Abstract
Background:
FOLFIRINOX (fluorouracil, folinic acid, irinotecan plus oxaliplatin) is an effective standard first-line treatment option for advanced pancreatic ductal adenocarcinoma (PDAC). There is no clear consensus on the second-line treatment following progression on FOLFIRINOX. In this multicenter retrospective analysis, we evaluated the efficacy and tolerability of second-line nab-P/Gem (nab-paclitaxel and gemcitabine) after progression on FOLFIRNOX in PDAC.
Methods:
Patients with unresectable or metastatic PDAC who received nab-P/Gem after progression on FOLFIRINOX between February 2016 and February 2019 were identified from five referral cancer centers in South Korea. Baseline characteristics, treatment history, survival outcomes, and toxicity profile were obtained retrospectively from medical records.
Results:
A total of 102 patients treated with second-line nab-P/Gem for advanced PDAC after progression on FOLFIRINOX were included. At the time of nab-P/Gem, the median age was 60 years, with males comprising 49.0%, and most (75.5%) had metastatic disease. Patients received a median of three cycles (range 1–12) of nab-P/Gem. The median overall survival (OS) and progression-free survival (PFS) from the start of second-line nab-P/Gem therapy were 9.8 (95% CI, 8.9–10.6) and 4.6 months (3.7–5.5), respectively. A partial response was achieved in 8.5%, and the disease control rate was 73.6%. From the start of first-line FOLFIRIOX, the OS1+2 and PFS1+2 were 20.9 (15.7–26.1) and 13.9 (10.8–17.0) months, respectively, with a 2-year survival rate of 45.1%. There was no treatment-related mortality and grade ⩾3 toxicity was observed in 60.2%.
Conclusion:
Our results showed that nab-P/Gem was an effective and tolerable second-line treatment option in medically fit patients with advanced PDAC who progressed on first-line FOLFIRNOX.
ClinicalTrials.gov identifier:
Keywords: FOLFIRINOX, gemcitabine, nab-paclitaxel, pancreatic cancer, second-line treatment
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is the 14th most common cancer and 7th leading cause of cancer-related deaths worldwide, with 459,000 new diagnoses and 432,000 deaths in 2018.1 Most patients present with advanced stages, either locally advanced or metastatic disease, and have a dismal prognosis, with a median overall survival (OS) of less than 2 years.2 There had been very limited progress in the treatment of PDAC until the 2000s, and single-agent gemcitabine had been used for the first-line treatment of advanced PDAC for more than 20 years.3 Beginning in 2011, the FOLFIRINOX regimen (fluorouracil, folinic acid, irinotecan plus oxaliplatin) became a new standard first-line treatment option for patients with advanced PDAC and good performance status.4 Subsequently, a combination of nab-paclitaxel and gemcitabine (nab-P/Gem) also succeeded in improving the OS of metastatic PDAC compared with conventional gemcitabine monotherapy from the MPACT trial.5
Several studies have been conducted to determine a second-line regimen for patients who progressed following first-line gemcitabine-based treatment, since gemcitabine monotherapy had been the standard first-line treatment for the last two decades.6–9 However, there is still no consensus on the optimal second-line treatment after progression on FOLFIRINOX because of the paucity of studies.
Although nab-P/Gem was proven to be more effective than gemcitabine monotherapy in the first-line setting, no randomized trial has assessed the efficacy of nab-P/Gem as a second-line therapy. In Korea, nab-P/Gem as salvage therapy after progression on first-line therapy for patients with advanced PDAC has not been approved. But its off-label use is officially granted by the health regulatory agency in some designated centers, although this is not reimbursed by Korean national health insurance. This multicenter retrospective analysis evaluated the efficacy and safety of second-line nab-P/Gem after progression on first-line FOLFIRINOX, or modified FOLFIRINOX (mFOLFIRINOX), in Korean patients with unresectable or metastatic PDAC.
Methods
Patients
All patients who were pathologically confirmed with PDAC and received second-line nab-P/Gem after progression on first-line (m)FOLFIRINOX were identified from five referral cancer centers in South Korea. Clinical data regarding baseline patient characteristics, treatment history, and survival outcomes were obtained retrospectively by reviewing the medical records. This study was approved by the Institutional Review Board (IRB) of each participating center (Asan Medical Center, 2019-1188; Ulsan University Hospital, 2019-11-037; CHA Bundang Medical Center, 2019-11-037; Chungnam National University Hospital, 2019-12-030; Haeundae Paik Hospital, 2019-03-001-001) and was performed in accordance with the ethical standards of the institutional research committee and the latest Declaration of Helsinki. IRBs waived the need for informed consent for this study owing to the non-requirement of consent in retrospective analysis covered by regulations in Korea.
Treatment and assessment
Nab-paclitaxel (125 mg/m2) and gemcitabine (1000 mg/m2) were administered intravenously on days 1, 8, and 15 of each 28-day cycle, as used in the first-line setting.5 Dose modification was made according to the treating physician’s discretion. Tumor assessment using computed tomography (CT) scanning and/or other imaging tools was performed every two cycles and whenever there was a sign or symptom suggesting tumor progression. Tumor response was determined according to the revised Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST 1.1).10 Toxicity profiles including laboratory values and symptoms were evaluated at every visit and graded by Common Terminology Criteria for Adverse Events version 5.0 (CTCAE 5.0).
Statistical analysis
Survival curves were estimated using the Kaplan–Meier method and compared by the log-rank test. OS was defined as the length of time from the start of second-line nab-P/Gem treatment to the date of death from any cause. Progression-free survival (PFS) was defined as the length of time from the start of second-line nab-P/Gem treatment to the date of tumor progression or death from any cause, whichever occurred first. OS1+2 was considered the time from the start of first-line (m)FOLFIRINOX to death from any cause, whereas PFS1+2 indicated the period between the start of first-line (m)FOLFIRINOX and second progression (progression on nab-P/Gem) or death from any cause, whichever came first. If progression or death was not observed, survival was censored at the time of patient’s last visit. Univariate and multivariate analyses for survival outcomes were analyzed using the Cox proportional hazards model. A p-value less than 0.05 was considered statistically significant. The statistical package for the Social Sciences version 22.0 (IBM, Armonk, NY, USA) was used for all statistical analyses.
Results
Patient characteristics
A total of 102 patients who were treated with second-line nab-P/Gem for their unresectable or metastatic PDAC and progressed following (m)FOLFIRINOX between February 2016 and February 2019 were included in this analysis, and their clinicopathologic characteristics are summarized in Table 1. The median age at the time of initiating second-line nab-P/Gem was 60 years (range, 35–76), with males comprising 49.0% (n = 50) of patients. In terms of tumor location, the pancreas head was most frequently involved, followed by the body and tail. With prior first-line (m)FOLFIRINOX, the overall response rate was 14.7%, and a median time-to-progression was 6.2 months (95% CI, 4.5–8.0 months). At the commencement of second-line nab-P/Gem, all but one patient had an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0 or 1, and most (n = 77, 75.5%) had metastatic disease. The most common metastatic site was the liver (n = 35, 34.3%), followed by the peritoneum (n = 31, 30.4%), distant lymph nodes (n = 30, 29.4%), lungs (n = 12, 11.8%), and bone (n = 2, 2.0%). Subsequent systemic treatment was administered to 59.4% of patients who progressed after second-line nab-P/Gem (n = 38/64). The detailed regimens are listed in Supplemental Table S1.
Table 1.
Baseline characteristics and treatment summary of all patients.
| Total
patients (n = 102) |
|
|---|---|
| Age at the time of nab-P/Gem, years | |
| Median (range) | 60 (35–76) |
| Sex | |
| Male | 50 (49.0%) |
| Female | 52 (51.0%) |
| Histology | |
| Adenocarcinoma | 102 (100%) |
| Primary tumor site | |
| Head | 63 (61.8%) |
| Body | 29 (28.4%) |
| Tail | 10 (9.8%) |
| Best overall response to first-line FOLFIRINOX | Measurable (n = 95) |
| CR | 1 (1.0%) |
| PR | 13 (13.7%) |
| SD (at least for 6 weeks) | 55 (57.9%) |
| PD | 26 (27.4%) |
| Previous surgery | |
| Curative resection | 22 (21.5%) |
| Palliative-intent surgery | 2 (2.0%) |
| ECOG PS at the time of second-line nab-P/Gem | |
| 0 | 18 (17.6%) |
| 1 | 83 (81.4%) |
| 2 | 1 (1.0%) |
| Disease extent at the time of second-line nab-P/Gem | |
| Locally advanced | 25 (24.5%) |
| Initially metastatic | 63 (61.8%) |
| Recurrent | 14 (13.7%) |
| No. of metastatic sites at the time of second-line nab-P/Gem | |
| <2 | 74 (72.5%) |
| ⩾2 | 28 (27.5%) |
| Metastatic site | |
| Distant LN | 30 (29.4%) |
| Liver | 35 (34.3%) |
| Peritoneum | 31 (30.4%) |
| Lung | 12 (11.8%) |
| Bone | 2 (2.0%) |
| Others | 8 (7.8%) |
| CA 19-9 at the time of second-line nab-P/Gem | |
| ⩽UNL | 15 (14.7%) |
| >UNL | 59 (57.8%) |
| Not available | 28 (27.5%) |
CA 19-9, cancer antigen 19-9; CR, complete response; ECOG PS, Eastern Cooperative Oncology Group performance status; FOLFIRINOX, fluorouracil, folinic acid, irinotecan and oxaliplatin; LN, lymph node; nab-P/Gem, nab-paclitaxel plus gemcitabine; PD, progressive disease; PR, partial response; RT, radiation therapy; SD, stable disease; UNL, upper normal limit.
Efficacy
Patients were treated with a median of three cycles (range, 1–12 cycles) of nab-P/Gem. The median OS and PFS from the start of second-line nab-P/Gem therapy were 9.8 (95% CI, 8.9–10.6) and 4.6 (95% CI, 3.7–5.5) months, respectively, with a median follow-up duration of 8.2 months (95% CI, 7.1–9.3) (Figure 1). The survival outcomes were not significantly different according to disease extent [locally advanced versus metastatic; PFS, median 5.1 (95% CI, 4.0–6.3) versus 4.2 (95% CI, 2.8–5.6) months, p = 0.115; OS, median 10.8 (95% CI, 8.3–13.3) versus 9.3 (95% CI, 5.6–13.0) months, p = 0.421]. Among the 94 patients with at least one measurable lesion at the baseline of second-line nab-P/Gem, 87 (92.6%) were available for response information. A partial response was achieved in eight patients (8.5%), and the disease control rate (DCR, the proportion of patients with a partial response and stable disease for ⩾6 weeks) was 73.6% (Table 2).
Figure 1.

Survival outcomes from the start of second-line nab-P/Gem.
The median OS (A) and PFS (B) from the start of second-line nab-P/Gem therapy were 9.8 (95% CI, 8.9–10.6) and 4.6 months (3.7–5.5), respectively.
CI, confidence interval; nab-P/Gem, nab-paclitaxel and gemcitabine; OS, overall survival; PFS, progression-free survival.
Table 2.
Response rate for second-line nab-P/Gem.
| Patients with measurable lesions | n = 94 |
|---|---|
| Best overall response | |
| CR | 0 |
| PR | 8 (8.5%) |
| SD | 57 (60.6%) |
| PD | 22 (23.4%) |
| Not evaluable | 7 (7.4%) |
| ORR | 8 (9.2%) |
| DCR | 64 (73.6%) |
CR, complete response; DCR, disease control rate; nab-P/Gem, nab-paclitaxel plus gemcitabine; ORR, overall response rate; PD, progressive disease; PR, partial response; SD, stable disease.
ORR included CR and PR among evaluable patients. DCR included CR, PR, and SD among evaluable patients.
After the initiation of first-line (m)FOLFIRINOX, the OS1+2 and PFS1+2 were 20.9 (95% CI, 15.7–26.1) and 13.9 (95% CI, 10.8–17.0) months in overall patients, respectively (Figure 2). By disease extent, there was no difference in OS1+2 [locally advanced versus metastatic, median 22.8 (95% CI, 16.6–29.0) versus 17.7 (95% CI, 13.8–21.6) months, p = 0.685] or PFS1+2 [median 13.9 (95% CI, 10.7–17.1) versus 11.5 (95% CI, 5.4–17.5) months), p = 0.827] between the locally advanced and metastatic patient groups. The 2-year survival rate from the start of first-line (m)FOLFIRINOX was 45.1% (locally advanced disease, 45.7%; metastatic disease, 41.6%).
Figure 2.
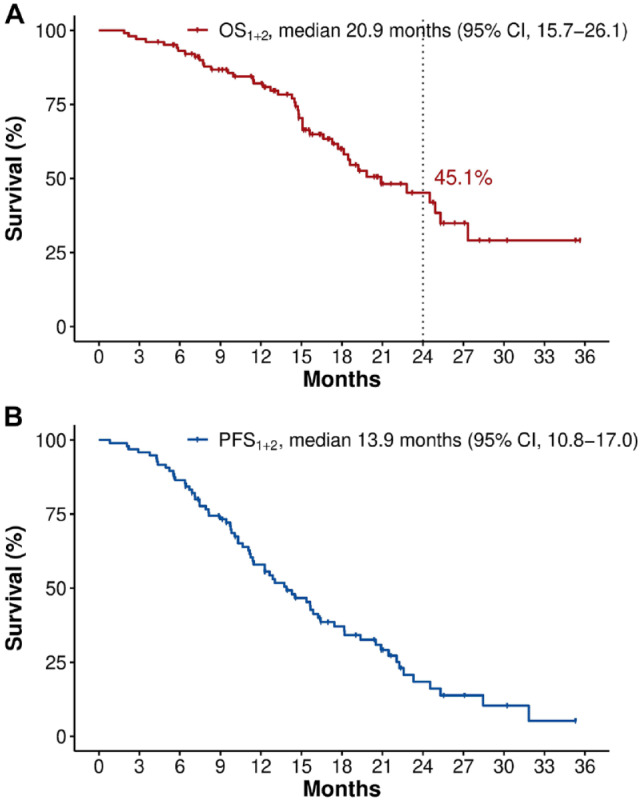
Survival outcomes from the start of first-line FOLFIRINOX.
From the start of first-line FOLFIRINOX, the median OS1+2 (A) and PFS1+2 (B) were 20.9 (95% CI, 15.7–26.1) and 13.9 (10.8–17.0) months, respectively, with a 2-year survival rate of 45.1%.
CI, confidence interval; FOLFIRINOX, fluorouracil, folinic acid, irinotecan plus oxaliplatin; OS, overall survival; PFS, progression-free survival.
Toxicity
During nab-P/Gem therapy, more than half of patients (60.2%) experienced a grade 3 or higher adverse event (Table 3). Neutropenia was the most common grade ≥3 adverse event (37.8%), but it rarely led to febrile neutropenic episodes (3.1%). In addition to neutropenia, all other adverse events of grade 3 or higher such as neurotoxicity, fatigue, nausea/vomiting, or diarrhea occurred in less than 10% of patients. Doses were reduced or modified in 60.2% of patients (n = 59), and 10.2% of patients (n = 10) discontinued the treatment for reasons other than disease progression. There was no treatment-related mortality.
Table 3.
Safety profile of second-line nab-P/Gem according to CTCAE 5.0.
| Adverse event | Grade | n = 98 |
|---|---|---|
| Any adverse event | All | 96 (98.0%) |
| Grade 3/4 | 59 (60.2%) | |
| Fatigue | All | 53 (54.1%) |
| Grade 3/4 | 5 (5.1%) | |
| Nausea | All | 20 (20.4%) |
| Grade 3/4 | 6 (6.1%) | |
| Vomiting | All | 14 (14.3%) |
| Grade 3/4 | 3 (3.1%) | |
| Diarrhea | All | 15 (15.3%) |
| Grade 3/4 | 3 (3.1%) | |
| Neurotoxicity | All | 31 (31.6%) |
| Grade 3/4 | 8 (8.2%) | |
| Mucositis | All | 9 (9.2%) |
| Grade 3/4 | 0 | |
| Neutropenia | All | 61 (62.2%) |
| Grade 3/4 | 37 (37.8%) | |
| Thrombocytopenia | All | 46 (46.9%) |
| Grade 3/4 | 5 (5.1%) | |
| Others | All | 13 (13.3%) |
| Grade 3/4 | 5 (5.1%) | |
| Febrile neutropenia episode | No | 95 (96.9%) |
| Yes | 3 (3.1%) | |
| Dose modification | No | 29 (29.6%) |
| Reduction/delay | 59 (60.2%) | |
| Cessation | 10 (10.2%) |
CTCAE 5.0, Common Terminology Criteria for Adverse Events version 5.0; nab-P/Gem, nab-paclitaxel plus gemcitabine.
Univariate and multivariate analysis for PFS and OS
Patients with two or more metastatic sites had a shorter PFS (versus <2 metastatic sites, median 3.4 versus 5.4 months, p < 0.001) and OS (versus <2 metastatic sites, median 6.2 versus 10.0 months, p = 0.024), but significance was not retained in the multivariate analysis. In terms of metastatic sites, lung metastasis was associated with a shorter PFS (versus absent, median 1.9 versus 5.1 months, p = 0.013), whereas bone metastasis had a tendency to be related to a shorter OS (versus absent, median 0.3 versus 9.9 months, p < 0.001); both remained significant in the multivariate analysis. Patients who had a time-to-progression (TTP) for first-line (m)FOLFIRINOX longer than the median (⩾6.2 months) tended to show rela-ted longer PFS [versus TTP for first-line (m)FOLFIRINOX < median, 5.1 versus 4.1 months; p = 0.097] and OS (11.6 versus 9.8 months; p = 0.212), but this association was not statistically significant in the univariate and multivariate analyses. The entire results of the univariate and multivariate analyses are provided in Supplemental Tables S2 and S3, respectively.
Discussion
According to current international guidelines,11–13 gemcitabine alone or in combination with nab-P, cisplatin (for patients with BRCA1/2 or PALB2 mutations) or erlotinib are recommended as second-line treatment options after progression on prior (m)FOLFIRINOX therapy. However, patterns of second-line treatment choices vary significantly between countries depending on each nation’s drug availability and reimbursement status,14 and there is no clear consensus on the standard of care after progression on (m)FOLFIRINOX.
Our results demonstrate that nab-P/Gem is effective and well tolerated as second-line treatment option for real-world patients with advanced PDAC who had previously been treated with (m)FOLFIRINOX. In the 102 patients included in the present analysis, the PFS and OS from the start of second-line nab-P/Gem were 4.6 and 9.8 months, respectively, with a response rate of 9.2% and a DCR (partial response or stable disease or at least 6 weeks) of 73.6%. As previous studies of second-line gemcitabine monotherapy after the progression on (m)FOLFIRINOX have shown the median PFS and OS of 2.1–2.5 and 3.6–5.7 months, respectively,15–18 our results suggest that the combination of nab-P and gemcitabine is likely to be more effective than gemcitabine monotherapy in patients who progressed on (m)FOLFIRINOX.
Our results are in line with prior studies investigating the nab-P/GEM as second-line therapy after progression on (m)FOLFIRINOX in patients with PDAC. In a French prospective multicenter cohort study of 57 patients,19 the median OS and PFS with second-line nab-P/Gem were 8.8 and 5.1 months, respectively, with a DCR of 58%. In a recent Japanese phase II study including 30 patients,20 second-line nab-P/Gem showed a median OS of 7.6 months and a PFS of 3.8 months. Although a single-center retrospective study in the United States (US) showed modest outcomes, with a median OS of 5.2 months and a DCR of 46%,21 this study may be limited because of the small number of patients (n = 28) (Table 4).
Table 4.
Previous reports on second-line nab-P/Gem after progression on FOLFIRINOX in advanced pancreatic cancer.
| Author | Second-line regimen | Design | Number of patients | ORR (%) | DCR (%) | Median OS (months) | Median PFS (months) |
|---|---|---|---|---|---|---|---|
| Sarabi et al.18 | Gem | Retrospective | 42 | N/A | 26.2 | 3.6 | N/A |
| Viaud et al.15 | Gem | Retrospective | 96 | 10 | 40 | 3.7 | 2.1 |
| da Rocha Lino et al.16 | Gem | Retrospective | 20 | N/A | N/A | 5.7 | 2.0 |
| Gilabert et al.17 | Gem | Retrospective | 72 | 11 | 35 | N/A | 2.5 |
| Zhang et al.21 | nab-P/Gem | Retrospective | 28 | 17.9 | 46 | 5.2 | 2.8 |
| Portal et al.19 | nab-P/Gem | Prospective cohort | 57 | 17.5 | 58 | 8.8 | 5.1 |
| Mita et al.20 | nab-P/Gem | Phase II | 30 | 13.3 | 46.7 | 7.6 | 3.8 |
DCR, disease control rate; Gem, gemcitabine monotherapy; FOLFIRINOX, fluorouracil, folinic acid, irinotecan and oxaliplatin; N/A; not available; nab-P/Gem, nab-paclitaxel plus gemcitabine; ORR, overall response rate; OS, overall survival; PFS, progression-free survival.
The treatment outcomes of nab-P/Gem as a second-line treatment were comparable with its benefits observed in a first-line setting.5,22 In the MPACT trial, the median PFS and OS of first-line nab-P/Gem in patients with metastatic PDAC were 5.5 and 8.5 months, respectively, and this was also validated in a daily practice setting (PFS, 5.1 months; OS, 9.6 months). Our patients who were treated with nab-P/Gem after (m)FOLFIRINOX showed similar PFS (4.6 months) and OS (9.8 months), implying that, as long as patients are medically fit for second-line nab-P/Gem, the survival outcome of nab-P/Gem after progression on FOLFIRINOX might be comparable with that of patients receiving nab-P/Gem as the first-line treatment. In our study population, from the initiation of first-line (m)FOLFIRINOX, median OS was 20.9 months, and the 2-year survival rate was 45.1%. This indicates that effective subsequent therapy, even after progression on first-line treatment may induce relatively long-term survival for unresectable or metastatic PDAC patients. This suggests that continuum of care approach, in which multiple active agents are considered and integrated as appropriate into a comprehensive treatment plan, is important in the management of pancreatic cancer, and a combination strategy should be actively considered for patients who progressed following FOLFIRINOX for their advanced PDAC, as long as they have a good performance status.
The safety profile for second-line nab-P/Gem was similar to those reported for first-line nab-P/Gem in the MPACT trial.5 In our study, grade 3–4 toxicity consisted mainly of hematologic adverse events (neutropenia 37.8%), but this rarely turned into a clinically critical condition (febrile neutropenia, 3.1%). There was no treatment-related mortality and most adverse events were well tolerated with appropriate dose modification and active supportive care.
In patients with fluoropyrimidine-refractory disease, several treatment options other than gemcitabine-based regimen are available in certain circumstances. An immune checkpoint inhibitor (pembrolizumab) can be considered as second-line therapy for patients who have microsatellite instability-high (MSI-H) or mismatch repair-deficient (MMR-D) tumor. Meanwhile, patients with NTRK fusion are indicated for tropomyosin receptor kinases inhibitors such as entrectinib and larotrectinib. However, most patients with advanced PDAC might not be applicable for those immunotherapy or target agents since MSI-H/MMR-D and NTRK fusion are observed in less than 1% of PDAC cases.23,24 Combination of liposomal irinotecan with 5-fluorouracil and folinic acid (nal-IRI plus 5-FU/LV) is regarded as one of the salvage treatment options according to recent guidelines, but it is indicated mainly for patients whose disease progressed following prior gemcitabine-based therapy. In addition, it should be noted that clinical outcomes of nal-IRI plus 5-FU/LV are known to be poorer in patients who previously received and progressed on conventional irinotecan, including the (m)FOLFIRINOX regimen.25
The limitations of this study include its retrospective design, and the heterogeneity of patients, with the inclusion of locally advanced and metastatic disease. However, this multicenter analysis included a larger number of patients than previous studies, and might be able to represent the real-world population and treatment setting.
In conclusion, the nab-P/Gem combination is well tolerated and effective as second-line therapy after progression on first-line (m)FOLFIRINOX in patients with unresectable or metastatic PDAC. Nab-P/Gem should be considered a feasible therapeutic option for patients who previously progressed after FOLFIRINOX and have a good general performance status.
Supplemental Material
Supplemental material, Supplemental_Information_proof for Efficacy and safety of second-line nab-paclitaxel plus gemcitabine after progression on FOLFIRINOX for unresectable or metastatic pancreatic ductal adenocarcinoma: multicenter retrospective analysis by Heejung Chae, Hyehyun Jeong, Jaekyung Cheon, Hong Jae Chon, Hyewon Ryu, Il-Hwan Kim, Myoung Joo Kang, Jae Ho Jeong, Baek-Yeol Ryoo, Kyu-pyo Kim and Changhoon Yoo in Therapeutic Advances in Medical Oncology
Footnotes
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by Celgene Corporation. The funding source had no role in study design, collection and analysis of data, or writing of the report.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Changhoon Yoo  https://orcid.org/0000-0002-1451-8455
https://orcid.org/0000-0002-1451-8455
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Heejung Chae, Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, South Korea; Center for Breast Cancer, National Cancer Center Korea, Goyang, Gyeonggi, South Korea.
Hyehyun Jeong, Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, South Korea.
Jaekyung Cheon, Division of Hematology and Oncology, Department of Internal Medicine, Ulsan University Hospital, University of Ulsan College of Medicine, Ulsan, South Korea.
Hong Jae Chon, Department of Medical Oncology, CHA Bundang Medical Center, CHA University, South Korea.
Hyewon Ryu, Division of Hematology and Oncology, Department of Internal Medicine, Chungnam National University Hospital, Chungnam National University College of Medicine, South Korea.
Il-Hwan Kim, Department of Oncology, Haeundae Paik Hospital, Cancer Center, Inje University College of Medicine, Busan, South Korea.
Myoung Joo Kang, Department of Oncology, Haeundae Paik Hospital, Cancer Center, Inje University College of Medicine, Busan, South Korea.
Jae Ho Jeong, Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, South Korea.
Baek-Yeol Ryoo, Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, South Korea.
Kyu-pyo Kim, Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, 88, Olympic-ro 43-gil, Songpa-gu, Seoul, 05505, Korea.
Changhoon Yoo, Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, 88, Olympic-ro 43-gil, Songpa-gu, Seoul, 05505, Korea.
References
- 1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68: 394–424. [DOI] [PubMed] [Google Scholar]
- 2. Rawla P, Sunkara T, Gaduputi V. Epidemiology of pancreatic cancer: global trends, etiology and risk factors. World J Oncol 2019; 10: 10–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Burris HA, III, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 1997; 15: 2403–2413. [DOI] [PubMed] [Google Scholar]
- 4. Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011; 364: 1817–1825. [DOI] [PubMed] [Google Scholar]
- 5. Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013; 369: 1691–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yoo C, Hwang JY, Kim JE, et al. A randomised phase II study of modified FOLFIRI.3 vs modified FOLFOX as second-line therapy in patients with gemcitabine-refractory advanced pancreatic cancer. Br J Cancer 2009; 101: 1658–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Neuzillet C, Hentic O, Rousseau B, et al. FOLFIRI regimen in metastatic pancreatic adenocarcinoma resistant to gemcitabine and platinum-salts. World J Gastroenterol 2012; 18: 4533–4541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zaniboni A, Aitini E, Barni S, et al. FOLFIRI as second-line chemotherapy for advanced pancreatic cancer: a GISCAD multicenter phase II study. Cancer Chemother Pharmacol 2012; 69: 1641–1645. [DOI] [PubMed] [Google Scholar]
- 9. Wang-Gillam A, Li CP, Bodoky G, et al. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): a global, randomised, open-label, phase 3 trial. Lancet 2016; 387: 545–557. [DOI] [PubMed] [Google Scholar]
- 10. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline; (version 1.1). Eur J Cancer 2009; 45: 228–247. [DOI] [PubMed] [Google Scholar]
- 11. Sohal DPS, Kennedy EB, Khorana A, et al. Metastatic pancreatic cancer: ASCO clinical practice guideline update. J Clini Oncol 2018; 36: 2545–2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pentheroudakis G. Recent eUpdates to the ESMO Clinical Practice Guidelines on hepatocellular carcinoma, cancer of the pancreas, soft tissue and visceral sarcomas, cancer of the prostate and gastric cancer. Ann Oncol 2019; 30: 1395–1397. [DOI] [PubMed] [Google Scholar]
- 13. National Comprehensive Cancer Network. Pancreatic adenocarcinoma (Version 1, 2020), https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf (2019, accessed November 26, 2019). [DOI] [PubMed]
- 14. Taieb J, Prager GW, Melisi D, et al. First-line and second-line treatment of patients with metastatic pancreatic adenocarcinoma in routine clinical practice across Europe: a retrospective, observational chart review study. ESMO Open 2020; 5: e000587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Viaud J, Brac C, Artru P, et al. Gemcitabine as second-line chemotherapy after Folfirinox failure in advanced pancreatic adenocarcinoma: a retrospective study. Dig Liver Dis 2017; 49: 692–696. [DOI] [PubMed] [Google Scholar]
- 16. da Rocha Lino A, Abrahao CM, Brandao RM, et al. Role of gemcitabine as second-line therapy after progression on FOLFIRINOX in advanced pancreatic cancer: a retrospective analysis. J Gastrointest Oncol 2015; 6: 511–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gilabert M, Chanez B, Rho YS, et al. Evaluation of gemcitabine efficacy after the FOLFIRINOX regimen in patients with advanced pancreatic adenocarcinoma. Medicine (Baltimore) 2017; 96: e6544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sarabi M, Mais L, Oussaid N, et al. Use of gemcitabine as a second-line treatment following chemotherapy with folfirinox for metastatic pancreatic adenocarcinoma. Oncol Lett 2017; 13: 4917–4924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Portal A, Pernot S, Tougeron D, et al. Nab-paclitaxel plus gemcitabine for metastatic pancreatic adenocarcinoma after Folfirinox failure: an AGEO prospective multicentre cohort. Br J Cancer 2015; 113: 989–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mita N, Iwashita T, Uemura S, et al. Second-line gemcitabine plus nab-paclitaxel for patients with unresectable advanced pancreatic cancer after first-line FOLFIRINOX failure. J Clin Med 2019; 8: 761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang Y, Hochster H, Stein S, et al. Gemcitabine plus nab-paclitaxel for advanced pancreatic cancer after first-line FOLFIRINOX: single institution retrospective review of efficacy and toxicity. Exp Hematol Oncol 2015; 4: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kang J, Hwang I, Yoo C, et al. Nab-paclitaxel plus gemcitabine versus FOLFIRINOX as the first-line chemotherapy for patients with metastatic pancreatic cancer: retrospective analysis. Invest New Drugs 2018; 36: 732–741. [DOI] [PubMed] [Google Scholar]
- 23. Cocco E, Scaltriti M, Drilon A. NTRK fusion-positive cancers and TRK inhibitor therapy. Nat Rev Clin Oncol 2018; 15: 731–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hu ZI, Shia J, Stadler ZK, et al. Evaluating mismatch repair deficiency in pancreatic adenocarcinoma: challenges and recommendations. Clin Cancer Res 2018; 24: 1326–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yoo C, Im HS, Kim KP, et al. Real-world efficacy and safety of liposomal irinotecan plus fluorouracil/leucovorin in patients with metastatic pancreatic adenocarcinoma: a study by the Korean Cancer Study Group. Ther Adv Med Oncol 2019; 11: 1758835919871126. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplemental_Information_proof for Efficacy and safety of second-line nab-paclitaxel plus gemcitabine after progression on FOLFIRINOX for unresectable or metastatic pancreatic ductal adenocarcinoma: multicenter retrospective analysis by Heejung Chae, Hyehyun Jeong, Jaekyung Cheon, Hong Jae Chon, Hyewon Ryu, Il-Hwan Kim, Myoung Joo Kang, Jae Ho Jeong, Baek-Yeol Ryoo, Kyu-pyo Kim and Changhoon Yoo in Therapeutic Advances in Medical Oncology


