Introduction
There are approximately 17 million cancer survivors living in the United States and by 2040 this estimate is predicted to increase to 26.1 million.1 Exercise provides a myriad of health benefits to individuals during and after cancer treatment by reducing treatment-related symptoms, improving functional status and quality of life, and lowering risk of disease recurrence.2,3 Despite the established benefits, an individual’s level of physical activity often decreases during treatment and does not return to pre-diagnosis levels after treatment completion.4,5 While exercise is regarded as safe and beneficial for individuals with cancer, promoting exercise for this population is complex. A patient-centered pathway is needed that can guide oncology and primary care professionals in efficient assessment of an individual’s condition and enable personalized referrals for exercise interventions that promote physical activity. The purpose of this manuscript is to provide a framework for clinical decision making that enables personalized condition assessment, risk stratification, and referral to optimal settings for exercise promotion for cancer survivors. Implementation strategies are also offered to support the integration of this model into an oncology clinical workflow.
With guidance from their medical provider, individuals are more likely to engage in exercise and maintain levels of physical activity during cancer treatments.6 However, the number of individuals with cancer who report receiving exercise-specific guidance from their health care providers is low.7 Of particular concern is the lack of knowledge and training among health care professionals about exercise prescription for this complex population.8
Condition Complexity and Exercise Prescription
The concurrence of cancer treatment-related side effects with pre-existing health conditions often makes it difficult for individuals to engage in physical activity and exercise.9–12 Furthermore, motivation,13 environmental constraints, and concerns about safety during cancer medical therapies are barriers that challenge exercise engagement.14,15 In the case of exercise, it is widely recognized that one size does not fit all. A safe and well-designed exercise prescription for an individual at one point in the treatment continuum may not be safe or tenable further into treatment. Cancer care is dynamic and warrants personalized treatment pathways that individualize interventions, particularly regarding exercise, as the individual’s medical status and personal needs change.16–18
Characterizing the individual’s prior level of function, exercise habits and lifestyle behaviors, preexisting comorbid conditions, and environment at the point of diagnosis provides important context to inform personalized exercise recommendations. A pragmatic approach to promote exercise is to then repeatedly screen for clinically meaningful changes in these baseline measures and refer for exercise prescription when indicated. Proactively prescribing exercise throughout cancer treatment may prevent the onset of some symptoms and mitigate the progressive severity of treatment-related functional impairments.19,20
Numerous care models promote proactive assessment and referral for exercise and rehabilitation interventions for individuals with cancer. These models address referral based on presence of physical and functional impairment,21–23 age-related senescence,24 adverse side effects of cancer treatments,25,26 and many propose skilled interventions based on level of risk for treatment-related functional decline27–30 or impairment burden.18,31 Our core author team (NS/JB/TM/AS) conducted an evidence review of these models and identified common components that promoted prospective assessment for rehabilitative and exercise referrals in oncology specifically identifying evidence for risk stratification, screening and assessment, triage concepts and pathways, and implementation strategies. These findings were reviewed with the entire author team over the course of two teleconference discussions. The decision was made by consensus to work in teams to synthesize the evidence to support an exercise clinical pathway focusing on (i) screening for risk stratification (JB/KBE/JM/JL), (ii) referral pathways (NS/CA/JS/DZ), and (iii) implementation (AS/KS/LN/AC). These three areas were prioritized as the most impactful to guiding oncology or primary care professionals in promoting exercise referral. The concept of individual assessment for exercise interventions (TM/AC/GC/KC) informed the final manuscript but was decided to be beyond the scope of this manuscript and will be addressed in future work. Based on this review, we propose five domains to inform a personalized exercise clinical pathway.
Five Domains to Guide Decision Making
The five domains provide perspective on the complexity of an individual’s condition, characterize risk for exercise-related complications, and guide clinical decision making for individualized recommendations. The domains include cardiometabolic status, oncologic factors, aging considerations, behavioral characteristics, and environmental elements. The confluence of presenting symptoms within and across domains influences the exercise prescription. Figure 1 identifies the domains and common symptoms and impairments that impact exercise prescription.
Figure 1.
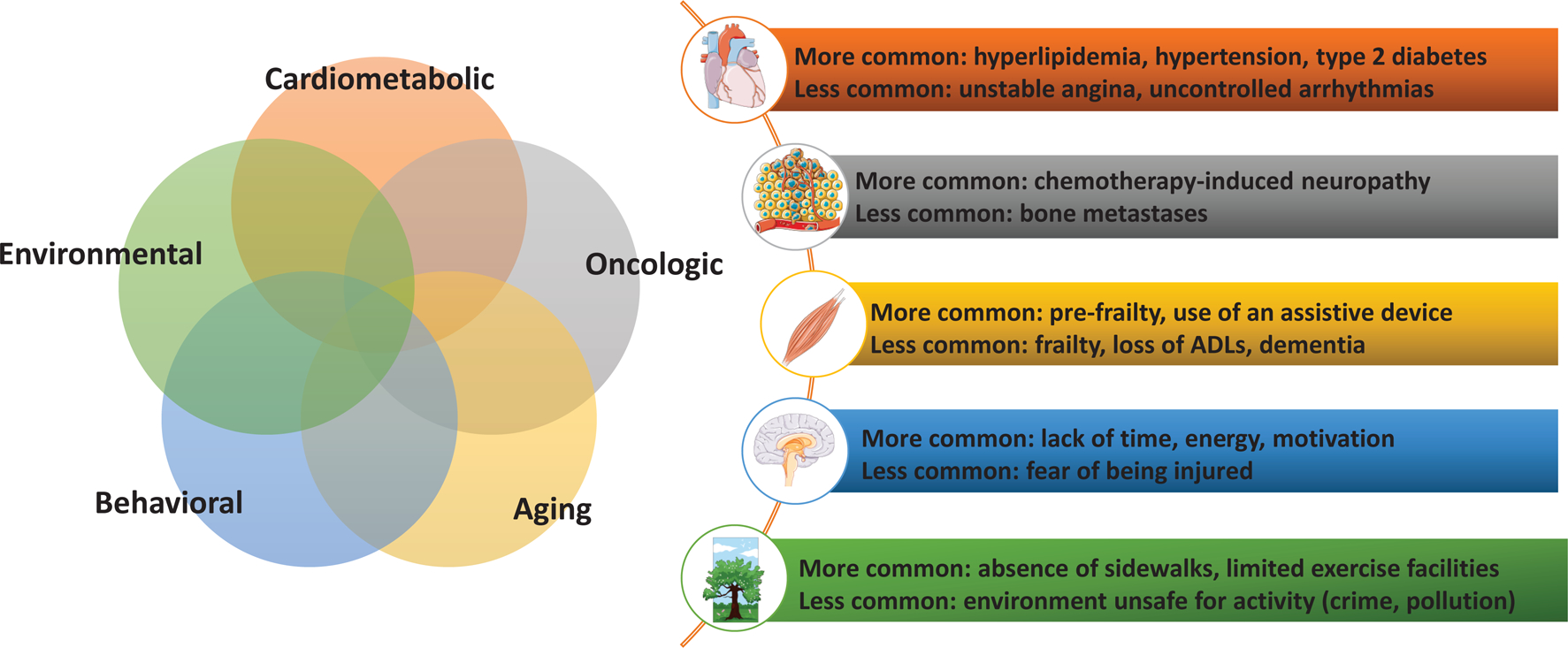
Five Domains that Inform Assessment for Exercise Referrals and Prescription
Cardiometabolic Status
Cardiometabolic conditions are common pre-existing conditions in individuals with cancer that influence exercise tolerance and safety.32–34 Pre-existing conditions may be exacerbated by cancer medical treatments and further suppress an individual’s ability to be physically active. Furthermore, cancer treatments may incite new cardiovascular risk factors and cardiovascular events in previously healthy individuals.35 In general, cardiovascular events, including stroke and myocardial infarction, are common causes of premature morbidity and mortality in cancer survivors.36–38 Risk assessment in this domain should consider the presence of cardiometabolic conditions, pre-existing and emerging, as well as the risk for cancer treatment-related cardiotoxicity. These conditions present barriers to exercise and introduce safety considerations when developing an exercise prescription. Risk assessment in this domain can determine if supervised exercise and clinical monitoring is indicated.39
Oncologic Factors
Cancer treatments impact multiple body systems and cause short and long-term sequelae. The nature and severity of side effects are quite varied across the cancer care continuum and differ substantially between individuals. Symptoms such as fatigue, restricted joint mobility, lymphedema, peripheral neuropathies, musculoskeletal arthralgias, sarcopenia, bone degradation and osseous fragility, incontinence, and many others are common40,41 and often cause physical impairments that challenge an individual’s tolerance to physical activity.42 While this symptom burden is anticipated during antineoplastic therapies, late effects such as pain, chronic fatigue, gait instability, and bone degradation also introduce challenges to physical activity participation and adherence beyond the completion of treatment.43
Risk assessment in this domain should consider the severity of treatment side effects, how they progress or regress during treatment, and should monitor for an accumulated burden of side effects over the course of treatment. This warrants ongoing, repeated assessment at medically important time points through the cancer continuum to inform adaptations to exercise interventions.
Age and Comorbidity
Older adults with cancer are more likely to have functional limitations compared to cancer-free controls10 and many of these limitations become more severe as a result of cancer treatment.11,12 Functional limitations precipitate additional barriers to exercise and participation in physical activity. The field of geriatric oncology recommends special consideration for older individuals44 regarding accelerated muscle loss, cognitive deficits, decreased aerobic capacity, and other geriatric syndromes such as frailty.45,46 Characterizing risk in this domain guides the selection of optimal interventions that enhance function and improve participation in physical activity. The exercise prescription needs to be tailored and gradually progressed in a supervised manner to maximize efficacy, safety, and tolerability.
Behavioral Characteristics
The most commonly cited reasons for not engaging in physical activity in the general population include lack of time, energy, and motivation.13 Individuals living with and beyond cancer experience even greater behavioral barriers to engaging in physical activity. Low energy, time stress from multiple appointments, and the stress of dealing with a potentially fatal condition exacerbate barriers to physical activity participation.47,48 Moreover, psychosocial factors such as motivational readiness, self-efficacy, and social support, contribute to whether an individual engages in physical activity.49 Assessment in this domain should evaluate the individual’s readiness to receive information about physical activity, confidence in their ability to exercise, perceived barriers, and preferences for exercise types. Some individuals may possess high self-efficacy, however their confidence is diminished by fears that exercise will harm them or worsen side effects. Conversely, some individuals have little or no experience with exercise and lack sufficient self-efficacy to independently adopt and sustain exercise habits. Assessment in this domain aligns individual preferences with evidence based exercise interventions.50
Environmental Elements
The environment in which individuals live influences their ability to adopt and sustain a physically active lifestyle. Environmental issues include the built environment in which one lives as well as their work environment, socioeconomic status, financial status, family support, health care insurance, access to care, and other social determinants that impact lifestyle and behavior.51,52 If an individual does not live in an accessible or safe community, is employed in multiple jobs working many hours, or has limited access to health and wellness facilities, they are at high risk for physical inactivity.53 Further complicating the environmental domain is the issue of access to and payment for medical care. Cancer treatment often incites financial toxicity54 which may limit the ability to afford copayments for exercise interventions or to pay for gym or recreational facility memberships.55 Risk assessment of environmental elements is important for promoting physical activity because the environment an individual lives and works in will influence their ability and willingness to engage in exercise. Assessment in this domain encourages exercise referrals according to individual needs and preferences that best fit the environmental circumstances.
Clinical Screening
The five domains offer a framework to simply and efficiently assess elements most relevant to identify decreasing physical activity and inform a clinical pathway for exercise referral. Oncology clinicians are in the ideal position to conduct repeated screenings with patients through the continuum of cancer care. Primary care and other advanced practice providers also play a critical role in recognizing changes across these domains that should prompt referral. Many individuals will have a constellation of factors that increase risk, and healthcare providers should consider the aggregate burden that exists at the confluence of these domains to inform their clinical decision.
The algorithm in Figure 2a provides simple screening questions across the five domains that open the conversation about exercise advice and enable referral. The algorithm accomplishes two important aspects of patient activation. First, it engages the individual in meaningful dialogue about the importance of exercise. All individuals should be counseled on the recommended physical activity guidelines and encouraged to maintain levels of activity during cancer treatment.56 When an oncology provider encourages exercise, patients are more likely to pursue the intervention.6,57 Second, it enables a quick screen of the five domains and a clinical decision about the appropriate exercise pathway most aligned with the individual’s needs.57 The goal of the algorithm is to enable a provider to quickly delineate between those who will benefit from an exercise prescription compared to those who will benefit from other services. More specific questions that characterize factors impacting the individual’s ability to and willingness to exercise would be introduced in a detailed assessment by the exercise or rehabilitation professional.
Figure 2a.
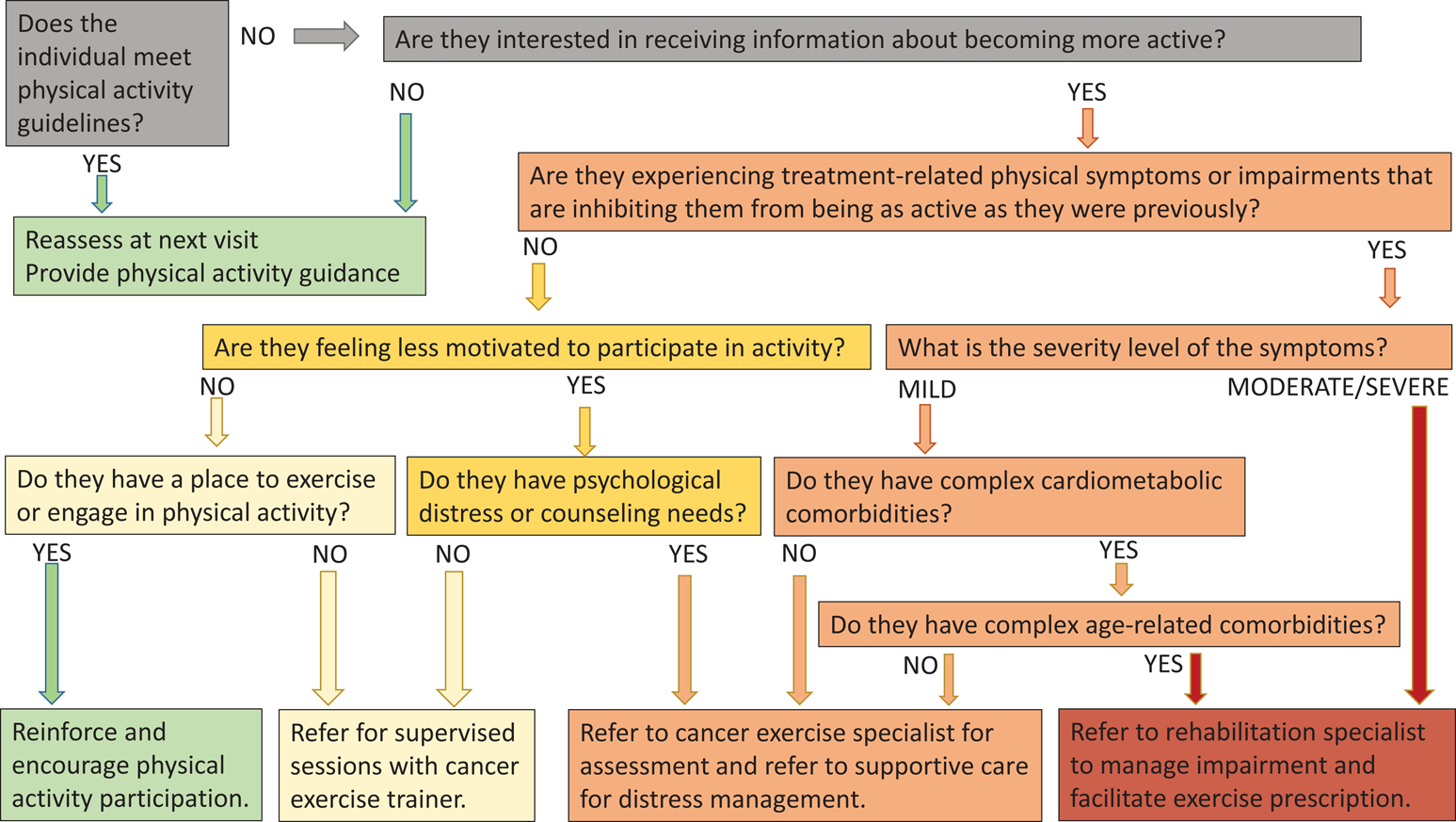
Algorithm for an exercise referral clinical pathway*
*The pathway is intended to stratify individuals to higher (red) or lower (green) condition complexity which provides insight to the level of supervision and guidance they may need to successfully engage in exercise and informs referrals to setting outlined in 2b.
The endpoints of the algorithm are intended to reflect the spectrum of complexity of the individual’s condition and the anticipated level of supervision and guidance that may be needed for successful exercise prescription with green representing lower complexity and risk/lower need for support and red representing higher complexity and risk/greater need for support. When treatment-related symptoms and impairments become persistent or severe they present barriers to exercise and may be an early sign of emerging functional morbidity.40,47 Screening for symptoms or impairments and assessing their severity provides insight on the level of intervention that is safe and effective to overcome barriers and promote improvements in physical activity. In situations of low complexity (green/yellow spectrum), independent exercise or supervised programs led by cancer exercise trainers are effective to increase exercise engagement.49 Moderate to high complexity situations (orange/red spectrum) pose barriers to exercise and warrant referral to an exercise or rehabilitation professional.23,58
The presence and severity of cancer treatment-related symptoms are routinely measured throughout disease treatment. Guidelines from the National Comprehensive Cancer Network, the American Society for Clinical Oncology, and others identify critical thresholds for intervention and suggest evidence-based pathways for symptom management, often including exercise.59–61 While it is beyond the scope of this manuscript to detail symptom management guidelines and referral thresholds, providers should be aware of these evidence-based recommendations and use them to objectively execute referrals along this exercise pathway.
Motivation and self-efficacy are important considerations when making exercise recommendations. Individuals may not be ready to change their behaviors nor interested in taking on exercise if they were not previously active. Acceptance of the individual’s preferences fosters better provider/patient relations that could eventually facilitate future health behaviors and prompt engagement in exercise.47 Safety with exercise and fear of doing harm to oneself by ‘overdoing it’ requires discussion with a cancer exercise specialist. Exercise and rehabilitative professionals have expertise in motivational strategies to enable individual self-activation towards exercise, as well as knowledge of safety considerations with exercise prescription, further supporting the need for referrals to exercise specialists. Undeniably, the suitability of these pathways is negated if the patient is not able to access or afford the prescribed care. Thus, assessing environmental and resource constraints and identifying resources that can overcome them is critical.
Referral Pathways
The screening algorithm can prompt advice and timely referrals for the most appropriate exercise intervention based on the individual’s presentation. Figure 2b describes the level of stepped care that is likely to be suited to the individual’s needs, based on the complexity of the individual’s condition, as identified through the screening algorithm, using the color scheme of green indicating low complexity and red indicating high complexity.
Figure 2b.
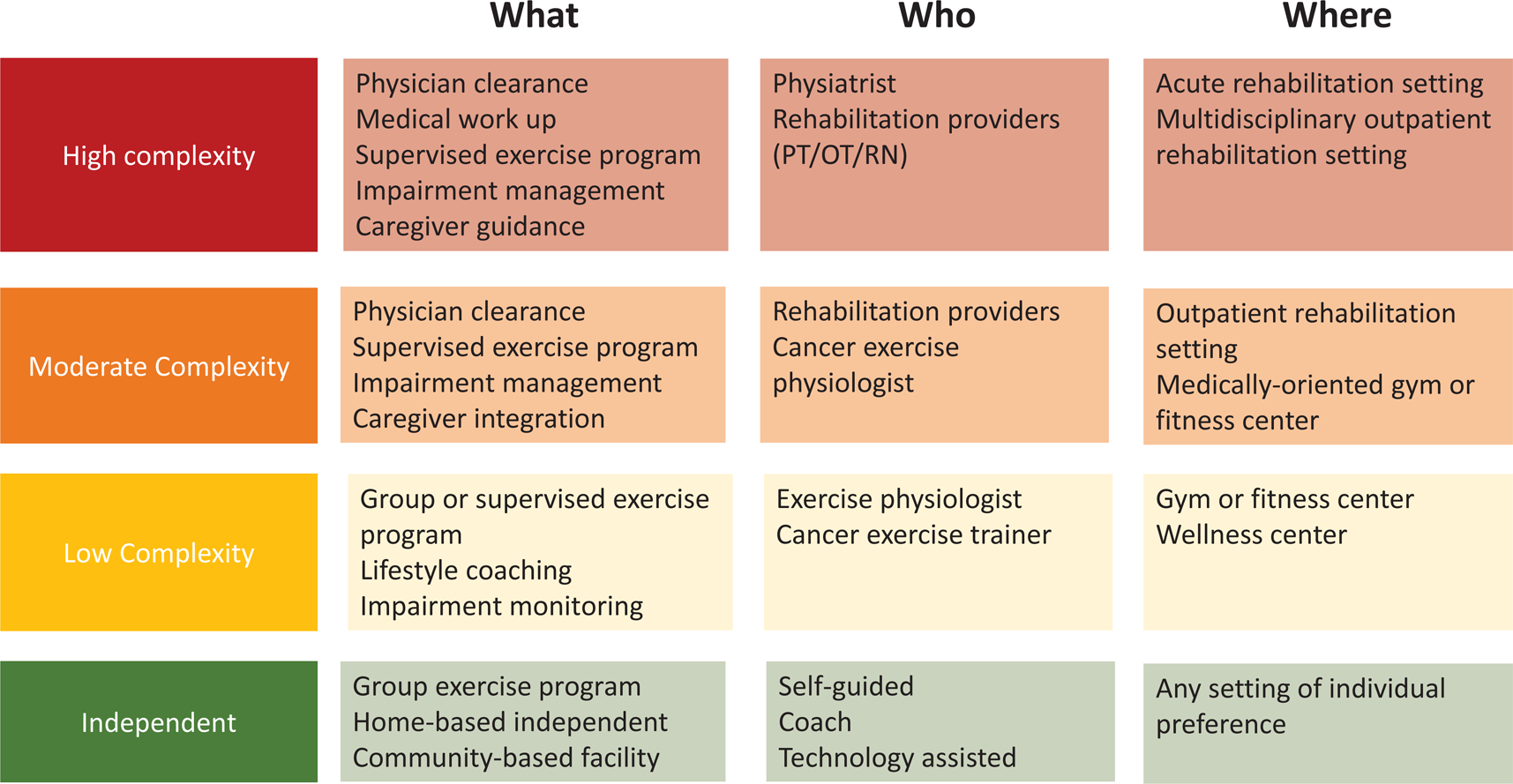
Suggested Exercise Settings and Supervision Based on Individual Condition Complexity and Risk for Decline.
Exercise Recommendations
Exercise is effective across many different disease types and positively impacts multiple body systems.20 Rehabilitative exercise that targets mobility, ADL and IADL performance, return to work, and role participation are well substantiated in the literature.62 It is beyond the scope of this paper to outline specific exercise interventions. The recently revised ACSM exercise guidelines for cancer survivors provide evidence-based recommendations for physical activity, outline preferred condition-based exercise prescriptions, and provide guidance for exercise implementation.56,57,63 Supportive care systems that include proactive exercise and rehabilitation enhance medical outcomes.64,65
Implementation
Achieving implementation of this framework requires enhancements to oncology clinical workflows, technology utilization, and professional education programs.65–68
Clinical Workflows
Survivorship care programs are evolving in clinical oncology care due to recent changes in accreditation standards.69 The survivorship care team is ideally positioned to use the screening algorithm throughout cancer care and to improve access to supportive care services including exercise. Implementation strategies to integrate the screening tool into oncology practice include leveraging patient navigation frameworks for screening and exercise referrals,70 use of patient reported outcomes measures to identify symptom changes that warrant referrals,71 and prospective supportive care services from the point of diagnosis.23,72 Co-located services with same day appointments for on-site supportive care intervention promotes earlier engagement and improves patient outcomes and satisfaction.73
Technology
Technology offers numerous opportunities for personalized exercise pathways. Electronic health records (EHR) can prompt use of the exercise screening tool and provide links to appropriate referrals based on findings. EHRs could be setup to prompt screening questions when entering vital signs and can integrate this information as a report to simplify assessment across the five domains.
Electronic assessment tools that use Item Response Theory (IRT)-based Computerized Adapted Testing (CAT) are gaining evidence base and clinical traction. These self-reported assessments are low burden and provide a precise reflection of the individual’s needs.74 IRT-based research demonstrates the ability to predict functional decline and disablement in advanced cancers, an important construct to support the prospective framework that we propose here.75,76 Precision exercise prescription can be driven by these tools’ ability to accurately characterize the individual’s level of function and promote tailored exercise recommendations.71
Telehealth and telecommunication technologies may be efficient options for delivering exercise interventions remotely.77 Studies in cardiac rehabilitation observe that physical activity increased with telehealth cardiac exercise programs.78 Mobile health (mHealth) using mobile devices, such as smart phones and wireless physiologic sensors, could deliver an exercise intervention any time and any place and allow remote monitoring of progress and physical measures, such as heart rate, enabling direct provider to patient interaction with feedback and support in near-real-time. These applications could reduce many barriers of face-to-face interactions, such as cost, transportation, access to an exercise facility, and geographic isolation.79
An ideal technology platform would offer individuals with cancer multiple, evidence-based options to meet their specific exercise needs and would adhere to their preferences with consideration for their current state of health and physical ability. A platform that provides individualized recommendations will likely enhance acceptability, care delivery, and engagement in exercise. However, technology can also negatively impact care by fragmenting services, increasing provider workloads, and contributing to burnout. Further it may frustrate patients if the interface is not user-friendly or if the output is not perceived as helpful. While it is outside of the scope of this commentary to review this literature, it is important to consider technology in the context of clinical workflows and in terms of the application’s acceptability to patients and providers.
Education
The education of oncology health care professionals must evolve regarding the current evidence and exercise guidelines if implementation is to succeed. Continuing education programs must train oncology health care professionals to know how and when to assess, and where to refer cancer survivors for exercise programs. Moreover, raising the knowledgebase of health care professionals across primary care and other disciplines is of paramount importance to assure that long-term needs are met.
Professional degree programs should incorporate cancer exercise evidence into their curriculum. Nurses, patient navigators, and community-based providers need stronger knowledge of the benefits and safety of exercise to counsel patients during and following treatment. Physicians and oncology advanced practice professionals need to be comfortable screening and referring appropriately. The algorithm herein identifies screening questions and provides prompts towards an exercise clinical pathway. Ideally, oncology professionals will discuss exercise with their patients, but this may not always be feasible and therefore the referral pathway to rehabilitation or exercise professionals may be optimal from a time management perspective.70
At present, most exercise science and rehabilitation discipline education curriculum do not have ample content in oncology and exercise prescription. There are emerging models for educational curriculum including Masters’ degree programs in Cancer Care* and oncology residency training programs in physiatry and physical therapy. Recently the Clinical Oncology Society of Australia (COSA) recommended that exercise become a standard of care in oncology across all disease states, incorporated in cancer care from the time of diagnosis.80 This has accelerated educational curriculum development and integrated exercise assessment into clinical workflows. ACSM’s Exercise is Medicine™ seeks to advance the dissemination and implementation of the cancer exercise guidelines through the Moving Through Cancer† initiative. However, significant changes are needed in exercise physiology curriculum to enhance knowledge and skills in cancer exercise among their graduates. Integrating curriculum changes, providing in-depth training opportunities and elevating awareness across disciplines are necessary steps to enhance implementation.
Integration to Practice: A Call to Action
A new standard of practice in cancer care is warranted due to the improvement in outcomes evident when exercise is integrated into cancer care from diagnosis through treatment. The value proposition of prospective personalized exercise clinical pathways in oncology is that they promote early detection of physical decline and prompt exercise interventions that mitigate or ameliorate many cancer treatment-related symptoms, reduce impairment and disability,81,82 enhance return to work and social roles,83,84 and positively influence health endpoints such as infection rates, hospitalization rates, and chemotherapy tolerability in some populations.85–87 The proposed five domains offer a framework for efficient and effective screening that enables exercise referrals best suited to an individual’s existing and evolving needs. The time is now for oncology professionals to adopt this framework and to start building the technical tools and systems to enhance healthcare professionals’ ability to engage patients around exercise and physical activity recommendations.
Footnotes
Conflict of Interest:
Catherine M. Alfano is employed by the American Cancer Society, which receives grants from private and corporate foundations, including foundations associated with companies in the health sector, for research outside the submitted work. Dr. Alfano is not funded by or key personnel for any of these grants, and her salary is solely funded through American Cancer Society funds. The other authors made no disclosures.
References
- 1.Bluethmann SM, Mariotto AB, Rowland JH. Anticipating the “Silver Tsunami”: Prevalence Trajectories and Comorbidity Burden among Older Cancer Survivors in the United States. Cancer Epidemiol Biomarkers Prev. 2016;25(7):1029–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schmitz KH, Courneya KS, Matthews C, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42(7):1409–1426. [DOI] [PubMed] [Google Scholar]
- 3.Friedenreich CM, Neilson HK, Farris MS, Courneya KS. Physical Activity and Cancer Outcomes: A Precision Medicine Approach. Clin Cancer Res. 2016;22(19):4766–4775. [DOI] [PubMed] [Google Scholar]
- 4.Chung JY, Lee DH, Park JH, et al. Patterns of physical activity participation across the cancer trajectory in colorectal cancer survivors. Support Care Cancer. 2013;21(6):1605–1612. [DOI] [PubMed] [Google Scholar]
- 5.Ferriolli E, Skipworth RJ, Hendry P, et al. Physical activity monitoring: a responsive and meaningful patient-centered outcome for surgery, chemotherapy, or radiotherapy? J Pain Symptom Manage. 2012;43(6):1025–1035. [DOI] [PubMed] [Google Scholar]
- 6.Kirkham AA, Van Patten CL, Gelmon KA, et al. Effectiveness of oncologist‐referred exercise and healthy eating programming as a part of supportive adjuvant care for early breast cancer. The oncologist. 2018;23(1):105–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pergolotti M, Deal AM, Lavery J, Reeve BB, Muss HB. The prevalence of potentially modifiable functional deficits and the subsequent use of occupational and physical therapy by older adults with cancer. Journal of geriatric oncology. 2015;6(3):194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ligibel JA, Jones LW, Brewster AM, et al. Oncologists’ Attitudes and Practice of Addressing Diet, Physical Activity, and Weight Management With Patients With Cancer: Findings of an ASCO Survey of the Oncology Workforce. Journal of oncology practice. 2019;15(6):e520–e528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones LW, Courneya KS, Mackey JR, et al. Cardiopulmonary function and age-related decline across the breast cancer survivorship continuum. J Clin Oncol. 2012;30(20):2530–2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ness KK, Wall MM, Oakes JM, Robison LL, Gurney JG. Physical performance limitations and participation restrictions among cancer survivors: a population-based study. Ann Epidemiol. 2006;16(3):197–205. [DOI] [PubMed] [Google Scholar]
- 11.Petrick JL, Foraker RE, Kucharska-Newton AM, et al. Trajectory of overall health from self-report and factors contributing to health declines among cancer survivors. Cancer Causes Control. 2014;25(9):1179–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petrick JL, Reeve BB, Kucharska-Newton AM, et al. Functional status declines among cancer survivors: trajectory and contributing factors. J Geriatr Oncol. 2014;5(4):359–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bauman AE, Reis RS, Sallis JF, Wells JC, Loos RJF, Martin BW. Correlates of physical activity: why are some people physically active and others not? The Lancet. 2012;380(9838):258–271. [DOI] [PubMed] [Google Scholar]
- 14.Giles-Corti B, Donovan RJ. The relative influence of individual, social and physical environment determinants of physical activity. Soc Sci Med. 2002;54(12):1793–1812. [DOI] [PubMed] [Google Scholar]
- 15.Thraen-Borowski KM, Gennuso KP, Cadmus-Bertram L. Accelerometer-derived physical activity and sedentary time by cancer type in the United States. PLoS One. 2017;12(8):e0182554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alfano CM, Mayer DK, Bhatia S, et al. Implementing personalized pathways for cancer follow-up care in the United States: Proceedings from an American Cancer Society-American Society of Clinical Oncology summit. CA Cancer J Clin. 2019;69(3):234–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mayer DK, Alfano CM. Personalized Risk-Stratified Cancer Follow-Up Care: Its Potential for Healthier Survivors, Happier Clinicians, and Lower Costs. J Natl Cancer Inst. 2019;111(5):442–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van der Leeden M, Huijsmans RJ, Geleijn E, et al. Tailoring exercise interventions to comorbidities and treatment-induced adverse effects in patients with early stage breast cancer undergoing chemotherapy: a framework to support clinical decisions. Disabil Rehabil. 2018;40(4):486–496. [DOI] [PubMed] [Google Scholar]
- 19.Kleckner IR, Dunne RF, Asare M, et al. Exercise for Toxicity Management in Cancer-A Narrative Review. Oncology & hematology review. 2018;14(1):28–37. [PMC free article] [PubMed] [Google Scholar]
- 20.Stout NL, Baima J, Swisher AK, Winters-Stone KM, Welsh J. A Systematic Review of Exercise Systematic Reviews in the Cancer Literature (2005–2017). PM R. 2017;9(9S2):S347–S384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Basen-Engquist K, Alfano CM, Maitin-Shepard M, et al. Agenda for Translating Physical Activity, Nutrition, and Weight Management Interventions for Cancer Survivors into Clinical and Community Practice. Obesity (Silver Spring). 2017;25 Suppl 2:S9–S22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McNeely ML, Dolgoy N, Onazi M, Suderman K. The Interdisciplinary Rehabilitation Care Team and the Role of Physical Therapy in Survivor Exercise. Clin J Oncol Nurs. 2016;20(6 Suppl):S8–S16. [DOI] [PubMed] [Google Scholar]
- 23.Stout NL, Binkley JM, Schmitz KH, et al. A prospective surveillance model for rehabilitation for women with breast cancer. Cancer. 2012;118(8 Suppl):2191–2200. [DOI] [PubMed] [Google Scholar]
- 24.Klepin HD, Mohile SG, Mihalko S. Exercise for older cancer patients: feasible and helpful? Interdiscip Top Gerontol. 2013;38:146–157. [DOI] [PubMed] [Google Scholar]
- 25.Jacobs LA, Shulman LN. Follow-up care of cancer survivors: challenges and solutions. Lancet Oncol. 2017;18(1):e19–e29. [DOI] [PubMed] [Google Scholar]
- 26.Okada M, Meeske KA, Menteer J, Freyer DR. Exercise recommendations for childhood cancer survivors exposed to cardiotoxic therapies: an institutional clinical practice initiative. J Pediatr Oncol Nurs. 2012;29(5):246–252. [DOI] [PubMed] [Google Scholar]
- 27.Berger AM, Mitchell SA, Jacobsen PB, Pirl WF. Screening, evaluation, and management of cancer-related fatigue: Ready for implementation to practice? CA Cancer J Clin. 2015;65(3):190–211. [DOI] [PubMed] [Google Scholar]
- 28.Cheville AL, Mustian K, Winters-Stone K, Zucker DS, Gamble GL, Alfano CM. Cancer Rehabilitation: An Overview of Current Need, Delivery Models, and Levels of Care. Phys Med Rehabil Clin N Am. 2017;28(1):1–17. [DOI] [PubMed] [Google Scholar]
- 29.Hayes SC, Johansson K, Alfano CM, Schmitz K. Exercise for breast cancer survivors: bridging the gap between evidence and practice. Transl Behav Med. 2011;1(4):539–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmitz KH. Exercise for secondary prevention of breast cancer: moving from evidence to changing clinical practice. Cancer Prev Res (Phila). 2011;4(4):476–480. [DOI] [PubMed] [Google Scholar]
- 31.Dalzell MA, Smirnow N, Sateren W, et al. Rehabilitation and exercise oncology program: translating research into a model of care. Curr Oncol. 2017;24(3):e191–e198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koene RJ, Prizment AE, Blaes A, Konety SH. Shared Risk Factors in Cardiovascular Disease and Cancer. Circulation. 2016;133(11):1104–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meijers WC, de Boer RA. Common risk factors for heart failure and cancer. Cardiovasc Res. 2019;115(5):844–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roy S, Vallepu S, Barrios C, Hunter K. Comparison of Comorbid Conditions Between Cancer Survivors and Age-Matched Patients Without Cancer. J Clin Med Res. 2018;10(12):911–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hwangbo Y, Kang D, Kang M, et al. Incidence of Diabetes After Cancer Development: A Korean National Cohort Study. JAMA Oncol. 2018;4(8):1099–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kenzik KM, Balentine C, Richman J, Kilgore M, Bhatia S, Williams GR. New-Onset Cardiovascular Morbidity in Older Adults With Stage I to III Colorectal Cancer. J Clin Oncol. 2018;36(6):609–616. [DOI] [PubMed] [Google Scholar]
- 37.Abdel-Qadir H, Austin PC, Lee DS, et al. A Population-Based Study of Cardiovascular Mortality Following Early-Stage Breast Cancer. JAMA Cardiol. 2017;2(1):88–93. [DOI] [PubMed] [Google Scholar]
- 38.Cheng H, Force T. Molecular mechanisms of cardiovascular toxicity of targeted cancer therapeutics. Circ Res. 2010;106(1):21–34. [DOI] [PubMed] [Google Scholar]
- 39.Thompson PD, Franklin BA, Balady GJ, et al. Exercise and acute cardiovascular events placing the risks into perspective: a scientific statement from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism and the Council on Clinical Cardiology. Circulation. 2007;115(17):2358–2368. [DOI] [PubMed] [Google Scholar]
- 40.Cheville AL, Beck LA, Petersen TL, Marks RS, Gamble GL. The detection and treatment of cancer-related functional problems in an outpatient setting. Support Care Cancer. 2009;17(1):61–67. [DOI] [PubMed] [Google Scholar]
- 41.Silver JK, Baima J, Mayer RS. Impairment-driven cancer rehabilitation: an essential component of quality care and survivorship. CA Cancer J Clin. 2013;63(5):295–317. [DOI] [PubMed] [Google Scholar]
- 42.Lieffers JR, Mourtzakis M, Hall KD, McCargar LJ, Prado CM, Baracos VE. A viscerally driven cachexia syndrome in patients with advanced colorectal cancer: contributions of organ and tumor mass to whole-body energy demands. Am J Clin Nutr. 2009;89(4):1173–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Winters-Stone KM, Horak F, Jacobs PG, et al. Falls, Functioning, and Disability Among Women With Persistent Symptoms of Chemotherapy-Induced Peripheral Neuropathy. J Clin Oncol. 2017;35(23):2604–2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rao AV, Hurria A, Kimmick G, Pinheiro S, Seo PH. Geriatric oncology: past, present, future. J Oncol Pract. 2008;4(4):190–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reid KF, Naumova EN, Carabello RJ, Phillips EM, Fielding RA. Lower extremity muscle mass predicts functional performance in mobility-limited elders. J Nutr Health Aging. 2008;12(7):493–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ness KK, Krull KR, Jones KE, et al. Physiologic frailty as a sign of accelerated aging among adult survivors of childhood cancer: a report from the St Jude Lifetime cohort study. J Clin Oncol. 2013;31(36):4496–4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Clifford BK, Mizrahi D, Sandler CX, et al. Barriers and facilitators of exercise experienced by cancer survivors: a mixed methods systematic review. Support Care Cancer. 2018;26(3):685–700. [DOI] [PubMed] [Google Scholar]
- 48.Yi JC, Syrjala KL. Anxiety and Depression in Cancer Survivors. Med Clin North Am. 2017;101(6):1099–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ormel HL, van der Schoot GGF, Sluiter WJ, Jalving M, Gietema JA, Walenkamp AME. Predictors of adherence to exercise interventions during and after cancer treatment: A systematic review. Psychooncology. 2018;27(3):713–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wong JN, McAuley E, Trinh L. Physical activity programming and counseling preferences among cancer survivors: a systematic review. International Journal of Behavioral Nutrition and Physical Activity. 2018;15(1):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gomez SL, Shariff‐Marco S, DeRouen M, et al. The impact of neighborhood social and built environment factors across the cancer continuum: current research, methodological considerations, and future directions. Cancer. 2015;121(14):2314–2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Paskett E, Thompson B, Ammerman AS, Ortega AN, Marsteller J, Richardson D. Multilevel interventions to address health disparities show promise in improving population health. Health Affairs. 2016;35(8):1429–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kirk MA, Rhodes RE. Occupation correlates of adults’ participation in leisure-time physical activity: a systematic review. Am J Prev Med. 2011;40(4):476–485. [DOI] [PubMed] [Google Scholar]
- 54.Zafar SY. Financial Toxicity of Cancer Care: It’s Time to Intervene. J Natl Cancer Inst. 2016;108(5). [DOI] [PubMed] [Google Scholar]
- 55.Dean LT, Schmitz KH, Frick KD, et al. Consumer credit as a novel marker for economic burden and health after cancer in a diverse population of breast cancer survivors in the USA. J Cancer Surviv. 2018;12(3):306–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Campbell KL, Winters-Stone KM, Wiskemann J, et al. Exercise guidelines for cancer survivors: consensus statement from International Multidisciplinary Roundtable. Medicine & Science in Sports & Exercise. 2019;51(11):2375–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schmitz KH, Campbell AM, Stuiver MM, et al. Exercise is medicine in oncology: engaging clinicians to help patients move through cancer. CA: A Cancer Journal for Clinicians. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Segal R, Zwaal C, Green E, et al. Exercise for people with cancer: a clinical practice guideline. Curr Oncol. 2017;24(1):40–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sanft T, Denlinger CS, Armenian S, et al. NCCN Guidelines Insights: Survivorship, Version 2.2019: Featured Updates to the NCCN Guidelines. Journal of the National Comprehensive Cancer Network. 2019;17(7):784–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Swarm RA, Paice JA, Anghelescu DL, et al. Adult Cancer Pain, Version 3.2019, NCCN Clinical Practice Guidelines in Oncology. Journal of the National Comprehensive Cancer Network. 2019;17(8):977–1007. [DOI] [PubMed] [Google Scholar]
- 61.Riba MB, Donovan KA, Andersen B, et al. Distress Management, Version 3.2019, NCCN Clinical Practice Guidelines in Oncology. Journal of the National Comprehensive Cancer Network. 2019;17(10):1229–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pergolotti M, Deal AM, Williams GR, et al. Older Adults with Cancer: A Randomized Controlled Trial of Occupational and Physical Therapy. J Am Geriatr Soc. 2019;67(5):953–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Patel AV, Friedenreich CM, Moore SC, et al. American College of Sports Medicine Roundtable report on physical activity, sedentary behavior, and cancer prevention and control. Medicine & Science in Sports & Exercise. 2019;51(11):2391–2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wisotzky E, Khanna A, Hanrahan N, Maltser S. Scope of practice in cancer rehabilitation. Current physical medicine and rehabilitation reports. 2017;5(1):55–63. [Google Scholar]
- 65.Demark-Wahnefried W, Rogers LQ, Alfano CM, et al. Practical clinical interventions for diet, physical activity, and weight control in cancer survivors. CA Cancer J Clin. 2015;65(3):167–189. [DOI] [PubMed] [Google Scholar]
- 66.Kirkham AA, Klika RJ, Ballard T, Downey P, Campbell KL. Effective Translation of Research to Practice: Hospital-Based Rehabilitation Program Improves Health-Related Physical Fitness and Quality of Life of Cancer Survivors. J Natl Compr Canc Netw. 2016;14(12):1555–1562. [DOI] [PubMed] [Google Scholar]
- 67.Demark-Wahnefried W, Morey MC, Sloane R, et al. Reach out to enhance wellness home-based diet-exercise intervention promotes reproducible and sustainable long-term improvements in health behaviors, body weight, and physical functioning in older, overweight/obese cancer survivors. J Clin Oncol. 2012;30(19):2354–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stout NL, Silver JK, Alfano CM, Ness KK, Gilchrist LS. Long-Term Survivorship Care After Cancer Treatment: A New Emphasis on the Role of Rehabilitation Services. Phys Ther. 2019;99(1):10–13. [DOI] [PubMed] [Google Scholar]
- 69.Cancer ACoSCo. Cancer Program Standards 2020: Optimal Resources for Cancer Care. Chicago, IL: 2020. [Google Scholar]
- 70.Stout NL, Sleight A, Pfeiffer D, Galantino ML, deSouza B. Promoting assessment and management of function through navigation: opportunities to bridge oncology and rehabilitation systems of care. Support Care Cancer. 2019;27(12):4497–4505. [DOI] [PubMed] [Google Scholar]
- 71.Ulrich CM, Himbert C, Boucher K, et al. Precision-Exercise-Prescription in patients with lung cancer undergoing surgery: rationale and design of the PEP study trial. BMJ Open. 2018;8(12):e024672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wong A, Vidal M, Prado B, et al. Patients Perspective of Timeliness and Usefulness of an Outpatient Supportive Care Referral at a Comprehensive Cancer Center. Journal of pain and symptom management. 2019. [DOI] [PubMed] [Google Scholar]
- 73.Kline RM, Brown M, Buescher N, et al. The Centers for Medicare & Medicaid Services Oncology Care Model Halfway Through–Perspectives from Diverse Participants. JNCI: Journal of the National Cancer Institute. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cheville AL, Yost KJ, Larson DR, et al. Performance of an item response theory-based computer adaptive test in identifying functional decline. Arch Phys Med Rehabil. 2012;93(7):1153–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cheville AL, Basford JR, Parney I, Yang P, Diehn FE. Nested cohort study to identify characteristics that predict near-term disablement from lung cancer brain metastases. Archives of physical medicine and rehabilitation. 2017;98(2):303–311. e301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cheville AL, Murthy NS, Basford JR, et al. Imaging and Clinical Characteristics Predict Near-Term Disablement From Bone Metastases: Implications for Rehabilitation. Arch Phys Med Rehabil. 2016;97(1):53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cheville AL, Moynihan T, Herrin J, Loprinzi C, Kroenke K. Effect of collaborative telerehabilitation on functional impairment and pain among patients with advanced-stage cancer: a randomized clinical trial. JAMA oncology. 2019;5(5):644–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rawstorn JC, Gant N, Direito A, Beckmann C, Maddison R. Telehealth exercise-based cardiac rehabilitation: a systematic review and meta-analysis. Heart. 2016;102(15):1183–1192. [DOI] [PubMed] [Google Scholar]
- 79.The Smartphone Difference Washington, DC: 2015. [Google Scholar]
- 80.Cormie P, Atkinson M, Bucci L, et al. Clinical Oncology Society of Australia position statement on exercise in cancer care. Medical Journal of Australia. 2018;209(4):184–187. [DOI] [PubMed] [Google Scholar]
- 81.Rafn BS, Hung S, Hoens AM, et al. Prospective surveillance and targeted physiotherapy for arm morbidity after breast cancer surgery: a pilot randomized controlled trial. Clinical rehabilitation. 2018;32(6):811–826. [DOI] [PubMed] [Google Scholar]
- 82.Kilgore LJ, Korentager SS, Hangge AN, et al. Reducing breast cancer-related lymphedema (BCRL) through prospective surveillance monitoring using bioimpedance spectroscopy (BIS) and patient directed self-interventions. Annals of surgical oncology. 2018;25(10):2948–2952. [DOI] [PubMed] [Google Scholar]
- 83.Gerber LH, Stout N, McGarvey C, et al. Factors predicting clinically significant fatigue in women following treatment for primary breast cancer. Supportive Care in Cancer. 2011;19(10):1581–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Levy EW, Pfalzer LA, Danoff J, et al. Predictors of functional shoulder recovery at 1 and 12 months after breast cancer surgery. Breast cancer research and treatment. 2012;134(1):315–324. [DOI] [PubMed] [Google Scholar]
- 85.Hijazi Y, Gondal U, Aziz O. A systematic review of prehabilitation programs in abdominal cancer surgery. International journal of surgery. 2017;39:156–162. [DOI] [PubMed] [Google Scholar]
- 86.Minnella EM, Bousquet-Dion G, Awasthi R, Scheede-Bergdahl C, Carli F. Multimodal prehabilitation improves functional capacity before and after colorectal surgery for cancer: a five-year research experience. Acta oncologica. 2017;56(2):295–300. [DOI] [PubMed] [Google Scholar]
- 87.Sebio Garcia R, Yanez Brage MI, Gimenez Moolhuyzen E, Granger CL, Denehy L. Functional and postoperative outcomes after preoperative exercise training in patients with lung cancer: a systematic review and meta-analysis. Interact Cardiovasc Thorac Surg. 2016;23(3):486–497. [DOI] [PubMed] [Google Scholar]


