The Argus II Retinal Prosthesis System (Second Sight Medical Products, Sylmar, CA) is currently approved for implantation in patients with retinitis pigmentosa. Although the proximity of the electrodes to the retina surface is crucial for optimal functioning of the device, the operating microscope alone does not allow for visualization of the distance of the electrodes from the retinal surface.
We demonstrate the enhanced visualization of the electrode to retina distance using images obtained with microscope-integrated OCT (MIOCT). Because 6.7% of devices required retacking in clinical trials, surgeons have reported visualizing the electrode array in cross-section (2-dimensional) with MIOCT to verify proper tacking and intraoperative position.1,2 Live volumetric (4-dimensional) intraoperative imaging using ultrafast swept-source MIOCT allows for real-time surgeon feedback to verify the ideal implantation position intraoperatively.
A video (available at www.ophthalmologyretina.org) demonstrates 4-dimensional live volumetric intraoperative imaging of the electrode array before tacking compared to a well-positioned device after tacking with the array in close contact with the retinal surface. Figure 1 shows B-scan imaging and still captures from 4-dimensional MIOCT imaging of the electrode array before tacking and after tacking again demonstrating an improved array-to-retina distance after tacking is completed.
Figure 1.
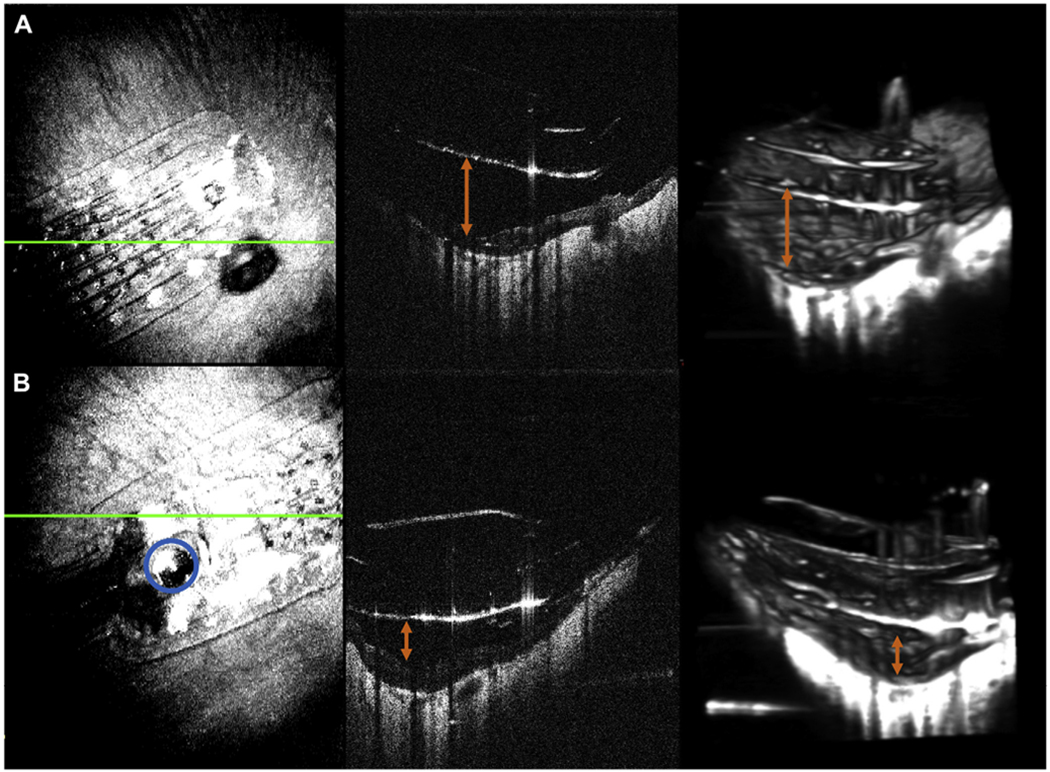
A, The electrode array before tacking including en face projection, B-scan imaging, and excerpts from 4-dimensional (4D) imaging. The orange arrow shows the distance of the array surface to the retina. B, Corresponding images after tacking. A blue outline highlights the tack in the en face and still from 4D imaging and the orange arrow shows the improvement in array to retina distance.
Supplementary Material
Acknowledgments
Funded by the NIH Bioengineering Research Partnership Grant: R01-EY023039 “Intraoperative OCT Guidance of Intraocular Surgery” (Izatt/Toth).
Financial Disclosures: The authors made the following disclosures:
L.V.: Research funding — Second Sight Medical Products, Inc.
J.I.: Intellectual property and commercial — Leica Microsystems.
C.A.T.: Royalties — Alcon; patent in OCT imaging and processing.
Footnotes
Human Subjects: This study includes a human subject. No animal subjects were used in this study. Study protocol was approved by the Duke University IRB. Informed consent was obtained from all human subjects. All tenets of the Declaration of Helsinki were followed.
References
- 1.da Cruz L, Dorn JD, Humayun MS, et al. Five-year safety and performance results from the Argus II Retinal Prosthesis System clinical trial. Ophthalmology. 2016;123:2248–2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grewal DS, Carrasco-Zevallos OM, Gunther R, et al. Intra-operative microscope-integrated swept-source optical coherence tomography guided placement of Argus II retinal prosthesis. Acta Ophthalmol. 2017;95:e431–e432. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


