Abstract
Background:
Although studies have observed several markers correlate with progression of prostate cancer (PCa), no specific markers have been identified that accurately predict the progression of this disease, even in African American (AA) men who are generally at higher risk than other ethnic groups. The primary goal of this study was to explore whether three markers could predict the progression of PCa.
Method:
We investigated protein expression of Annexin 2 (ANX2), serine peptidase inhibitor, kazal type 1(SPINK1)/tumor-associated trypsin inhibitor (TATI), and heat shock protein 60 (Hsp60) in 79 archival human prostate trans-rectal ultrasound (TRUS) biopsy tissues according to a modified World Health Organization (WHO) classification: normal (WHO1a), Gleason Score (GS6 (WHO1b), GS7 subgroups (WHO2 = 3 + 4, WHO3 = 4 + 3), GS8 (WHO4), and GS9–10 (WHO5). AA men aged 41–90 diagnosed from 1990 to 2013 at Howard University were included. Automated staining assessed expression of each biomarker. Spearman correlation assessed the direction and relationship between biomarkers, WHO and modified WHO GS, age, and 5-year survival. A two-tailed t-test and ANOVA evaluated biomarkers expression in relationship to WHO normal and other GS levels, and between WHO GS levels. A logistic and linear regression analysis examined the relationship between biomarker score and WHO GS categories. Kaplan-Meier curves graphed survival.
Results:
ANX2 expression decreased monotonically with the progression of PCa while expression of SPINK1/TATI and Hsp60 increased but had a more WHO GS-specific effect; SPINK1/TATI differed between normal and GS 2–6 and HSP60 differed between GS 7 and GS 2–6. WHO GS was found to be significantly and negatively associated with ANX2, and positively with SPINK1/TATI and Hsp60 expression. High SPINK1/TATI expression together with the low ANX2 expression at higher GS exhibited a bi-directional relationship that is associated with PCa progression and survival.
Conclusion:
Importantly, the data reveal that ANX2, and SPINK1/TAT1 highly associate with WHO GS and with the transition from one stage of PrCa to the next in AA men. Future research is needed in biracial and larger population studies to confirm this dynamic relationship between ANX2 and SPINK1 as independent predictors of PCa progression in all men.
Keywords: 5 year free survival, Gleason score, tumor-associated trypsin inhibitor, World Health Organization
1 |. INTRODUCTION
Prostate cancer (PCa) is the most prevalent cancer and the second leading cause of cancer death among Western males.1 In particular, African American (AAs) men, have the highest death rate and shortest survival for prostate cancer compared to other ethnic groups in the US.2 It is estimated that 161 360 American men will be diagnosed with PCa in 2017 and over 26 730 will die from the disease.1 AA men are disproportionately affected by the disease.2 Although mortality rates have decreased across all age and ethnic groups, AA men continue to have the highest incidence and death rates. The clinical potential of PCa ranges from relative indolence to a highly aggressive phenotype, where progression occurs rapidly and 30% of men diagnosed with PCa have locally advanced or metastatic disease,3 largely affecting AA men. Therefore, identifying biomarkers of aggressive PCa among AA men could provide a public health advance for early detection or for predicting aggressiveness.
Recent biomarkers studies of TMPRSS2-ERG fusion gene, androgen receptor, and other molecular markers demonstrated a correlation with aggressive PCa phenotype.4–11 Accordingly, these markers which show promise as predictors of aggressive disease have the potential to provide valuable information that could inform the PCa community. However, these molecular markers have been primarily studied in Caucasian men. As a result there has been a concerted effort to discover biomarkers that can predict aggressive disease in PCa among AA men.12
The purpose of the present study is to contribute to current prognostic screening methodology by identifying highly sensitive and specific biomarker signatures of aggressive cancer, leading to a biomarker risk prediction model that successfully characterizes PCa prognosis and informs a more effective therapeutic approach. We have chosen to evaluate by immunohistochemistry (IHC) three promising candidate biomarkers ANX2, SPINK1/TATI, and Hsp60 in relation to bifurcated WHO GS status and 5 year-free survival.
ANX2 is a heavy chain protein. It belongs to a family of Ca2+ dependent phospholipid and membrane binding proteins called annexins and contains a conserved repeating domain of approximately 70 amino acids.13 It is up-regulated in response to physiological stress and plays multiple roles in regulating cellular, including angiogenesis, proliferation, apoptosis, cell migration, invasion, and adhesion.14,15 Most importantly, studies have shown that the protein of ANX2 was specifically lost in primary adenocarcinoma of the prostate as opposed to other types of cancers and the majority of prostate intraepithelial neoplasia.16
SPINK1/TATI was originally isolated from the pancreas and its primary function is inhibition of serine proteases, such as trypsin, in the pancreas and small intestines.17 It encodes for a 56 amino acid peptide which is secreted in the prostate gland.17,18 SPINK1/TATI gene is located on 5q32 containing approximately 7.5 kb and four exons. A 40 bp DNA fragment located between kb −3.84 and −3.80 carries the element responsible for both transcriptional activity and IL-6-induced gene expression.17 SPINK1/TATI appears to play an important role in both cell survival and prevention of apoptosis by several different pathways in normal tissues.19,20 It has been shown that SPINK1 is also expressed in the prostate and its expression increases with increasing tumor grade.21 Moreover, SPINK1 expression in the urine and serum has shown to be a significant predictor of PrCa.21 Thus, measurement of SPINK1 in serum may be useful for identification of suitable patients and for monitoring of response to treatment.
Heat shock proteins (HSPs) are evolutionarily conserved, act as molecular chaperones in all cells at physiological temperatures and may be considered to have clinical utility as prognostic markers.22 These chaperone proteins are ubiquitous and function to assist a protein to attain its functional conformation, to mediate interaction with other proteins and to prevent non-functional side reactions.23–26 In addition, these proteins ensure metabolic homeostasis, and participate in a diverse range of pathogenic processes.27–29 For instance, these proteins are expressed under non-stress conditions in a cell cycle-dependent manner.30 In neoplasias, HSPs have been implicated in multidrug resistance, in regulation of apoptosis, and as modulators of p53 function.31–33 There are five principal mammalian heat shock protein families classified according to their electrophoretic characteristics—HSP90, HSP70, HSP60, HSP40 and small HSPs, like HSP27.22,34 High molecular weight HSPs are ATP-dependent, whereas small HSPs are ATP-independent.22 Although each protein has been shown to be expressed in PrCa, HSP60, has shown a unique expression pattern in PrCa.34,35 Hsp60 is a mitochondrial chaperonin that is responsible for the transportation and refolding of proteins from the cytoplasm into the mitochondrial matrix. Studies have linked Hsp60 to diabetes, stress response, cancer, and certain types of immunological disorders. HSP60 protein levels were observed to be elevated in poorly differentiated PCas and demonstrated a strong association with prognostic clinical parameters.36 In addition, HSP60 was shown to predict biochemical recurrence after radical prostatectomy.36 These studies demonstrate that Hsp60 may have prognostic value.
Despite the significant breakthrough with these known biomarkers, some men are still over-diagnosed with indolent PCa while others die from aggressive disease that progresses rapidly or is diagnosed too late. Although these markers have reasonable operating characteristics, none of the markers taken alone seems ideal. Because of PCa heterogeneity, a combination of biomarkers may provide better prediction. Therefore, this study seeks to evaluate whether combination of ANX2, SPINK1, and/or Hsp60 have significant predictive capacity as PCa progresses.
2 |. MATERIALS AND METHODS
2.1 |. Patient selection
The cases and controls were selected from patients seen between 1990 and 2013. There was uniform data collection and case completeness of tumor registry records as they relate to PCa during this time period. Samples were retrieved from the Howard University Pathology Department and identified by a pre-assigned and de-identified surgery accession number. Patient characteristics include age at diagnosis, vital status, and date of last contact. The years of cancer free survival were evaluated in relation to GS to illustrate the relationship between the two.
Based on the work of D’Amico37 and Borley38 a combination of pre-therapy PSA, GS, and clinical stage have been used to stratify patients into low grade (T1-T2a, GS 2–6, and PSA <10 ng/mL), intermediate grade (T2b-T2c, GS 7, or PSA 10–20 ng/mL), high grade (T3a or GS8–10 or PSA >20 ng/mL), and locally advanced (T3b-T4) groups that predict risk for both biochemical recurrence and survival following definitive local therapy (radical prostatectomy or radiation). The WHO has furthered clarified clinical GS status (WHO1 = < = 6, WHO2 = 3 + 4, WHO3 = 4 + 3, WHO4 = 4 + 4, WHO5 = 4 + 5, and 5 + 5). A case-control study of African American men with five cancer groups or normal tissue was assembled using modified WHO categories: (i) 15 normal prostate tissue (WHO1a); (ii) 15 newly diagnosed with non-aggressive low grade GS 2–6 (WHO1b); (iii) 25 with pre-advanced cancer or intermediate GS7 (3 + 4 [WHO2] and 4 + 3 [WHO3]); (iv) 12 advanced cancer or high grade GS 8 (WHO4); and (v) 12 aggressive GS9 and GS10 (WHO5). We separated out WHO1 into WHO1a and WHO1b group because of observable staining differences.
2.2 |. Immunohistochemistry assay
Optimally formalin-fixed paraffin-embedded (FFPE) specimens from a total of 79 patients each from WHO1a, WHO1b, WHO 2, WHO 3, WHO 4, and WHO 5 GS categories were selected for the study. From each patient sample 4–5 μm sections of the tissue samples were stained to assess protein expression of ANX2 (clone C-10, Santa Cruz; monoclonal mouse anti human 1:10 000; high pH), SPINK1/TATI (clone E-2, Santa Cruz, mouse anti-human, monoclonal, 1:50; high pH), and Hsp60 (clone LK1, Santa Cruz, mouse anti-human, monoclonal, 1:50; high pH). The polymer-HRP system was utilized for immuno-staining. Staining intensity and extent of staining were scored by two Howard University pathologists based on the system reported by other researchers.14,15 The following scoring system was used for extent of staining; 1 = less than 25% staining; 2 = 25–50%; 3 = 50–75% staining, and 4 = 75–100% tumor cells positive. For intensity of staining: 0 = no staining, 1 = weak staining, 2 = medium staining, 3 = strong staining was used. H score was determined by multiplying the extent score by intensity score.
2.3 |. Statistical analysis
Each observation was assigned to cancer groups as described above for the analysis. The staining scores for ANX2, SPINK1/TATI, and Hsp60 were coded as 0 for no staining and 1 for positive staining. WHO GS categories were also treated as a continuous variable to indicate the severity of the disease. Spearman’s correlation with covariates ANX2, SPINK1, and Hsp60 with and without adjustment for age was carried out. T-test was used to compare the staining scores between each WHO GS group and normal biopsies as well as between the different WHO GS categories. Linear regression was performed to assess biomarker regression on aggregated WHO levels or ordinal WHO2 levels. In addition, logistic multi-nominal models were performed to evaluate biomarker regression on modified WHO category levels compared to normals. The difference between date of last contact and the date of initial diagnosis in years was used as the years of survival. Kaplan Meier method was used to detect the difference in survival time between WHO GS categories, ANX2, Hsp60, and SPINK1 groups.
3 |. RESULTS
A visual comparison of the expression of the three proteins shows distinct features that distinguish low grade and high grade tumors (Figure 1). ANX2 is expressed in the normal prostate glands but is expressed neither in LGPCA (GS6) (E) nor in HGPCA (GS8–10) (F). Hsp60 is expressed in normal glands (G) and shows patchy expression in LGPCA (H) and increased strong expression in HGPCA (I). SPINK1/TATI is not expressed in normal glands (J) but shows strong diffused cytoplasm expression in LGPCA (K) and patchy strong dot-like expression in HGPCA (L). H & Es are included to display the broad range of cytoplasmic, nuclear, and extracellular matrix features in the normal, low grade, and high grade cancers.
FIGURE 1.
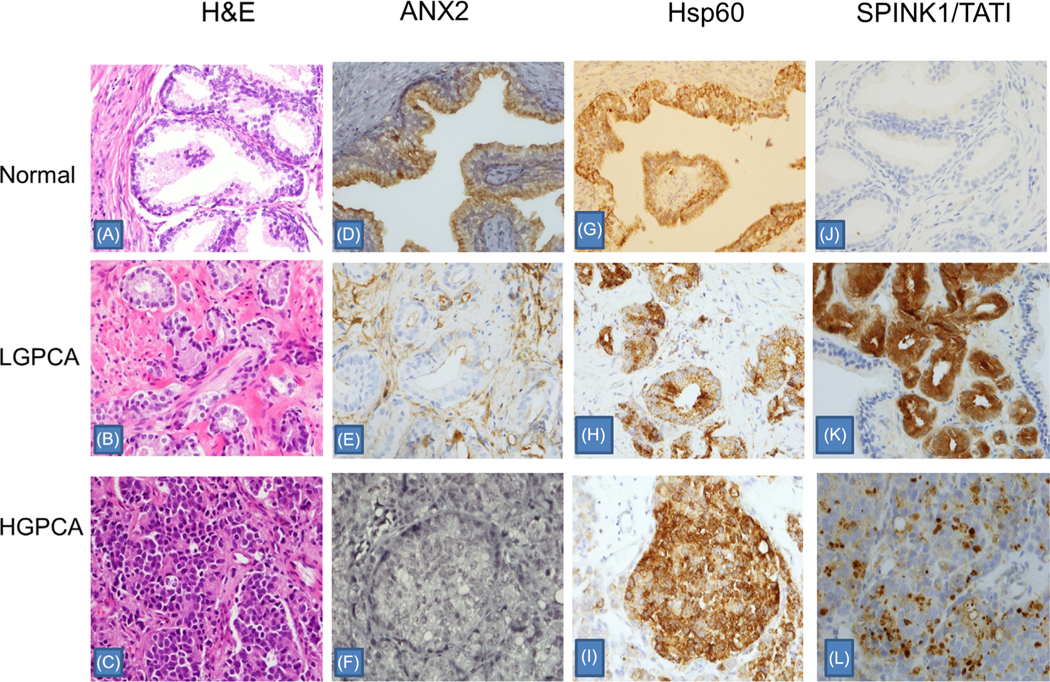
Comparison of Annexin (ANX2), Heat Shock Protein 60 (HSP60), and SPINK1/TATI expression in normal prostate, low grade prostate carcinoma (LGPCA) (Gleason score 6, Grade I), and high grade prostate carcinoma (HGPCA) (Gleason score 10, Grade V). Normal prostate with presence of the basal layer (A). Low grade PCA with well-defined microacinar glands demonstrating absence of basal layer and perineural involvement (B). High grade PCA with sheets of individual cells (C). ANX2 is expressed in the normal prostate glands (D) but not expressed in LGPCA (E) or HGPCA (F). HSP60 is expressed in normal glands (G) and shows patchy expression in LGPCA (H) and increased strong expression in HGPCA (I). SPINK1/TATI is not expressed in normal glands (J) but shows diffuse cytoplasmic expression in LGPCA (K) and patchy strong dot-like expression in HGPCA (L). Each slide is presented at 20× magnification. [Color figure can be viewed at wileyonlinelibrary.com]
Spearman’s correlation (rank correlation/nonparametric correlation) showed significant association between WHO and modified WHO GS categories, and two out of the three proteins and with all three proteins (Table 1) respectively. ANX2 and Hsp60 were significantly (P < 0.05) correlated with WHO, and modified WHO while SPINK1 only correlated (P < 0.05) with modified WHO. Among the proteins, ANX2 was negatively correlated with Hsp60, reaching statistical significance; SPINK1/TATI was not correlated with ANX2, but correlated with Hsp60 (Table 1). ANX 2 had a negative correlation with WHO and modified WHO GS. The largest correlations were observed between WHO and modified WHO and ANX2 expression (Table 1). SPINK1 had a positive significant association with modified WHO status. We showed that HSP60 had a positive significant correlation with WHO and modified WHO GS categories. Furthermore, it was established that WHO GS category is significantly correlated with modified WHO GS category. Age is neither correlated with WHO or modified WHO GS categories nor with ANX2, SPINK1/TATI, or Hsp60 expression (Table 1).
TABLE 1.
Spearman’s correlation among expression of proteins, WHO (Gleason score) categories, age, and 5-year survival
| SPINK1 score | Annexin2 score | HSP60 score | WHO | Modified WHO | Age | Survived 5 years | |
|---|---|---|---|---|---|---|---|
| SPINK1 score | 1.000 | ||||||
| Annexin2 score | −0.1935** | 1.000 | |||||
| HSP60 score | 0.2641* | −0.2965* | 1.000 | ||||
| WHOa | 0.1477 | −0.7610* | 0.3671* | 1.000 | |||
| Modified WHOb | 0.2384* | −0.7926* | 0.3809* | 0.9794* | 1.000 | ||
| Age | −0.0091 | −0.1421 | −0.0194 | 0.2060** | 0.1608 | 1.000 | |
| Survived 5 years | −0.0682 | 0.1838 | 0.0485 | −0.2614* | −0.2311* | −0.1101 | 1.000 |
Indicates correlation is significant at P ≤ 0.05, P ≤ 0.10.
WHO (1:GS 0–6, 2: 3 + 4, 3: 4 + 3, 4: GS 4 + 4, 5: GS 4 + 5 + 5 + 5).
Modified WHO (1A: GS = 0, 1B: GS = 2–6, 2: GS = 3 + 4, 3: GS = 4+3, 4 = 4+4, 5 = 4+ 5 + 5 + 5).
T-tests of WHO GS levels showed significant differences in ANX2, SPINK1/TATI, and Hsp60 expression (Table 2). Ordered logistic regression and logistic regression show comparable findings for ANX2 and Hsp60, though the ordered logistic regression is not as good a fit for SPINK1. In addition, T-tests further demonstrated that ANX2 intensity of expression differed significantly between normal (WHO1a) and pre-malignancy (WHO1b) and between intermediate (WHO 3) and advanced (WHO 4) stage of cancer (Table 3). They demonstrated that SPINK1/TATI significantly different between normal and GS 2–6 (Table 3). ANX2 held its significance comparing normal to all cancer levels as exhibited by a steady decline from normal to aggressive. The strength of association between normal and other GS levels was strongest, differentiating normal from GS 2–6, GS7 (4 + 3) and GS 8 for ANX2 protein after controlling for age (Table 3). HSP60 expression was statistically significant in the comparison of modified WHO 1b (GS 2–6) to WHO 2 (GS 3 + 4).
TABLE 2.
Mean ANX2, SPINK1, and Hsp60 scores by modified WHO (Gleason Score Categories) levels
| Group comparisons | Annexin 2 H score mean (95%CI) | SPINK H score mean (95%CI) | HSP60 H score mean (95%CI) |
|---|---|---|---|
| WHO 1a | 11.07 (9.48–12.65) | 1.93 (0.45–3.42) | 4.70 (3.29–6.11) |
| WHO 1b | 5.30 (3.64–6.96) | 7.90 (5.89–9.91) | 6.17 (4.13–8.21) |
| WHO 2 | 3.38 (2.22–4.55) | 7.12 (5.03–9.20) | 9.38 (7.47–11.15) |
| WHO 3 | 2.63 (1.46–3.79) | 5.92 (3.33–8.50) | 7.71 (4.66–10.75) |
| WHO 4 | 1.23 (0.85–1.61) | 5.35 (3.02–7.67) | 9.85 (7.76–11.93) |
| WHO 5 | 1.33 (0.82–1.84) | 6.83 (4.00–9.68) | 9.38 (6.56–12.19) |
| P (ANOVA) | P < 0.0000 | P = 0.0014 | P = 0.0034 |
| Logistic coefficient (controlling for age) | −0.63 (−0.93 to −0.34) P < 0.000 | 0.48 (0.15–0.80) P = 0.005 | 0.23 (0.07–0.39) P = 0.006 |
| Ordered logistic coefficient (controlling for age) | −0.62 (−0.81 to −0.43) P < 0.000 | 0.082 (−0.01 to 0.17) P = 0.081 | 0.17 (0.073–0.27) P = 0.001 |
WHO1a, Normal; WHO1b, GS 2–6.
TABLE 3.
ANX2, SPINK1, and Hsp60 expression assessment of adjacent modified WHO levels or WHO levels
| Group comparisons | Annexin 2 H score mean (95%CI) | SPINK1 H score mean (95%CI) | HSP60 H score mean (95%CI) |
|---|---|---|---|
| WHO 1a (Normal) | 11.1 (9.4–12.8) | 1.93 (0.33–3.5) | 4.7 (3.2–6.2) |
| WHO 1b (Gleason 2–6) | 5.3 (3.5–7.1) | 7.90 (5.7–10.1) | 6.17 (4.0–8.4) |
| P(difference*) | P < 0.0000 | P < 0.0001 | P = 0.25 |
| WHO 1b (Gleason 2–6) | 5.3 (3.5–7.1) | 7.9 (5.7–10.1) | 6.2 (4.0–8.4) |
| WHO 2 (Gleason 3 + 4) | 3.4 (2.1–4.7) | 7.1 (4.8–9.4) | 9.3 (7.3–11.3) |
| P(difference*) | P = 0.08 | P = 0.60 | P = 0.03 |
| WHO 2 (Gleason 3 + 4) | 3.4 (2.1–4.7) | 7.1 (4.8–9.4) | 9.3 (7.3–11.3) |
| WHO 3 (Gleason 4 + 3) | 2.6 (1.3–3.9) | 5.9 (3.1–8.8) | 7.7 (4.3–11.1) |
| P(difference*) | P = 0.37 | P = 0.48 | 0.37 |
| WHO 3 (Gleason 4 + 3) | 2.6 (1.3–3.9) | 5.9 (3.1–8.8) | 7.7 (4.3–11.1) |
| WHO 4 (Gleason 4 + 4) | 1.2 (0.81–1.7) | 5.3 (2.8–7.9) | 9.8 (7.6–12.1) |
| P(difference*) | P = 0.02 | P = 0.75 | 0.25 |
| WHO 4 (Gleason 4 + 4) | 1.2 (0.81–1.7) | 5.3 (2.8–7.9) | 9.8 (7.6–12.1) |
| WHO 5 (Gleason 9–10) | 1.3 (0.77–1.9) | 6.8 (3.7–10.0) | 9.4 (6.3–12.5) |
| P(difference*) | P = 0.75 | P = 0.43 | P = 0.79 |
| All Levels of WHO | |||
| Category | 0.7122 | 0.2139 | 0.1980 |
| P(F test)** | P < 0.0000 | P = 0.0028 | P = 0.0052 |
T test (Stata) two tailed test.
One way ANOVA two tailed test.
Linear regression analysis, adjusted for age, clearly showed significant associations between WHO categories and the expressions of ANX2 (Table 4). In the model of ANX2, SPINK1, HSP60 and age predicting WHO1a:WHO1b, WHO1b:WHO2–3, and WHO2–3:WHO4–5, only ANX2 response was negative and significant (−0.062, 95%CI: −0.062 to −0.14). SPINK1 response was positive and significant for only WHO1a:WHO1b (0.061, 95%CI: 0.036–0.085). The models further showed that Hsp60 response was positive and significant for only WHO1b:WHO 2–3 (0.028, 95%CI: 0.19–2.1).
TABLE 4.
Linear regression models: Biomarkers regressed on aggregated modified WHO levels
| WHO 1a:1b | WHO 1b:WHO 2–3 | WHO 2–3:WHO 4–5 | |
|---|---|---|---|
| Age | 0.079 (−0.022 to 0.12) | 0.0096 (−0.0060 to 0.025) | 0.0080 (−0.0044 to 0.020) |
| ANX2 H score | −0.062* (−0.087 to −0.036) | −0.062* (−0.12 to −0.0078) | −0.14* (−0.21 to −0.064) |
| SPINK1 H score | 0.061* (0.036–0.085) | −0.015 (−0.053 to 0.023) | −0.0074 (−0.037 to 0.23) |
| HSP60 H score | −0.0075 (−0.039 to 0.024) | 0.028* (−0.0054 to 0.62) | 0.018 (−0.012 to 0.048) |
WHO1 has been split for this analysis into 1a (Gleason normal) and 1b (Gleason 1–6).
P < 0.05.
In Table 5 we demonstrate a logistical multi-nominal regression model for age, ANX2, SPINK1, and Hsp60 intensity of expression on modified WHO levels. The data reveal that ANX2 is significant across all pairwise modified WHO cancer categories compared to WHO1a. However, SPINK1 was only significant between WHO1b and WHO1a with borderline significance across remaining pairwise WHO cancer categories. Furthermore, we demonstrated that Hsp60 expression was only significant between WHO1b:WHO2–3. Concerning age there was no significance among modified WHO GS categories. The logistical ordinal regression model for ANX2, SPINK1, and Hsp60 expression controlling for age (Supplementary Table S1) demonstrated similar association pattern with the WHO categories to the multi-nominal model.
TABLE 5.
Multinomial models: Biomarkers regressed on modified WHO levels
| WHO 1a | WHO 1b | WHO 2 | WHO 3 | WHO 4 | WHO 5 | |
|---|---|---|---|---|---|---|
| Age | Reference | 0.027 (−0.41 to 0.10) | 0.41 (−0.023 to 0.11) | 0.05 (−0.01 to 0.12) | 0.07 (0.002–0.15) | −0.07 (−0.005 to 0.14) |
| ANX2 H score | Reference | −1.29 (−2.64 to 0.055**) | −1.68 (−3.08 to −0.29)* | −1.81 (−3.21 to −0.41)* | −2.85 (−4.53 to −1.17)* | −2.65 (−4.23 to −1.06)* |
| SPINK1 H score | Reference | 1.31 (0.019–2.59)* | 1.21 (−0.09 to 2.51)** | 1.18 (−0.12 to 2.48)** | 1.12 (−0.19 to 2.42)** | 1.20 (−0.10 to 2.51)** |
| HSP60 H score | Reference | 0.46 (−0.22 to 1.14) | 1.21 (−0.05 to 1.33)** | 0.60 (−0.09 to 1.29)** | 0.70 (0.003–1.41) | 0.66 (−0.04 to 1.36)** |
Indicates correlation is significant at P ≤ 0.05, P ≤ 0.10.
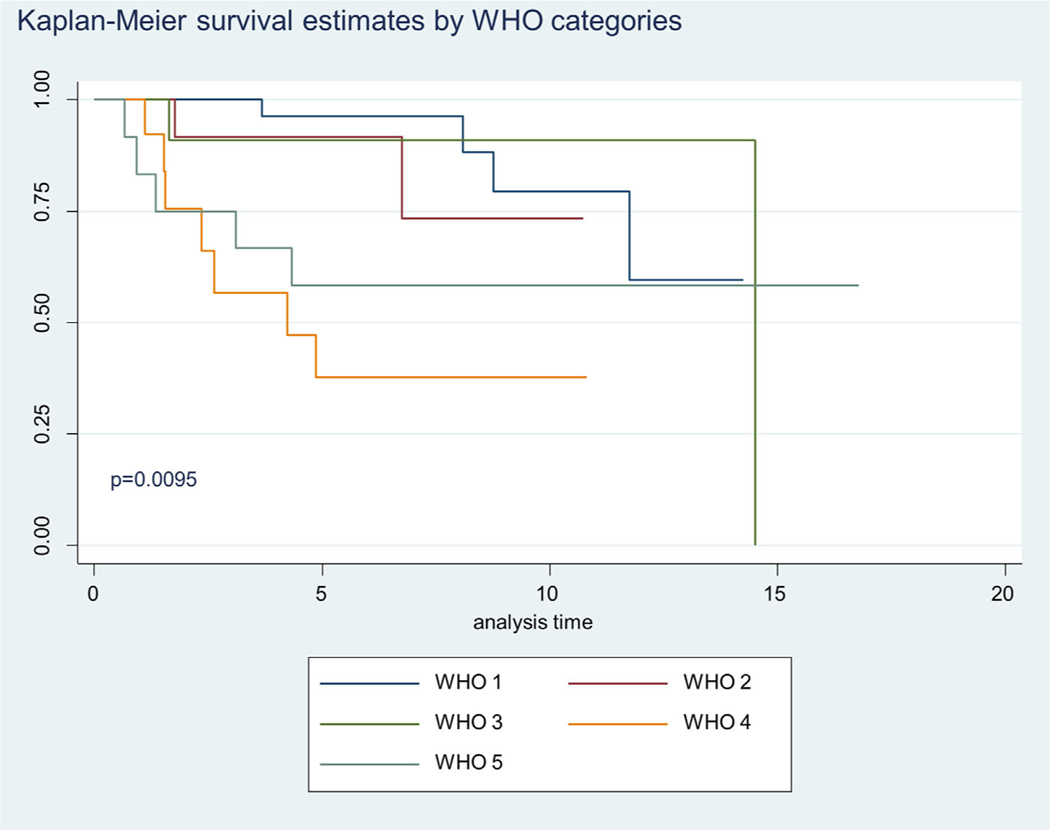
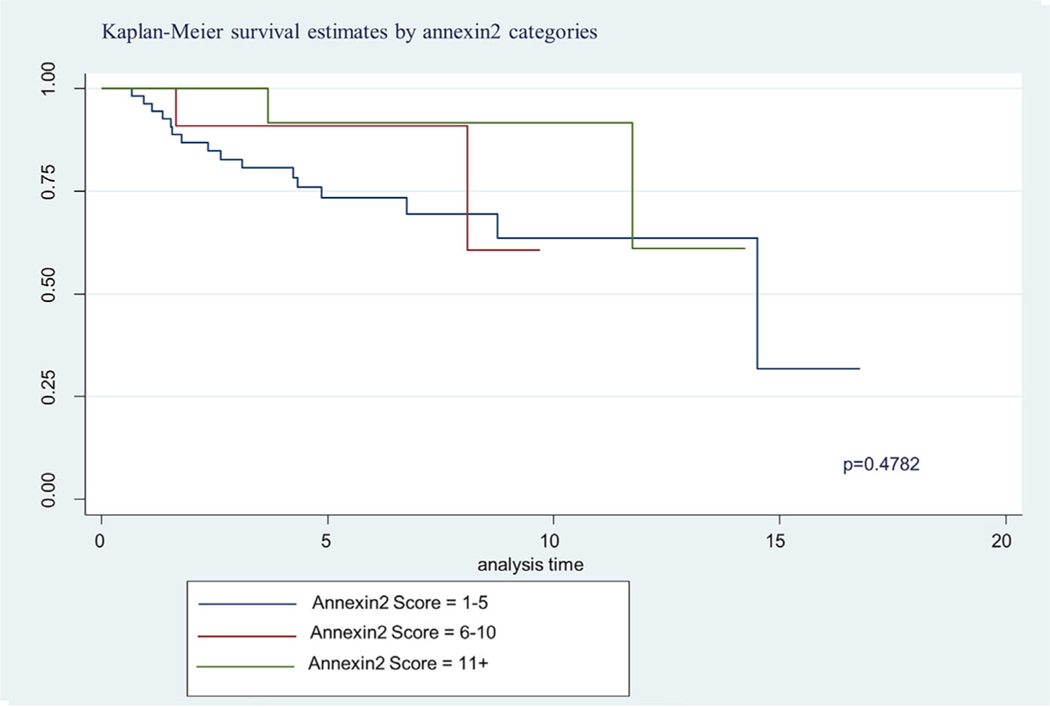
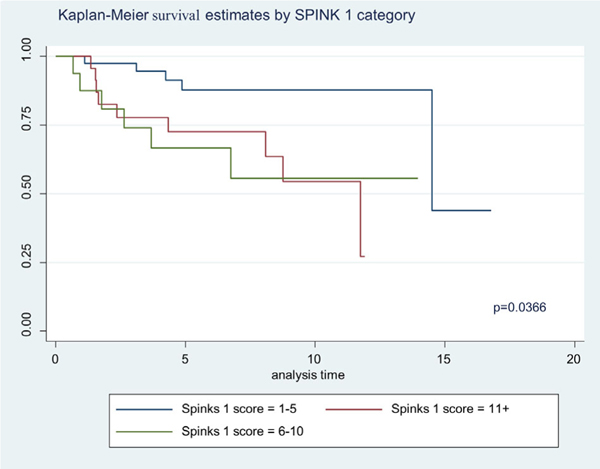
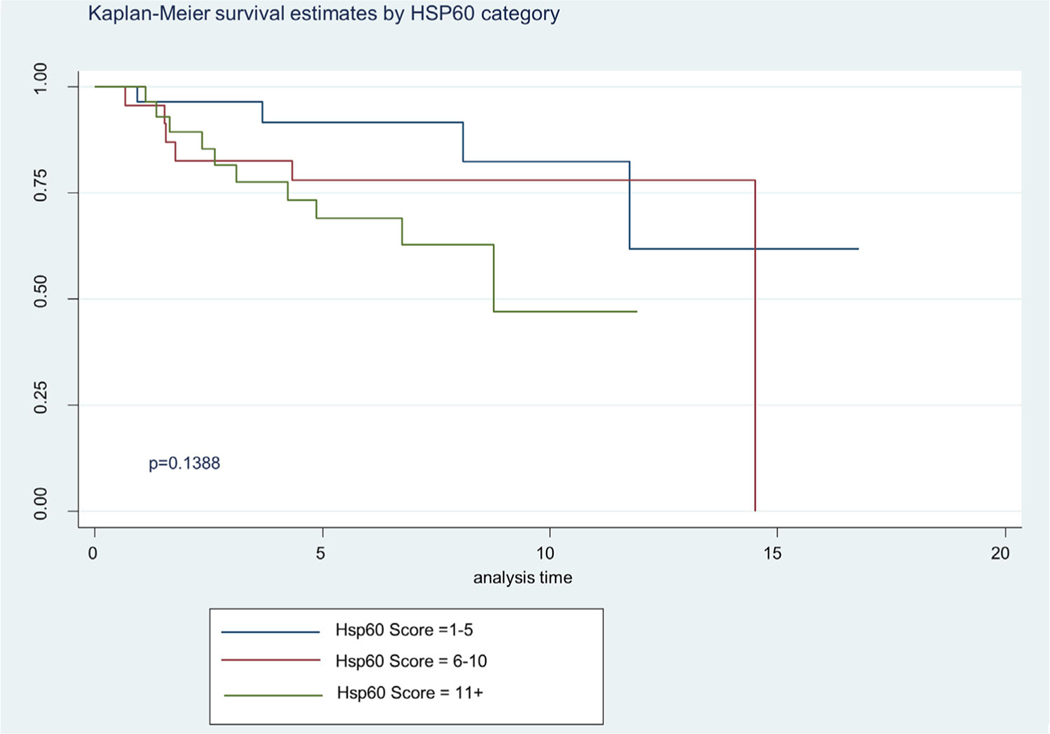
The Kaplan Meier 5-year survival curves by WHO GS categories are shown in Figure 2. In Figure 2 we show significant difference in survival between WHO categories, specifically the advance stages (WHO 4 and 5) (P = 0.0095). About 87% of the patient samples had a 5 year survival for GS2–6 and the combined GS7 groups had 48% 5 year survival. The GS 8 displayed a 39% 5 year survival and GS9–10, a 54% 5 year survival. Although ANX2 intensity of expression did not differ by Kaplan Meier survival, we show in Figure 3 where ANX2 illustrates intriguing but nonsignificant differences in survival between ANX2 expression levels (low = 1–5, high = 11+). However, Figure 4 shows a significant difference (P = 0.0366) in Kaplan Meier survival for SPINK1 expression levels (low = 1–5, high = 6–10, and 11+). Moreover, Figure 5 shows a borderline significance (P = 0.1388) for Hsp60 expression levels (low = 1–5, high = 6–10, 11+) and survival.
FIGURE 2.
Demonstrates Kaplan-Meier 5 year survival estimates for WHO1, WHO2, WHO3, WHO4, and WHO5 Gleason score categories. Of all the pairwise comparison WHO4 and WHO1 Gleason score categories showed significant differences at P = 0.0095. [Color figure can be viewed at wileyonlinelibrary.com]
FIGURE 3.
Demonstrates Kaplan-Meier 5 year survival estimate based on ANX2 expression categories. The data reveals a trend where those with high level expression (6–10, 11+) survive longer than those with low expression (1–5). [Color figure can be viewed at wileyonlinelibrary.com]
FIGURE 4.
Illustrates Kaplan Meier of SPINK1 expression categories on survival (Low = 1–5, High = 6–10, 11+). The significant difference was revealed between the low and high (P = 0.0366). [Color figure can be viewed at wileyonlinelibrary.com]
FIGURE 5.
Illustrates Kaplan Meier of Hsp60 expression categories on survival (low = 1–5, high = 6–10, 11+). Demonstrated borderline significance between low expression and high (P = 0.1388).
4 |. DISCUSSION
The inverse relationship between ANX2 expression and GS score is shown by the negative correlation coefficient (−) with a significant trend (Table 3, P < 0.0000). This result clearly showed that ANX 2 more and more under-expressed as degree of cancer progresses. This protein is strongly expressed in normal prostatic epithelial glands. Our results on the expression of ANX2 are in agreement with those reported by Yee et al14 who showed reduced expression in high grade prostate cancer. Another study carried out by Liu et al15 also showed that ANX2 expression is reduced or lost in primary adenocarcinoma of the prostate as opposed to other types of cancers and in the majority of prostate intraepithelial neoplasia. They suggested that ANX2 may be an endogenous suppressor of prostate cancer cell migration and their reduced or lost expression may contribute to prostate cancer development and progression. Similarly, Chetcuti et al13 and Banerjee et al16 found high expression of ANX2 in normal and benign hyperplastic glandular and basal epithelium but was immunohisto-chemically lost in cancer tissues. Furthermore, in glands involved by prostate intraepithelial neoplasia, 65% lost ANX2 in glandular epithelial cells, whereas basal cells were all positively stained. Alternatively, a recent study by Inokuchi39 had demonstrated a correlation between high ANX2 levels and a more aggressive prostate cancer phenotype. Biochemically, it appears that ANX2 declines as GS increases, with a plateau at WHO 4–5.
SPINK1/TATI is known to be produced in the prostate and in prostate cancer, and its production increases with a higher tumor grade.20,40 Strongly increased SPINK1 expression is found in about 10% of all prostate cancers, and serum concentrations of SPINK1 are increased in more than 40% of patients with advanced prostate cancer.41–43 Increased SPINK1/TATI expression was observed in our cancer groups compared to controls (normals). In addition, our data show that modifiedWHO2 GS status is a more accurate predictor and correlative of SPINK1 protein expression. Its coefficient and P-value indicates that there is a positive relationship between expression of the protein and GS. It is probable that higher ordered pathological GS value, which is an indicator for higher disease status and provides a model for bifurcating GS with biomarker status in assessing normal versus modified WHO 1b (GS 2–6). SPINK1 expression results from our study are in agreement with those reported by others.41,42 These studies showed high level SPINK1 expression in prostate cancer patients that is associated with a greater rate of cancer recurrence. Paju et al41 showed that strong expression of tumor associated trypsinogen1 (TATI) which is equivalent to SPINK1 was associated with higher GS grade as well. With these findings validated in two independent studies across multiple independent cohorts, this research group also concluded that SPINK1 promoted prostate tumor growth in part by signaling through the epidermal growth factor receptor (EGFR).17 Studies from this group have also shown that SPINK1/TATI may be involved in prostate cancer progression.17 In our patient series, the main effect of SPINK1/TATI is manifested in the transition from normal (modified WHO 1a) to GS 2–6 (WHO 1b).
With regard to Hsp60 expression, several studies have shown that the protein is overexpressed in prostate cancer. Castilla et al36 showed that the expression level of Hsp60 was significantly increased in tumors with high GS. The researchers observed that well-differentiated, low, and moderate GS tumors showed low levels of Hsp60 immunostaining, while high GS7–10 tumors exhibited elevated Hsp60 expression. Our results showed relatively higher Hsp60 expression for GS 7 (3 + 4) (WHO 2) compared to GS 2–6 (modified WHO 1b). However, the differences in expression between the normal and the cancer groups were not significant due to the unusually high (70%) expression in the normal tissues. This high Hsp60 expression is contrary to the absent expression in the normal tissues reported by others.36 Nevertheless, there was a positive correlation coefficient (0.37) between Hsp60 and WHO GS status. Cappello et al44 reported that the overexpression of Hsp60 occurs as an early event of prostate cancer development. Lianos et al45 suggested that the over-expression of Hsp60 protein may be associated with therapeutic resistance and poor survival. Cornfold et al46 found no association between the level of Hsp60 expression and GS, in either the early or the advanced prostate cancers. Therefore, our study confirms the variability of Hsp60 expression in prostate cancer and its inability to be used as a stand-alone marker of progression in AA men. Nevertheless, considering a staged progression to advanced disease, the rise in our patients took place solely in the transition to WHO 2.
It is worth noting that the result for GS7 relative to GS 2–6 was striking. Both ANX2 and Hsp60 proteins showed unusually high levels of expression for this cancer group. According to Corn et al47 and Balacescui et al48 GS 7 cancers (ie, 3 + 4 or 4 + 3) represent a biological and clinical heterogeneous group with variable biologic potential and clinical outcomes. They reported that some low-risk tumors rapidly progress while some high-risk tumors are relatively indolent. Our statistical analysis has also shown that the GS7 group does not show a clear response pattern regardless of bifurcated WHO 2–3 GS 7 categories (3 + 4 or 4 + 3). This presentation of WHO 2–3 GS 7 categories certainly aligns with the biological presentation reported by Corn et al47 and Balacescui et al.48
The Kaplan Meier Survival estimate showed a higher survival rate with an increased expression of ANX2. The probability of having 5 years of cancer free recurrence survival for patients with higher ANX2 expression is about 2.5 times that of those who lost ANX2 expression. Alternatively, expressions of SPINK1 and Hsp60 resulted in 5 years of recurrence free survival for most of the patients with high GS. Overall, about 87% of patients had 5 years of recurrence free survival for GS2–6 while only 46% had 5 years of recurrence free survival for GS8–10. The highest survival rate was observed from Hsp60 expression followed by ANX2; and the highest number of deaths found from SPINK1 expression. According to Axel Glassgen et al49 recurrence-free survival in patients with strong Hsp60 staining was shorter than in those with weak expression. They found Hsp60 to be an independent predictor of biochemical recurrence in multivariate analysis of patients with radical prostatectomy. In another study, survival curves calculated by the Kaplan-Meier method and analyzed using the log-rank test showed that the survival rates of the patients were significantly related with the down-regulation of ANX2.50 Multivariate analysis also showed that GS, recurrence, distant metastasis and the expression level of ANX2 had an independent prognostic effect on overall survival. Flavin et al51 found no association between SPINK1 expression and biochemical recurrence and cancer mortality. They concluded that SPINK1 protein expression may not be a predictor of recurrence or lethal prostate cancer amongst men treated by radical prostatectomy. However, others were able to show a significant association of SPINK1 expression and aggressive PCa phenotype in AA men.52
5 |. CONCLUSION
A comparison of the expression of the three proteins showed distinct features that distinguish low grade and high grade tumors. ANX2 is expressed in the normal prostate glands but is not expressed in the low grade or high grade tumors. Alternatively, SPINK1/TATI is not expressed in normal glands but showed cytoplasm expression in the low grade and high grade tumors. Hsp60 expression was more evident in the high grade tumors. Standard regression analysis clearly showed significant correlations between GS and the expressions of ANX2 and SPINK1. Significant positive correlations were also obtained between SPINK1, Hsp60, and GS. The negative correlation between age and SPINK1 compared with the positive age and ANX2 correlation is a clear indication of the bidirectional response from the two proteins. This bidirectional relationship is also shown by the Kaplan Meier Survival estimates. The Kaplan Meier Survival estimates showed a higher survival rate with an increased expression of ANX2, while SPINK1 and Hsp60 expressions resulted in shorter years of recurrence free survival.
Our findings demonstrate an important bidirectional relationship that can be used to predict prostate progression in African Americans using total and bifurcated WHO GS categories. The expressions of ANX2 and SPINK1 by immunohistochemistry clearly showed that these two bi-directional proteins have the potential to predict the clinical outcome of prostate cancer in African Americans. The expression of Hsp60 can be used to validate the expression of SPINK1 in GS2–6 and GS8–10 patients. However, Hsp60 by itself does not appear to be a good predictor of overall cancer progression.
We have observed in a limited dataset individualistic patterns of progression from one WHO GS level to the next, though these observations must be confirmed in additional datasets and across a broad range of demographics. ANX2 appears to decline continuously across the range of WHO GS levels. SPINK1 exerts its effect in the transition from normal (WHO1a) to GS 2–6 (WHO 1b) and WHO 2 GS category is more correlated with SPINK1 protein expression. Hsp60 has its effect in transitions from GS 2–6 to subsequent stages with the greatest effect observed in GS 2–6 to GS 7. Although, the data were limited to a smaller population of AA men, this study provides an avenue to further confirm an association of WHO GS with current and additional bi-directional biomarkers on biracial and larger population of men.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank Dr Yasmine Kanaan from Howard University for the use of the microtome. In addition, we thank Dr Chaun Suman from the VA for assistance in the procurement of prostate paraffin blocks. This study was supported by funds provided by Veterans Administration-Historically Black Colleges and University Research Training Grant, Grant number: 1lK2RX001114–01. Dr Kanarek is supported by the Maryland Cigarette Restitution Fund and the Comprehensive Cancer Center Grant (P30 CA006973).
Funding information
Veterans Administration-Historically Black Colleges and University Research Training Grant, Grant number: 1lK2RX001114–01; Maryland Cigarette Restitution Fund and the Comprehensive Cancer Center Grant, Grant number: P30 CA006973
Footnotes
SUPPORTING INFORMATION
Additional Supporting Information may be found online in the supporting information tab for this article.
CONFLICT OF INTEREST
None.
REFERENCES
- 1.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics. CA Cancer J Clin. 2011;61:212–236. [DOI] [PubMed] [Google Scholar]
- 2.American Cancer Society: Cancer Facts and Figures for African Americans, 2017. Atlanta, GA, American Cancer Society, 2017. [Google Scholar]
- 3.Paller CJ. Management of biochemically recurrent prostate cancer after local therapy: evolving standards of care and new directions. Clin Adv Hematol Oncol. 2013;11:14–23. [PMC free article] [PubMed] [Google Scholar]
- 4.Perner S, Hofer MD, Kim R, et al. Prostate specific membrane antigen expression as a predictor of prostate cancer progression. Hum Pathol. 2007;38:696–701. [DOI] [PubMed] [Google Scholar]
- 5.Perner S, Mosquera JM, Demichelis F, et al. TMPRSS2-ERG fusion prostate cancer: an early molecular event associated with invasion. Am J Surg Pathol. 2007;31:882–888. [DOI] [PubMed] [Google Scholar]
- 6.Berney DM, Gopalan A, Kudahetti S, et al. Ki-67 and outcome in clinically localized prostate cancer: analysis of conservatively treated prostate cancer patients from the Trans-Atlantic Prostate Group study. Br J Cancer. 2009;100:888–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li R, Wheeler T, Dai H, et al. High level of androgen receptor is associated with aggressive clinicopathologic features and decreased biochemical recurrence-free survival in prostate: cancer patients treated with radical prostatectomy. Am J Surg Pathol. 2004; 28:928–934. [DOI] [PubMed] [Google Scholar]
- 8.Attard G, Clark J, Ambroisine L, et al. Duplication of the fusion of TMPRSS2 to ERG sequences identifies fatal human prostate cancer. Oncogene. 2008;27:253–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fine SW, Gopalan A, Leversha MA, et al. TMPRSS2-ERG gene fusion is associated with low Gleason scores and not with high-grade morphological features. Mod Pathol. 2010;23:1325–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bjartell AS, Al-Ahmadie H, Serio AM, et al. Association of cysteine-rich secretory protein 3 and-microseminoprotein with outcome after radical prostatectomy. Clin Cancer Res. 2007;13:4130–4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee WH, Morton RA, Epstein JI, et al. Cytidine methylation of regulatory sequences near the pi class glutathione S-transferase gene accompanies human prostatic carcinogenesis. Proc Natl Acad Sci USA. 1994;91:11733–11737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khani F, Mosquera JM, Park K, et al. Evidence for molecular differences in prostate cancer between African American and Caucasian men. Clin Cancer Res. 2014;20:4925–4934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chetcuti A, Margan SH, Russell P, et al. Loss of annexin II heavy and light chains in prostate cancer and its precursors. Cancer Res. 2001;61:6331–6334. [PubMed] [Google Scholar]
- 14.Yee DS, Narula N, Ramzy l, et al. Reduced annexin 2 protein expression in high-grade prostatic intraepithelial neoplasia and prostate cancer. Arch Pathol Lab Med. 2007;13:902–908. [DOI] [PubMed] [Google Scholar]
- 15.Liu JW, Shen JJ, Tanzillo-Swarts A, et al. Annexin II expression is reduced or lost in prostate cancer cells and its re-expression inhibits prostate cancer migration. Oncogene. 2003;22:1475–1485. [DOI] [PubMed] [Google Scholar]
- 16.Banerjee AG, Liu J, Yuan Y, et al. Expression of biomarkers modulating prostate cancer angiogenesis: differential expression of annexin II in prostate carcinomas from India and USA. Mol Cancer. 2003;2:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ateeq B, Tomlins SA, Laxman B, et al. Therapeutic targeting of SPINK 1 —positive prostate cancer. Sci. Transl Med. 2011;3:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhalla R, Kunju LP, Tomlins SA, et al. Novel dual-color immunohistochemical methods for detecting ERG–PTEN and ERG–SPINK1 status in prostate carcinoma. Modern Pathol. 2013;26:835–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stenman UH. Role of the tumor-associated trypsin inhibitor SPINK1 in cancer development. Asian J Androl. 2011;13:628–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bjartell A, Paju A, Zhang WM, et al. Expression of tumor-associated trypsinogens (TAT-1 and TAT-2) in prostate cancer. Prostate. 2005;64:29–39. [DOI] [PubMed] [Google Scholar]
- 21.Flavin R, Pettersson A, Hendrickson WK, et al. SPINK 1 Protein Expression and Prostate Cancer Progression. Clin Cancer Res. 2014;20:4904–4911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.So A, Hadaschik B, Sowery R, Gleave M. The role of stress proteins in prostate cancer. Curr Genomics. 2007;8:252–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ellis RJ, van der Vies SM. Molecular chaperones. Annu Rev Biochem. 1991;60:321–347. [DOI] [PubMed] [Google Scholar]
- 24.Georgopoulos C, Welch WJ. Role of the major heat shock proteins as molecular chaperones. Annu Rev Cell Biol. 1993;9:601–634. [DOI] [PubMed] [Google Scholar]
- 25.Lindquist S, Craig EA. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. [DOI] [PubMed] [Google Scholar]
- 26.Cornford PA, Dodson AR, Parsons KF, et al. Heat shock protein expression independently predicts clinical outcome in prostate cancer. Cancer Res. 2000;60:7099–7105. [PubMed] [Google Scholar]
- 27.Raine CS, Wu E, Ivanyi J, Katz D, Brosnan CF. Multiple sclerosis: a protective or a pathogenic role for heat shock protein 60 in the central nervous system? Lab Invest. 1996;75:109–123. [PubMed] [Google Scholar]
- 28.Slater S, Jalleh R, Gilbertson J, Lampert I, Williamson R, Foster CS. Expression of heat shock proteins in chronic pancreatitis: protective or pathogenic roles? Lab Invest. 1997;76:533–545. [PubMed] [Google Scholar]
- 29.Morimoto RI, Tisseres A, Georgopoulos C. Stress Proteins in Biology and Medicine. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1990. [Google Scholar]
- 30.Ciocca DR, Clark GM, Tandon AK, Fuqua SA, Welch WJ, McGuire WL. Heat shock protein hsp70 in patients with axillary lymph node-negative breast cancer: prognostic implications. J Natl Cancer Inst. 1993;85:570–574. [DOI] [PubMed] [Google Scholar]
- 31.Levine AJ, Momand J, Finlay CA. The p53 tumour suppressor gene. Nature. 1991;351:453–456. [DOI] [PubMed] [Google Scholar]
- 32.Tomei LD, Cope FO. Apoptosis: The Molecular Basis of Cell Death. Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1991. [Google Scholar]
- 33.Adams DJ, Hajj H, Edwards DP, Bjercke RJ, McGuire WL. Detection of a Mr 24,000 estrogen-regulated protein in human breast cancer by monoclonal antibodies. Cancer Res. 1983;43:4297–4301. [PubMed] [Google Scholar]
- 34.Foster CS, Dodson AR, Ambroisine L, et al. Hsp-27 expression at diagnosis predicts poor clinical outcome in prostate cancer independent of ETS-gene rearrangement. Br J Cancer. 2009;101:1137–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gibbons NB, Watson RW, Coffey RN, Brady HP, Fitzpatrick JM. Heat-shock proteins inhibit induction of prostate cancer cell apoptosis. Prostate. 2000;45:58–65. [DOI] [PubMed] [Google Scholar]
- 36.Castilla C, Congregado B, Conde JM, et al. Immunohistochemical expression of Hsp60 correlates with tumor progression and hormone resistance in prostate cancer. Urology. 2010;76:1017.e1–1017.e6. [DOI] [PubMed] [Google Scholar]
- 37.D’Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280:969–974. [DOI] [PubMed] [Google Scholar]
- 38.Borley N, Feneley MR. Prostate cancer: diagnosis and staging. Asian J Androl. 2009;11:74–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Inokuchi J, Narula N, Yee DS, et al. Annexin A2 positively contributes to the malignant phenotype and secretion of IL-6 in DU145 prostate cancer cells. Int J Cancer. 2009;124:68–74. [DOI] [PubMed] [Google Scholar]
- 40.Paju A, Bjartell A, Zhang WM, et al. Expression and characterization of trypsinogen produced in the human male genital tract. Am J Pathol. 2000;157:2011–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paju A, Hotakainen K, Cao Y, et al. Increased expression of tumor-associated trypsin inhibitor, TATI, in prostate cancer and in androgen-independent 22Rv1 cells. Eur Urol. 2007;52:1670–1679. [DOI] [PubMed] [Google Scholar]
- 42.Leinonen KA, Tolonen TT, Bracken H, et al. Association of SPINK1 expression and TMPRSS2:ERG fusion with prognosis in endocrine-treated prostate cancer. Clin Cancer Res. 2010;16:2845–2851. [DOI] [PubMed] [Google Scholar]
- 43.Ateeq B1, Kunju LP, Carskadon SL, et al. Molecular profiling of ETS and non-ETS aberrations in prostate cancer patients from northern India. Prostate. 2015;75:1051–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cappello F, Rappa F, David S, et al. Immunohistochemical evaluation of PCNA, p53, Hsp60, Hsp10 and MUC-2 presence and expression in prostate carcinogenesis. Anticancer Res. 2003;23:1325–1331. [PubMed] [Google Scholar]
- 45.Lianos GD, Alexiou GA, Mangano A, et al. The role of heat shock proteins in cancer. Cancer Lett. 2015;360:114–118. [DOI] [PubMed] [Google Scholar]
- 46.Cornfold PA, Dodson AR, Parsons KF, et al. Heat shock protein expression independently predicts clinical outcome in prostate cancer. Cancer Res. 2000;60:7099–7105. [PubMed] [Google Scholar]
- 47.Corn PG, Thompson TC. Identification of a novel prostate cancer biomarker, Caveolin-1: implications and potential clinical benefit. Cancer Manag Res. 2010;2:111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Balacescui L, Crisan N, Fetica B, et al. Identifying molecular features for prostate cancer with Gleason 7 based on microarray gene expression profiles. Rom J Morphol Embryol. 2011;52:1195–1202. [PubMed] [Google Scholar]
- 49.Glaessgen A, Jonmarker S, Lindberg A, et al. Heat shock proteins 27, 60 and 70 as prognostic markers of prostate cancer. APMIS. 2008;116:888–895. [DOI] [PubMed] [Google Scholar]
- 50.Ding T, Yang L, Wang Y, et al. Down-regulation of annexin II in prostate cancer is associated with Gleason score, recurrence, metastasis and poor prognosis. Mol Med Rep. 2010;3:781–787. [DOI] [PubMed] [Google Scholar]
- 51.Thomas Jefferson University. “Genomic fingerprint may predict aggressive prostate cancer in African Americans.” ScienceDaily. ScienceDaily, 20 July 2015. www.sciencedaily.com/releases/2015/07/150720161906.htm [Google Scholar]
- 52.Yamoah K, Johnson MH, Choeurng V, et al. Novel biomarker signature that may predict aggressive disease in african american men with prostate cancer. J Clin Oncol. 2015;33:2789–2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.