Abstract
Skin cancer is a common malignant tumor in China and throughout the world, and the rate of recurrence is considerably high, thus endangering the quality of life and health of patients, and increasing the economic burden and pressure to the families of those afflicted. Due to the limitations of traditional drug treatments, it is difficult to achieve the desired therapeutic effect of complete removal. However, targeted gene therapy may be a novel means of treating skin cancer, as the targeted nature of treatment may improve therapeutic outcomes. However, targeted gene therapy requires physicians to select the appropriate gene, which means suitable genetic biomarkers must be identified from complex genetic data. In the present study, the least absolute shrinkage and selection operator regression analysis method was used with 10-fold cross verification to reduce the dimensions of gene data in patients with skin cancer, and subsequently, 20 gene biomarkers were screened. A prognostic model was constructed using these 20 gene biomarkers, and the validity of the model was assessed using a training set and a verification set, which showed that the model performed well. Finally, gene function analysis of these 20 gene biomarkers was determined. Relevant studies were found to show that the genetic biomarkers identified in this paper may possess value for the follow-up clinical treatment of skin cancer.
Keywords: skin cancer, metastatic, least absolute shrinkage and selection operator, gene biomarker, prognosis, data mining
Introduction
Skin cancer is a malignant tumor that afflicts individuals all over the world. Skin cancer is divided into malignant melanoma and non-melanoma skin cancer (NMSC) (1,2). According to global cancer statistics in 2018, there were 142,056 new cases of NMSC, accounting for 5.8% of global cancer cases, and 65,155 NMSC-related deaths, accounting for 0.7% of global cancer mortality. There were 779,723 new cases of skin malignant melanoma, accounting for 1.6% of global cancer cases, and 60,712 skin melanoma-related deaths, accounting for 0.6% of global cancer mortality (3).
According to the 2015 Chinese Cancer Statistics Report, the incidence of skin cancer in China was 8/1,000 and the mortality rate was 3.22/1,000 (4,5). Compared with other types of cancer, skin cancer has a higher recurrence rate, with a 35% probability of recurrence in the first 3 years and a 50% probability of recurrence in the first 5 years. Recurrent skin cancer is usually the same sub-type as the original cancer (6). Although the mortality rate of skin cancer is extremely low and the cure rate is high, its high incidence and recurrence rate constitute a significant economic burden to health services. Furthermore, skin cancer located on the head and other highly visible areas may affect a patients mental wellbeing and quality of life (7,8).
Cutaneous metastases of malignant tumors can be caused by malignant tumor cells traveling through the blood or lymphatic system, tissue interstitial diffusion (tissue gap diffusion), or surgical implantation (9). The higher the malignancy of the tumor, the more likely it is to metastasize, and skin metastasis is often the end-stage manifestation of a malignant tumor (10). Once metastasis occurs, the prognosis of cancer is poor (11). Unfortunately, skin metastasis may be the first clinical manifestation of a malignant tumor (12). Therefore, a suitable prognosis serves an important role in the recurrence and treatment of metastatic skin cancer. The aim of the present study was to identify potential prognostic biomarkers of metastatic skin cancer, which may be used in a clinical setting, through data mining and analysis of skin cancer prognosis genes.
With the development of gene chip technology and next-generation sequencing technology (13), and the constant revision of the viewpoints of individualized medical treatment and precision medical care, understanding skin cancer from the perspective of the genome and proposing more effective genetic biomarkers provides more relevant information for drug development and clinical decision-making (14-18). Traditional statistical methods often yield unstable results and excessive errors when applied to gene expression analysis (19). Furthermore, in high-throughput gene expression experiments, the number of variables is generally much higher than the sample size, which is called the Curse of Dimensionality (20). Therefore, in the present study, least absolute shrinkage and selection operator (LASSO) was used to mine genomic data to minimize the instability caused by high-dimensional data (21-27).
Materials and methods
Data collection
Using The Cancer Genome Atlas, data on skin cancer from the Xena Functional Genomics Explorer (xenabrowser.net/datapages/) was obtained, including the number of patients (n=481) and the number of genes assessed (n=20,530). Among these, the number of primary samples (Primary Tumor) was 105, the number of metastatic (Metastatic) samples was 368, and the number of other types of samples was 8 (28-32).
Data preprocessing
First, 219 samples with missing survival attribute values were removed from 473 primary and metastatic samples and 255 patient samples were retained. These data were stratified into primary tumor samples (n=72) and metastatic samples (n=183) by matching the patients' samples in the gene expression spectrum. Finally, using the ‘sample’ function in R version 3.5.2(33), the metastatic samples were randomly divided into two groups; training samples (n=91) and test samples (n=92).
Clinical data analysis
Several clinical factors affect the prognosis of patients with skin cancer, so it is necessary for data analysis to consider multiple attribute values in the sample as influencing factors. Based on previous studies, clinical variables including age, sex, history of radiotherapy, Tumor-Node-Metastasis pathological stage (34) and cancer status were used to analyze the related clinical factors in the subsequent data mining analysis (35-38).
Analysis of gene data
First, the ‘Limma’ package version 3.42.2(39) in R was used to analyze the differentially expressed genes of the 91 training set samples and 72 primary tumor samples. According to the adjusted P-value (adj.P.val<0.001), 783 genes were considered to be differentially expressed genes.
The ‘survival’ package (rdocumentation.org/packages/survival; version 3.1-8) in R was used to perform Cox regression analysis of the differentially expressed genes and the Cox coefficient, the hazard ratio (HR) and the P-values of the Wald test of each gene were calculated using the Kaplan-Meier method (40), and the genes that were significantly associated with the survival of the patients (P<0.05) were screened using the ‘survivalROC’ package (version number 1.0.3; rdocumentation.org/packages/survivalROC).
Finally, the screened genes were analyzed again using LASSO in the R package ‘glmnet’ (rdocumentation.org/packages/glmnet; version 3.0-2), to obtain more critical genes. Through 10-fold cross validation, 20 risk genes, which were closely related to survival, were identified (41-49). Additionally, the Gene Ontology (GO) Cell Component Ontology Method (50,51) was used to analyze pathway involved in protein activity.
Prognostic index (PI) calculation
As an important indicator of the integration of risk genes, a PI value can be determined for each patient with skin cancer. The PI was obtained by linearly fitting the product of the expression and the coefficient corrected by LASSO of each gene. The formula for calculating the PI was: PI=β1 X1+β2X2+…+βiXi…+βnXn; where Xi is the expression of the ith gene and βi is the coefficient corrected by LASSO of the ith gene.
Data validation
By extrapolating genes with a P<0.05, the 20 risk genes obtained were verified using the test sample using the same methods described above.
Statistical analysis of clinical variables
From the clinical information of 255 patient samples, the patients' age, sex, radiation therapy, pathological T-stage and cancer status were taken as single variables. Cox regression analysis was performed, and the P-value of the Log-rank test and the HR value of each clinical factor were calculated. P<0.05 was considered to indicate a statistically significant difference.
Results
Clinicopathological variables
Age, pathologic T-stage and cancer status were all significantly associated with the recurrence of skin cancer, suggesting that these three clinical factors may be used as independent prognostic indicators (Table I).
Table I.
Clinicopathological characteristics of the patients.
| Characteristics | Number of patients (relapse) | P-value | HR (95% CI) |
|---|---|---|---|
| Age | 0.030 | 1.669 (1.037-2.688) | |
| >57 | 123(35) | ||
| ≤57 | 132(36) | ||
| Sex | 0.200 | 1.359 (0.8058-2.291) | |
| Male | 160(49) | ||
| Female | 95(22) | ||
| Cancer status | |||
| Unknown | 3(1) | 7x10-10 | |
| With tumor | 160(18) | 0.802 (0.1089-5.908) | |
| Tumor free | 92(52) | 0.1572 (0.0206-1.201) | |
| Radiation therapy | 0.200 | 1.7204 (0.851-3.478) | |
| Unknown | 1 (0) | ||
| Yes | 38(9) | ||
| No | 216(62) | ||
| Pathological T-stage | 0.009 | 2.003 (1.175-3.412) | |
| T1-T3 | 165(49) | ||
| T4 | 90(22) |
HR, hazard ratio; CI, confidence interval.
Genetic data analysis
After determining differential gene expression, 44 genes were considered significantly associated with the survival of patients using survival analysis. Cox regression analysis and LASSO analysis were performed, and 20 risk genes associated with skin cancer were identified (Table II).
Table II.
Prognostic genes.
| Gene symbol | Name | Univariate HR (95% CI) | Coefficient | P-value | LASSO HR (95% CI) |
|---|---|---|---|---|---|
| TMEM45B | Transmembrane protein 45B | 0.899 (0.822-0.982) | -0.086 | 1.88x10-2 | 0.918 (0.864-0.975) |
| CDKN1B | Cyclin-dependent kinase inhibitor 1B (p27, Kip1) | 0.928 (0.886-0.972) | -0.029 | 1.55x10-3 | 0.971 (0.914-1.032) |
| PKHD1L1 | Polycystic kidney and hepatic disease 1 (autosomal recessive)-like 1 | 0.938 (0.886-0.993) | -0.010 | 2.73x10-2 | 0.990 (0.932-1.052) |
| PLCL2 | Phospholipase C-like 2 | 0.961 (0.929-0.994) | -0.006 | 2.14x10-2 | 0.994 (0.936-1.056) |
| CKMT1A | Creatine kinase, mitochondrial 1A | 1.024 (1.000-1.049) | 0.001 | 4.90x10-2 | 1.001 (0.942-1.063) |
| SPINK5 | Serine peptidase inhibitor, Kazal type 5 | 1.027 (1.002-1.052) | 0.007 | 3.31x10-2 | 1.008 (0.948-1.070) |
| CST6 | Cystatin E/M | 1.027 (1.000-1.054) | 0.011 | 4.67x10-2 | 1.011 (0.952-1.074) |
| FABP5 | Fatty acid binding protein 5 (psoriasis-associated) | 1.037 (1.007-1.067) | 0.007 | 1.53x10-2 | 1.007 (0.948-1.070) |
| PTK6 | Protein tyrosine kinase 6 | 1.047 (1.010-1.084) | 0.029 | 1.11x10-2 | 1.030 (0.969-1.094) |
| TXNDC17 | Thioredoxin domain containing 17 | 1.047 (1.002-1.095) | 0.004 | 4.02x10-2 | 1.004 (0.945-1.066) |
| LTB4R | Leukotriene B4 receptor | 1.050 (1.007-1.096) | 0.018 | 2.39x10-2 | 1.018 (0.958-1.082) |
| FAM100A | Family with sequence similarity 100, member A | 1.050 (1.008-1.094) | 0.015 | 1.90x10-2 | 1.016 (0.956-1.079) |
| KRT10 | keratin 10 | 1.057 (1.025-1.091) | 0.016 | 4.33x10-4 | 1.016 (0.956-1.080) |
| PPP1R13L | Protein phosphatase 1, regulatory subunit 13 like | 1.059 (1.010-1.109) | 0.000 | 1.65x10-2 | 1.000 (0.941-1.063) |
| DLX3 | Distal-less homeobox 3 | 1.063 (1.025-1.102) | 0.008 | 9.40x10-4 | 1.008 (0.949-1.071) |
| KPRP | Keratinocyte proline rich protein | 1.064 (1.015-1.115) | 0.044 | 9.68x10-3 | 1.045 (0.984-1.111) |
| VSIG10L | V-set and immunoglobulin domain containing 10 like | 1.066 (1.011-1.123) | 0.021 | 1.87x10-2 | 1.021 (0.961-1.085) |
| MYOM3 | Myomesin 3 | 1.073 (1.015-1.134) | 0.040 | 1.25x10-2 | 1.041 (0.979-1.106) |
| NMU | Neuromedin U receptor 1 | 1.092 (1.042-1.144) | 0.072 | 2.22x10-4 | 1.075 (1.012-1.142) |
| PIGW | Phosphatidylinositol glycan anchor biosynthesis, class W | 1.113 (1.044-1.188) | 0.002 | 1.08x10-3 | 1.002 (0.943-1.064) |
HR, hazard ratio; CI, confidence interval.
Gene PI analysis
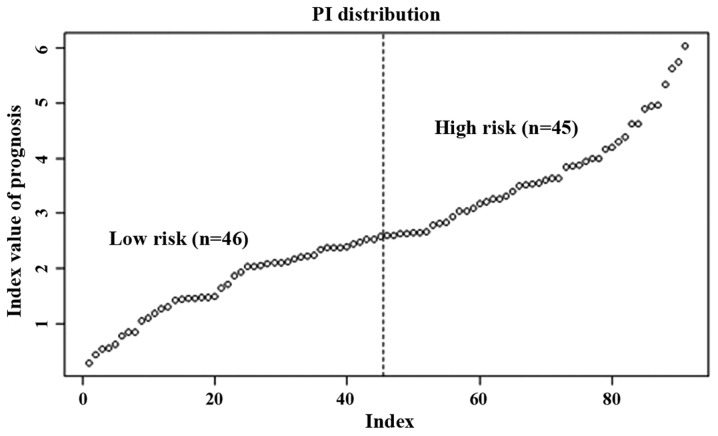
Through linear fitting of the product of expression and regression coefficient of the 20 genes in each sample, the PI of each patient was calculated, and the patients were sorted from lowest to highest according to their PI value. Based on the median PI value, the patients were divided into high-risk and low-risk groups (Fig. 1). The lower the PI value, the lower the risk of recurrent survival, and the higher the PI value, the higher the risk of recurrent survival.
Figure 1.
Stratification of patients with skin cancer into high-risk and low-risk groups using the prognostic gene biomarkers. PI, prognostic index.
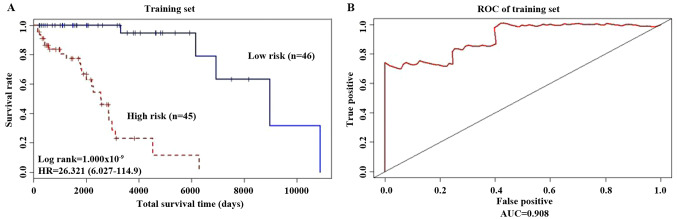
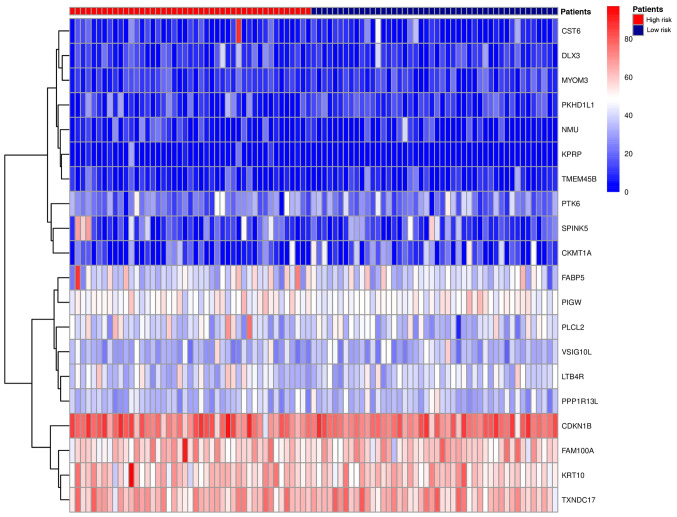
Using the Kaplan Meier method, the survival curves for the two groups of patients, are presented in Fig. 2A; patients considered low risk exhibited significantly longer overall survival times (P<0.001; HR=26.321). Using the 5-year survival rates, a Receiver Operating Characteristic (ROC) curve was drawn (Fig. 2B) and analysis was performed using the ‘survivalROC’ package. The advantages and disadvantages of the model constructed using the 20 gene biomarkers were determined based on the Area Under the Curve (AUC) value. The results showed that AUC was equal to 0.908 (AUC >0.5 indicates a suitable model). The heat map of risk gene expression profiles in high-risk and low-risk patients were plotted (Fig. 3). The high-risk group was clearly distinguished from the low-risk group. This indicated that the models constructed by these 20 gene biomarkers performed well.
Figure 2.
Integrative model for predicting outcome. (A) Survival curves of the high-risk and low-risk patients distinguished according to the prognostic index value. There was a significant difference in survival between the two groups. (B) ROC curve analysis of gene marker models. ROC, Receiver Operating Characteristic; AUC, area under the curve.
Figure 3.
Heat map of the expression profile of the risk genes in the high-risk and low-risk patients.
Based on the experimental results, the 20 gene prognostic biomarkers could be used to significantly classify the patients with skin cancer in the training samples into two groups: High risk and low risk. In order to further verify the accuracy of the test results, the 20 genetic biomarkers were used to validate the test samples. As shown in Fig. 4, these genetic biomarkers could still classify patients with skin cancer in the test sample into high-risk and low-risk categories (P=0.004, HR=1.194). The AUC of the ROC curve was 0.855.
Figure 4.
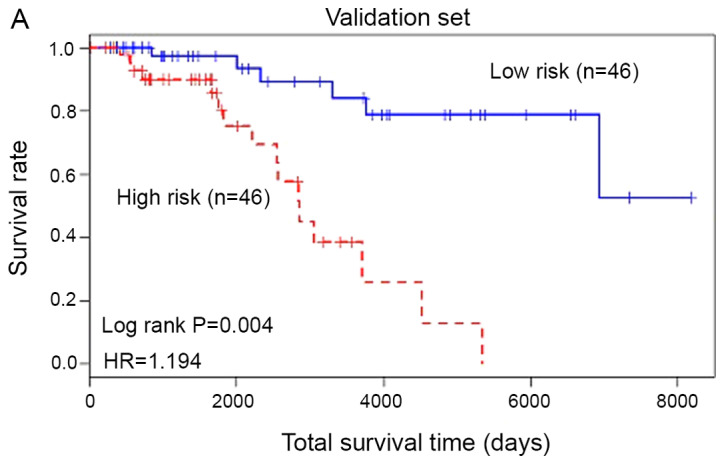
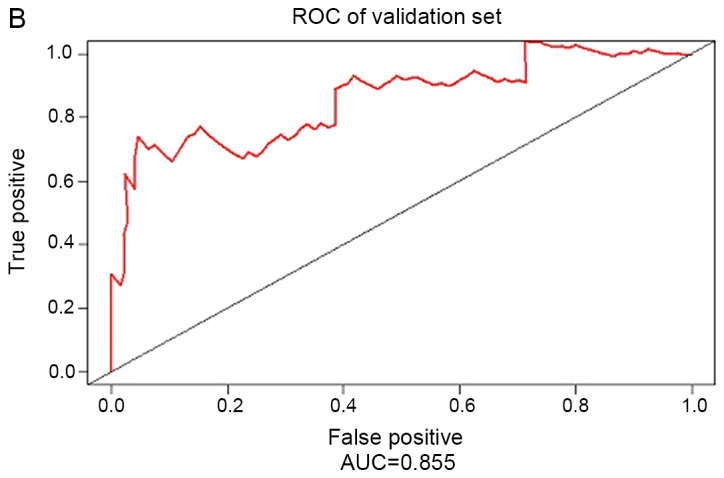
Integrative model for validating the results. (A) Survival curves of patients in the validation set stratified by the PI value. (B) ROC curve analysis of gene marker models. ROC, Receiver Operating Characteristic; AUC, area under the curve.
Prognostic gene function analysis
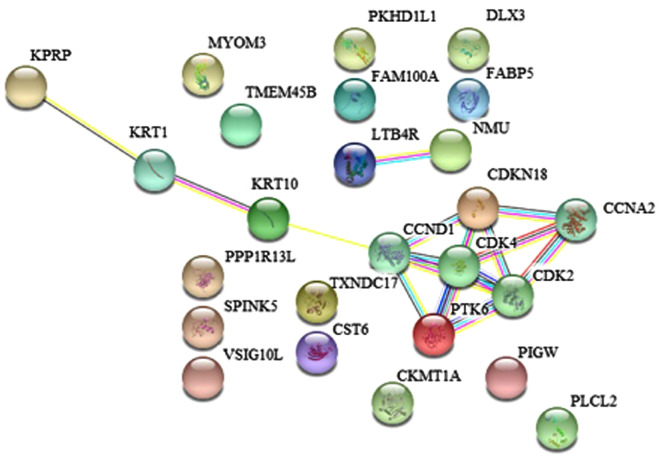
The online genetic analysis tool STRING (52) was used to analyze and study the association between the identified gene biomarkers and their associated protein synthesis pathways (Fig. 5; Table III) Gene Ontology (GO) cellular component ontology analysis identified a pathway of which involved a cyclin-dependent protein kinase holoenzyme complex, which included three proteins (CCND1, CDK2 and CDK4).
Figure 5.
Protein network of CDK4, CDKN18, CCNA2, CCND1, PTK6 and CDK2.
Table III.
GO biological processes associated with the prognostic genes.
| Pathway ID | Pathway description | Gene count | False discovery rate | Matching proteins |
|---|---|---|---|---|
| GO.0010948 | Negative regulation of cell cycle process | 5 | 0.0219 | CCNA2, CCND1, CDK2, CDK4, CDKN1B |
| GO.0031100 | Organ regeneration | 4 | 0.0219 | CCNA2, CCND1, CDK2, CDK4 |
| GO.0044773 | mitotic DNA Damage checkpoint | 4 | 0.0219 | CCNA2, CCND1, CDK2, CDKN1B |
| GO.0071156 | Regulation of cell cycle arrest | 4 | 0.0219 | CCND1, CDK2, CDK4, CDKN1B |
| GO.1901990 | Regulation of mitotic cell cycle phase transition | 5 | 0.0219 | CCNA2, CCND1, CDK2, CDK4, CDKN1B |
| GO.0032355 | Response to estradiol | 4 | 0.0238 | CCNA2, CCND1, CDK2, CDKN1B |
| GO.0010389 | Regulation of G2/M transition of mitotic cell cycle | 3 | 0.0277 | CCNA2, CCND1, CDK4 |
| GO.0044772 | Mitotic cell cycle phase transition | 5 | 0.0277 | CCNA2, CCND1, CDK2, CDK4, CDKN1B |
| GO.0097305 | Response to alcohol | 5 | 0.0277 | CCNA2, CCND1, CDK2, CDK4, CDKN1B |
| GO.1901991 | Negative regulation of mitotic cell cycle phase transition | 4 | 0.0287 | CCNA2, CCND1, CDK2, CDKN1B |
| GO.0000082 | G1/S transition of mitotic cell cycle | 4 | 0.0326 | CCND1, CDK2, CDK4, CDKN1B |
| GO.0048513 | Organ development | 11 | 0.0345 | CCNA2, CCND1, CDK2, CDK4, CDKN1B, DLX3, KRT1, KRT10, PLCL2, PPP1R13L, SPINK5 |
| GO.0060429 | Epithelium development | 7 | 0.0457 | CCND1, CST6, DLX3, FABP5, KRT10, PTK6, SPINK5 |
| GO.0048545 | Response to steroid hormone | 5 | 0.0482 | CCNA2, CCND1, CDK2, CDK4, CDKN1B |
GO, Gene Ontology.
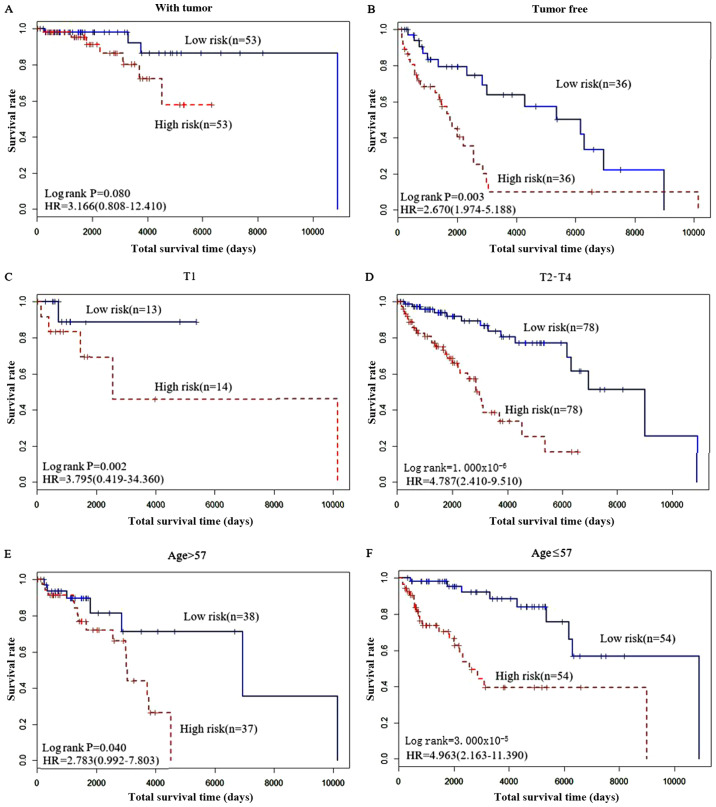
Performance of the biomarkers in clinical subtypes
Among the clinical factors, pathological T stage (T1 and T2-4), cancer status (with tumor and tumor free) and age (>57 years vs. ≤57 years) were all significantly associated with the recurrence status of patients with skin cancer (Fig. 6). Therefore, these 20 genes should to be considered in different clinical types to determine which clinical state they are more suitable for.
Figure 6.
Survival curves of patients. Survival curves of the patients based on (A and B) cancer status, (C and D) pathological T-stage or (E and F) age, stratified by risk. HR, hazard ratio.
The results in Fig. 6 shows the predictive effect of these 20 gene biomarkers. Patients who were tumor free had improved survival compared with those with tumor. Similarly, patients with a T-stage of T1-3 exhibited improved survival compares with patients classed as T4, and patients ≤57 years old had improved survival compared with patients >57 years old.
Discussion
In the present study, a variety of statistical analysis methods were used (including LASSO regression, single-factor survival analysis, multi-factor Cox proportional hazards regression model and ROC curve analysis) to identify differences in the gene expression profiles of patients with skin cancer. A supervised cluster analysis method was used and 20 prognostic genes from the training samples were identified and verified against a test sample. The results showed that these 20 genes can stratify patients with skin cancer as high-risk and low-risk, which shows the feasibility of the mining method used in the present study.
From a biological point of view, the 20 prognostic genes identified in the present study successfully divided the patients with cancer according to the risk of recurrence, which may have important reference value for the treatment of recurrence of metastatic skin cancer, and may influence future clinical studies of skin cancer and drug development.
Among the 20 prognostic genes identified in the present study, several are closely associated with skin cancer and metastatic skin cancer, including DLX3, PTK6 and CST6 genes. For example, physical interaction between DLX3 and P53 on P21 promoter can enhance the expression of P21(53). Increasing DLX3 expression in keratinocytes produces a G1-S blockade associated with P53 signature transcriptional profiles (54).
Deletion of DLX3 promotes a mitogenic phenotype associated with constitutive activation of ERK (55). The loss of DLX3 expression in human skin cancer suggests that a DLX3-P53 interaction may be a primary regulatory axis of epidermis differentiation and that DLX3 may be a regulator of skin cancer development. Protein tyrosine kinase 6 (PTK6) is expressed in ~70% of cases of triple-negative breast cancer, in which it serves an important role in promoting metastatic lung colonization and survival (56). PTK6 inhibits the inhibition of metastasis of triple-negative breast cancer via SNAIL-dependent regulation of E-cadherin expression (57). Epigenetic changes associated with upregulation of CST6 gene expression may be accompanied by metastatic diffusion of primary tumor sites, and current studies have shown that methylation-dependent epigenetic silencing of CST6 represents an important mechanism for loss of CST6 during the development and/or progression to metastasis (58). Other gene prognostic biomarkers identified in the present study have potential research value and need further exploration and research.
Among these, several genes, including PLCL2, serine protease inhibitor Kazal type-5 (SPINK5) and KRT10 are associated with skin diseases. Inosine strongly enhances proliferation of human C32 melanoma cells through the PLCL2 and PI3K pathways (59). SPINK5 serves a crucial role in the timing of desquamation of the skin (60). Biallelic KRT10 mutations result in skin fragility caused by self-improving epidermolytic ichthyosis (61). Several other genes, such as transmembrane protein 45B (TMEM45B), CDKN1B, CKMT1A, FABP5 and PPP1R13L have also been shown to be associated with several types of cancer. TMEM45B has been shown to be abnormally expressed in gastric tumors and serves an important role in gastric tumorigenesis (62). The CCND1-A870G and CDKN1B-C79T polymorphisms are associated with breast cancer risk (63). The n335586/miR-924/CKMT1A axis contributes to migration and invasion of hepatocellular carcinoma cells (64). FABP5 serves an important role in the carcinogenesis and metastasis of cervical cancer, and may be a novel predictor for prognostic assessment of patients with cervical cancer (65). Polymorphisms of ERCC1, CD3EAP and PPP1R13L in chromosomal region 19q13.2-3 have previously been shown to exhibit a synergistic effect on apoptosis and DNA repair pathways (66).
In summary, the 20 gene biomarkers identified based on the LASSO algorithm can effectively predict the risk of patients with skin cancer and may be more convenient as a model of prognosis.
Acknowledgements
Not applicable.
Funding
This work was supported by the Key Project of China Ministry of Education for Philosophy and Social Science: Big Data Driven Risk Research on City's Public Safety (grant no. 16JZD023) and the Fundamental Research Funds for the Central Universities: Big Data Driven Risk Pre-Warning Research on City's Public Safety (grant no. 17LZUJBWZD012).
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
GL analyzed and interpreted the patient data was a major contributor in writing the manuscript. CL analyzed the data. HZ made substantial contributions to acquisition of data. ZZ was a major contributor in interpreting the data and in writing the manuscript. YS made substantial contributions to analysis and interpretation of data. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Diepgen TL, Mahler V. The epidemiology of skin cancer. Br J Dermatol. 2002;146 (Suppl 61):S1–S6. doi: 10.1046/j.1365-2133.146.s61.2.x. [DOI] [PubMed] [Google Scholar]
- 2.Lai V, Cranwell W, Sinclair R. Epidemiology of skin cancer in the mature patient. Clin Dermatol. 2018;36:167–176. doi: 10.1016/j.clindermatol.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 3.Pilgrim W, Hayes R, Hanson DW, Zhang B, Boudreau B, Leonfellner S. Skin Cancer (Basal Cell Carcinoma, Squamous Cell Carcinoma, and Malignant Melanoma): New cases, treatment practice, and health care costs in New Brunswick, Canada, 2002-2010. J Cutan Med Surg. 2014;18:320–331. doi: 10.2310/7750.2014.13162. [DOI] [PubMed] [Google Scholar]
- 4.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in china, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 5.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 6.Karagas MR, Stukel TA, Greenberg ER, Baron JA, Mott LA, Stern RS. Risk of subsequent basal cell carcinoma and squamous cell carcinoma of the skin among patients with prior skin cancer. Skin cancer prevention study group. JAMA. 1992;267:3305–3310. [PubMed] [Google Scholar]
- 7.Jerant AF, Johnson JT, Sheridan CD, Caffrey TJ. Early detection and treatment of skin cancer. Am Fam Physician. 2000;62:357–368. 375-376, 381-382. [PubMed] [Google Scholar]
- 8.Gauci J, Muscat G. A local perspective on basal cell carcinoma: Frequency of subsequent skin tumours. Malta Med J. 2017;1:46–55. [Google Scholar]
- 9.Gillner J, Kirchberg K, Korge B, Hunzelmann N, Krieg T, Scharffetter-Kochanek K. Cutaneous metastases from a leiomyosarcoma of the testicular tunica albuginea. Hautarzt. 2000;51:41–45. doi: 10.1007/s001050050009. (In German) [DOI] [PubMed] [Google Scholar]
- 10.Hoyt BS, Cohen PR. Cutaneous scrotal metastasis: Origins and clinical characteristics of visceral malignancies that metastasize to the scrotum. Int J Dermatol. 2013;52:398–405. doi: 10.1111/j.1365-4632.2012.05717.x. [DOI] [PubMed] [Google Scholar]
- 11.Bremnes RM, Veve R, Hirsch FR, Franklin WA. The E-cadherin cell-cell adhesion complex and lung cancer invasion, metastasis, and prognosis. Lung Cancer. 2002;36:115–124. doi: 10.1016/s0169-5002(01)00471-8. [DOI] [PubMed] [Google Scholar]
- 12.Zhen Y, Wu C, Jiang J. Analysis of clinicopathological features and prognostic factors in cutaneous metastases of malignant tumors. Chin Clin Oncol. 2014;19:152–155. [Google Scholar]
- 13.Niu JX, Meng XK, Ren JJ. Studied microRNA gene expression in human hepatocellular carcinoma by microRNA microarray techniques. World J Gastroenterol. 2015;21:12605–12611. doi: 10.3748/wjg.v21.i44.12605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hou X, Peng JX, Hao XY, Cai JP, Liang LJ, Zhai JM, Zhang KS, Lai JM, Yin XY. DNA methylation profiling identifies EYA4 gene as a prognostic molecular marker in hepatocellular carcinoma. Ann Surg Oncol. 2014;21:3891–3899. doi: 10.1245/s10434-013-3401-z. [DOI] [PubMed] [Google Scholar]
- 15.Pivarcsi A, Sonkoly E. Skin cancer associated microRNAs. US Patent 12/994,734. Filed June 4, 2009; issued May 5, 2011. [Google Scholar]
- 16.Glavač D, Ravnik-Glavač M. Essential role of microRNA in skin physiology and disease. Adv Exp Med Biol. 2015;888:307–330. doi: 10.1007/978-3-319-22671-2_16. [DOI] [PubMed] [Google Scholar]
- 17.Leffell DJ. The scientific basis of skin cancer. J Am Acad Dermatol. 2000;42 (Suppl):S18–S22. doi: 10.1067/mjd.2000.103340. [DOI] [PubMed] [Google Scholar]
- 18.Thyagarajan A, Shaban A, Sahu RP. MicroRNA-directed cancer therapies: Implications in melanoma intervention. J Pharmacol Exp Ther. 2018;364:1–12. doi: 10.1124/jpet.117.242636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Z, Chen D, Xu Y, Liu J. Logistic support vector machines and their application to gene expression data. Int J Bioinform Res Appl. 2005;1:169–182. doi: 10.1504/IJBRA.2005.007576. [DOI] [PubMed] [Google Scholar]
- 20.Yongchao GE, Sealfon SC, Speed TP. Multiple testing and its applications to microarrays. Stat Methods Med Res. 2009;18:543–563. doi: 10.1177/0962280209351899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okimoto G, Zeinalzadeh A, Wenska T, Loomis M, Nation JB, Fabre T, Tiirikainen M, Hernandez B, Chan O, Wong L, Kwee S. Joint analysis of multiple high-dimensional data types using sparse matrix approximations of rank-1 with applications to ovarian and liver cancer. BioData Min. 2016;9(24) doi: 10.1186/s13040-016-0103-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiong J, Xiong K, Bing Z. Clinical and RNA expression integrated signature for urothelial bladder cancer prognosis. Cancer Biomark. 2018;21:535–546. doi: 10.3233/CBM-170314. [DOI] [PubMed] [Google Scholar]
- 23.Tang RX, Chen WJ, He RQ, Zeng JH, Liang L, Li SK, Ma J, Luo DZ, Chen G. Identification of a RNA-Seq based prognostic signature with five lncRNAs for lung squamous cell carcinoma. Oncotarget. 2017;8:50761–50773. doi: 10.18632/oncotarget.17098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao X, Wu Y, Yu W, Li H. Identification of a seven-miRNA signature as prognostic biomarker for lung squamous cell carcinoma. Oncotarget. 2016;7:81670–81679. doi: 10.18632/oncotarget.13164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Malenstein H, Gevaert O, Libbrecht L, Daemen A, Allemeersch J, Nevens F, Van Cutsem E, Cassiman D, De Moor B, Verslype C, van Pelt J. A seven-gene set associated with chronic hypoxia of prognostic importance in hepatocellular carcinoma. Clin Cancer Res. 2010;16:4278–4288. doi: 10.1158/1078-0432.CCR-09-3274. [DOI] [PubMed] [Google Scholar]
- 26.Nakazawa H, English D, Randell PL, Nakazawa K, Martel N, Armstrong BK, Yamasaki H. UV and skin cancer: Specific p53 gene mutation in normal skin as a biologically relevant exposure measurement. Proc Natl Acad Sci USA. 1994;91:360–364. doi: 10.1073/pnas.91.1.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Linck L, Liebig J, Völler D, Eichner N, Lehmann G, Meister G, Bosserhoff A. MicroRNA-sequencing data analyzing melanoma development and progression. Exp Mol Pathol. 2018;105:371–379. doi: 10.1016/j.yexmp.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 28.Goldman M, Craft B, Swatloski T, Ellrott K, Cline M, Diekhans M, Ma S, Wilks C, Stuart J, Haussler D, Zhu J. The UCSC Cancer Genomics Browser: Update 2013. Nucleic Acids Res. 2013;41 (Database Issue):D949–D954. doi: 10.1093/nar/gks1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cline MS, Craft B, Swatloski T, Goldman M, Ma S, Haussler D, Zhu J. Exploring TCGA Pan-Cancer Data at the UCSC Cancer Genomics Browser. Sci Rep. 2013;3(2652) doi: 10.1038/srep02652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goldman M, Craft B, Swatloski T, Cline M, Morozova O, Diekhans M, Haussler D, Zhu J. The UCSC cancer genomics browser: Update 2015. Nucleic Acids Res. 2015;43 (Database Issue):D812–D817. doi: 10.1093/nar/gku1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Turner N, Ware O, Bosenberg M. Genetics of metastasis: Melanoma and other cancers. Clin Exp Metastasis. 2018;35:379–391. doi: 10.1007/s10585-018-9893-y. [DOI] [PubMed] [Google Scholar]
- 32.Kong J, Zhang Y, Zhao B. A clinicopathological analysis of 104 cases with metastatic tumors of the skin. Chin J Diagnostic Pathol. 1995;2:211–213. [Google Scholar]
- 33. R Core Team: R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, 2006. [Google Scholar]
- 34.Loh KC, Greenspan FS, Gee L, Miller TR, Yeo PP. Pathological Tumor-Node-Metastasis (pTNM) staging for papillary and follicular thyroid carcinomas: A retrospective analysis of 700 patients. J Clin Endocrinol Metab. 1997;82:3553–3562. doi: 10.1210/jcem.82.11.4373. [DOI] [PubMed] [Google Scholar]
- 35.Lezcano C, Shoushtari AN, Ariyan C, Hollmann TJ, Busam KJ. Primary and metastatic melanoma with NTRK fusions. Am J Surg Pathol. 2018;42:1052–1058. doi: 10.1097/PAS.0000000000001070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rhee JS, Matthews BA, Neuburg M, Logan BR, Burzynski M, Nattinger AB. The skin cancer index: Clinical responsiveness and predictors of quality of life. Laryngoscope. 2007;117:399–405. doi: 10.1097/MLG.0b013e31802e2d88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morgan M, McCreedy R, Simpson J, Hay RJ. Dermatology quality of life scales: A measure of the impact of skin diseases. Br J Dermatol. 1997;136:202–206. [PubMed] [Google Scholar]
- 38.Rogers EM, Connolly KL, Nehal KS, Dusza SW, Rossi AM, Lee E. Comorbidity scores associated with limited life expectancy in the very elderly with nonmelanoma skin cancer. J Am Acad Dermatol. 2018;78:1119–1124. doi: 10.1016/j.jaad.2017.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, Smyth GK. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43(e47) doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nagy A, Lánczky A, Menyhárt O, Győrffy B. Validation of miRNA prognostic power in hepatocellular carcinoma using expression data of independent datasets. Sci Rep. 2018;8(9227) doi: 10.1038/s41598-018-27521-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zaorsky NG, Lee CT, Zhang E, Galloway TJ. Skin CanceR Brachytherapy vs External beam radiation therapy (SCRiBE) meta-analysis. Radiother Oncol. 2018;126:386–393. doi: 10.1016/j.radonc.2017.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Riker AI, Enkemann SA, Fodstad O, Liu S, Ren S, Morris C, Xi Y, Howell P, Metge B, Samant RS, et al. The gene expression profiles of primary and metastatic melanoma yields a transition point of tumor progression and metastasis. BMC Med Genomics. 2008;1(13) doi: 10.1186/1755-8794-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lawrence MS, Stojanov P, Polak P, Kryukov GV, Cibulskis K, Sivachenko A, Carter SL, Stewart C, Mermel CH, Roberts SA, et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature. 2013;499:214–218. doi: 10.1038/nature12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wiltgen M, Gerger A, Smolle J. Tissue counter analysis of benign common nevi and malignant melanoma. Int J Med Inform. 2003;69:17–28. doi: 10.1016/s1386-5056(02)00049-7. [DOI] [PubMed] [Google Scholar]
- 45.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Esteva A, Kuprel B, Novoa RA, Ko J, Swetter SM, Blau HM, Thrun S. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542:115–118. doi: 10.1038/nature21056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Esteva A, Kuprel B, Novoa RA, Ko J, Swetter SM, Blau HM, Thrun S. Corrigendum: Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;546(686) doi: 10.1038/nature22985. [DOI] [PubMed] [Google Scholar]
- 48.Masood A, Al-Jumaily AA. Computer aided diagnostic support system for skin cancer: A review of techniques and algorithms. Int J Biomed Imaging. 2013;2013(323268) doi: 10.1155/2013/323268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chang P, Bing Z, Tian J, Zhang J, Li X, Ge L, Ling J, Yang K, Li Y. Comprehensive assessment gene signatures for clear cell renal cell carcinoma prognosis. Medicine (Baltimore) 2018;97(e12679) doi: 10.1097/MD.0000000000012679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, et al. Gene ontology: Tool for the unification of biology The Gene Ontology Consortium. Nat Genet. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.The Gene Ontology Resource: 20 years and still GOing strong. Nucleic Acids Res. 2019;47:D330–D338. doi: 10.1093/nar/gky1055. The Gene Ontology Consortium. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Szklarczyk D, Gable AL, Lyon D, Junge A, Wyder S, Huerta-Cepas J, Simonovic M, Doncheva NT, Morris JH, Bork P, et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019;47:D607–D613. doi: 10.1093/nar/gky1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Panno ML, Giordano F, Mastroianni F, Morelli C, Brunelli E, Palma MG, Pellegrino M, Aquila S, Miglietta A, Mauro L, et al. Evidence that low doses of Taxol enhance the functional transactivatory properties of p53 on p21 waf promoter in MCF-7 breast cancer cells. FEBS Lett. 2006;580:2371–2380. doi: 10.1016/j.febslet.2006.03.055. [DOI] [PubMed] [Google Scholar]
- 54.Palazzo E, Kellett MD, Cataisson C, Bible PW, Bhattacharya S, Sun HW, Gormley AC, Yuspa SH, Morasso MI. A novel DLX3-PKC integrated signaling network drives keratinocyte differentiation. Cell Death Differ. 2017;24:717–730. doi: 10.1038/cdd.2017.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jiang L, Campagne C, Sundström E, Sousa P, Imran S, Seltenhammer M, Pielberg G, Olsson MJ, Egidy G, Andersson L, Golovko A. Constitutive activation of the ERK pathway in melanoma and skin melanocytes in Grey horses. BMC Cancer. 2014;14(857) doi: 10.1186/1471-2407-14-857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Palazzo E, Kellett M, Cataisson C, Gormley A, Bible PW, Pietroni V, Radoja N, Hwang J, Blumenberg M, Yuspa SH, Morasso MI. The homeoprotein DLX3 and tumor suppressor p53 co-regulate cell cycle progression and squamous tumor growth. Oncogene. 2016;35:3114–3124. doi: 10.1038/onc.2015.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ito K, Park SH, Nayak A, Byerly JH, Irie HY. PTK6 Inhibition suppresses metastases of triple-negative breast cancer via SNAIL-dependent E-cadherin regulation. Cancer Res. 2016;76:4406–4417. doi: 10.1158/0008-5472.CAN-15-3445. [DOI] [PubMed] [Google Scholar]
- 58.Rivenbark AG, Livasy CA, Boyd CE, Keppler D, Coleman WB. Methylation-dependent silencing of CST6 in primary human breast tumors and metastatic lesions. Exp Mol Pathol. 2007;83:188–197. doi: 10.1016/j.yexmp.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Soares AS, Costa VM, Diniz C, Fresco P. Inosine strongly enhances proliferation of human C32 melanoma cells through PLC-PKC-MEK1/2-ERK1/2 and PI3K pathways. Basic Clin Pharmacol Toxicol. 2015;116:25–36. doi: 10.1111/bcpt.12280. [DOI] [PubMed] [Google Scholar]
- 60.Le NA, Katsuyama M, Demura M, Tanii H, Katsuyama H, Saijoh K. Regulation of serine protease inhibitor Kazal type-5 (SPINK5) gene expression in the keratinocytes. Environ Health Prev Med. 2014;19:307–313. doi: 10.1007/s12199-014-0393-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Frommherz L, Küsel J, Zimmer A, Fischer J, Has C. Skin fragility caused by biallelic KRT10 mutations: An intriguing form of self-improving epidermolytic ichthyosis. Br J Dermatol. 2020;182:780–785. doi: 10.1111/bjd.18325. [DOI] [PubMed] [Google Scholar]
- 62.Shen K, Yu W, Yu Y, Liu X, Cui X. Knockdown of TMEM45B inhibits cell proliferation and invasion in gastric cancer. Biomed Pharmacother. 2018;104:576–581. doi: 10.1016/j.biopha.2018.05.016. [DOI] [PubMed] [Google Scholar]
- 63.Canbay E, Eraltan IY, Cercel A, Isbir T, Gazioglu E, Aydogan F, Cacina C, Cengiz A, Ferahman M, Zengin E, Unal H. CCND1 and CDKN1B polymorphisms and risk of breast cancer. Anticancer Res. 2010;30:3093–3098. [PubMed] [Google Scholar]
- 64.Fan H, Lv P, Mu T, Zhao X, Liu Y, Feng Y, Lv J, Liu M, Tang H. LncRNA n335586/miR-924/CKMT1A axis contributes to cell migration and invasion in hepatocellular carcinoma cells. Cancer Lett. 2018;429:89–99. doi: 10.1016/j.canlet.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 65.Wang W, Chu HJ, Liang YC, Huang JM, Shang CL, Tan H, Liu D, Zhao YH, Liu TY, Yao SZ. FABP5 correlates with poor prognosis and promotes tumor cell growth and metastasis in cervical cancer. Tumor Biol. 2016;37:14873–14883. doi: 10.1007/s13277-016-5350-1. [DOI] [PubMed] [Google Scholar]
- 66.Chae YS, Kim JG, Kang BW, Lee SJ, Jeon HS, Park JS, Choi GS, Lee WK. PPP1R13Lvariant associated with prognosis for patients with rectal cancer. J Cancer Res Clin Oncol. 2013;139:465–473. doi: 10.1007/s00432-012-1346-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.