Abstract
OBJECTIVES:
Irritable bowel syndrome (IBS) is a common gastrointestinal condition with a heterogeneous pathophysiology. An altered gut microbiome has been identified in some IBS patients, and fecal microbiota transplantation (FMT) has been suggested to treat IBS. We performed meta-analyses and systematic review of available randomized controlled trials (RCTs) to evaluate the efficacy of FMT in IBS.
METHODS:
We performed a systematic literature search of MEDLINE, EMBASE, Cochrane Central Register of Controlled Trials, and Web of Science. Selection criteria included RCTs of FMT vs placebo using FMT excipients or autologous FMT in IBS. Meta-analyses were conducted to evaluate the summary relative risk (RR) and 95% confidence intervals (CIs) of combined studies for primary outcome of improvement in global IBS symptoms as measured by accepted integrative symptom questionnaires or dichotomous responses to questions of overall symptom improvement.
RESULTS:
Among 742 citations identified, 7 were deemed to be potentially relevant, of which 4 studies involving 254 participants met eligibility. No significant difference in global improvement of IBS symptoms was observed at 12 weeks in FMT vs placebo (RR = 0.93; 95% CI 0.48–1.79). Heterogeneity among studies was significant (I2 = 79%). Subgroup analyses revealed benefits of single-dose FMT using colonoscopy and nasojejunal tubes in comparison with autologous FMT for placebo treatment (number needed to treat = 5, RR = 1.59; 95% CI 1.06–2.39; I2 = 0%) and a reduction in likelihood of improvement of multiple-dose capsule FMT RCTs (number needed to harm = 3, RR = 0.54; 95% CI 0.34–0.85; I2 = 13%).Placebo response was 33.7%in nonoral FMT RCTs and 67.8% in capsule FMT RCTs. The Grading of Recommendations Assessment, Development and Evaluation quality of the body of evidence was very low.
DISCUSSION:
Current evidence from RCTs does not suggest a benefit of FMT for global IBS symptoms. There remain questions regarding the efficacy of FMT in IBS as well as the lack of a clean explanation on the discrepant results among RCTs in subgroup analyses.
INTRODUCTION
Irritable bowel syndrome (IBS) is a symptom-based functional bowel disorder characterized by abdominal pain and altered bowel habits in the absence of detectable structural or biochemical abnormalities (1,2). IBS is the most commonly diagnosed gastrointestinal (GI) condition, with pooled regional prevalence ranging from 5.8% to 17.5% worldwide (3). A chronic disorder with fluctuating symptom severity, it often overlaps with other functional disorders and psychiatric conditions (4), and can significantly impair quality of life (QOL), resulting in high health care costs (5,6). The pathogenesis of IBS is heterogeneous, which has created significant challenges in the development of effective therapeutic strategies (7). Thus, although various dietary, lifestyle, medical, and behavioral interventions have proven effective in randomized controlled trials (RCTs), most IBS patients remain symptomatic despite treatment (7,8).
Recent studies have demonstrated a disturbance of the gut microbiota in patients with IBS, with decreased diversity compared with healthy patients (9–13). Manipulation of the gut microbiota has been proposed as a treatment strategy for IBS, and supported by accumulating evidence from clinical studies using antibiotics, probiotics, and dietary modifications (14–16). Fecal microbiota transplantation (FMT) targets gut dysbiosis, and has been proven an effective treatment for recurrent Clostridium difficile infection (CDI) (17). Recent observational studies of FMT in IBS have been encouraging. A systematic review including an analysis of case reports and case series of IBS found clinical improvement with FMT in 48% (28/48) of patients. Results from RCTs have been inconsistent, however (18–22). The aim of this study was to conduct meta-analyses and a systematic review of RCTs to estimate the efficacy and safety of FMT for the treatment of IBS, with subgroup analyses by delivery method.
METHODS
Meta-analyses were conducted according to the published Meta-analysis of Observational Studies in Epidemiology and Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (23,24).
Literature search
We performed a systematic search of the literature using MEDLINE (1946–June 2018), EMBASE (1947–July 2018), Cochrane Central Register of Controlled Trials (1993–July 2018), and Web of Science (1900–July 2018). Bibliographies from national and international gastroenterology conferences from 2008 to 2018, including Digestive Disease Week, the American College of Gastroenterology Annual Meeting, and United European Gastroenterology Week, were searched manually.
Search terms used for fecal microbiota transplantation were “faecal” or “fecal” or “feces” or “faeces” or “stool” or “microbiota” or “microflora” or “fecal flora” or “faecal flora,” and “transplant*” or “transfusion” or “implant*” or “instillation” or “donor*” or “enema” or “reconstitution or infusion*” or “transfer*” or “FMT” or “bacteriotherapy.” The results were combined with key words for IBS (“IBS” or “irritable bowel syndrome”). These search terms were used both as Medical Subject Headings terms and as free text. No language limits were used. In addition, references from identified articles were reviewed to identify any missed studies.
Study selection
Study references and citations were collected in EndNote X8 software (Thomson Reuters, New York, NY). Two reviewers (D.X. and V.L.C.) independently reviewed the titles and abstracts of all citations identified by the literature search. Potentially relevant studies were retrieved, their references were reviewed and included if relevant, and the selection criteria were applied.
Studies were considered for inclusion if they met the following criteria: (i) prospective, randomized, double-blind, placebo-controlled trials (parallel group or first arm of cross-over); (ii) adult patients older than 16 years with IBS defined by accepted symptom-based criteria including Manning, Kruis, Rome I, Rome II, Rome III, or Rome IV; (iii) compared FMT with placebo consisting of only the FMT excipients (no microbiota) or an autologous FMT; (iv) primary outcome of improvement in global IBS symptoms; and (v) minimum duration of 8-week follow-up.
When identified clinical trials were not yet published as full manuscripts in the peer-reviewed literature, we collected data published in abstract form and contacted the respective authors for additional data. If raw event numbers were unavailable, data were extrapolated from reported percentages of relevant outcomes and total sample size.
Outcome of interest
The primary outcome was the short-term global improvement in IBS symptoms assessed between 8 and 12 weeks after FMT. This is the recommended duration for the assessment of short-term response to therapy in functional GI disorders (25,26).
Global improvement was assessed either as response to the dichotomous question of global improvement of IBS symptoms or clinically meaningful improvement assessed by accepted integrative symptom questionnaires, such as IBS Severity Scoring System instrument and Functional Bowel Disease Symptom Index (25). Secondary outcomes included QOL, microbiota profiles, and adverse events (AEs).
Data extraction and quality assessment
Two physician authors (D.X. and V.L.C.) abstracted data independently from each study. We collected publication year, study design, country of origin, study population, study site, sample size, IBS criteria, subtypes, primary and secondary study outcomes, fecal microbiota and placebo preparation, FMT route, frequency and duration, length of follow-up, and AEs. Disagreements in trial eligibility or data extraction were resolved by consensus among authors. Data were extracted from all studies for intention-to-treat analyses. Treatment failure was assumed for all cases with incomplete follow-up or missing data.
The Cochrane risk-of-bias tool was used to evaluate the quality of each eligible study for randomization, allocation, blinding of participants, personnel and outcome assessment, complete outcome data addressed, selective outcome reporting, and other sources of bias (27).
Assessment of quality of evidence
The quality of evidence was assessed by means of Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology (28). Two authors (D.X. and V.L.C.) independently assessed risk of bias, inconsistency, indirectness, imprecision, and publication bias. Overall quality was graded using GRADEPro Guideline Development Tool (29).
Data synthesis and statistical analyses
Data analyses were performed using STATA 14 (StataCorp, College Station, TX). Weighted random-effects meta-analysis was performed to compare FMT with placebo. Our principal summary measure was the relative risk (RR) of each outcome. Results were displayed as forest plots using the R software version 3.5.0 (R Foundation for Statistical Computing, Vienna, Austria). Heterogeneity was measured with I2 values. I2 > 50% was considered to be significant heterogeneity. Subgroup analyses were performed in methodology of FMT, differences in study setting, diagnostic criteria, IBS subtype, and risk of bias. Tests for funnel plot asymmetry were considered, but not used to assess for publication bias, as the number of studies identified was fewer than 10 (30).
RESULTS
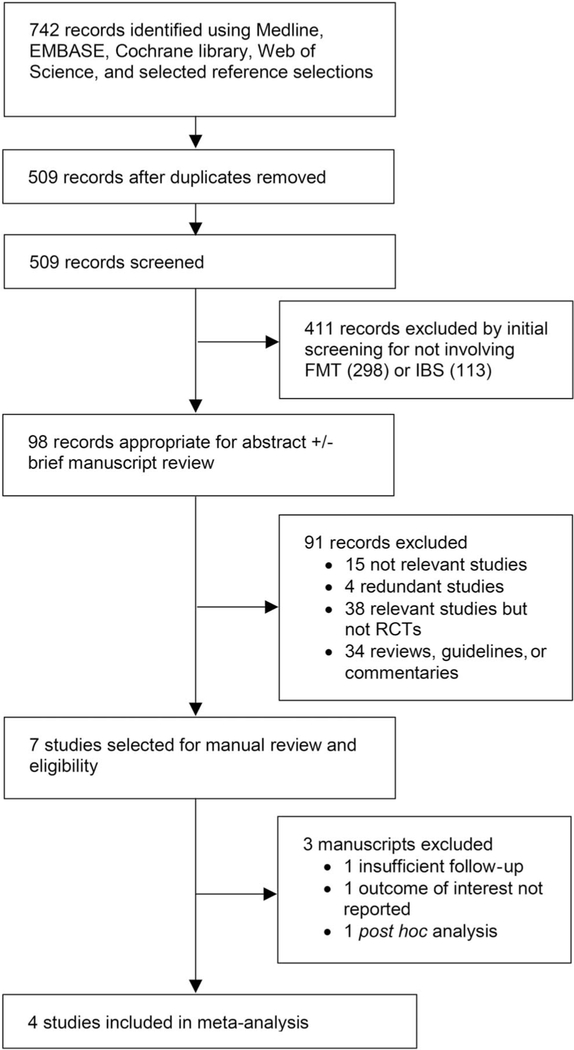
The literature search identified 742 citations, narrowed to 509 after duplicates were removed. Of these, 411 abstracts were excluded as not relevant in initial screening, resulting in 98 abstracts for review. The reviewers then examined the abstracts and manuscripts based on previously determined eligibility criteria, further excluding 91 references. Figure 1 provides the Preferred Reporting Items for Systematic Reviews and Meta-Analyses flowchart. Of the 7 remaining citations, careful full-text review excluded 3 articles because of insufficient follow-up (31), outcome of interest not reported (18), and post hoc analysis (32). Therefore, 4 RCTs (including 2 abstracts not yet published as full manuscripts) were eligible and included in our analysis (19–22).
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flowchart of study selection for the systematic review and meta-analyses. FMT, fecal microbiota transplantation; IBS, irritable bowel syndrome; RCT, randomized controlled trial.
Supplementary Table 1 (see Supplementary Digital Content 1, http://links.lww.com/AJG/A160) summarizes the risk of bias across studies using the Cochrane Collaboration tool. One RCT was low risk. Three trials were unclear of risk.
Detailed characteristics of the included RCTs are summarized in Table 1. All studies used Rome III criteria for the diagnosis of IBS. One study included IBS-D only (22), 2 studies included IBS without constipation (19,20), and 1 study included all 3 subtypes of IBS (21). FMT was administered using colonoscopy in1study(19),nasojejunal tube in 1 study (20), and oral capsules in 2 studies (21,22). The 2 nonoral route studies performed single-dose administration of donor or autologous fecal microbiota preparation (19,20) and the 2 oral capsule FMT studies used multiple doses (3 and 12 doses) of donor fecal microbiota or placebo consisting of FMT excipients alone (no microbiota) (21,22).
Table 1.
Characteristics of RCTs of FMT vs placebo in IBS
| Study, year | Study population | Setting | Study site | Sample size | IBS criteria | IBS subtypes | Primary outcome |
|---|---|---|---|---|---|---|---|
| Johnsen et al., 2017 (19) | Single center | Primary care | Norway | 90 | Rome III | IBS-D 53% IBS-M 47% |
Decrease in IBS-SSS > 75 points at 3 mo |
| Holvoet et al., 2018 (20) | Single center | Tertiary care | Belgium | 64 | Rome III | Predominant bloating and non-C | Yes to question of improvement in overall symptoms and abdominal bloating at 12 wk |
| Aroniadis et al., 2018 (22) | Multi-center | Primary, secondary, and tertiary care | USA | 48 | Rome III | IBS-D | Decrease in IBS-SSS ≥ 50 points at 12 wk |
| Halkjaer et al., 2018 and Aroniadis et al., 2018 (21,22) | Two centers | Tertiary care | Denmark | 52 | Rome III | All subtypes 33.3% IBS-C 29.4% IBS-D 37.3% IBS-M |
Decrease in IBS-SSS ≥ 50 points at 3 mo |
| Study, Year | Secondary outcomes | Fecal microbiota preparation | Placebo | FMT route | Frequency and duration | Follow-up |
|---|---|---|---|---|---|---|
| Johnsen et al., 2017(19) | Decrease in IBS-SSS > 75 points at 12 mo | 50–80 g pooled donor feces (2 donors) mixed with 200 mL isotonic saline and 50 mL 85% glycerol | Patients’ own feces | Colonoscopy | Once | 12 mo |
| Holvoet et al., 2018(20) | Change in IBS symptom scores by using daily diary, IBS-QOL, microbiota composition | Feces from 2 healthy donors | Patients’ own feces | Nasojejunal tube | Once | 12 mo |
| Aroniadis, 2018(22) | IBS-QOL, HADS, Bristol stool scale scores, microbiome profiles, AEs | 75 FMT capsules containing 50 g feces from 1 of 4 donors | Placebo capsules not containing fecal microbiota | Water soluble gelatin oral capsules | 25 capsules daily × 3 d | 24 wk |
| Halkjaer, 2018(21) | Change in IBS-QOL at 3 mo, changes in microbiota diversity | 300 FMT capsules containing 144 g fecal matter derived from 600 g pooled donor feces (4 donors) | Placebo capsules not containing fecal microbiota | Acid-resistant oral capsules (capsugel DRcaps) | 25 capsules daily × 12 d | 6 mo |
AE, adverse event; FMT, fecal microbiota transplantation; HADS, Hospital Anxiety and Depression Scale; IBS, irritable bowel syndrome; IBS-C, -D, -M, non-C, IBS with predominant constipation, predominant diarrhea, predominant irregular bowel habits (mixed diarrhea/constipation), non-constipation; IBS-SSS, IBS Severity Scoring System; IBS-QOL, Irritable Bowel Syndrome-Quality of Life Measure; RCT, randomized controlled trial.
Global improvement in IBS symptoms
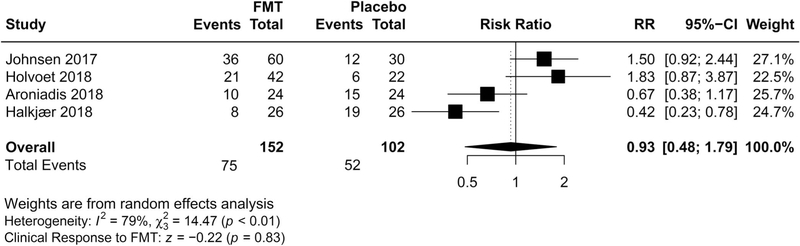
All 254 participants were included in the intention-to-treat analysis of the primary outcome, of whom 152 received FMT and 102 received placebo. The overall clinical response rate at 12 weeks was 49.3% (75/152) in patients assigned to donor FMT, and 51.0% in patients assigned to placebo (52/102) (Figure 2). No significant difference in global improvement of IBS symptoms was observed in patients receiving donor FMT compared with placebo (RR 0.93; 95% confidence interval (CI) 0.48–1.79, P = 0.83 from random effects), with a wide CI, and with significant heterogeneity identified across the studies (I2 = 79%). Statistical assessment for publication bias was not performed because only 4 included trials were inadequate for funnel plots or regression based assessments.
Figure 2.
Forest plot of all studies for efficacy of FMT vs placebo on global improvement of IBS symptoms. CI, confidence interval; FMT, fecal microbiota transplantation; IBS, irritable bowel syndrome; RR, risk ratio.
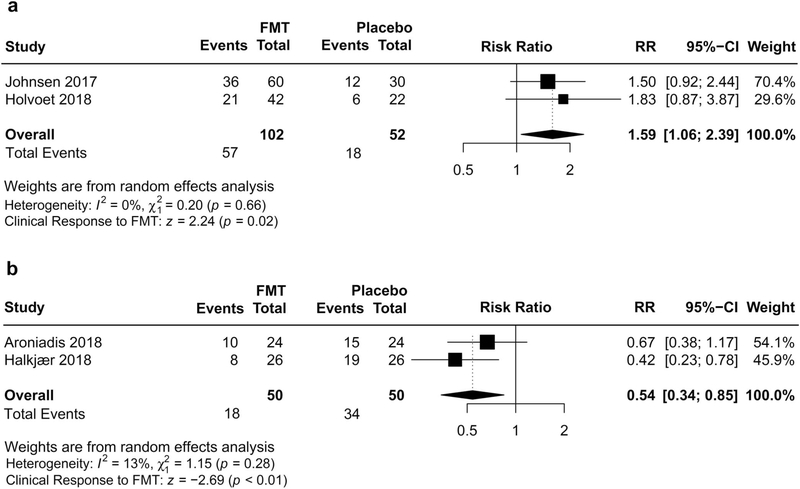
Given significant heterogeneity, the wide 95% CI, and the relatively small number of trials, we performed subgroup analyses to explore possible explanations for the striking inconsistency (Table 2). Among studies using single-dose FMT administration, and autologous FMT by means of colonoscopy and nasojejunal tube for placebo intervention, FMT was associated with improvement of global IBS symptoms compared with placebo, with low heterogeneity (RR 1.59; 95% CI 1.06–2.39; I2 = 0%) (Figure 3). Among studies using oral capsules administered in multiple doses, FMT was associated with a reduction in likelihood of global improvement compared with capsule excipients as placebo, also with a low heterogeneity (RR 0.54; 95% CI 0.34–0.85; I2 = 13%) (Figure 4). Mean placebo response rate was 33.7% (27.3% and 40.0%, respectively) in RCTs using single-dose FMT and autologous FMT as placebo, whereas 67.8% was observed in RCTs using multiple-dose FMT capsules with capsule excipients as placebo (62.5% and 73.1%, respectively).
Table 2.
Subgroup analyses of comparisons of FMT vs placebo in IBS
| No. of RCTs | No. of patients | RR of global improvement (95% CI) | NNT (95% CI) | I2 | |
|---|---|---|---|---|---|
| All studies | 4 | 254 | 0.93 (0.48–1.79) | Not estimable | 79% |
| Route of administration | |||||
| Colonoscopy | 1 | 90 | 1.50 (0.82–2.44) | Not estimable | Not applicablea |
| Nasojejunal tube | 1 | 64 | 1.83 (0.87–3.87) | Not estimable | Not applicablea |
| Oral capsules | 2 | 100 | 0.54 (0.34–0.85) | 3 (2–0)b | 13% |
| Placebo treatment | |||||
| Autologous FMT | 2 | 154 | 1.59 (1.06–2.39) | 5 (2–48) | 0% |
| FMT excipients (no microbiota) | 2 | 100 | 0.54 (0.34–0.85) | 3 (2–10)b | 13% |
| FMT frequency | |||||
| Single | 2 | 154 | 1.59 (1.06–2.39) | 5 (2–48) | 0% |
| Multiple | 2 | 100 | 0.54 (0.34–0.85) | 3 (2–10)b | 13% |
| Setting | |||||
| Tertiary care only | 2 | 116 | 0.87 (0.20–3.69) | Not estimable | 89% |
| Primary, secondary, and tertiary care | 2 | 138 | 1.01 (0.46–2.25) | Not estimable | 78% |
| IBS subtypes | |||||
| Without constipation | 3 | 202 | 1.20 (0.66–2.20) | Not estimable | 68% |
| All subtypes | 1 | 52 | 0.42 (0.23–0.78) | 2 (1–6) | Not applicablea |
| Risk of bias | |||||
| Low | 1 | 90 | 1.50 (0.92–2.44) | Not estimable | Not applicablea |
| Unclear | 3 | 164 | 0.78 (0.36–1.72) | Not estimable | 77% |
CI, confidence interval; FMT, fecal microbiota transplantation; IBS, irritable bowel syndrome; NNT, number needed to treat; RCTs, randomized controlled trial; RR, risk ratio.
Numberof studies insufficient to assess heterogeneity.
Number needed to harm.
Figure 3.
Individual forest plots of subgroups based on FMT frequency and placebo treatment for FMT. (a) Efficacy of single-dose FMT administration with autologous FMT as placebo on global improvement of IBS symptoms. (b) Efficacy of multiple-dose FMT capsules with capsule excipients as placebo on global improvement of IBS symptoms. CI, confidence interval; FMT, fecal microbiota transplantation; IBS, irritable bowel syndrome; RR, risk ratio.
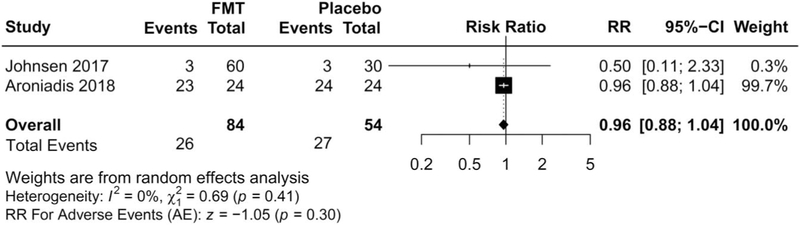
Figure 4.
Forest plot of adverse events with FMT vs placebo in irritable bowel syndrome (IBS). AE, adverse event; CI, confidence interval; FMT, fecal microbiota transplantation; RR, risk ratio.
QOL
Two studies demonstrated improvement of IBS-QOL within groups from baseline to 12 weeks after treatment (mean difference = 3; 95% CI = −7.2 to 13.2 (22), and 16% improvement; P = 0.03 (20), but no significant difference was seen between the FMT and control groups at 12 weeks. One study reported less improvement in IBS-QOL in FMT than in placebo at 3 months, favoring the placebo (mean difference = 9.3; 95% CI = 3.7–14.8). However, data could not be extracted from the study for cumulative analysis because of discrepant reporting between the stated less improvement in QOL and the presented higher IBS-QOL scores in FMT vs placebo group (21).
Microbiota analyses
Fecal microbiota analyses were conducted in 3 of 4 RCTs (20–22). One study demonstrated an increase in richness (α-diversity) and a shift of recipients’ microbial community composition toward donors’ microbial communities (β-diversity) that was maintained up to 6 months after FMT treatment, implicating engraftment of donors’ gut microbiota (21). These microbial changes were, however, not associated with clinical responses to FMT (21). One study reported no differences in microbial composition (β-diversity) assessed by Shannon diversity and Jensen–Shannon divergence between FMT responders and nonresponders (22). Finally, the study conducted by Holvoet et al. (20) performed supervised principal component analysis and demonstrated a significant difference in post-FMT fecal microbiota between successful active treatment and unsuccessful placebo treatment.
Safety and AEs
AE data were available for 3 studies (19,21,22). Overall, FMT was well tolerated. Two serious AEs were reported. One serious AE of transient vertigo and nausea developed after the FMT procedure, requiring a few hours of observation in the hospital (19). One serious AE of suicide in the month following FMT occurred in the placebo group (20). No other serious AEs were reported.
Data were pooled from 2 trials, which included 26 AEs of 84 participants (30.9%) assigned to FMT, compared with 27 AEs in the 54 assigned to placebo (50.0%) (Figure 3). No significant difference in the total number of AEs was observed in patients receiving donor FMT compared with control patients (RR 0.96; 95% CI 0.88–1.04, P = 0.30 from random effects, I2 = 0%) (20,22).
The study conducted by Halkjaer et al. (21), which used 12 days of FMT capsules, reported that a number of patients experienced side effects. The data could not be pooled with the other 2 trials for analyses of AEs. In the FMT group, 84.6% (22/26) of patients and in the placebo group 57.7% (15/26) experienced side effects (P = 0.07). Diarrhea was more frequent in the FMT group (23.1%; 6/26) compared with placebo (0%; 0/26) (P = 0.03); all episodes developed during the FMT therapy period (21). No significant difference in other individual symptoms was found between FMT and control groups in this particular study.
GRADE quality of evidence
Supplementary Table 2 (see Supplementary Digital Content 1, http://links.lww.com/AJG/A160) summarizes the assessment of quality of evidence using GRADE methodology. The quality of the current body of evidence was “very low” because of heterogeneity in the methodology of FMT and placebo interventions between studies, and imprecision of effect estimate.
DISCUSSION
We performed a systematic review and meta-analyses to evaluate the efficacy and safety of FMT as compared with placebo in patients with IBS. To date, these are the first meta-analyses of FMT in IBS using RCTs.
Using the endpoint of global improvement in IBS symptoms at 12 weeks after FMT, 4 RCTs involving 254 participants for evaluation of FMT in IBS have yielded statistically inconclusive results, with no significant difference in global improvement between FMT and placebo, and significant inconsistency of results. The benefit or harm of FMT seems to be associated with the methodology of FMT and placebo because the 2 RCTs using single-dose FMT through colonoscopy and nasojejunal tubes demonstrated a clinically significant improvement in global IBS symptoms in comparison with autologous FMT, whereas the 2 multiple-dose oral capsule FMT studies showed not just lack of benefit, but potential harm to the subjects when compared with capsule excipients only. The reasons for these differences may be explained by placebo effect, potential dose differences in beneficial bacteria delivered to the GI tract, or due to route of administration; delivery of fecal bacteria to the upper GI tract (through oral administration) may inadvertently cause an exacerbation of underlying functional GI symptoms.
The fecal microbiota after FMT was different in responders compared with nonresponders in 1 study (20), implicating the role of stable engraftment of donors’ gut microbiota in the success of FMT. However, this was not proven in 2 other studies, which demonstrated no relationship of post-FMT gut microbial diversities with clinical responses (21,22). The effect of fresh and frozen FMT on IBS-Severity Scoring System in a post hoc analysis after adjustment for functional comorbidities was similar (19).
This systematic review and meta-analysis has several methodological limitations. All 4 RCTs are relatively small studies and include 2 conference abstracts with an unclear risk of bias because of missing information regarding methodology. Given the small number of studies included, we did not perform statistical tests to assess for publication bias. The risk of publication bias is still suspected and reflected in the assessment of GRADE quality of evidence for unpublished small size studies and the possibility of lag bias (early publication of positive results). The Meta-analysis of Observational Studies in Epidemiology guidelines were followed to extract all the data. We extracted data from all studies for an intention-to-treat analysis. To further explore the explanation of the heterogeneity, besides interventions, we also analyzed the differences in study setting, subgroups of IBS, and risk of bias of RCTs. Heterogeneity was not associated with these factors.
The apparent difference between oral and nonoral FMT in IBS is in stark contrast to the observation that capsule FMT is highly effective and not inferior to nonoral FMT in prevention of recurrent CDI (33,34). The difference derived from the post hoc analysis should be interpreted with caution. Because of the known limitations of subgroup analysis, along with the small sample size within each trial, the statistical power of the current subgroup analyses is substantially reduced and risk of false-positive findings is increased. Furthermore, previous probability affects the positive predictive value of the subgroup analysis. Whether FMT capsules are symptomatically and/or physiologically harmful in IBS is currently unclear; however, FMT capsules were associated with abdominal cramping and bloating in 30% (6/20) of patients with CDI, and fewer GI AEs occurred with FMT administered using colonoscopy (33–35).
Last, distinct from CDI, the pathogenesis of IBS involves multiple central and peripheral pathophysiological factors. The mechanism by which gut dysbiosis contributes to the development of IBS is not entirely clear. Both FMT excipients and autologous FMT are not truly inert placebos and may introduce bias against FMT through their own biological effects on IBS and gut microbiota. Twenty-five large size 00 capsule excipients daily for 3 and 12 days may increase the magnitude of placebo effects through psychological and neurobiological mechanisms (36). Human fecal microbiota is significantly different from luminal and mucosal source in the upper and lower GI tract (37,38) and may impact gut microbiota when given as an autologous FMT (19,20). Laxatives, used for bowel preparation before FMT may alter gut microbiota (38,39), and glycerol, used as a cryoprotectant, may potentially affect the composition of colonic microbiota (40). The placebo response rates in autologous FMT trials are comparable to previously reported pooled placebo response rate of 37.5% in RCTs in IBS (19,20,41), however, are markedly higher in the capsule FMT, suggesting different placebo effects, probably related to the methodology of placebo treatment.
The apparent clinical benefit of single-dose FMT using colonoscopy and nasojejunal tubes appears more promising than the effect seen with oral capsules. The clinical response rate of 55.9% with number needed to treat of 5 is comparable to a previously published summary of case reports and case series (58%; 28/48) (42). Currently, the overall GRADE quality of evidence for FMT in IBS is very low due to the relatively small number of trials, the high heterogeneity of results, and imprecision of effect estimate. This is expected to improve and become more robust with time as ongoing RCTs are added to the body of evidence.
In summary, our results report that the current evidence from available RCTs does not suggest an overall clinical benefit from FMT for global IBS symptoms. A discrepancy in efficacy of FMT for IBS in subgroup analyses may be related to the differences in route of administration, placebo treatment, and FMT frequency among the RCTs. There remains uncertainty about the efficacy of FMT in IBS, as well as the lack of a clear explanation on the discrepant results among RCTs in subgroup analysis. The clinical benefits of FMT for IBS need to be further evaluated in high-quality clinical trials that involve comparison of FMT with an appropriate control.
Supplementary Material
Study Highlights.
WHAT IS KNOWN
-
✓
IBS is a heterogeneous disorder. Disturbance of gut microbiota has been found in patients with IBS.
-
✓
Observational studies in IBS showed clinical improvement with FMT targeting gut dysbiosis.
-
✓
Results from RCTs have been inconsistent. No previous meta-analysis has assessed the RCT data.
WHAT IS NEW HERE
-
✓
Meta-analysis of 4 RCTs does not show a clinical benefit of FMT to improve global IBS symptoms.
-
✓
Subgroup analyses reveal discrepancy in efficacy among the RCTs, which may be related to the differences in route of administration, placebo treatment, and FMT frequency.
Acknowledgments
Financial support: NIH funding support: NIH 5T32 DK094775, 2P30 DK0349331, 5R01 DK0589, T32 DK062708.
Footnotes
CONFLICTS OF INTERESTS
Potential competing interests: None.
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/AJG/A160
REFERENCES
- 1.Owyang C. Irritable bowel syndrome In: Kasper D, Fauci A, Hauser S, et al. (eds). Harrison’s Principles of Internal Medicine, 19th edn McGrawHill Education: New York, NY, 2015, pp 1965–70. [Google Scholar]
- 2.Mearin F, Lacy BE, Chang L, et al. Bowel disorders. Gastroenterology 2016;150:1393–407.e5. [DOI] [PubMed] [Google Scholar]
- 3.Sperber AD, Dumitrascu D, Fukudo S, et al. The global prevalence of IBS in adults remains elusive due to the heterogeneity of studies: A Rome foundation working team literature review. Gut 2017;66:1075–82. [DOI] [PubMed] [Google Scholar]
- 4.Ford AC, Bercik P, Morgan DG, et al. Characteristics of functional bowel disorder patients: A cross-sectional survey using the Rome III criteria. Aliment Pharmacol Ther 2014;39:312–21. [DOI] [PubMed] [Google Scholar]
- 5.Gralnek IM, Hays RD, Kilbourne A, et al. The impact of irritable bowel syndrome on health-related quality of life. Gastroenterology 2000;119:654–60. [DOI] [PubMed] [Google Scholar]
- 6.Peery AF, Crockett SD, Barritt AS, et al. Burden of gastrointestinal, liver, and pancreatic diseases in the United States.Gastroenterology 2015;149:1731–41.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chey WD, Kurlander J, Eswaran S. Irritable bowel syndrome: A clinical review. JAMA 2015;313:949–58. [DOI] [PubMed] [Google Scholar]
- 8.El-Serag HB, Pilgrim P, Schoenfeld P. Systemic review: Natural history of irritable bowel syndrome. Aliment Pharmacol Ther 2004;19:861–70. [DOI] [PubMed] [Google Scholar]
- 9.Carroll IM, Ringel-Kulka T, Keku TO, et al. Molecular analysis of the luminal- and mucosal-associated intestinal microbiota in diarrhea predominant irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol 2011;301:G799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carroll IM, Ringel-Kulka T, Siddle JP, et al. Alterations in composition and diversity of the intestinal microbiota in patients with diarrhea-predominant irritable bowel syndrome. Neurogastroenterol Motil 2012;24:521–30, e248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhattarai Y, Muniz Pedrogo DA, Kashyap PC. Irritable bowel syndrome: A gut microbiota-related disorder? Am J Physiol Gastrointest Liver Physiol 2017;312:G52–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tap J, Derrien M, Tornblom H, et al. Identification of an intestinal microbiota signature associated with severity of irritable bowel syndrome. Gastroenterology 2017;152:111–23.e8. [DOI] [PubMed] [Google Scholar]
- 13.Menees S, Chey W. The gut microbiome and irritable bowel syndrome.F1000Res 2018;7:F1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foxx-Orenstein AE, Chey WD. Manipulation of the gut microbiota asa novel treatment strategy for gastrointestinal disorders. Am J Gastroenterol Suppl 2012;1:41–6. [Google Scholar]
- 15.Menees SB, Maneerattannaporn M, Kim HM, et al. The efficacy andsafety of rifaximin for the irritable bowel syndrome: A systematic review and meta-analysis. Am J Gastroenterol 2012;107:28–36; quiz 36. [DOI] [PubMed] [Google Scholar]
- 16.Chey J, Ford AC, Yuan Y, et al. A systematic review and meta-analysis evaluating the efficacy of a gluten-free dietanda low FODMAPs diet in treating symptoms of irritable bowelsyndrome.AmJGastroenterol2018;113:1290–300. [DOI] [PubMed] [Google Scholar]
- 17.Kassam Z, Lee CH, Yuan Y, et al. Fecal microbiota transplantation for Clostridium difficile infection: Systematic review and meta-analysis. Am J Gastroenterol 2013;108:500–8. [DOI] [PubMed] [Google Scholar]
- 18.Hunt S, Brummer RJ, Repsilber D, et al. Fecal microbiota transplantation in irritable bowel syndrome and a randomized placebo-controlled trial. Gastroenterology 2017;152:S101–2. [Google Scholar]
- 19.Johnsen PH, Hilpüsch F, Cavanagh JP, et al. Faecal microbiota transplantation versus placebo for moderate-to-severe irritable bowel syndrome: A double-blind, randomised, placebo-controlled, parallel group, single-centre trial. Lancet Gastroenterol Hepatol 2018;3:17–24. [DOI] [PubMed] [Google Scholar]
- 20.Holvoet T, Joossens M, Jerina B, et al. Fecal microbiota transplantation in irritable bowel syndrome with predominant abdominal bloating: Results from a double blind, placebo-controlled clinical trial. Gastroenterology 2018;154:S130. [DOI] [PubMed] [Google Scholar]
- 21.Halkjaer SI, Christensen AH, Lo BZS, et al. Faecal microbiota transplantation alters gut microbiota in patients with irritable bowel syndrome: Results from a randomised, double-blind placebo-controlled study. Gut 2018;67:2107–15. [DOI] [PubMed] [Google Scholar]
- 22.Aroniadis OC, Brandt LJ, Oneto C, et al. A double-blind, randomized, placebo-controlled trial of fecal microbiota transplantation capsules (FMTC) for the treatment of diarrhea-predominant irritable bowel syndrome (IBS-D). Gastroenterology 2018;154:S154–55. [Google Scholar]
- 23.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studiesin epidemiology: A proposal for reporting. Meta-analysis of observational studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008–12. [DOI] [PubMed] [Google Scholar]
- 24.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann Intern Med 2009;151:264–9, W64. [DOI] [PubMed] [Google Scholar]
- 25.Irvine EJ, Tack J, Crowell MD, et al. Design of treatment trials for functional gastrointestinal disorders. Gastroenterology 2016;150:1469–80.e1. [DOI] [PubMed] [Google Scholar]
- 26.Veldhuyzen van Zanten SJ, Talley NJ, Bytzer P, et al. Design of treatment trials for functional gastrointestinal disorders. Gut 1999;45(Suppl 2):II69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 2011;64:383–94. [DOI] [PubMed] [Google Scholar]
- 29.GRADEpro GDT: GRADEpro guideline development tool [software]. McMaster University,2015(developed by Evidence Prime, Inc., gradepro.org). [Google Scholar]
- 30.Sterne JA, Sutton AJ, Ioannidis JP, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011;343:d4002. [DOI] [PubMed] [Google Scholar]
- 31.Bruno G, Cicerone C, Auria S, et al. Fecal microbiota transplantation inpatients with irritable bowel syndrome unresponsive to standard treatments: Transplant protocol via retention enema and preliminary results. Dig Liver Dis 2018;50:e175. [Google Scholar]
- 32.El-SalhyM Holger JohnsenP, Mazzawi T, et al. Effect of faecal microbiota transplantation on the enteroendocrine cells of the colon in patients with irritable bowel syndrome (IBS):Double blinded-placebo controlled study. Neurogastroenterol Motil 2017;29:71. [Google Scholar]
- 33.Youngster I, Russell GH, Pindar C, et al. Oral, capsulized, frozen fecal microbiota transplantation for relapsing Clostridium difficile infection. JAMA 2014;312:1772–8. [DOI] [PubMed] [Google Scholar]
- 34.Wang S, Xu M, Wang W, et al. Systematic review: Adverse events of fecal microbiota transplantation. PLoS One 2016;11:e0161174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kao D, Roach B, Silva M, et al. Effect of oral capsule- vs colonoscopy delivered fecal microbiota transplantation on recurrent Clostridium difficile infection: A randomized clinical trial. JAMA 2017;318:1985–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shah E, Pimentel M. Placebo effect in clinical trial design for irritable bowel syndrome. J Neurogastroenterol Motil 2014;20:163–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marteau P, Pochart P, Dore J, et al. Comparative study of bacterial groupś within the human cecal and fecal microbiota. Appl Environ Microbiol 2001;67:4939–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zmora N, Zilberman-Schapira G, Suez J, et al. Personalized gut mucosal colonization resistance to empiric probiotics is associated with unique host and microbiome features. Cell 2018;174:1388–405.e21. [DOI] [PubMed] [Google Scholar]
- 39.Tropini C, Moss EL, Merrill BD, et al. Transient osmotic perturbation causes long-term alteration to the gut microbiota. Cell 2018;173:1742–1754.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cleusix V, Lacroix C, Vollenweider S, et al. Glycerol induces reuterin production and decreases Escherichia coli population in an in vitro model of colonic fermentation with immobilized human feces. FEMS Microbiol Ecol 2008;63:56–64. [DOI] [PubMed] [Google Scholar]
- 41.Ford AC, Moayyedi P. Meta-analysis: Factors affecting placebo response rate in the irritable bowel syndrome. Aliment Pharmacol Ther 2010;32:144–58. [DOI] [PubMed] [Google Scholar]
- 42.Halkjaer SI, Boolsen AW, Gunther S, et al. Can fecal microbiota transplantation cure irritable bowel syndrome? World J Gastroenterol 2017;23:4112–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.