Abstract
Deubiquitinases are enzymes that remove ubiquitin moieties from the vast majority of cellular proteins, controlling their stability, interactions, and localization. The expression and activity of deubiquitinases are critical for physiology and can go awry in various diseases, including cancer. Based on recent findings in human blood cancers, we discuss the functions of selected deubiquitinases in acute leukemia and efforts to target these enzymes with the aim of blocking leukemia growth and improving disease outcomes. We focus on the emergence of the newest generation of preclinical inhibitors by discussing their modes of inhibition and their effects on leukemia biology.
Defective Deubiquitination and Its Role in Blood Cancers
Ubiquitination (the addition of ubiquitin moieties) is a post-translational modification linked to biological processes such as protein degradation, signal transduction, protein–protein interactions, cellular localization, and others (Boxes 1 and 2). Ubiquitin moieties are removed via the activity of deubiquitinating enzymes [deubiquitinases (DUBs) (see Glossary)], and recent work has indicated that DUBs participate in various pathways related to leukemogenesis and disease maintenance in mice and humans, especially those linked to cellular energetics, sustained proliferation, and genome instability (Figure 1) [1–7]. Moreover, the dependency of these hematological malignancies on certain DUBs renders these enzymes excellent candidates for putative targeted therapy. For instance, inhibition of the proteasome-related DUBs ubiquitin-specific protease (USP) 14 and ubiquitin C-terminal hydrolase (UCH) L5 (UCHL5) using the small inhibitors b-AP15 and VLX1570 has decreased tumor growth and extended the survival of mice using in vivo xenograft models of acute myeloid leukemia (AML) and multiple myeloma (MM) [8,9]. Given the efficiency shown by these USP14/UCHL5 inhibitors, VLX1570 in combination with dexamethasone previously progressed into a Phase I/II clinical trial that was ultimately terminated due to toxicity (NCT02372240; see later)i.
Box 1. Human DUB Families and Functions.
Ubiquitin is a 76-amino-acid, 8-kDa protein that can be added to other proteins via an isopeptide bond formed between the C-terminal glycine on ubiquitin and, most commonly, a lysine on the target protein [118–120]. A single ubiquitin moiety (monoubiquitination), many moieties of single ubiquitin molecules (multimonoubiquitination), or multiple ubiquitin moieties (polyubiquitination) can be added to a single protein. In polyubiquitination, ubiquitin is added to either the first methionine of the first ubiquitin moiety or to different lysines - K6, K11, K27, K29, K33, K48, K63 - on ubiquitin [121]. Usually, K48 and K11 ubiquitin branches are responsible for protein degradation through the proteasome, whereas other polyubiquitin and monoubiquitin marks can control signaling, protein–protein interactions, cellular localization, or activity [121–123].
In contrast to the N600 E3 ligase enzymes that add ubiquitin, there are only ~100 DUBs, clustered in seven families: the USP family, the UCH family, the OTU family, the Machado–Joseph disease protease (MJD) (or Josephins) family, the motif interacting with ubiquitin-containing novel DUB (MINDY) family, the zinc finger-containing ubiquitin peptidase (ZUP1) family, and the JAB1/MPN/Mov34 metalloenzyme (JAMM) family. The first six families belong to the cysteine protease family, while JAMM proteins belong to the zinc-dependent metalloproteinase family (Box 2). There are approximately 58 USPs, 4 UCHs, 14 OTUs, 5 MJDs, 5 MINDYs, 1 ZUP1, and 14 JAMMs.
Deregulation of DUB activity can lead to aberrant ubiquitination signaling. Since the addition of ubiquitin regulates various cellular processes, including proteasomal degradation, transcription, and DNA repair, its deregulation can lead to cancer [124]. Modifications in the number, linkage nature, or branching of the ubiquitin moieties on a protein can lead to drastic changes in protein localization, interactions, activity, and abundance [117].
Box 2. DUB Chemistry and Inhibitors.
The cysteine protease family of DUBs, which contains six of the seven DUB families, relies on three crucial amino acid residues in the catalytic pocket [125]. The most critical amino acid is the cysteine in the active site. The thiol group on this cysteine performs a nucleophilic attack on the isopeptide bond between the amino group on the lysine of the substrate (or ubiquitin itself) and the carboxyl group on glycine 76 of ubiquitin. Deprotonation of this catalytic cysteine is assisted by an adjacent histidine, which, in turn, is polarized by an aspartate residue. Based on the conformational changes seen in crystal structures, it was postulated that ubiquitin binding triggers the activation of the enzyme; USP7 activation involves a conformational change that enables the interaction between histidine (H) 464, aspartate (D) 481, and the catalytic cysteine (C) 223 [126]. In the absence of ubiquitin, various USPs display an apo or inactive conformation, but when they are bound to the ubiquitin C terminus their holo or active conformations can be superimposed. For example, USP7 [127] and USP14 [128] rest in catalytically inactive states or apo conformations in the absence of substrate or cofactor binding and become active only after binding to ubiquitin. Structural knowledge of apo and active conformations as well as the protein sequences and structures surrounding the catalytic pockets of DUBs has facilitated the development of increasingly specific inhibitors.
Until recently, the lack of solved crystal structures hampered the development of specific inhibitors. The structural characteristics of some DUBs have been recently studied, providing mechanistic insights into their catalytic mechanisms and modes of action. Previous efforts were mainly focused on the development of covalent inhibitors acting at the catalytic center, but it has recently become possible to develop allosteric inhibitors with potency and selectivity similar to those of covalent inhibitors [127].
Figure 1. Aspects of Leukemia Biology Influenced by Deubiquitinases (DUBs).
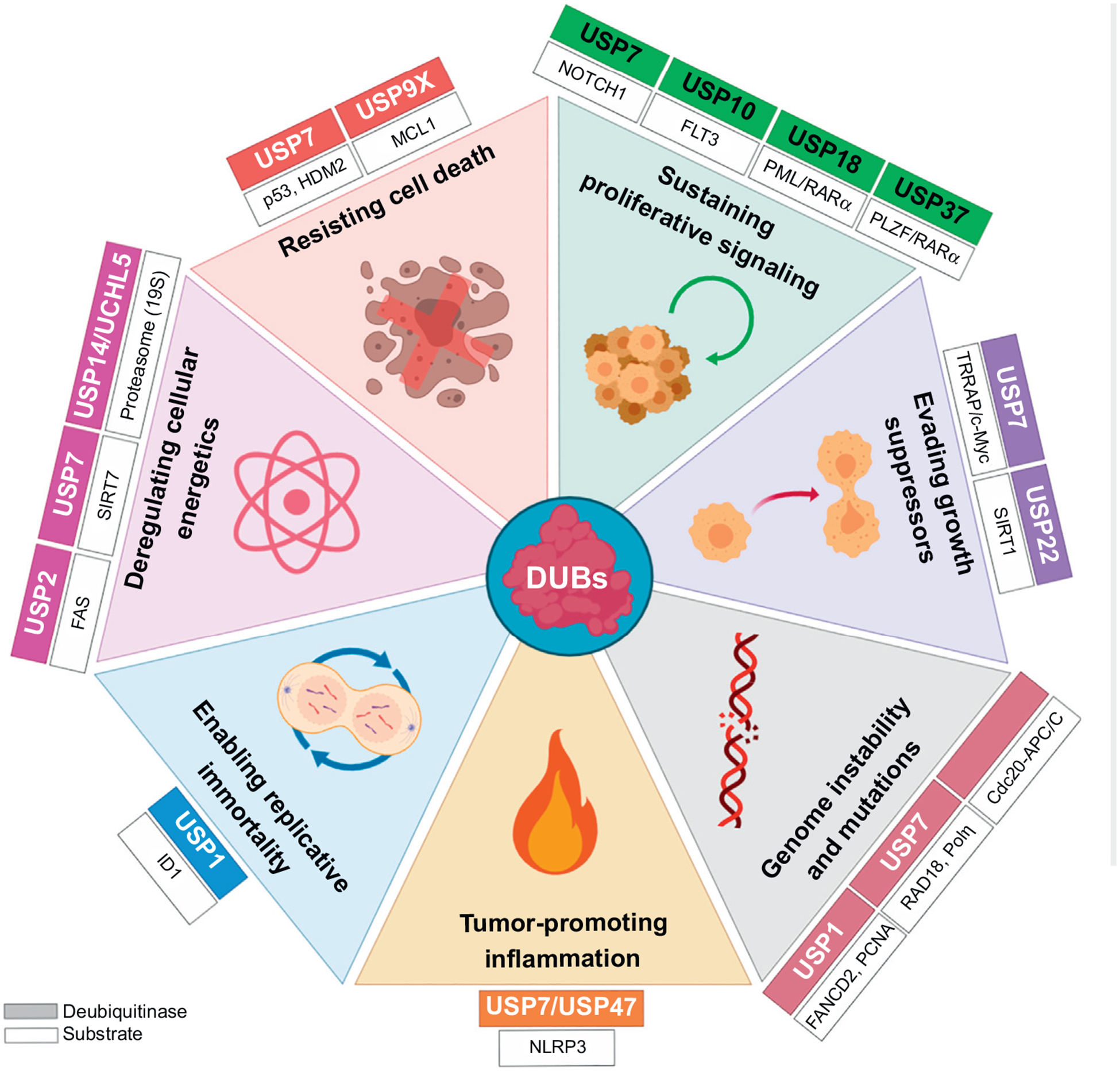
The different DUBs can maintain an oncogenic phenotype in leukemia through the stabilization, activation, and relocalization of their targets. Abbreviations: USP, ubiquitin-specific protease; RARα, retinoic acid receptor alpha; PLZF, promyelocytic leukemia zinc finger; TRRAP, transformation/transcription domain associated protein; SIRT, sirtuin; ID1, inhibitor of DNA binding 1.
Consistent with this trend, the use of USP7 and USP1 inhibitors has also shown promising results in the treatment of MM, AML, acute lymphoblastic leukemia (ALL), and other hematological malignancies in various in vitro and in vivo preclinical models [10–14]. DUBs can control and influence chromatin function and gene expression, which is relevant as hematological malignancies are considered epigenetic diseases [12,15–21]. For example, the regulation of histone ubiquitination has been linked to myeloid leukemogenesis in murine c-Kit+ cells expressing BRCA1 associated protein-1 (BAP1) DUB as well as to a mutant form of the chromatin remodeling protein additional sex combs like 1 (ASXL1), where H2AK119Ub removal induces the upregulation of genes related to myeloid progenitors [22]. In this work, researchers demonstrated that CRISPR– Cas9 depletion of BAP1 impaired leukemogenesis, as shown by decreased colony-forming activity and the extended survival of mice using RUNX1-ETO and MLL-AF9 models [22]. USP7, another DUB, has been deemed to regulate the maintenance and inheritance of DNA methylation via physical association with the DNA methyl transferase 1 (DNMT1) and ubiquitin-like containing PHD and RING finger domains (UHRF) proteins [23–25]; these findings have suggested the existence of a tripartite USP7-DNMT1-UHRF1 protein complex that can repress genes through increased DNA methylation in a human hepatoblastoma cell line [26] (other examples of DUBs that have been associated with leukemogenesis are further discussed in Box 3).
Box 3. Other DUBs in Leukemogenesis.
DUBs can also drive leukemogenesis and alter gene expression through the stabilization or regulation of key transcription factors. For instance, USP37 can stabilize the transcription factor promyelocytic leukemia zinc finger/retinoic acid receptor alpha (PLZF/RARA) in acute promyelocytic leukemia (APL) and sustain continuous gene transcription of its targets [129]. Similarly, USP7 binds and increases the activity of NOTCH1, a key transcription factor, and its cofactor histone demethylase JMJD3 to promote T cell ALL (T-ALL) [12,13].
Mechanisms of resistance in acute disease remain poorly characterized; however, some studies have shown evidence that DUBs can ultimately affect treatment responses. Examples of this evidence include USP9X’s function in the control of glucocorticoid resistance in B cell ALL (B-ALL) [2] and USP7 activity in bortezomib resistance in MM [11].
Many other DUBs have targeting potential, although to date there are no available inhibitors. UBP43, also known as USP18, plays an important role in hematopoietic cell differentiation [130] and knockdown of this DUB reduces cell growth and induces apoptosis in APL [131], possibly through destabilization of the transcription factor PML/RARα [4]. BAP1, together with a mutant form of ASXL1, can accelerate leukemogenesis [22]. USP37, USP42, and USP44 are other DUBs with possible protumor roles in APL, AML, and T-ALL, respectively [7,129,132].
Thus, there is collective evidence that DUB inhibitors might serve as new therapeutic tools to treat certain hematological malignancies, particularly when considering the combinatorial use of DUB inhibitors with systemic or targeted therapies. Given such recent advances, in this review we discuss such putative therapeutic approaches, focusing on five DUBs (USP1, USP2, USP7, USP14, and UCHL5). These DUB enzymes harbor pro-oncogenic functions in human hematological diseases and have been the subject of intense targeting efforts with small-molecule inhibitors.
USP14 and UCHL5: Roles in Proteasome Function and Associated Targeting Efforts
We discuss ubiquitin-specific peptidase 14 (USP14) and UCHL5 together, due to their role in the 19S regulatory subunit of the proteasome. These enzymes are believed to perform a quality control function in a variety of human cells, ensuring that substrates with small or nondegradable ubiquitin chains are not processed by the proteasome [27]. These two DUBs are thought to trim ubiquitin from the distal end of the ubiquitin chains (exo-DUB activity) [27]. Moreover, in vitro degradation assays using cyclin B1, a canonical substrate of USP14, recently demonstrated that this DUB harbors limited ability to deubiquitinate single chains regardless of their linkage type; instead, it can remove many ubiquitin moieties en bloc [28]. The importance of abnormal proteasome function in hematological malignancies became evident when proteasome inhibitors such as bortezomib, carfilzomib, and ixazomib were shown to be successful in MM at clinical stages, eliciting a deep initial response and improved outcome in MM patients [29]. Since USP14 and UCHL5 belong to the proteasomal complex, these DUBs were also suspected to play a role in tumor progression. Later, inhibition of these DUBs using small-molecule inhibitors such as b-AP15 and auranofin was found to decrease tumor cell invasion and lead to regression of tumor growth in xenograft murine models of AML [8,30]. Both proteins are highly expressed in MM patients compared with normal plasma cells, and inhibition of their activity using b-AP15 or gene knockdown decreases the viability and proliferation of MM cell lines and patient cells [31]. Administration of this inhibitor also impeded tumor growth in vivo, prolonged survival in MM xenograft models, resensitized cells to bortezomib, and synergized with first-line chemotherapy drugs used in MM (lenalidomide, dexamethasone) [31]. Inhibition of USP14 has also been shown to be detrimental in T-ALL cell lines growth [32].
Given the success of proteasome inhibitors in MM treatment, there is great interest in the development of small-molecule inhibitors that can target UCHL5 and USP14. The first molecule to be described as an inhibitor of these two enzymes was b-AP15 (Table 1, Key Table). This drug, initially thought to target the proteasome, inhibited USP14 and UCHL5 instead. Binding of this compound was observed to be reversible, its IC50 was in the low-micromolar range, and in in vitro treatments it was more selective for USP14 and UCHL5 than for other DUBs of the USP and UCH families [8]. However, newer evidence for VLX1570, a next-generation analog with a chemical structure similar to that of b-AP15, showed that this inhibitor has an electrophilic nature and can form a covalent bond with the cysteine at the catalytic centers of USP15 and UCHL5 [33]. Although unconfirmed, this finding suggests that b-AP15 might presumably also form a covalent bond with those enzymes. The inhibitor b-AP15 has been used in vivo and was shown to be effective in decreasing growth in MM and AML mouse xenograft models [8,31]. VLX1570 exhibits improvements in potency and aqueous solubility and is thus suitable for intravenous injection (Table 1); like its predecessor, VLX1570 has extended mouse survival in vivo in MM and B lymphoma xenograft models [9,34]. These compounds also exhibit low-micromolar IC50 in cellular assays, but in biochemical assays with the isolated 19S proteasome their IC50 can be tenfold higher than in cellular assays [33]. The more potent activity of the compound in the cellular assays has been attributed to rapid drug uptake - resulting in drug enrichment within the cells - as opposed to off-target effects [35]. Profiling of 10-μM VLX1570 against 211 kinases and 41 DUBs showed a minimum level of inhibition activity towards those enzymes, with the exception of cyclin-dependent kinase 4 (CDK4) [33]. However, these results are difficult to interpret because USP14 and UCHL5 were not inhibited under those screening conditions [33]. In 2014, the US FDA approved the use of VLX1570 in clinical Phase I/II to determine the safety and efficacy of this drug co-administered with low-dose dexamethasone for the treatment of relapsed and refractory MM, marking the first progression of a compound to the clinic on the basis of its DUB-inhibitory activity (NCT02372240)i. This open-label single-group assignment study intended to measure the maximum tolerated dose of VLX1570 and the clinical benefit rate (minimal response or better) in 15 MM patients. However, the study was terminated and put on clinical hold due to dose-limiting toxicity. Auranofin, an FDA-approved drug for patients with chronic lymphocytic leukemia (CLL), was also shown to target UCHL5/USP14 [36]. This drug induced cytotoxicity in AML patient samples and inhibited tumor growth in an in vivo AML mouse model [30]. In addition, auranofin induced apoptosis in chronic myeloid leukemia (CML) cells resistant to imatinib, presumably via a proteasome-dependent process [37]. However, its enhanced specificity over UCHL5/USP14 is questionable because, although this compound has some inhibitory selectivity for DUB activity in the proteasome, it also interferes with the activity of other cytoplasmic DUBs [30].
Key Table Table 1.
Selective DUB Inhibitors in Preclinical Testing
| Target | Compound (structure) |
Mechanism of inhibition | IC50 | Specificity (activity inhibition) | Refs |
|---|---|---|---|---|---|
| USP1 | ML323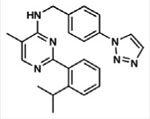
|
Noncompetitive binding, noncovalent | 76 nM | No activity against 18 DUBs, 70 proteases, and 451 kinases | [92] |
| USP2 | ML364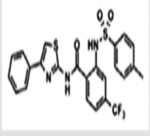
|
Noncovalent | 1.1 pM | No activity against 102 kinases, 4 proteases, and USP20 Significant activity against USP8 |
[107] |
| USP7 | P5091 P22077 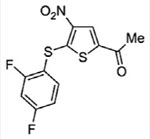 P50429 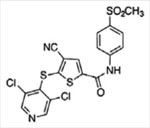
|
Unknown Competitive, covalent Competitive, covalent |
4.2 pM 8 pM 0.42 pM | No activity against 9–49 DUBs and 5 proteases Significant activity against USP47 | [11,71,127] |
| USP7 | P217564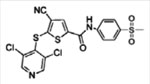
|
Competitive, covalent | 0.48 pM | No activity against 4 DUBs and 4 proteases | [76] |
| USP7 | GNE-6640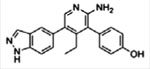 GNE-6776 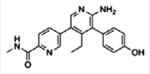
|
Competitive binding, noncovalent | 0.75 pM 1.34 pM | No activity against 44–47 DUBs | [14] |
| USP7 | C4
|
Noncompetitive binding, reversible | 6 nM | No activity against 44 DUBs, 63 proteases, and 49 kinases | [77] |
| USP7 | XL188 |
Noncovalent | 90–193 nM | No activity against 41 DUBs | [79] |
| USP7 | FT671 FT827 
|
Noncovalent Covalent |
0.1–2 pM | No activity against 38 DUBs | [78] |
| USP14/UCHL5 | b-AP15
|
Reversible | 2.1 pM | No activity against 6 DUBs | [8] |
In addition to its effect on cancer, USP14 inhibition by drugs of the IU2 family can reduce viral yield in HEK293T and HeLa cells infected with flavivirus and enhance the clearance of aggregation-prone brain proteins in MEF cells overexpressing Tau protein [38,39]. For these reasons, some research groups have focused on developing selective USP14 inhibitors. One group identified compound IU1 from a high-throughput screen of 63 000 compounds: IU1 had an IC50 of 4–5 μM and was selective over six other DUBs, including UCHL5, as evidenced by an in vitro Ub-AMC essay measuring the hydrolysis of Ub linkages in the presence or absence of the inhibitor [40]. A close analog, IU1–47, was ten times more potent than IU1 in the Ub-AMC assay, while retaining selectivity (Table 1) [41]. Later, these two compounds were shown to bind to a steric binding site that is unique among DUBs and essential for USP14 activity [42].
USP7 Is Included in Multiple Protein Complexes and Plays Context-Specific Roles in Blood Cancers
USP7 is one of the best-studied disease-associated DUBs, and several groups have reviewed USP7’s roles in physiological and pathological contexts [43,44]. This enzyme can cleave all polyubiquitin linkages (K6, K11, K27, K29, K33, K48, K63) but the linear linkage [45]. Moreover, this enzyme is capable of removing monoubiquitin, a post-translational mark usually associated with signaling [46]. Although USP7 is associated with the HDM2–p53 axis in epithelial and fibroblast human cells [47–52] (Figure 2A), this DUB has numerous substrates in addition to mediators of programmed cell death [43]. USP7 can regulate the balance between DNA damage tolerance and mutagenesis by controlling the localization and stability of Rad18, Pol η, and PCNA in many human epithelial and fibroblast cell lines [53–55]. USP7 is also an important epigenetic regulator, as it can remove monoubiquitin from histone H2B and is a component of the noncanonical Polycomb repressive complex 1 (PRC1), which deubiquitinates histone H2A [56–59]. Moreover, USP7 plays a role in maintenance methylation by stabilizing UHRF1 and controlling DNMT1 binding to DNA targets in vertebrates [23,24,60]. Other USP7-related functions in cancer include control of glycolysis by inhibition of sirtuin 7 (SIRT7) activity and decreased glucose 6-phosphatase transcription in HCT116 cells and increased c-Myc transcription through transformation/transcription domain associated protein (TRRAP) in HEK293 cells and by enhanced inflammasome activation and proinflammatory cytokine production (IL-1β and IL-18) in human macrophages [61–63].
Figure 2. Molecular Functions of Ubiquitin-Specific Protease 7 (USP7) and Inhibition by Small-Molecule Compounds.
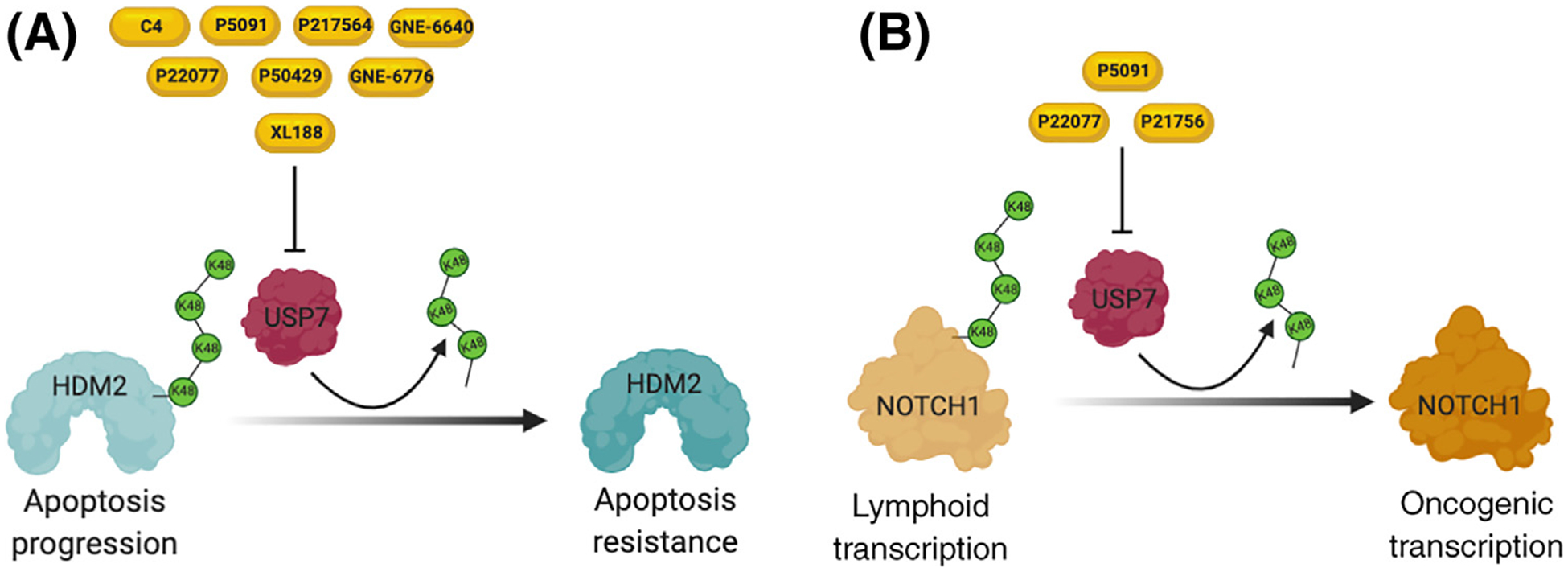
(A) Under stress conditions, USP7 can bind and stabilize HDM2 through the removal of lysine 48 (K48) ubiquitin chains, leading to apoptosis resistance. (B) High USP7 expression increases NOTCH1 activity, resulting in hyperactivation of leukemia transcriptional programs.
In the hematopoietic system, USP7 expression is generally associated with aberrant proliferation and a leukemic phenotype [11–13,64,65]. High USP7 protein expression has been reported to be present in MM patients compared with normal peripheral blood mononuclear cells and, in turn, this high expression is associated with decreased survival [11]. Administration of the USP7 inhibitor P5091 blocked tumor growth, prolonged survival, and inhibited angiogenesis in human-mouse xenograft models of MM [11]. Moreover, P5091 reversed resistance to bortezomib and synergized with the activity of lenalidomide, glucocorticoids (dexamethasone), and the histone deacetylase inhibitor vorinostat, as evidenced by increased apoptosis using MTT assays relative to controls [11]. Two independent studies showed that, in T-ALL cells, USP7 was highly expressed relative to mononuclear cells and other blood cancers and controlled the stability and transcription of NOTCH1, an important driver of T-ALL oncogenesis (Figure 2B) [12,13]. Both groups also found that inhibition of this USP by small-molecule drugs like P217564, P22077, and P5091 or by gene silencing using small hairpin RNAs compromised the growth of T-ALL cell lines and increased animal survival in human-mouse xenografts relative to controls [12,13]. Although these findings indicate that USP7 can be a driver of leukemogenesis, patient data also indicate that USP7 can be mutated in 6–13% of T-ALL cases that also present with high expression of TAL1, a T cell-specific transcription factor crucial for T cell leukemogenesis [66–69]. This finding indicates that the role of USP7 might be context specific, and thus additional mechanistic studies are needed to integrate these observations. Context specificity may be in part responsible for potentially paradoxical roles of DUBs as tumor suppressors or oncogenes, depending on the leukemia subtype or stage (diagnosis vs relapse). For instance, there could be critical transcriptional programs or specific protein-protein networks necessary for leukemia initiation that rely on a specific DUB to work properly. However, it is also possible that those programs, necessary for disease initiation, are detrimental if expressed in later stages of disease and thus the activity of the same DUB is then detrimental for leukemia development. Similar to USP7, these types of behaviors have been shown for other proteins, such as EZH2, which can act as a tumor suppressor during AML initiation and function as an oncogene at later stages of the disease [70]. In addition to its effect on MM and T-ALL, in vitro luminescent cell-viability assays have shown that the GNE-6640 and GNE-6776 USP7 inhibitors decrease the viability of AML cell lines relative to controls [14].
Over the past 2 years, there has been significant development of novel USP7 inhibitors. Systematic efforts initially led to specific USP7 inhibitors (Table 1). P5091 and its analogs P22077 and P50429 belong to first-generation inhibitors [11,71]. These compounds have an IC50 in the low-micromolar range and do not interfere with the activity of a large number of DUBs; however, they can inhibit USP47 [72], an enzyme in the USP family with close domain and structural resemblance to USP7 [73]. Two of these compounds have been utilized in vivo: P5091 inhibited tumor growth and prolonged survival in a human MM xenograft model [11], while P22077 was used and exhibited significant growth inhibitory effects by inducing p53-dependent cell death in a orthotopic neuroblastoma xenograft model [74]. Although the inhibitory mechanism of these two compounds is not completely characterized, it is known that P22077 can interact with the catalytic domain of USP7, suggesting that both compounds can destabilize the catalytic core [75]. Twenty-one thiazole derivatives were produced using P22077 as the lead compound. At least one of these derivatives, compound 7, bound to the catalytic domain of USP7 and inhibited the enzyme with an IC50 similar to that of P22077; however, the selectivity of these derivatives was not discussed [75]. P217564 belongs to the second generation of USP7 inhibitors developed by the same company [76]. Its mechanisms of action involve the formation of a covalent adduct in the active site of USP7 [76]. This compound is more potent (with a tenfold-lower IC50 value) and selective than its precursors; however, P217564 also targets USP47 activity. Mass spectrometry-based activity assays for GNE-6640 and GNE-6776 (Table 1) demonstrated that these drugs inhibited USP7 at low-micromolar concentrations, showing negligible inhibition across a panel of 36 DUBs including USP47 [14]. These inhibitors bind to a pocket near the catalytic domain and block USP7 activity by weakening the interactions at the catalytic domain, thus inhibiting ubiquitin binding and generally preventing the transition of the enzyme to the active conformation [14]. Although the plasma concentration (bioavailability) in mice administrated with GNE-6776 proved to be suitable for oral administration and produced a modest delay in tumor growth in an in vivo lymphoid xenograft model, the authors concluded that it was necessary to develop inhibitors with improved drug-like properties [14]. Two highly potent and selective USP7 inhibitors, compound 2 (C2) and compound 4 (C4) (Table 1), present an IC50 in the nanomolar range and are selective, as they have inhibited the activity of USP7 only, among a panel of 39 other DUBs [77]. Mechanistically, these two drugs bind near the catalytic domain in a noncompetitive manner, disrupting the catalytic triad [77]. Noncovalent FT671 and covalent FT827 inhibitors have also been developed (Table 1). both molecules are selective for USP7 (against 38 other DUBs) and bind near the catalytic domain of USP7, exhibiting an IC50 in the range 0.1–2 μM [78]. Studies show that FT671 can stabilize p53 and decreased the amounts of USP7 target proteins such as MDM2, UHRF, and DNMT1 relative to the untreated cells [78]. FT671 was used in an in vivo MM xenograft mouse model and shown to increase the amounts of p53 in tumors in a dose-dependent manner, as evidenced by western blotting [78]. One group developed XL188 (Table 1), a compound that binds a pocket near the USP7 catalytic domain, has an IC50 in the low-nanomolar range, and is selective across a panel of 41 DUBs [79]. These researchers used the crystal structure of USP7 bound to a previously patented compound [80] as a guide to develop XL188. As previously shown for other inhibitors, XL188 reduced HDM2 and increased p53 and p21 protein amounts in MCF7 cells [79]. This compound has the longest half-life among USP7 inhibitors (31 min in mouse liver microsomes); however, the use of this drug in in vivo models has not yet been described [79].
USP1’s Roles in DNA Damage Response Pathways and Its Therapeutic Implications in Blood Cancers
USP1 is a DUB capable of degrading a broad range of polyubiquitin chains, including K48, K63, and K11 linkages [45]. It is an attractive candidate for targeted therapy because it participates in multiple DNA repair programs, including the Fanconi anemia pathway [81] and translesion DNA synthesis [82]. Moreover, USP1 stabilizes the protein expression of inhibitor of DNA binding 1 (ID1), a downstream target of the oncogenic BCR-ABL and TEL-ABL kinases, presumably via the removal of K48 polyubiquitin chains [83,84]. ID1 immortalized hematopoietic progenitors in vitro and promoted myeloproliferative disease in an in vivo mouse model [85]. ID1 expression in AML patient samples has been found to be highly correlated with poor prognosis [86].
Deubiquitination of the ID family members by USP1 was first described in human osteosarcoma cell lines and was later described in leukemias [84]. One group found that, relative to controls, USP1 inhibition decreased cell viability, induced apoptosis, and sensitized AML cell lines as well as patient cell samples to other chemotherapeutic agents such as etoposide [10]; this presumably occurred through ID1 degradation.
USP1 shows poor enzymatic activity alone, but binding of its cofactor USP1-associated factor 1 (UAF1) significantly enhances its DUB activity relative to controls [87]. Therefore, the USP1/UAF1 complex has been used to develop small-molecule inhibitors of USP1, one of the DUBs with the most inhibitors, including two patented compounds [88,89]. One group identified pimozide as one of the top inhibitors among a collection of bioactive molecules in a quantitative high-throughput screening (HTS) assay [90]. Pimozide did not disrupt the interaction of USP1/UAF1 and bound to this complex independently of the presence of substrate. Moreover, pimozide showed superior selectivity for USP1 (IC50 2 μM) over other USPs including USP46, which also bound to UAF1 [90]. However, pimozide was later shown to also inhibit other DUBs, with an affinity similar to that of its binding to USP1 [91]. Subsequent studies led to the development of the ML323 compound (Table 1). ML323 does not disrupt the USP1/UAF1 complex and exerts its effect through allosteric inhibition [92]. This compound is substantially selective, showing no effect against 18 DUBs, 70 proteases, and 451 kinases in in vivo assays; however, it has a short half-life of 15 min in rat liver microsomes [93]. Another group has also worked on developing effective USP1/UAF1 inhibitors. A screening assay was performed on a library of 150 000 compounds, identifying C527 as a potent USP1 inhibitor with an IC50 of 0.88 μM; however, this compound also affects USP5 and UCH-L3 activities [10]. SJB3–019A, a close analog of C527, showed high promiscuity among DUBs when used at 3 μM [91]. Due to this cross-reactivity, C527 and SJB3–019A have been proposed to be redox compounds that oxidize cysteines at the catalytic site of USP1 and other DUBs, resulting in the observed nonspecific inhibition of DUB activity [94].
USP2 Is an Oncogenic Player in MLL-Rearranged (MLL-r) Leukemia
USP2 is a DUB that removes polyubiquitin chains with K48 and K63 linkages [95] and its different isoforms have been extensively studied in the cancer field. USP2 is a key regulator in the cell cycle and apoptosis pathways and hyperactivation of this DUB is associated with a cancerous phenotype, especially in human prostate tumors [96]. MDM2, Aurora kinase A, cyclin D1, and fatty acid synthase are some of its well-characterized targets associated with prostate cancer and breast adenocarcinoma [97–100]. Although the role of USP2 is largely unexplored in blood cancers, recent findings indicate that this DUB could play a role in MLL-r leukemia. The first evidence came from a mutational landscape study performed in samples from pediatric MLL-r patients, where USP2 was identified via RNA-seq as a novel fusion partner of genes for the MLL1 protein (e.g., KMT2A) [101]. Other studies indicated that the number of MLL–USP2 fusions was underestimated, mainly because of the use of techniques that detect rearrangements occurring in the MLL breakpoint cluster region (BRC-1) only, although most translocations occur outside that area [102,103]. According to this study, a customized PCR panel was developed for MLL-r evaluation in 109 pediatric patients and MLL-USP2 translocations were present in ~15% of all rearrangements [102,103]. Although the clinical outcome associated with this rearrangement remains unknown, and given the ability of DUBs to impede protein degradation, a MLL–USP2 chimeric protein has been proposed to drive leukemogenesis through USP2-mediated stabilization of the MLL1/MLL complex and/or by USP2-mediated stabilization of its substrates, specifically cyclin D1 [104].
Given the role that USP2 plays in oncogenic transformation, there are several available inhibitors for this DUB. One class of inhibitors is the patented 2-cyano-pyrimidines for the treatment of various proliferative diseases including MM and non-Hodgkin’s lymphoma [105]. These inhibitors have a potency range of 100 nM to 50 μM against USP2; however, they also target UCHL3, another DUB, in the same concentration range [105]. Although the mechanism of action has not been described for these inhibitors, co-crystal structures of compounds in the same family, solved using other cysteine proteases, suggest that they might form reversible covalent links with active-site cysteine residues [106]. The compound ML364 (Table 1) was developed: it forms reversible, noncovalent bonds with USP2 and inhibits DUB activity with an IC50 of 1.1 μM for K48-linked substrates and 1.7 μM for K63-linked substrates [107]. This small-molecule inhibitor significantly decreases cyclin D1 protein expression and induces cell cycle arrest in Mino cells, a non-Hodgkin’s lymphoma B cell line [107]. Moreover, ML364 displays similar potency against USP2 and USP8, given the similarity of their active sites [107]. This compound does not affect the activity of USP20 or that of four other proteases or 102 kinases involved in cell cycle regulation, as demonstrated by in vitro kinase, caspase, and protease activity assays in the presence or absence of the inhibitor [107], although the selectivity of ML364 for USP2 and USP8 over other DUBs warrants further investigation. LCAHA, a derivate of lithocholic acid, can also interfere with the activity of one of the USP2 isoforms (USP2a) [108]. Ubiquitination assays demonstrated that LCAHA binds to USP2a in a noncompetitive manner and inhibits this DUB’s activity at low-micromolar concentrations [108]. Functional studies indicated that LCAHA-treated HCT116 cells (a human colon cancer cell line used in therapeutic research and drug screenings), decreased cyclin D1 and Aurora kinase A protein expression relative to the untreated group [108]. However, this compound is not specific for USP2 since it inhibits 13 other USPs and some DUBs in the Otubain protease (OTU) family [108]. 6-Thioguanine (6TG) is an old drug clinically used to treat certain leukemias and was shown to inhibit USP2 activity (IC50 24.6 μM) [109]. 6TG binds to its target in a noncompetitive manner and this reaction is irreversible, suggesting that the compound forms a covalent bond with the DUB [109]. Although it is well known that this drug has a broad spectrum of activity, whether 6TG can affect the activity of other DUBs has not been addressed [109].
General Considerations for Targeting DUBs and Therapeutic Implications
To date, several DUBs, especially those in the USP family, have been successfully targeted with small-molecule inhibitors and have shown cytotoxic effects in in vitro and in vivo models. The use of USP1 inhibitors, such as C527, decrease aberrant ID1 signaling by promoting the degradation of this protein in AML cell lines and patient cells; in vitro treatment with this drug also increases apoptosis and sensitizes HeLa and U2OS cells to DNA crosslinking chemotherapy agents such as etoposide, camptothecin, and mitomycin C [10]. In MM, USP7 inhibition reverses resistance to the proteasomal inhibitor bortezomib as well as glucocorticoids, two drugs commonly used in relapsed MM; this has also led to increased survival of mice bearing human plasmacytoma xenografts and SCID-hu mice bearing MM tumors [11]. In T-ALL models, the combination of USP7 inhibitor and glucocorticoids can affect leukemia growth in vivo and in vitro [12]. The approval of proteasome inhibitors for MM treatment and the debut of the USP14/UCHL5 inhibitor VLX1570 in MM clinical trials are illustrative examples of possible therapeutic uses of ubiquitin-proteasome system interventions in blood cancers.
Besides the potential therapeutic implications, the use of inhibitors has significantly contributed to the understanding of the role played by DUBs in various processes. However, targeting DUBs is not an easy task because: (i) ubiquitination and deubiquitination are intracellular processes that cannot be targeted by biologicals such as monoclonal antibodies and, at least currently, can be blocked only via the use of small molecules; (ii) DUBs exhibit complex structural characteristics in their catalytic pockets as well as similarities among family members that render their specific targeting by small molecules difficult; (iii) DUBs undergo substantial conformational changes on ubiquitin binding, suggesting flexibility of their active sites, which constitutes an additional challenge in predicting and producing efficient and specific inhibitors as well as in designing accurate tests to measure inhibitor efficiency; (iv) not only the structure but also the mechanisms of action of these enzymes are complex, involving the regulation of catalytic activity through allosteric and/or substrate-mediated catalysis; moreover, (v) DUBs exist in complexes with other DUB enzymes or E3 ligases, and the dynamics of those complexes are not yet well understood; and finally, (vi) DUBs catalyze ubiquitin transfer via a reactive thiol and most inhibitor screenings are likely to select for nonspecific redox or alkylating agents that can target other cysteine proteases.
Of note, first-generation DUB inhibitors have shown low bioavailability and stability in contrast to the long half-life of DUBs. However, the use of new and better technologies has demonstrated that the development of highly selective inhibitors with good stability may be possible, as shown for USP7 inhibitors (Table 1) [14,71,76,78]. Thus, overall, DUBs possess characteristics that render them suitable therapeutic targets. For instance, in the ubiquitin cascade, DUBs are considered better targets than E3 ligases because the latter lack a defined catalytic residue, while most DUBs possess a conserved catalytic cysteine that can be targeted [110]. Additionally, DUBs can be differentially expressed in healthy and cancerous cells [11,13,96].
Concluding Remarks
The study of DUBs has progressed significantly over the past few years. It has been widely documented that a substantial number of DUBs play important roles in cancer development, especially in hematological malignancies [11,111].
Although DUBs seem to play an important role in tumor progression, there are systems where they display both oncogenic and antitumor properties, shown for USP9X in B-ALL [2,112–115] and USP22 in AML [6,116]. This dual role suggests that the phenotypic outcome related to DUB activity might be dependent on the substrates present in a given cellular background, under specific stress conditions, or even at a certain cancer stage, as demonstrated for other enzymes, including epigenetic modulators such as members of the Polycomb complex in various AML settings [70]. Moreover, we need to continue investigating and understanding ubiquitination signaling. Research in recent years has indicated that the ubiquitin chain cleavage mode, the chain linkage specificity of DUBs, and the ubiquitin chain architecture on substrate proteins are key factors that can determine the outcome of the different biological functions [117]; thus, this knowledge might be exploited to fine-tune the design of future generations of inhibitors. Additionally, the development of inhibitors better suited for in vivo experiments, as well as more comprehensive toxicity experiments with carefully planned pharmacokinetic and pharmacodynamic studies, are some of the crucial points that need to be addressed to facilitate the clinical translation of these compounds.
Overall, advances in the deubiquitination field are paving the way for targeted therapy (see Outstanding Questions) and the development of DUB inhibitors can inform translational studies that may eventually lead to clinical benefits for leukemic patients.
Outstanding Questions.
Could DUBs be used as putative prognostic markers in blood cancers? DUB expression is frequently elevated in a variety of cancers (especially blood cancers) and has been frequently associated with poor prognosis, rendering DUBs potential prognostic markers of certain malignancies.
Could DUB inhibitors be therapeutically effective in combination with other anti-cancer treatments? DUBs affect many processes, including DNA methylation, DNA replication, the DNA damage response, and apoptosis. This functional diversity paves the way for testing the combinatorial use of several regimens, such as proapoptotic drugs and cell cycle inhibitors, currently in clinical trials.
What is the toxicity of DUB inhibitors in normal tissues? Although DUBs are differentially expressed between healthy and cancerous tissues, they participate in many developmental and homeo-static processes, and as a result, a careful assessment of toxicity should be performed.
What is the specificity of DUB inhibitors for other members of DUB families and proteases? The catalytic center of DUBs exhibits certain similarities across members of DUB families and some proteases. Current studies towards a better understanding of DUB structures may facilitate the development of specific inhibitors. Of relevance, the spectrum of activity of DUB compounds against related enzymes, including desumoylases and deneddylases, warrants further investigation.
What are the challenges in the development and design of allosteric DUB inhibitors? Many DUBs have active sites that do not contain binding pockets shaped like small molecules or that might exhibit extensive active-site flexibility; thus, the development of allosteric inhibitors might be an attractive alternative. Elucidation of the structure of the DUB enzyme of interest in complex with inhibitors and ubiquitinated substrates is a crucial step in the design of specific allosteric inhibitors.
Highlights.
Deubiquitinases (DUBs) can act as both pro-oncogenic factors and tumor suppressors in cancer.
Recent structural studies have elucidated aspects of deubiquitination chemistry and DUB structures, thus facilitating the development of more specific targeting compounds.
Inhibition of deubiquitination can be a disease-specific therapeutic avenue in malignancies, potentially in combination with other systemic and targeted treatments.
As DUBs exist in complexes with multiple proteins, more accurate biochemical studies are required to understand the functional stoichiometry of the complexes for therapeutic reasons.
As DUBs influence many targets, further evaluation of DUB inhibitor toxicity to normal tissues should be performed.
Acknowledgments
The cover image was designed by Elizabeth Gutierrez. We want to thank all members of the Ntziachristos laboratory for their comments and critical review of the manuscript. All figures were created using BioRender (https://biorender.com/). The cover image was designed by Elizabeth Gutierrez (behance.net/elizabegutirr1). B.T.G-D. was supported in part by the US Department of State and COMEXUS via a Fulbright Garcia-Robles scholarship and by an NIH/NCI training grant (T32 CA009560). Related work in the Ntziachristos laboratory has been supported by the National Cancer Institute (R00CA188293), the Leukemia Research Foundation, the St. Baldrick’s Foundation, the Zell Foundation, and the Elsa U. Pardee Foundation.
Glossary
- Acute lymphoblastic leukemia (ALL)
type of blood cancer characterized by lymphocyte transformation and rapid disease progression; most common form of leukemia in children. If the leukemia originates in the B cell compartment, it is denominated B-ALL. If it occurs within the T cell subpopulation, it is called T-ALL
- Acute myeloid leukemia (AML)
type of blood cancer characterized by a rapid increase in the number of myeloid cells and arrest in their maturation. It is one of the most common leukemias in adults
- Apo structure
from the Greek apo-(‘away, off, apart’); the structure of a protein when it is not bound to its substrate or cofactor (inactive conformation)
- Deubiquitinases (DUBs)
proteases cleaving peptide or isopeptide bonds between ubiquitin molecules or between ubiquitin and a modified protein
- DNA methyl transferase 1 (DNMT1)
enzyme involved in the maintenance of DNA methylation patterns via the addition of methyl group to the newly synthesized daughter DNA strand during replication
- Exo-DUB
exo-deubiquitinase activity where the peptidase starts cleaving the ubiquitin chain from the distal end of the chain. This is in contrast to the endo-deubiquitinase activity where degradation is initiated from an internal position in the ubiquitin chain
- H2AK119Ub
ubiquitylation of lysine 119 histone H2A. This post-translational histone modification is linked to gene silencing
- Holo structure
from the Greek olos-(‘whole’); structural conformation of a protein when it is bound to its ligand (active)
- Inhibitor, competitive
compound binding to the same enzymatic site as the substrate. Competitive inhibitors usually compete with the substrate for occupation of the active site
- Inhibitor, noncompetitive
compound binding to an enzyme in the presence or absence of its substrate. Noncompetitive inhibitors bind at a location different from the active site and alter the conformation of the enzyme, hampering its function
- K11 linkage
binding of ubiquitin to lysine residue 11 of another ubiquitin. Similar to proteins tagged with K48 chains, proteins tagged with this type of linkage mainly signal for proteasomal degradation
- K48 linkage
binding of ubiquitin to lysine residue 48 of another ubiquitin moiety. This type of linkage targets proteins for proteasomal degradation and is the most abundant connection between ubiquitin molecules
- K63 linkage
binding of ubiquitin to lysine residue 63 of another ubiquitin. K63-linked chains work as platforms that allow rapid and reversible formation of signaling complexes. This type of linkage is associated mainly with NF-κB and DNA repair signaling
- MLL-rearranged (MLL-r) leukemia
type of acute leukemia characterized by chromosomal rearrangements involving the human MLL/KMT2A histone methyltransferase gene
- Multiple myeloma (MM)
type of blood cancer forming in plasma B lymphocytes
- Ubiquitin-like containing PHD and RING finger domains 1 (UHRF1)
E3 ligase essential in maintaining genomic DNA methylation. It binds to hemimethylated DNA and histone H3K9 trimethylation and recruits DNMT1 for chromatin localization
Footnotes
Resource
iThis trial is listed at https://clinicaltrials.gov/ct2/show/NCT02372240
References
- 1.Huang X et al. (2009) Deubiquitinase USP9X stabilizes MCL1 and promotes tumour cell survival. Nature 463, 103–107 [DOI] [PubMed] [Google Scholar]
- 2.Zhou M et al. (2015) Targeting of the deubiquitinase USP9X attenuates B-cell acute lymphoblastic leukemia cell survival and overcomes glucocorticoid resistance. Biochem. Biophys. Res. Commun 459, 333–339 [DOI] [PubMed] [Google Scholar]
- 3.Weisberg EL et al. (2017) Inhibition of USP10 induces degradation of oncogenic FLT3. Nat. Chem. Biol 13, 1207–1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guo Y et al. (2010) Blockade of the ubiquitin protease UBP43 destabilizes transcription factor PML/RARa and inhibits the growth of acute promyelocytic leukemia. Cancer Res. 70, 9875–9885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shih H-M and Yang W-C. Academia Sinica. USP37 inactivation as a treatment for PLZF/RARA-associated acute promyelocytic leukemia, US9353422B2 [Google Scholar]
- 6.Li L et al. (2014) SIRT1 activation by a c-MYC oncogenic network promotes the maintenance and drug resistance of human FLT3-ITD acute myeloid leukemia stem cells. Cell Stem Cell 15, 431–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Y et al. (2011) Overexpression of ubiquitin specific protease 44 (USP44) induces chromosomal instability and is frequently observed in human T-cell leukemia. PLoS One 6, e23389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D’Arcy P et al. (2011) Inhibition of proteasome deubiquitinating activity as a new cancer therapy. Nat. Med 17, 1636–1640 [DOI] [PubMed] [Google Scholar]
- 9.Wang X et al. (2016) The proteasome deubiquitinase inhibitor VLX1570 shows selectivity for ubiquitin-specific protease-14 and induces apoptosis of multiple myeloma cells. Sci. Rep 6, 26979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mistry H et al. (2013) Small-molecule inhibitors of USP1 target ID1 degradation in leukemic cells. Mol. Cancer Ther 12, 2651–2662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chauhan D et al. (2012) A small molecule inhibitor of ubiquitin-specific protease-7 induces apoptosis in multiple myeloma cells and overcomes bortezomib resistance. Cancer Cell 22, 345–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jin Q et al. (2018) USP7 cooperates with NOTCH1 to drive the oncogenic transcriptional program in T cell leukemia. Clin. Cancer Res 25, 222–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shan H et al. (2018) USP7 deubiquitinates and stabilizes NOTCH1 in T-cell acute lymphoblastic leukemia. Signal Transduct. Target. Ther 3, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kategaya L et al. (2017) USP7 small-molecule inhibitors interfere with ubiquitin binding. Nature 550, 534–538 [DOI] [PubMed] [Google Scholar]
- 15.Gong Y et al. (2018) Stratification of TAD boundaries reveals preferential insulation of super-enhancers by strong boundaries. Nat. Commun 9, 542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lazaris C et al. (2017) HiC-bench: comprehensive and reproducible Hi-C data analysis designed for parameter exploration and benchmarking. BMC Genomics 18, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ntziachristos P et al. (2016) Emerging concepts of epigenetic dysregulation in hematological malignancies. Nat. Immunol 17, 1016–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arcipowski KM et al. (2016) Histone demethylases in physiology and cancer: a tale of two enzymes, JMJD3 and UTX. Curr. Opin. Genet. Dev 36, 59–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ntziachristos P et al. (2014) Contrasting roles of histone 3 lysine 27 demethylases in acute lymphoblastic leukaemia. Nature 514, 513–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ntziachristos P et al. (2014) From fly wings to targeted cancer therapies: a centennial for Notch signaling. Cancer Cell 25, 318–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Woods BA and Levine RL (2015) The role of mutations in epigenetic regulators in myeloid malignancies. Immunol. Rev 263, 22–35 [DOI] [PubMed] [Google Scholar]
- 22.Asada S et al. (2018) Mutant ASXL1 cooperates with BAP1 to promote myeloid leukaemogenesis. Nat. Commun 9, 2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qin W et al. (2011) Usp7 and Uhrf1 control ubiquitination and stability of the maintenance DNA methyltransferase Dnmt1. J. Cell. Biochem 112, 439–444 [DOI] [PubMed] [Google Scholar]
- 24.Yamaguchi L et al. (2017) Usp7-dependent histone H3 deubiquitylation regulates maintenance of DNA methylation. Sci. Rep 7, 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Felle M et al. (2011) The USP7/Dnmt1 complex stimulates the DNA methylation activity of Dnmt1 and regulates the stability of UHRF1. Nucleic Acids Res. 39, 8355–8365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Längst G et al. (2018) Overexpression of UHRF1 promotes silencing of tumor suppressor genes and predicts outcome in hepatoblastoma. Clin. Epigenetics 10, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.D’Arcy P and Linder S (2012) Proteasome deubiquitinases as novel targets for cancer therapy. Int. J. Biochem. Cell Biol 44, 1729–1738 [DOI] [PubMed] [Google Scholar]
- 28.Lee B-H. et al. (2016) USP14 deubiquitinates proteasome-bound substrates that are ubiquitinated at multiple sites. Nature 532, 398–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manasanch EE and Orlowski RZ (2017) Proteasome inhibitors in cancer therapy. Nat. Rev. Clin. Oncol 14, 417–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu S et al. (2015) Clinically used antirheumatic agent auranofin is a proteasomal deubiquitinase inhibitor and inhibits tumor growth. Oncotarget 5, 5453–5471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang X et al. (2013) A novel small molecule inhibitor of deubiquitylating enzyme USP14 and UCHL5 induces apoptosis in multiple myeloma and overcomes bortezomib resistance. Blood 123, 706–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Song C et al. (2017) The deubiquitinating enzyme USP14 regulates leukemic chemotherapy drugs-induced cell apoptosis by suppressing ubiquitination of Aurora kinase B. Cell. Physiol. Biochem 42, 965–973 [DOI] [PubMed] [Google Scholar]
- 33.Wang X et al. (2015) Synthesis and evaluation of derivatives of the proteasome deubiquitinase inhibitor b-AP15. Chem. Biol. Drug Des 86, 1036–1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paulus A et al. (2016) Coinhibition of the deubiquitinating enzymes, USP14 and UCHL5, with VLX1570 is lethal to ibrutinib-or bortezomib-resistant Waldenstrom macroglobulinemia tumor cells. Blood Cancer J. 6 (11). 10.1038/bcj.2016.93e492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang X et al. (2014) The 19S deubiquitinase inhibitor b-AP15 is enriched in cells and elicits rapid commitment to cell death. Mol. Pharmacol 85, 932–945 [DOI] [PubMed] [Google Scholar]
- 36.Fiskus W et al. (2014) Auranofin induces lethal oxidative and endoplasmic reticulum stress and exerts potent preclinical activity against chronic lymphocytic leukemia. Cancer Res. 74, 2520–2532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen X et al. (2015) Anti-rheumatic agent auranofin induced apoptosis in chronic myeloid leukemia cells resistant to imatinib through both Bcr/Abl-dependent and -independent mechanisms. Oncotarget 5, 9118–9132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Finley DJ et al. Harvard College, Health Research, Inc. USP14 inhibitors for treating or preventing viral infections, US9849135B2 [Google Scholar]
- 39.Finley DJ et al. Harvard College. Tricyclic proteasome activity enhancing compounds, US20180265519A1 [Google Scholar]
- 40.Lee BH et al. (2010) Enhancement of proteasome activity by a small-molecule inhibitor of USP14. Nature 467, 179–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boselli M et al. (2017) An inhibitor of the proteasomal deubiquitinating enzyme USP14 induces Tau elimination in cultured neurons. J. Biol. Chem 292, 19209–19225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang Y et al. (2018) Small molecule inhibitors reveal allosteric regulation of USP14 via steric blockade. Cell Res. 28, 1186–1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rawat R et al. (2019) Nuclear deubiquitination in the spotlight: the multifaceted nature of USP7 biology in disease. Curr. Opin. Cell Biol 58, 85–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bhattacharya S et al. (2018) Emerging insights into HAUSP (USP7) in physiology, cancer and other diseases. Signal Transduct. Target. Ther 3, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Faesen AC et al. (2011) The differential modulation of USP activity by internal regulatory domains, interactors and eight ubiquitin chain types. Chem. Biol 18, 1550–1561 [DOI] [PubMed] [Google Scholar]
- 46.Hoeller D et al. (2006) Regulation of ubiquitin-binding proteins by monoubiquitination. Nat. Cell Biol 8, 163–169 [DOI] [PubMed] [Google Scholar]
- 47.Li M et al. (2002) Deubiquitination of p53 by HAUSP is an important pathway for p53 stabilization. Nature 416, 648–653 [DOI] [PubMed] [Google Scholar]
- 48.Li M et al. (2004) A dynamic role of HAUSP in the p53–Mdm2 pathway. Mol. Cell 13, 879–886 [DOI] [PubMed] [Google Scholar]
- 49.Hu M et al. (2006) Structural basis of competitive recognition of p53 and MDM2 by HAUSP/USP7: implications for the regulation of the p53–MDM2 pathway. PLoS Biol. 4, 228–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kon N et al. (2010) Inactivation of HAUSP in vivo modulates p53 function. Oncogene 29, 1270–1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tavana O et al. (2016) HAUSP deubiquitinates and stabilizes N-Myc in neuroblastoma. Nat. Med 22, 1180–1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tavana O et al. (2018) Targeting HAUSP in both p53 wildtype and p53-mutant tumors. Cell Cycle 17, 823–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zlatanou A et al. (2016) USP7 is essential for maintaining Rad18 stability and DNA damage tolerance. Oncogene 35, 965–976 [DOI] [PubMed] [Google Scholar]
- 54.Qian J et al. (2015) USP7 modulates UV-induced PCNA monoubiquitination by regulating DNA polymerase η stability. Oncogene 34, 4791–4796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kashiwaba S et al. (2015) USP7 is a suppressor of PCNA ubiquitination and oxidative-stress-induced mutagenesis in human cells. Cell Rep. 13, 2072–2080 [DOI] [PubMed] [Google Scholar]
- 56.Maertens GN et al. (2010) Ubiquitin-specific proteases 7 and 11 modulate Polycomb regulation of the INK4a tumour suppressor. EMBO J. 29, 2553–2565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lecona E et al. (2015) USP7 cooperates with SCML2 to regulate the activity of PRC1. Mol. Cell. Biol 35, 1157–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vidal M and Starowicz K (2017) Polycomb complexes PRC1 and their function in hematopoiesis. Exp. Hematol 48, 12–31 [DOI] [PubMed] [Google Scholar]
- 59.Hu B et al. (2014) HSCARG, a novel regulator of H2A ubiquitination by downregulating PRC1 ubiquitin E3 ligase activity, is essential for cell proliferation. Nucleic Acids Res. 42, 5582–5593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yarychkivska O et al. (2018) Independent functions of DNMT1 and USP7 at replication foci. Epigenetics Chromatin 11, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jiang L et al. (2017) Ubiquitin-specific peptidase 7 (USP7)-mediated deubiquitination of the histone deacetylase SIRT7 regulates gluconeogenesis. J. Biol. Chem 292, 13296–13311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bhattacharya S and Ghosh MK (2015) HAUSP regulates c-MYC expression via de-ubiquitination of TRRAP. Cell. Oncol 38, 265–277 [DOI] [PubMed] [Google Scholar]
- 63.Palazón-Riquelme P et al. (2018) USP7 and USP47 deubiquitinases regulate NLRP3 inflammasome activation. EMBO Rep. 19, e44766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xia J et al. (2020) NEK2 induces autophagy-mediated bortezomib resistance by stabilizing Beclin-1 in multiple myeloma. Mol. Oncol Published online January 19, 2020. 10.1002/1878-0261.12641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.He Y et al. (2019) The deubiquitinase USP7 stabilizes Maf proteins to promote myeloma cell survival. J. Biol. Chem Published online December 10, 2019. 10.1074/jbc.RA119.010724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Seki M et al. (2017) Recurrent SPI1 (PU.1) fusions in high-risk pediatric T cell acute lymphoblastic leukemia. Nat. Genet 49, 1274–1281 [DOI] [PubMed] [Google Scholar]
- 67.Richter-Pechańska P et al. (2017) Identification of a genetically defined ultra-high-risk group in relapsed pediatric T-lymphoblastic leukemia. Blood Cancer J. 7, e523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Qian M et al. (2019) Genome-wide association study of susceptibility loci for T-cell acute lymphoblastic leukemia in children. J. Natl. Cancer Inst 111, 1350–1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Huether R et al. (2014) The landscape of somatic mutations in epigenetic regulators across 1,000 paediatric cancer genomes. Nat. Commun 5, 3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Basheer F et al. (2019) Contrasting requirements during disease evolution identify EZH2 as a therapeutic target in AML. J. Exp. Med 216, 966–981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Altun M et al. (2011) Activity-based chemical proteomics accelerates inhibitor development for deubiquitylating enzymes. Chem. Biol 18, 1401–1412 [DOI] [PubMed] [Google Scholar]
- 72.Weinstock J et al. (2012) Selective dual inhibitors of the cancer-related deubiquitylating proteases USP7 and USP47. ACS Med. Chem. Lett 3, 789–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rigden DJ et al. (2013) Deubiquitylases from genes to organism. Physiol. Rev 93, 1289–1315 [DOI] [PubMed] [Google Scholar]
- 74.Fan YH et al. (2013) USP7 inhibitor P22077 inhibits neuroblastoma growth via inducing p53-mediated apoptosis. Cell Death Dis. 4, e867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen C et al. (2017) Synthesis and biological evaluation of thiazole derivatives as novel USP7 inhibitors. Bioorg. Med. Chem. Lett 27, 845–849 [DOI] [PubMed] [Google Scholar]
- 76.Wang F et al. (2017) Active site-targeted covalent irreversible inhibitors of USP7 impair the functions of Foxp3+ T-regulatory cells by promoting ubiquitination of Tip60. PLoS One 12, e0189744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gavory G et al. (2017) Discovery and characterization of highly potent and selective allosteric USP7 inhibitors. Nat. Chem. Biol 14, 118–125 [DOI] [PubMed] [Google Scholar]
- 78.Turnbull AP et al. (2017) Molecular basis of USP7 inhibition by selective small-molecule inhibitors. Nature 550, 481–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lamberto I et al. (2017) Structure-guided development of a potent and selective non-covalent active-site inhibitor of USP7. Cell Chem. Biol 24, 1490–1500.e11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Colland F and Gourdel M-E. Selective and reversible inhibitors of ubiquitin specific protease 7, WO2013030218 [DOI] [PubMed] [Google Scholar]
- 81.Brummelkamp TR et al. (2005) The deubiquitinating enzyme USP1 regulates the Fanconi anemia pathway. Mol. Cell 17, 331–339 [DOI] [PubMed] [Google Scholar]
- 82.Huang TT et al. (2006) Regulation of monoubiquitinated PCNA by DUB autocleavage. Nat. Cell Biol 8, 339–347 [DOI] [PubMed] [Google Scholar]
- 83.Tam WF et al. (2008) Id1 is a common downstream target of oncogenic tyrosine kinases in leukemic cells. Blood 112, 1981–1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Williams SA et al. (2011) USP1 deubiquitinates ID proteins to preserve a mesenchymal stem cell program in osteosarcoma. Cell 146, 918–930 [DOI] [PubMed] [Google Scholar]
- 85.Suh HC et al. (2008) Id1 immortalizes hematopoietic progenitors in vitro and promotes a myeloproliferative disease in vivo. Oncogene 27, 5612–5623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pardo J et al. (2009) High Id1 expression is associated with poor prognosis in 237 patients with acute myeloid leukemia. Blood 114, 2993–3000 [DOI] [PubMed] [Google Scholar]
- 87.Yang K et al. (2008) A UAF1-containing multisubunit protein complex regulates the Fanconi anemia pathway. Mol. Cell 28, 786–797 [DOI] [PubMed] [Google Scholar]
- 88.D’Andrea AD et al. Brigham and Women’s Hospital, Dana Farber Cancer Institute, Inc. Small molecule inhibitors of USP1 deubiquitinating enzyme activity, US9518032B2 [Google Scholar]
- 89.Maloney DJ et al. US Department of Health and Human Services (HHS), University of Delaware. Inhibitors of the usp1/uaf1 deubiquitinase complex and uses thereof, US20150344443A1 [Google Scholar]
- 90.Chen J et al. (2011) Selective and cell-active inhibitors of the USP1/UAF1 deubiquitinase complex reverse cisplatin resistance in non-small cell lung cancer cells. Chem. Biol 18, 1390–1400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ritorto MS et al. (2014) Screening of DUB activity and specificity by MALDI-TOF mass spectrometry. Nat. Commun 5, 4763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liang Q et al. (2014) A selective USP1–UAF1 inhibitor links deubiquitination to DNA damage responses. Nat. Chem. Biol 10, 298–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Simeonov A et al. (2014) Synthesis and structure–activity relationship studies of N-benzyl-2-phenylpyrimidin-4-amine derivatives as potent USP1/UAF1 deubiquitinase inhibitors with anticancer activity against nonsmall cell lung cancer. J. Med. Chem 57, 8099–8110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kemp M (2016) Recent Advances in the Discovery of Deubiquitinating Enzyme Inhibitors (1st edn), Elsevier; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ohtake F et al. (2016) The K48–K63 branched ubiquitin chain regulates NF-κB signaling. Mol. Cell 64, 251–266 [DOI] [PubMed] [Google Scholar]
- 96.Priolo C et al. (2006) The isopeptidase USP2a protects human prostate cancer from apoptosis. Cancer Res. 66, 8625–8632 [DOI] [PubMed] [Google Scholar]
- 97.Stevenson LF et al. (2007) The deubiquitinating enzyme USP2a regulates the p53 pathway by targeting Mdm2. EMBO J. 26, 976–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Luo Y et al. (2011) Ubiquitin-specific cysteine protease 2a (USP2a) regulates the stability of Aurora-A. J. Biol. Chem 286, 38960–38968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shan J et al. (2009) Suppression of cancer cell growth by promoting cyclin D1 degradation. Mol. Cell 36, 469–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Graner E et al. (2004) The isopeptidase USP2a regulates the stability of fatty acid synthase in prostate cancer. Cancer Cell 5, 253–261 [DOI] [PubMed] [Google Scholar]
- 101.Andersson AK et al. (2015) The landscape of somatic mutations in infant MLL-rearranged acute lymphoblastic leukemias. Nat. Genet 47, 330–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Meyer C et al. (2018) MLL-USP2: an underestimated new entity of MLL-rearranged leukemia identified by NGS analysis. Blood 132, 3920 [Google Scholar]
- 103.Meyer C et al. (2019) Human MLL/KMT2A gene exhibits a second breakpoint cluster region for recurrent MLL–USP2 fusions. Leukemia 33, 2306–2340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ikeda J et al. (2019) Whole transcriptome sequencing reveals a KMT2A–USP2 fusion in infant acute myeloid leukemia. Genes Chromosomes Cancer 58, 669–672 [DOI] [PubMed] [Google Scholar]
- 105.Flohr S et al. Novartis AG. 2-Cyano-pyrimidines and-triazines as cysteine protease inhibitors, US7700605B2 [Google Scholar]
- 106.Rankovic Z et al. (2010) Design and optimization of a series of novel 2-cyano-pyrimidines as cathepsin K inhibitors. Bioorg. Med. Chem. Lett 20, 1524–1527 [DOI] [PubMed] [Google Scholar]
- 107.Davis MI et al. (2016) Small molecule inhibition of the ubiquitin-specific protease USP2 accelerates cyclin D1 degradation and leads to cell cycle arrest in colorectal cancer and mantle cell lymphoma models. J. Biol. Chem 291, 24628–24640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Magiera K et al. (2017) Lithocholic acid hydroxyamide destabilizes cyclin D1 and induces G0/G1 arrest by inhibiting deubiquitinase USP2a. Cell Chem. Biol 24, 458–470.e18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chuang SJ et al. (2018) 6-Thioguanine is a noncompetitive and slow binding inhibitor of human deubiquitinating protease USP2. Sci. Rep 8, 3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Huang X and Dixit VM (2016) Drugging the undruggables: exploring the ubiquitin system for drug development. Cell Res. 26, 484–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jin Q et al. (2019) USP7 cooperates with NOTch1 to drive the oncogenic transcriptional program in T-cell leukemia. Clin. Cancer Res 25, 222–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Aveic S et al. (2011) BAG1: the guardian of anti-apoptotic proteins in acute myeloid leukemia. PLoS One 6, e26097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kapoor S et al. (2018) Concurrent inhibition of Pim and FLT3 kinases enhances apoptosis of FLT3-ITD acute myeloid leukemia cells through increased Mcl-1 proteasomal degradation. Clin. Cancer Res 24, 234–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Vetere A et al. (2017) Suppressors and activators of JAK-STAT signaling at diagnosis and relapse of acute lymphoblastic leukemia in Down syndrome. Proc. Natl. Acad. Sci. U. S. A 114, E4030–E4039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Healy J et al. (2016) Genomic characterization of pediatric T-cell acute lymphoblastic leukemia reveals novel recurrent driver mutations. Oncotarget 7, 65485–65503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Melo-Cardenas J et al. (2018) USP22 deficiency leads to myeloid leukemia upon oncogenic Kras activation through a PU.1-dependent mechanism. Blood 132, 423–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Clague MJ et al. (2019) Breaking the chains: deubiquitylating enzyme specificity begets function. Nat. Rev. Mol. Cell Biol 20, 338–352 [DOI] [PubMed] [Google Scholar]
- 118.Komander D et al. (2009) Breaking the chains: structure and function of the deubiquitinases. Nat. Rev. Mol. Cell Biol 10, 550–563 [DOI] [PubMed] [Google Scholar]
- 119.Komander D and Rape M (2012) The ubiquitin code. Annu. Rev. Biochem 81, 203–229 [DOI] [PubMed] [Google Scholar]
- 120.Mevissen TET and Komander D (2017) Mechanisms of deubiquitinase specificity and regulation. Annu. Rev. Biochem 86, 159–192 [DOI] [PubMed] [Google Scholar]
- 121.Yau R and Rape M (2016) The increasing complexity of the ubiquitin code. Nat. Cell Biol 18, 579–586 [DOI] [PubMed] [Google Scholar]
- 122.Komander D (2009) The emerging complexity of protein ubiquitination. Biochem. Soc. Trans 37, 937–953 [DOI] [PubMed] [Google Scholar]
- 123.Rape M (2017) Ubiquitylation at the crossroads of development and disease. Nat. Rev. Mol. Cell Biol 19, 59–70 [DOI] [PubMed] [Google Scholar]
- 124.Davis MI and Simeonov A (2015) Ubiquitin-specific proteases as druggable targets. Drug Target Rev 2, 60–64 [PMC free article] [PubMed] [Google Scholar]
- 125.Cstorer A and Ménard R (1994) Catalytic mechanism in papain family of cysteine peptidases. Methods Enzymol. 244, 486–500 [DOI] [PubMed] [Google Scholar]
- 126.Hu M et al. (2002) Crystal structure of a UBP-family deubiquitinating enzyme in isolation and in complex with ubiquitin aldehyde. Cell 111, 1041–1054 [DOI] [PubMed] [Google Scholar]
- 127.Pozhidaeva A et al. (2017) USP7-specific inhibitors target and modify the enzyme’s active site via distinct chemical mechanisms. Cell Chem. Biol 24, 1501–1512 [DOI] [PubMed] [Google Scholar]
- 128.Hu M et al. (2005) Structure and mechanisms of the proteasome-associated deubiquitinating enzyme USP14. EMBO J. 24, 3747–3756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yang WC and Shih HM (2013) The deubiquitinating enzyme USP37 regulates the oncogenic fusion protein PLZF/RARA stability. Oncogene 32, 5167–5175 [DOI] [PubMed] [Google Scholar]
- 130.Liu L-Q. et al. (1999) A novel ubiquitin-specific protease, UBP43, cloned from leukemia fusion protein AML1–ETO-expressing mice, functions in hematopoietic cell differentiation. Mol. Cell. Biol 19, 3029–3038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yan M et al. (2007) Ubp43 regulates BCR-ABL leukemogenesis via the type 1 interferon receptor signaling. Blood 110, 305–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Paulsson K et al. (2006) A novel and cytogenetically cryptic t(7;21)(p22;q22) in acute myeloid leukemia results in fusion of RUNX1 with the ubiquitin-specific protease gene USP42. Leukemia 20, 224–229 [DOI] [PubMed] [Google Scholar]


