and α-SMA) increased under TGF-β1 stimulation. Asiaticoside alleviated TGF-β1-induced expressions of Vimentin and α-SMA, with an increase in the expression of E-Cadherin. These results were accompanied by enhanced phosphorylated Smad2/3 (p-Smad2/3), a Smad signaling pathway activated marker protein, suggesting that Smad2/3 was activated in TGF-β1-induced MMT. Furthermore, after pretreatment with the TGF-β receptor kinase inhibitor LY2109761, both asiaticoside and LY2109761 attenuated MMT and the Smad signaling pathway, indicating that asiaticoside has similar effects as TGF-β inhibitor (Fig. 3d-e). In addition, the migratory ability of HPMCs was enhanced under the TGF-β1 (10 ng/cm3) condition, whereas the addition of asiaticoside reduced the migration of HPMCs. These results were further confirmed by transwell migration assay and wound healing assay (Fig. 4a-b). These observations suggested that the anti-MMT effect of asiaticoside may be due to the downregulation of Smad signaling in TGF-β1-stimulated HPMCs.
Fig. 3.
Effect of asiaticoside on TGF-β1-induced activation of Smad2/3 and MMT in HPMCs. Notes: (A) After treatment with TGF-β1 (10 ng/cm3) or/and asiaticoside (150 μM), the morphologic alterations of HPMCs were observed using a microscope (scale bar = 50 μm). (B) HPMCs were treated with TGF-β1 (10 ng/cm3) for various times (0, 6, 12, 24 h) and subjected to immunoblot for Smad-related proteins. (C) HPMCs were treated with asiaticoside at various concentrations (0, 50, 100, 150 μM) with or without TGF-β1 (10 ng/cm3) treatment for 24 h. The expression of Smad2/3 and p-Smad2/3 was detected by immunoblotting. (D) Immunoblot showed relative levels of Smad2/3 phosphorylation at 1 h and 24 h post-treatment with LY2109761. (E) HPMCs were divided into a vehicle group, an asiaticoside group (treated with 150 μM asiaticoside), a TGF-β1 group (treated with 10 ng/ cm3 TGF-β1), a TGF-β1 + asiaticoside group (treated with 150 μM asiaticoside + 10 ng/cm3 TGF-β1) and a LY2109761 + TGF-β1 group (pretreated with LY2109761 at 4 μM 1 h prior to 10 ng/cm3 TGF-β1). After incubation for 24 h, immunoblot was performed to detect relative proteins. β-actin was used as a loading control. The densitometric analysis of the expression of MMT markers, Smad2/3 and p-Smad2/3 are shown as unique figures. Data are expressed as mean ± SEM, *p < 0.05 vs. control; **p < 0.01 vs. control; #p < 0.05 vs. TGF-β1 treatment; ##p < 0.01 vs. TGF-β1 treatment. Abbreviations: AS, asiaticoside; TGF-β1, transforming growth factor-β1; LY, TGF-β receptor kinase inhibitor (LY2109761); p-Smad2/3, phosphorylated Smad2/3
Fig. 4.
Effect of asiaticoside on TGF-β1-induced migration ability of HPMCs. Notes: HPMCs were exposed to asiaticoside (150 μM) with or without TGF-β1 (10 ng/cm3) and subjected to microscope to visualize the lateral (A) and longitudinal (B) migration of cells. Quantitative measurements of the fluorescence intensities were conducted using ImageJ software. Five random fields for each insert were counted, and three independent experiments were performed in each group. n = 3. Abbreviations: AS, asiaticoside; TGF-β1, transforming growth factor-β1
Asiaticoside inhibited ROS production independently of TGF-β/Smad signaling
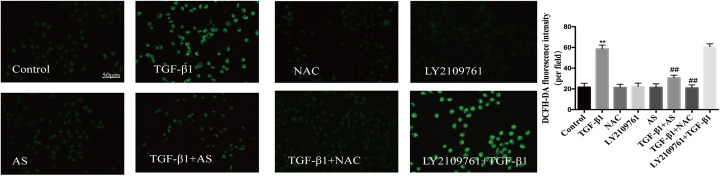
ROS levels were markedly increased after treatment with 10 ng/cm3 TGF-β1 for 24 h. Both asiaticoside and NAC (oxygen radical scavenger, 5 mM) attenuated this increase in TGF-β1-exposed HPMCs, which indicated the antioxidant effect of asiaticoside. In addition, LY2109761 treatment did not affect the TGF-β1-induced ROS production in HPMCs (Fig.5). These results suggested that asiaticoside-mediated attenuation of ROS is independent of TGF-β/Smad signaling pathway.
Fig. 5.
Effect of asiaticoside on reactive oxygen species (ROS) production in TGF-β1-treated HPMCs. Notes: HPMCs were divided into a vehicle group, an asiaticoside group (treated with 150 μM asiaticoside), a TGF-β1 group (treated with 10 ng/cm3 TGF-β1), a TGF-β1 + asiaticoside group (treated with 150 μM asiaticoside + 10 ng/cm3 TGF-β1), an NAC group (treated with 5 mM NAC), an NAC + TGF-β1 group (pretreated with NAC at 5 mM 2 h prior to 10 ng/cm3 TGF-β1), a LY2109761 group (treated with 4 μM LY2109761), and a LY2109761 + TGF-β1 group (pretreated with LY2109761 at 4 μM 1 h prior to 10 ng/cm3 TGF-β1). After incubation for 24 h, DCFH-DA fluorescence in cultured cells was analyzed by fluorescence microscopy (scale bar = 50 μm). Quantitative measurements of the fluorescence intensities were conducted using ImageJ software. Abbreviations: AS, asiaticoside; TGF-β1, transforming growth factor-β1; DCFH-DA, 2′,7′-dichlorodihydrofluorescein diacetate; NAC, n-acetylcysteine; LY2109761, TGF-β receptor kinase inhibitor
Nrf2 was activated by asiaticoside in HPMCs
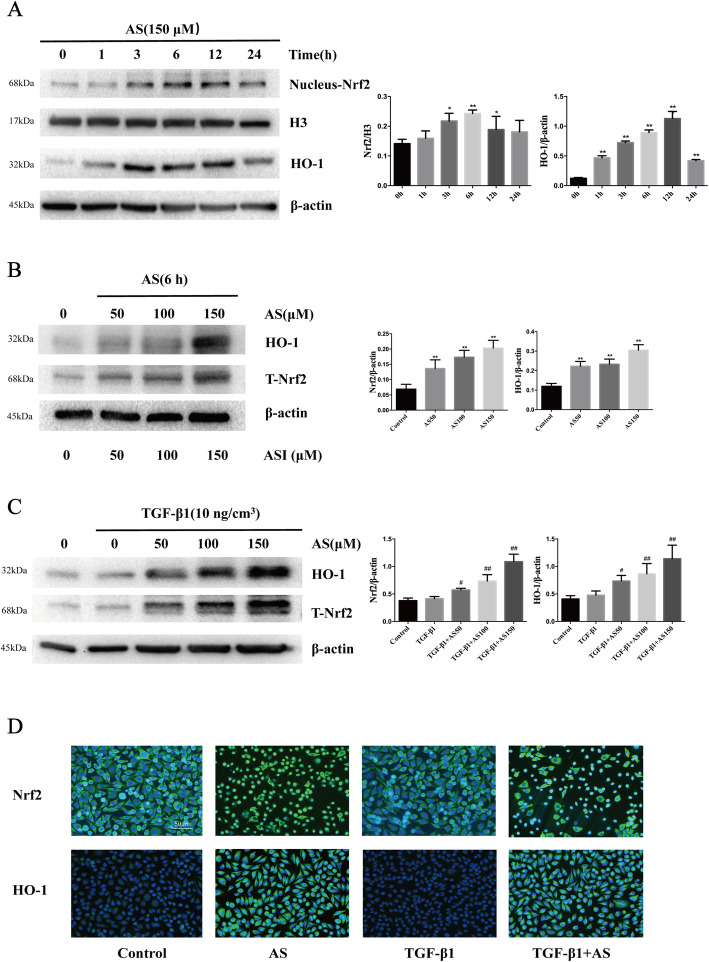
Previous studies have reported that this traditional Chinese medicine plant Centella asiatica has antioxidant effects resulting from the activation of Nrf2 and the expression of its downstream antioxidant enzymes in different disease areas. To examine the optimal moment for asiaticoside to activate Nrf2, we treated HPMCs with asiaticoside (150 μM) at different times. Nuclear Nrf2 (nNrf2) levels markedly increased after treatment with asiaticoside for 3–6 h. However, the nuclear translocation of Nrf2 was reduced 12 h after asiaticoside treatment. Consistent with this, asiaticoside increased the expression of HO-1, which is a well-studied Nrf2 target gene (Fig. 6a). After treatment with various doses of asiaticoside (50–150 μM) for 6 h, both total Nrf2 (T-Nrf2) and HO-1 levels were significantly increased in a dose-dependent manner (Fig. 6b). These results suggest that Nrf2 could be activated by different concentrations of asiaticoside at 6 h. Accordingly, asiaticoside intervention of 150 μM concentration for 6 h was chosen for the subsequent experiments. After treatment with 10 ng/cm3 TGF-β1 for 24 h and intervention with 150 μM asiaticoside for 6 h, T-Nrf2 and HO-1 levels in TGF-β1-stimulated HPMCs were both increased in dose-dependent manners (Fig. 6c). Immunofluorescence assay further confirmed the phenomenon that asiaticoside promoted Nrf2 to enter the nucleus and enhanced the expression of the downstream antioxidant enzyme HO-1 (Fig. 6d). All the above results suggested that asiaticoside activated the Nrf2/HO-1 signaling pathway, which may exert an antioxidant effect in inhibiting TGF-β1-induced ROS.
Fig. 6.
Effect of asiaticoside on Nrf2 activation in HPMCs. Notes: (A) After asiaticoside (150 μM) treatment for various times (0, 1, 3, 6, 12, 24 h), the expression of Nrf2 in the nucleus and HO-1 expression were detected by immunoblotting. Histone H3 and β-actin were used as the loading controls for the nucleus and cytosol, respectively. (B) After treatment with various doses of asiaticoside (0, 50, 100, 150 μM) for 6 h, Nrf2 and HO-1 expression were detected by immunoblotting. (C) After treated with asiaticoside at various concentrations (0, 50, 100, 150 μM) and TGF-β1 (10 ng/cm3) treatment for 6 h, Nrf2 and HO-1 expression were detected by immunoblotting. β-actin was used as a loading control. Densitometric analysis of the expression of Nrf2 and HO-1 are shown as unique figures. Data are expressed as the mean ± SEM, *p < 0.05 vs. control, **p < 0.01 vs. control; #p < 0.05 vs. TGF-β1 treatment; ##p < 0.01 vs. TGF-β1 treatment. (D) Immunofluorescence assays were performed to observe the subcellular translocation of Nrf2. Nrf2 expression in the membrane, cytoplasm and nucleus were visualized based on the green fluorescent signal obtained by fluorescence microscopy (scale bar = 50 μm). Abbreviations: AS, asiaticoside; TGF-β1, transforming growth factor-β1; Nrf2, nuclear factor erythroid-2-related factor 2; HO-1, heme oxygenase 1
Nrf2 played a key role in the inhibitory effect of asiaticoside on TGF-β1-induced MMT and ROS production
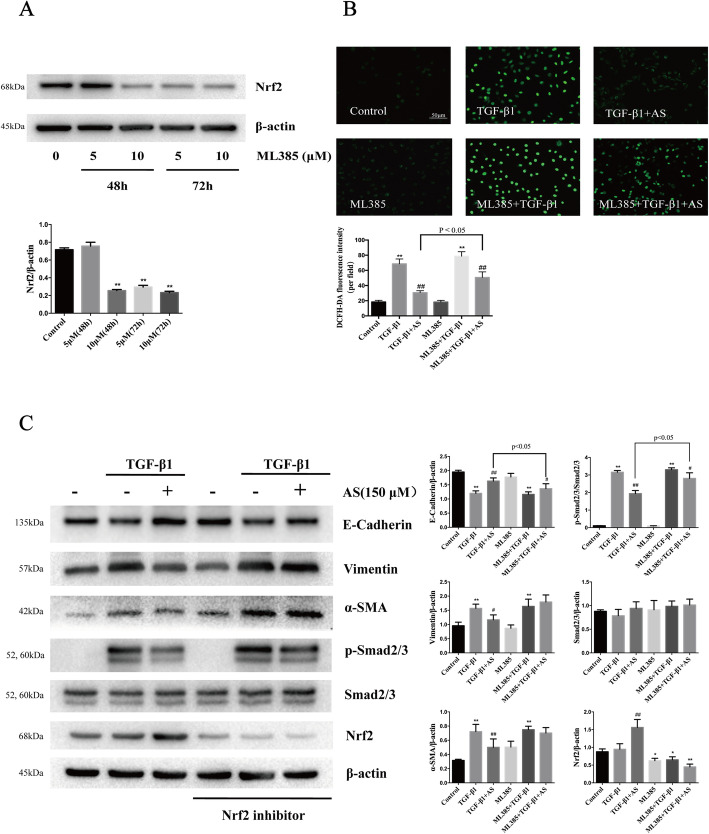
To verify the role of asiaticoside-mediated Nrf2 activation in HPMCs, we measured TGF-β1-mediated ROS generation after treatment with Nrf2 inhibitor (ML385) in HPMCs. Although asiaticoside still inhibited TGF-β1-induced ROS production after treatment with ML385, the inhibitory effect was partially reversed. However, compared with Nrf2 inhibited cells, the inhibitory effect of TGF-β1 plus asiaticoside treatment was still significant (Fig. 7a-b). In addition, it was verified by immunoblotting that inhibition by Nrf2 partially alleviated the inhibition effect of asiaticoside on MMT and p-Smad2/3 (Fig. 7c). These results suggested that asiaticoside had antioxidant effects against TGF-β1-induced ROS production through the Nrf2/HO-1 pathway, leading to the inhibition of fibrogenic gene expression.
Fig. 7.
The role of Nrf2 activation in the inhibitory effect of asiaticoside on TGF-β1-induced MMT and ROS production in HPMCs. Notes: (A) After HPMCs treatment with two concentration of ML385 (5, 10 μM) and subsequently incubation for 48 h and 72 h, Nrf2 was analyzed by immunoblotting. Data are shown as the mean ± SEM, *p < 0.05 vs. control, **p < 0.01 vs. Control. (B) HPMCs were divided into a vehicle group, a TGF-β1 group (treated with 10 ng/ml TGF-β1), a TGF-β1 + asiaticoside group (treated with 150 μM asiaticoside + 10 ng/cm3 TGF-β1), a ML385 group (treated with 10 μM ML385 for 72 h), a ML385 + TGF-β1 group (pretreated with ML385 at 10 μM 72 h prior to 10 ng/cm3 TGF-β1), and a ML385 + TGF-β1 + asiaticoside group (pretreated with ML385 at 10 μM 72 h prior to 150 μM asiaticoside + 10 ng/cm3 TGF-β1). After incubation for 24 h, DCFH-DA fluorescence in cultured cells was analyzed by fluorescence microscopy (scale bar = 50 μm). Quantitative measurements of the fluorescence intensities were conducted using ImageJ software. (C) Immunoblot was performed to detect MMT markers, Smad-related proteins and Nrf2 expression. Data are shown as the mean ± SEM, *p < 0.05 vs. control, **p < 0.01 vs. control; #p < 0.05 vs. TGF-β1 treatment; ##p < 0.01 vs. TGF-β1 treatment. Abbreviations: AS, asiaticoside; TGF-β1, transforming growth factor-β1; Nrf2, nuclear factor erythroid-2-related factor 2; p-Smad2/3, phosphorylated Smad2/3; ML385, Nrf2 inhibitor; ROS, reactive oxygen species
Discussion
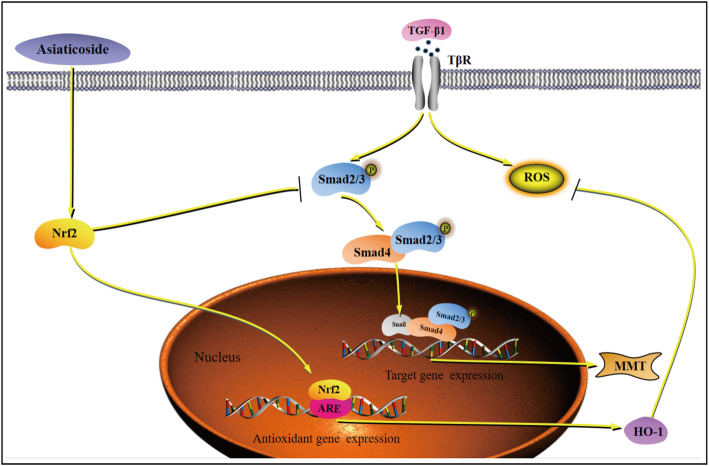
In this study, it was found that (i) asiaticoside inhibits MMT and ROS generation in TGF-β1-treated HPMCs; (ii) the beneficial effect of asiaticoside on MMT is attributable to inhibition of TGF-β/Smad signaling, which has no effect on ROS; (iii) asiaticoside inhibited the TGF-β1-induced oxidative stress and Smad signaling via Nrf2 activation, thus preserving peritoneal membrane function and preventing PF (Fig. 8).
Fig. 8.
Schematic diagram illustrating the mechanism by which asiaticoside inhibited MMT and ROS generation
MMT is an important cellular process strongly associated with PF observed in long-term PD patients [17]. Pathological changes of PMCs include exfoliation of mesothelial cells and thickening of the submesothelial layer, which is characterized by increased myofibroblasts, collagen deposition, and new blood vessels. PMCs acquire a mesenchymal phenotype, and directly participate in the pathogenesis of PF. Therefore, prevention and/or reversal of the regulation of MMT can be an effective method for treating PF. TGF-β1 plays a central role in MMT of HPMCs [18]. Many TGF-β1 blockers are used for anti-MMT action in vivo and vitro, such as TGF-β1 receptor blocker and neutralizing antibody. In the last years, many authors have focused on the beneficial effects of natural herbs or their active ingredients on maintaining peritoneal integrity [19, 20]. But none of them have been used clinically, and the safety is unknown.
The most important finding of this study is validation of the anti-MMT effect of asiaticoside in HPMCs. It is already known that TGF-β1 forms a complex with the TGF-β type II receptor, and then the activated TGF-β type I receptor phosphorylates the downstream Smad2/3 proteins. Next, phosphorylated Smad2/3 and Smad4 combine to transmit signaling to the nucleus, where they work in concert with transcription factors such as Snail and Twist to suppress the expression of mesothelial cell markers and to activate the expression of mesenchymal cell markers [21]. Starting from this evidence, our study found that TGF-β1 induced MMT with activation of Smad2/3 by phosphorylation in HPMCs. We confirmed activation of the TGF-β/Smad signaling pathway and MMT assessed by immunoblotting, which was blocked by asiaticoside and LY2109761. Furthermore, transwell and wound-healing assays illustrated that asiaticoside treatment reduced transmigration in TGF-β1-treated cells to a certain extent. All the above results confirmed that asiaticoside can inhibit MMT via TGF-β/Smad signaling. Earlier studies have revealed the anti-MMT effect of asiaticoside in keloid fibroblasts and lung epithelial cells [14]. However, there have been no reports in the context of PD. This study is the first one to demonstrate the regulatory effect of asiaticoside on PMCs MMT by Smad signaling.
It is generally accepted that oxidative stress is associated with the occurrence and development of PF in PD patients, which is related to RRF. The composition of the PD solution (low pH, high glucose, elevated osmolality, advanced glycation end products and glucose degradation products) is responsible for the accumulation of oxidation products. Therefore, oxidative stress has been considered as an important factor in PF [22]. Asiaticoside has been reported as an antioxidant agent that ameliorates cell apoptosis and alleviates fibrotic change by inhibiting ROS production. Under the stimulation of TGF-β1, ROS generation was significantly increased in HPMCs. Similar to the effect of NAC, asiaticoside eliminated the overproduction of ROS. Accumulated studies have demonstrated that TGF-β1 stimulated the production of ROS in various cell types, which in turn activated TGF-β and mediated many fibrogenic effects. Some studies have shown that TGF-β/Smad signaling plays a vital role in oxidative stress [23]. We investigated the relationship between TGF-β/Smad signaling and oxidative stress in PMCs; however, LY2109761 treatment did not decrease ROS production, indicating that TGF-β1-induced ROS in HPMCs does not depend on TGF-β/Smad signaling. While TGF-β1-induced Smad signaling is a critical event in the progression of oxidative stress, the role of non-Smad mechanisms in the overproduction of ROS is still critical [24, 25]. These results revealed asiaticoside to be an antioxidant agent through non-Smad pathways, and a multi-target compound with anti-MMT and anti-ROS mechanisms.
In order to further study the specific mechanism and antioxidant target of asiaticoside, we clarified the transduction of Nrf2/HO-1 signaling. Nrf2 is a redox-sensitive transcription factor which provides an antioxidant defense mechanism by regulating the induction of genes encoding antioxidant proteins and phase2 detoxifying enzymes through the activation of AREs [26]. Previous studies have reported that activation of Nrf2 and its downstream gene HO-1 plays an antioxidant role in many cell lines [12]. In the last years, many researchers have focused on the antioxidant effects of natural products and their extracts [27–29]. In our study, interestingly, both nNrf2 and HO-1 levels increased rapidly after asiaticoside treatment for 3–6 h. After treatment with various doses of asiaticoside (50–150 μM) for 6 h, both T-Nrf2 and HO-1 levels were significantly increased. These results confirmed that asiaticoside acts as an Nrf2 activator in a dose-dependent manner. After treatment with 10 ng/cm3 TGF-β1 for 24 h, asiaticoside intervention for 6 h was still able to increase the expressions of T-Nrf2 and HO-1 in a dose-dependent manner. Immunofluorescence assay further confirmed that asiaticoside promoted Nrf2 nuclear translocation. These results indicated that asiaticoside served an antioxidant role via activating Nrf2/HO-1 signaling in HPMCs.
Furthermore, a specific role of Nrf2 in asiaticoside-mediated suppression of ROS was verified using the Nrf2/HO-1 inhibitor ML385. ML385 can directly interact with Nrf2 protein and binds to the Neh1 binding region of Nrf2, thus preventing binding of the Nrf2-MAFG complex to the promoter ARE sequence and reducing the transcriptional activity. After treatment with ML385, asiaticoside’s effect on TGF-β1-induced ROS generation was partly ameliorated. These results confirmed that TGF-β1-induced ROS generation in HPMCs was dependent on Nrf2 activation. To our knowledge, few data have demonstrated the activation of Nrf2/HO-1 signaling inhibiting ROS production and MMT in HPMCs. However, it was limited to phenomenon observation and lacked mechanism research and efficacy verification [30]. In our study, we illustrated for the first time that asiaticoside can inhibit ROS production by targeting the Nrf2/HO-1 signaling pathway in the PD field. Previous studies have found that Nrf2 activation inhibits TGF-β/Smad signaling and MMT in tissue fibrosis [31]. Adenovirus-mediated overexpression of Nrf2 prevented MMT and ROS generation in TGF-β1-treated liver and renal cells, which was accompanied by decreased expression of p-Smad2/3. In contrast, Nrf2 knockdown by siRNA prevented this alteration [32, 33]. To verify whether asiaticoside can inhibit MMT and TGF-β/Smad signaling pathways by activating Nrf2, we applied ML385 pretreatment and the experimental results showed that pretreatment with Nrf2 inhibitor partly reversed the inhibitory effect of asiaticoside on the TGF-β1-stimulated MMT and p-Smad2/3 expression. These data indicated that Nrf2 is a key target working in concert with asiaticoside on TGF-β/Smad signaling and oxidative stress, leading to the inhibition of MMT and ROS generation.
To our knowledge, asiaticoside is a large molecule and the studies so far have provided no evidence about a specific receptor or potential binding site. Although it seems difficult to enter the cytosol, we have good reason to believe that asiaticoside indeed interacts with cytoplasmic proteins according to the database. The associations between asiaticoside and relative cytoplasmic/nuclear proteins have been described in many articles (21 studies for TNF-α, 13 for IL-6 and 7 for Smad2). Furthermore, anti-inflammatory activities in LPS-stimulated mouse RAW264.7 cells were assessed as the nitrite level at 1 to 100 μM after 24 h by the Griess method [34]. It is noted that asiaticoside (Centella asiatica extract) is more often used in dermatology, while our study demonstrates for the first time its protection of human peritoneum. With the progression of pharmaceutical research, more specific targets/analogs/functional structure are wanted to elucidate the properties of the new drug.
Taken together, our results suggest that asiaticoside effectively suppresses TGF-β1-induced MMT and ROS generation via Nrf2 activation in HPMCs. This result indicates a potential therapeutic effect of asiaticoside on PF.
Acknowledgments
The study was supported by the Renal Division, Affiliated Hospital of Nanjing University of Chinese Medicine, Nanjing 200029, Jiangsu Province, China.
Abbreviations
- AS
asiaticoside
- CCK-8
cell counting kit-8
- DAPI
4′,6-diamidino-2-phenylindole
- DCFH-DA
2′,7′-dichlorodihydrofluorescein diacetate
- DMSO
dimethyl sulfoxide
- ESRD
end-stage renal disease
- HD
hemodialysis
- HO-1
heme oxygenase 1
- HPMCs
human peritoneal mesothelial cells
- LY
TGF-β receptor kinase inhibitor (LY2109761)
- ML385
Nrf2 inhibitor
- MMT
mesothelial-mesenchymal transition
- NAC
n-acetylcysteine
- Nrf2
nuclear factor erythroid-2-related factor 2
- PBS
phosphate buffer saline
- PD
peritoneal dialysis
- PDS
peritoneal dialysis solution
- PF
peritoneal fibrosis
- PMCs
peritoneal mesothelial cells
- p-Smad2/3
phosphorylated Smad2/3
- ROS
reactive oxygen species
- RRF
residual renal function
- TGF-β1
transforming growth factor-β1
Authors’ contributions
Conceptualization and Supervision, Meixiao Sheng; Data curation, Junyi Zhao and Yilin Zhu; Investigation, Junyi Zhao and Jun Shi; Methodology, Yun Shan, Manshu Yu and Xiaolin Zhu; Resources, Li Liu; Writing, review and editing, Jun Shi. The authors read and approved the final manuscript.
Funding
This work was supported by grants from the National Natural Science Foundation of China (No.81774253, 81904121), a grant from the Natural Science Foundation of Jiangsu Province (BK20171514, BK20191087), Leading Talent Project in Jiangsu Province (SLJ0205). Summit Scholar Project in Jiangsu Province Hospital of Chinese Medicine (y2018rc08).
Availability of data and materials
All data from this study are available in this published article.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Junyi Zhao and Jun Shi contributed equally to this work.
References
- 1.Li Philip Kam-tao, Chow Kai Ming. Peritoneal Dialysis–First Policy Made Successful: Perspectives and Actions. American Journal of Kidney Diseases. 2013;62(5):993–1005. doi: 10.1053/j.ajkd.2013.03.038. [DOI] [PubMed] [Google Scholar]
- 2.Wong Ben, Ravani Pietro, Oliver Matthew J., Holroyd-Leduc Jayna, Venturato Lorraine, Garg Amit X., Quinn Robert R. Comparison of Patient Survival Between Hemodialysis and Peritoneal Dialysis Among Patients Eligible for Both Modalities. American Journal of Kidney Diseases. 2018;71(3):344–351. doi: 10.1053/j.ajkd.2017.08.028. [DOI] [PubMed] [Google Scholar]
- 3.Raffaele S, Roberto MV, Cecilia B, Carla C, Valeria N, Laura A, Alessandra M, Miguel A, Marco T. Molecular mechanisms underlying peritoneal EMT and fibrosis. Stem Cells Int 2016; 4: 1–11. http://dx.doi.org/10.1155/2016/3543678. [DOI] [PMC free article] [PubMed]
- 4.Krediet Raymond T., Struijk Dirk G. Peritoneal changes in patients on long-term peritoneal dialysis. Nature Reviews Nephrology. 2013;9(7):419–429. doi: 10.1038/nrneph.2013.99. [DOI] [PubMed] [Google Scholar]
- 5.Liu Yu, Dong Zheng, Liu Hong, Zhu Jiefu, Liu Fuyou, Chen Guochun. Transition of Mesothelial Cell to Fibroblast in Peritoneal Dialysis: EMT, Stem Cell or Bystander? Peritoneal Dialysis International: Journal of the International Society for Peritoneal Dialysis. 2015;35(1):14–25. doi: 10.3747/pdi.2014.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kriz Wilhelm, Kaissling Brigitte, Le Hir Michel. Epithelial-mesenchymal transition (EMT) in kidney fibrosis: fact or fantasy? Journal of Clinical Investigation. 2011;121(2):468–474. doi: 10.1172/JCI44595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kojiro T, Keiko I, Etsuro H, Shinji U. Controversies over the epithelial-to-mesenchymal transition in liver fibrosis. J Clin Med 2016; 5(1): 9–15. http://dx.doi.org/10.3390/jcm5010009. [DOI] [PMC free article] [PubMed]
- 8.Selgas Rafael, Bajo Auxiliadora, Jiménez-Heffernan José A., Sánchez-Tomero Jose A., del Peso Gloria, Aguilera Abelardo, López-Cabrera Manuel. Epithelial-to-mesenchymal transition of the mesothelial cell—its role in the response of the peritoneum to dialysis. Nephrology Dialysis Transplantation. 2006;21(suppl_2):ii2–ii7. doi: 10.1093/ndt/gfl183. [DOI] [PubMed] [Google Scholar]
- 9.Shin HS, Ko J, Kim DA, Ryu ES, Ryu HM, Park SH, Kim YL, Oh ES, Kang DH. Metformin ameliorates the phenotype transition of peritoneal mesothelial cells and peritoneal fibrosis via a modulation of oxidative stress. Sci Rep 2017; 7(1): 5690. http://dx.doi.org/10.1038/s41598-017-05836-6. [DOI] [PMC free article] [PubMed]
- 10.Gonzalez D. M., Medici D. Signaling mechanisms of the epithelial-mesenchymal transition. Science Signaling. 2014;7(344):re8–re8. doi: 10.1126/scisignal.2005189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi Yigong, Massagué Joan. Mechanisms of TGF-β Signaling from Cell Membrane to the Nucleus. Cell. 2003;113(6):685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen Truyen, Nioi Paul, Pickett Cecil B. The Nrf2-Antioxidant Response Element Signaling Pathway and Its Activation by Oxidative Stress. Journal of Biological Chemistry. 2009;284(20):13291–13295. doi: 10.1074/jbc.R900010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nezu Masahiro, Souma Tomokazu, Yu Lei, Suzuki Takafumi, Saigusa Daisuke, Ito Sadayoshi, Suzuki Norio, Yamamoto Masayuki. Transcription factor Nrf2 hyperactivation in early-phase renal ischemia-reperfusion injury prevents tubular damage progression. Kidney International. 2017;91(2):387–401. doi: 10.1016/j.kint.2016.08.023. [DOI] [PubMed] [Google Scholar]
- 14.Wu Xin, Bian Difei, Dou Yannong, Gong Zhunan, Tan Qian, Xia Yufeng, Dai Yue. Asiaticoside hinders the invasive growth of keloid fibroblasts through inhibition of the GDF-9/MAPK/Smad pathway. Journal of Biochemical and Molecular Toxicology. 2017;31(8):e21922. doi: 10.1002/jbt.21922. [DOI] [PubMed] [Google Scholar]
- 15.Jiang Jing Zhi, Ye Jing, Jin Guang Yu, Piao Hong Mei, Cui Hong, Zheng Ming Yu, Yang Jin Shi, Che Nan, Choi Yun Ho, Li Liang Chang, Yan Guang Hai. Asiaticoside Mitigates the Allergic Inflammation by Abrogating the Degranulation of Mast Cells. Journal of Agricultural and Food Chemistry. 2017;65(37):8128–8135. doi: 10.1021/acs.jafc.7b01590. [DOI] [PubMed] [Google Scholar]
- 16.Xing Ying, Ji Qiuhe, Li Xiaomiao, Ming Jie, Zhang Nana, Zha Dingjun, Lin Ying. Asiaticoside protects cochlear hair cells from high glucose-induced oxidative stress via suppressing AGEs/RAGE/NF-κB pathway. Biomedicine & Pharmacotherapy. 2017;86:531–536. doi: 10.1016/j.biopha.2016.12.025. [DOI] [PubMed] [Google Scholar]
- 17.Aroeira Luiz S., Aguilera Abelardo, Sánchez-Tomero José A., Bajo M. Auxiliadora, del Peso Gloria, Jiménez-Heffernan José A., Selgas Rafael, López-Cabrera Manuel. Epithelial to Mesenchymal Transition and Peritoneal Membrane Failure in Peritoneal Dialysis Patients: Pathologic Significance and Potential Therapeutic Interventions. Journal of the American Society of Nephrology. 2007;18(7):2004–2013. doi: 10.1681/ASN.2006111292. [DOI] [PubMed] [Google Scholar]
- 18.Zhou Qin, Bajo M-Auxiliadora, del Peso Gloria, Yu Xueqing, Selgas Rafael. Preventing peritoneal membrane fibrosis in peritoneal dialysis patients. Kidney International. 2016;90(3):515–524. doi: 10.1016/j.kint.2016.03.040. [DOI] [PubMed] [Google Scholar]
- 19.Lupinacci S., Perri Anna, Toteda G., Vizza D., Puoci F., Parisi O. I., Giordano F., Lofaro D., La Russa A., Bonofiglio M., Bonofiglio R. Olive leaf extract counteracts epithelial to mesenchymal transition process induced by peritoneal dialysis, through the inhibition of TGFβ1 signaling. Cell Biology and Toxicology. 2018;35(2):95–109. doi: 10.1007/s10565-018-9438-9. [DOI] [PubMed] [Google Scholar]
- 20.Xu Weijia, Shao Xinghua, Tian Lei, Gu Leyi, Zhang Minfang, Wang Qin, Wu Bei, Wang Ling, Yao Jufang, Xu Xiaoping, Mou Shan, Ni Zhaohui. Astragaloside IV Ameliorates Renal Fibrosis via the Inhibition of Mitogen-Activated Protein Kinases and Antiapoptosis In Vivo and In Vitro. Journal of Pharmacology and Experimental Therapeutics. 2014;350(3):552–562. doi: 10.1124/jpet.114.214205. [DOI] [PubMed] [Google Scholar]
- 21.Rodrigues-Diez R, Rodrigues-Diez RR, Lavoz C, Carvajal G, Droguett A, Garcia-Redondo AB, Rodriguez I, Ortiz A, Egido J, Mezzano S, Ruiz-Ortega M. Gremlin activates the Smad pathway linked to epithelial mesenchymal transdifferentiation in cultured tubular epithelial cells. Biomed Res Int. 2014: 802–841. http://dx.doi.org/10.1155/2014/802841. [DOI] [PMC free article] [PubMed]
- 22.Liakopoulos Vassilios, Roumeliotis Stefanos, Gorny Xenia, Eleftheriadis Theodoros, Mertens Peter R. Oxidative Stress in Patients Undergoing Peritoneal Dialysis: A Current Review of the Literature. Oxidative Medicine and Cellular Longevity. 2017;2017:1–14. doi: 10.1155/2017/3494867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jeong Bo Young, Park Se-Ra, Cho Sungkwon, Yu Seong-Lan, Lee Hoi Young, Park Chang Gyo, Kang Jaeku, Jung Da-Young, Park Moon Hyang, Hwang Won-Min, Yun Sung-Ro, Jung Ju-Young, Yoon Se-Hee. TGF-β-mediated NADPH oxidase 4-dependent oxidative stress promotes colistin-induced acute kidney injury. Journal of Antimicrobial Chemotherapy. 2018;73(4):962–972. doi: 10.1093/jac/dkx479. [DOI] [PubMed] [Google Scholar]
- 24.Samarakoon Rohan, Dobberfuhl Amy D., Cooley Catherine, Overstreet Jessica M., Patel Samik, Goldschmeding Roel, Meldrum Kirstan K., Higgins Paul J. Induction of renal fibrotic genes by TGF-β1 requires EGFR activation, p53 and reactive oxygen species. Cellular Signalling. 2013;25(11):2198–2209. doi: 10.1016/j.cellsig.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 25.Jiang M, Zhang H, Zhai L, et al. ALA/LA ameliorates glucose toxicity on HK-2 cells by attenuating oxidative stress and apoptosis through the ROS/p38/TGF-β1 pathway. Lipids Health Dis 2017; 16(1): 216. http://dx.doi.org/10.1186/s12944-017-0611-6. [DOI] [PMC free article] [PubMed]
- 26.Yagishita Yoko, Uruno Akira, Fukutomi Toshiaki, Saito Ritsumi, Saigusa Daisuke, Pi Jingbo, Fukamizu Akiyoshi, Sugiyama Fumihiro, Takahashi Satoru, Yamamoto Masayuki. Nrf2 Improves Leptin and Insulin Resistance Provoked by Hypothalamic Oxidative Stress. Cell Reports. 2017;18(8):2030–2044. doi: 10.1016/j.celrep.2017.01.064. [DOI] [PubMed] [Google Scholar]
- 27.Liu Mandy, Ravula Ranadheer, Wang Zhijun, Zuo Zhong, Chow Moses SS, Thakkar Arvind, Prabhu Sunil, Andresen Bradley, Huang Ying. Traditional Chinese medicinal formula Si-Wu-Tang prevents oxidative damage by activating Nrf2-mediated detoxifying/antioxidant genes. Cell & Bioscience. 2014;4(1):8. doi: 10.1186/2045-3701-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Xin, Ye Xiao-long, Liu Rui, Chen Hong-Li, Bai Hua, Liang Xin, Zhang Xiao-Di, Wang Zhao, Li Wen-li, Hai Chun-Xu. Antioxidant activities of oleanolic acid in vitro: Possible role of Nrf2 and MAP kinases. Chemico-Biological Interactions. 2010;184(3):328–337. doi: 10.1016/j.cbi.2010.01.034. [DOI] [PubMed] [Google Scholar]
- 29.Liang B, Peng L, Li R, Li H, Mo Z, Dai X, Jiang N, Liu Q, Zhang E, Deng H, Li Z, Zhu H. Lycium barbarum polysaccharide protects HSF cells against ultraviolet-induced damage through the activation of Nrf2. Cell Mol Biol Lett 2018. 23: 18. http://dx.doi.org/10.1186/s11658-018-0084-2. [DOI] [PMC free article] [PubMed]
- 30.Gao LL, Fan Y, Zhang XL, Yang LN, Huang WY, Hang TY, Li MY, Du SY, Ma JF. Zinc supplementation inhibits the high glucose-induced EMT of peritoneal mesothelial cells by activating the Nrf2 antioxidant pathway. Mol Med Rep 2019; 20(1): 655–663. http://dx.doi.org/10.3892/mmr.2019.10260. [DOI] [PMC free article] [PubMed]
- 31.Oh Chang Joo, Kim Joon-Young, Min Ae-Kyung, Park Keun-Gyu, Harris Robert A., Kim Han-Jong, Lee In-Kyu. Sulforaphane attenuates hepatic fibrosis via NF-E2-related factor 2-mediated inhibition of transforming growth factor-β/Smad signaling. Free Radical Biology and Medicine. 2012;52(3):671–682. doi: 10.1016/j.freeradbiomed.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 32.Chen Q, Zhang H, Cao Y, Li Y, Zhang G. Schisandrin B attenuates CCl4 induced liver fibrosis in rats by regulation of Nrf2-ARE and TGF-β/Smad signaling pathways. Drug Des Devel Ther 2017; 11: 2179–2191. http://dx.doi.org/10.2147/DDDT.S137507. [DOI] [PMC free article] [PubMed]
- 33.Oh Chang Joo, Kim Joon-Young, Choi Young-Keun, Kim Han-Jong, Jeong Ji-Yun, Bae Kwi-Hyun, Park Keun-Gyu, Lee In-Kyu. Dimethylfumarate Attenuates Renal Fibrosis via NF-E2-Related Factor 2-Mediated Inhibition of Transforming Growth Factor-β/Smad Signaling. PLoS ONE. 2012;7(10):e45870. doi: 10.1371/journal.pone.0045870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hafiz Zetty Zulikha, Amin Muhammad ‘Afif Mohd, Johari James Richard Muhammad, Teh Lay Kek, Salleh Mohd Zaki, Adenan Mohd Ilham. Inhibitory Effects of Raw-Extract Centella asiatica (RECA) on Acetylcholinesterase, Inflammations, and Oxidative Stress Activities via In Vitro and In Vivo. Molecules. 2020;25(4):892. doi: 10.3390/molecules25040892. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data from this study are available in this published article.