Abstract
Objective:
Smoking among individuals with serious mental illness is a critical public health problem. Although guidelines recommend bupropion for these smokers, many do not want to use medications for smoking cessation, express ambivalence about identifying a “quit date,” and do not have access to behavioral smoking cessation services integrated with mental health care.
Methods:
Individuals with serious mental illness who smoked 10 or more cigarettes per day (N = 178) were randomized to either a multifaceted behavioral group intervention or a supportive group intervention, both of which were integrated within outpatient mental health services at three VA medical centers. Participants attended twice-weekly meetings for 12 weeks, provided information on their smoking at each meeting, and completed baseline and post-treatment assessments conducted by an assessor who was blind to condition. Primary outcomes collected at post-treatment included 1-week abstinence, number of cigarettes smoked per day during the last week, and number of quit attempts during the treatment period. Outcomes examined for a subset of participants who attended at least one intervention meeting (n = 152) included smoking abstinence for 1-, 2-, and 4-week blocks during the treatment period. Analyses conducted on those participants who attended three or more intervention meetings (n = 127) included time to 50% reduction in the number of cigarettes smoked and time to first quit attempt.
Results:
Sixteen participants achieved abstinence (11.8%), smoking quantity was significantly reduced (baseline M = 15.2, SD = 9.8 to post-treatment M = 7.5, SD = 7.7, p < .0001), and most reported making a quit attempt (n = 88, 72.7%). There were no differences by study condition on any abstinence or reduction outcomes. Significant reductions in number of cigarettes smoked generally took place within the first two weeks; however, these reductions did not often translate into abstinence.
Conclusions:
Many participants reduced their smoking and sampled quitting during the study. Implementing smoking cessation services in mental health treatment settings is feasible and, if delivered in line with best practices, either a behavioral or a supportive approach can be helpful. Future research should examine ways to facilitate the transition from reduction to abstinence. This study was part of a clinical trial registered as NCT#00960375 at www.clinicaltrials.gov
Keywords: smoking cessation, serious mental illness, psychosocial interventions, RCT
Smoking among individuals with serious mental illness is a critical public health problem. Between 55% and 70% of individuals with serious mental illness smoke, and while tobacco dependence has declined overall, rates have remained stable among smokers with serious mental illness (Dickerson, Stallings, Origoni, Vaughan et al., 2013; Fiore et al., 2008; Leonard & Adams, 2006; Smith, Mazure, & McKee, 2014). Smoking is the largest contributor to early mortality in serious mental illness (Auquier, Lancon, Rouillon, Lader, & Holmes, 2006; Bushe, Taylor, & Haukka, 2010; Carney & Jones, 2006; Carney, Jones, & Woolson, 2006; Dickerson, Stallings, Origoni, Schroeder et al., 2013; Dixon et al., 2007; Hennekens, Hennekens, Hollar, & Casey, 2005; Himelhoch et al., 2004; Jeste, Gladsjo, Lindamer, & Lacro, 1996; Kelly et al., 2009; Kelly et al., 2010; Kelly et al., 2012; Miller, Paschall, & Svendsen, 2006; Muir-Cochrane, 2006; Newcomer, 2006; Sokal et al., 2004). The single change that could do the most to decrease mortality risk and improve health-related quality of life for individuals with serious mental illness is for those who smoke to quit (Compton, Daumit, & Druss, 2006; Williams, Steinberg, Griffiths, & Cooperman, 2013).
Smoking cessation interventions help some smokers with serious mental illness quit in the short term, but relapse rates are high (Bennett, Wilson, Genderson, & Saperstein, 2013; Dixon et al., 2010; Tsoi, Porwal, & Webster, 2013a, 2013b). Individuals with serious mental illness experience many barriers— from a lack of attention to smoking within mental health services (Himelhoch & Daumit, 2003; Himelhoch, Riddle, & Goldman, 2014; Lawn & Campion, 2013; Lucksted, Dixon, & Sembly, 2000; Prochaska, 2010a; Ratschen, Britton, & McNeill, 2011), to biological connections that link nicotine with improvements in cognitive functioning (Shim et al., 2012), to low confidence in the ability to quit (Mann-Wrobel, Bennett, Weiner, Buchanan, & Ball, 2010)—that make cessation exceptionally challenging. Medications such as bupropion and nicotine replacement therapies have been found to be beneficial (Bennett et al., 2013; Dixon et al., 2010; Tsoi, Porwal, & Webster, 2013a, 2013b) and are the recommended smoking cessation intervention for this group (Buchanan et al., 2010), yet many with serious mental illness experience additional medications as burdensome and/or report negative opinions of or experiences with nicotine replacement therapy (Esterberg & Compton, 2005). Others may experience medical conditions that preclude use of smoking cessation medications. In addition, cognitive or social deficits that are experienced by many individuals with serious mental illness can compromise the effective use of medications as well as interfere with learning skills and strategies for coping without smoking. For example, deficits in attention, memory, and problem-solving ability, as well as social impairments such as asociality, may make it difficult for people with serious mental illness to use smoking cessation medications accurately (e.g., not using medications appropriately, early discontinuation, running out of medication and not getting prescriptions refilled, putting a new nicotine patch on each morning) or to ask for help if needed. These factors may keep many with serious mental illness from trying to quit. For individuals with serious mental illness who are unwilling or unable to use medications, other options for smoking cessation must be available.
There are few other evidence-based options for smoking cessation for individuals with serious mental illness. Telephone quit lines are widely available and there is evidence for their effectiveness in general population smokers (Centers for Disease Control and Prevention, 2014), but there are fewer data in serious mental illness, and mental health service providers rarely refer individuals with serious mental illness to them (Himelhoch et al., 2014). Research focused on predominantly psychosocial interventions for smoking cessation in serious mental illness is sparse, and there is no psychosocial intervention program that has a sufficient evidence base to recommend it (Dixon et al., 2010). Current best practices for smoking cessation emphasize offering counseling, including support for quitting, assistance with setting a quit date, and discussion about ways to cope with cravings, to all smokers (Fiore et al., 2008). The literature on psychosocial interventions for smoking cessation in serious mental illness has included behavioral and motivational strategies, both alone and in combination with bupropion and/or nicotine replacement therapy, and found such interventions beneficial in terms of generating change. Behavioral interventions that involve learning skills for coping with negative affect and problem solving high-risk situations are effective with primary substance abusers (Bickel, Amass, Higgins, Badger, & Esch, 1997; Epstein, Hawkins, Covi, Umbricht, & Preston, 2003), substance abusers with dual diagnoses (Bellack, Bennett, Gearon, Brown, & Yang, 2006; Bennett, Bellack, & Gearon, 2001), and general population smokers (Stitzer, 1999). Bennett et al. (2013) reviewed a range of psychosocial treatment studies for smoking cessation in schizophrenia and concluded that the literature to date supports the use of behavioral strategies such as psychoeducation, relapse prevention, cognitive behavioral therapy, and skills building to help individuals with serious mental illness change their smoking in meaningful ways, although such interventions show equivalent outcomes to psychosocial interventions based on support (George et al., 2000), medication management (Baker et al., 2006), or routine care (Williams et al., 2010) in schizophrenia samples. In addition, there is evidence that incentives and contingency management procedures can reduce cigarette smoking as measured by either breath carbon monoxide or urine cotinine (Ferron, Alterman, McHugo, Brunette, & Drake, 2009; Sigmon & Patrick, 2012). Developing and testing psychosocial interventions for smoking cessation is of critical importance. Such interventions are appropriate for all individuals with serious mental illness regardless of interest in or ability to use bupropion/nicotine replacement therapy or level of readiness to quit. Those who are ambivalent or are unwilling to set a quit date may be willing to attend a behavioral intervention in which they learn and practice skills for now or in the future. Many behavioral strategies have shown promise in reducing smoking among people with serious mental illness (Bennett et al., 2013; Ziedonis et al., 2008). Because individuals with serious mental illness often have long smoking histories and high levels of nicotine dependence, they may require more extended interventions (Ziedonis et al., 2008). Combining behavioral strategies into an intensive program for smoking cessation, with frequent offers to use bupropion/nicotine replacement therapy and assistance with their use as needed, may offer the best chance to affect smoking abstinence.
An additional complication for individuals with serious mental illness is that most smoking cessation programs are not integrated within mental health service settings (Prochaska, 2010b), despite the recognition that integrated services for substance use and mental health disorders are recommended for individuals with serious mental illness (Drake, Mueser, & Brunette, 2007). Integrating smoking cessation within mental health care can be done by locating services in mental health clinics, promoting smoking as a focus of intervention by mental health professionals, and coordinating smoking cessation with mental health care. Such strategies promote smoking cessation, support individuals’ efforts at change, and highlight the ways that smoking cessation can foster improvements in health and well-being that can have important benefits for mental health recovery for those with serious mental illness. Unfortunately, this level of integration is rarely provided.
In the present study, we examined the effectiveness of a multifaceted, tailored behavioral intervention that was integrated within outpatient mental health services at three VA medical centers in a sample of smokers with serious mental illness. Participants were randomly assigned to either the behavioral intervention or a supportive comparison intervention that provided smoking cessation services in line with best practices. We examined group differences in treatment engagement, attendance, and smoking abstinence and reduction outcomes. We hypothesized that the behavioral intervention would be associated with better engagement and attendance and would be superior to the supportive condition on abstinence, number of cigarettes smoked, levels of nicotine dependence, and making quit attempts. Exploratory analyses in a subsample of participants who attended three or more intervention meetings examined changes in self-efficacy over time and when during the study period participants reported meaningful reductions in smoking and a first quit attempt.
METHODS
Participants
Participants were veterans with serious mental illness attending outpatient mental health programs at the Baltimore, Perry Point, and Washington, DC, Veterans Administration Medical Centers. Participants had a DSM-IV diagnosis of schizophrenia spectrum disorders, affective psychosis, other psychotic disorder, major depression with psychotic features, or post-traumatic stress disorder (PTSD; Department of Veterans Affairs, Veterans Health Administration, 2012). To ensure functional impairment in line with serious mental illness, participants with PTSD had to have worked less than 25% of the past year or received disability payments for PTSD. Other inclusion criteria were age 18 to 75 and nicotine dependence defined by self-reported smoking 10 or more cigarettes/day or a score of 5 or higher on the Fagerström Test of Nicotine Dependence (FTND; Heatherton, Kozlowski, Frecker, & Fagerström, 1991). Exclusion criteria included current problem drinking or drug use, defined as a score of 5 or higher on the Michigan Alcohol Screening Test (Selzer et al., 1971) or 6 or higher on the Drug Abuse Screening Test (Skinner et al., 1982), and a documented history of serious neurological disorder, head trauma, or severe/profound intellectual disability. Participants continued their regular mental health care including use of psychoactive medications. Study staff maintained contact with participants’ mental health treatment teams to discuss issues as needed.
Figure 1 shows the study consort diagram. Overall, 412 individuals were screened for preliminary eligibility, while 178 (43.20%) signed informed consent, completed baseline assessments, and were randomized to study condition. Of these, 136 (76.4%) completed the post-treatment assessment.
FIGURE 1.
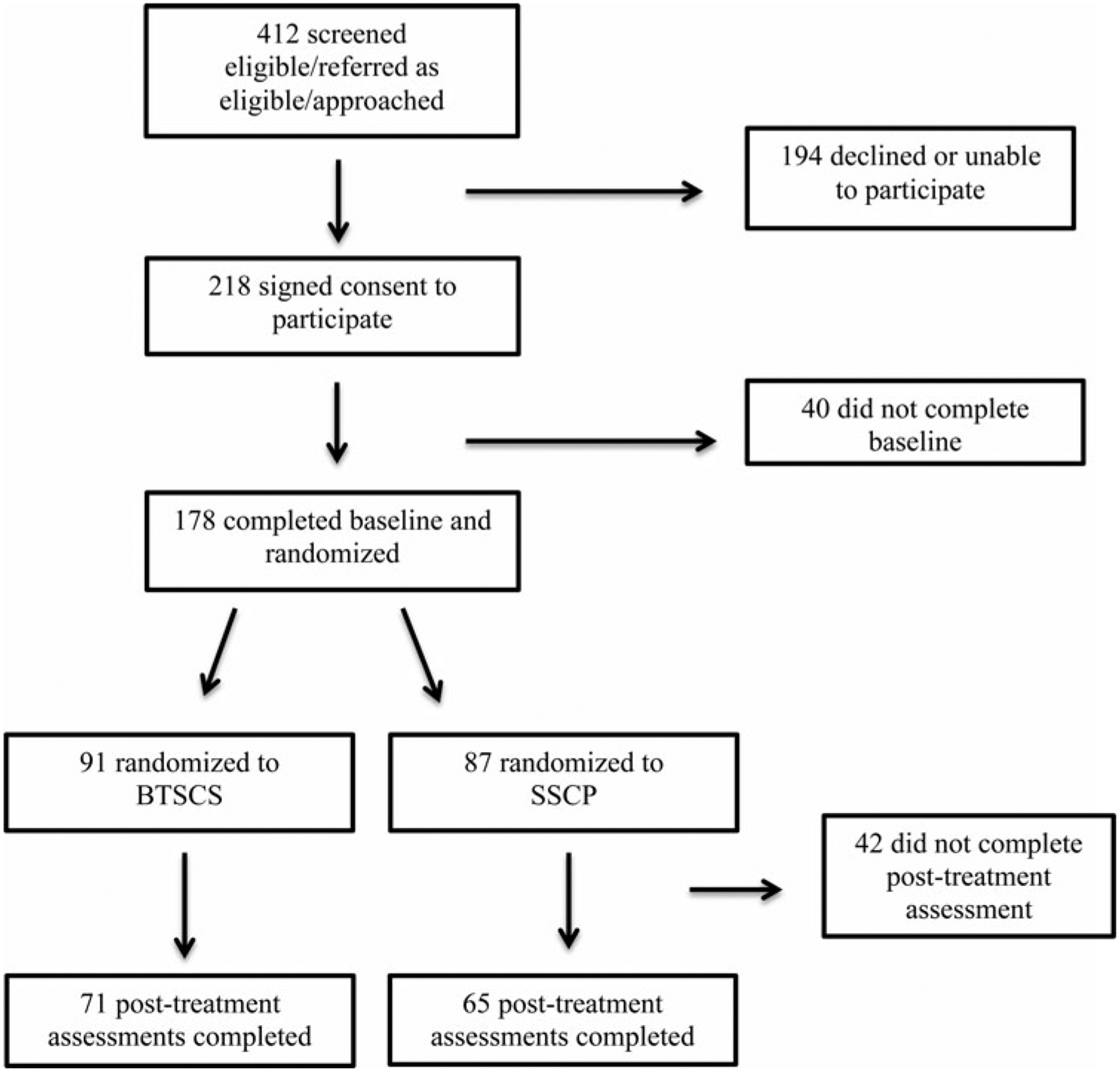
Study consort diagram.
Interventions
Behavioral Treatment of Smoking Cessation in Serious Mental Illness (Bellack et al., 2006; Bennett, Dixon, & Dickerson, 2008) is a multifaceted intervention for individuals with seri ous mental illness. Participants completed an individual meeting at the start of the intervention to review personal negative consequences from smoking and identify personally meaningful reasons for change. Each group meeting started with breath carbon monoxide monitoring in which participants received a small financial reward for values less than 10 ppm. Participants were tested on site and the results were told to the participant immediately. Those with test results less than 10 ppm indicating no smoking during the last six hours received social (i.e., praise and support) and financial reinforcement that began at $1.50 and was increased by $0.25 (to a maximum of $3.50) each time the participant achieved negative test results in two successive scheduled meetings; it was reset to $1.50 after a positive result. While this modest sum would not drive behavior change, the combined financial and social reinforcement and attention to nonsmoking was intended to provide a tangible reward for success, increase the salience of goals for reduced use, secure a valid report of recent smoking, and encourage the development of peer support. Groups also included goal setting, in which participants created a concrete goal to work on between meetings, and skills training aimed at helping people cope with quitting, refusing offers to smoke, and coping with high-risk situations. The behavioral treatment offered ongoing assistance with use of bupropion or nicotine replacement therapy if desired by the participant.
The Supportive Smoking Cessation Program is an active comparison group that provides support for quitting within a nonjudgmental atmosphere. The supportive program groups were modeled after those of Addington (1998) and modified using educational materials from the American Cancer Society Fresh Start Program (Weiner, Ball, Summerfelt, Gold, & Buchanan, 2001). Each meeting included a topic (e.g., support for quitting; harm from smoking; smoking as a habit; barriers and confidence) that was addressed via discussion, education, and assistance with planning to quit. Participants completed a breath carbon monoxide test at the start of each meeting with no feedback of results or financial reinforcement.
Admission was done on a rolling basis. Both conditions were delivered by trained interventionists with substantial experience working with individuals with serious mental illness and substance use; different interventionists led each group. Both conditions offered 24 twice-weekly group meetings, one of which was focused on the negative health consequences of smoking and the health benefits of quitting. Across conditions, participants were asked to set a quit date at each meeting but were not required to do so. Both conditions included two weeks of engagement-focused outreach with reminder calls and assistance with transportation if needed. Both also included basic education on medication options for smoking cessation provided by a trained study nurse or physician assistant; extended bupropion/nicotine replacement therapy support was a component of the behavioral treatment and not the supportive program. Basic education on medication use included providing information and encouragement to use bupropion and/or nicotine replacement therapy to aid in smoking cessation. Participants were not required to use medications; the emphasis of the basic education and the more extended support provided in the behavioral treatment condition was one of psychoeducation, facilitation, and monitoring/problem solving. In the behavioral treatment condition, study personnel could prepare a participant to talk with his/her prescriber on his/her own, provide assistance in setting up an appointment with their prescriber, and/or follow up as needed to ensure that the prescription was filled and obtained.
Bupropion and nicotine replacement therapy were not prescribed by study personnel. For those participants who expressed an interest in using either bupropion or nicotine replacement therapy, study personnel provided assistance contacting the participant’s mental health treatment team, who would do the prescribing. The ultimate decision about a participant’s medical appropriateness to use medications for smoking cessation, as well as monitoring for safety of these medications, resided with the treatment teams who regularly worked with and prescribed medications for these participants. Participants could obtain these medications free of charge when prescribed by their treatment team.
The basic education about medications and the more extended support provided in the behavioral treatment condition did not include information about varenicline, as varenicline was not listed as a first- or second-line intervention for smoking cessation within the VA pharmacy at the start of the study period. Participants who inquired about varenicline were instructed to speak with their mental health treatment team to receive additional information. Participants who were taking varenicline prescribed by their mental health treatment team could continue in the study, and we would assist with their use of varenicline as we did for other smoking cessation medications.
Measures
Diagnostic, Demographic, and Clinical Information
Demographic information collected via self-report included age, gender, race, marital status, years of education, and employment status. Psychiatric diagnosis was obtained from medical records. If the diagnosis was unclear, the participant’s treatment team was consulted for additional information so a determination could be made. Psychiatric symptoms were assessed at baseline and post-treatment with the Brief Psychiatric Rating Scale (Overall & Gorham, 1962), a reliable and valid 20-item interviewer-rated scale to assess change in severity of psychopathology with an emphasis on symptoms of psychotic illnesses. Items are rated on a 7-point scale from not present to extremely severe.
Smoking and Attendance Outcome Variables
The Smoking History Form (SHF) assessed smoking and quitting variables at baseline and post-treatment. SHF items are commonly used; some are drawn from the National Health and Nutrition Examination Survey (Department of Health and Human Services, 1998). The FTND (Heatherton et al., 1991) measured nicotine dependence at both time points. Biological assessment of smoking was measured by expired carbon monoxide with the EC 50 Micro III Smokerlyzer Breath Carbon Monoxide Monitor (Bedfont Instruments). These measures were used to assess four smoking-related outcomes: abstinence (self-reported no smoking in the last 7 days + expired carbon monoxide ≤ 10 ppm), number of cigarettes smoked per day during the last 7 days, nicotine dependence (FTND total score), and whether the participant made at least one quit attempt between baseline and post-treatment (yes, no).
At each treatment meeting, the following data were collected: attendance (yes, no), self-reported smoking since the last meeting (yes, no); average number of cigarettes smoked per day since the last meeting; expired carbon monoxide; use of nicotine replacement therapy (patch, gum, lozenges) or medication (bupropion) since the last meeting (yes, no); and approximately how many times each was used. Completion of outreach and medication education meetings was also tracked. These items were used to measure (1) abstinence since last meeting (defined as self-reported no smoking since the last group meeting + expired carbon monoxide ≤ 10 ppm) and (2) abstinence for 1-, 2-, and 4-week blocks (defined as self-reported no smoking since the last group meeting + expired carbon monoxide ≤ 10 ppm for either 2, 4, or 8 consecutive meetings).
Self-Efficacy for Quitting
The Temptation to Smoke Scale and the Abstinence Self-Efficacy Scale, Smoking Version (DiClemente, Prochaska, & Gibertinin, 1985) were completed at both time points to assess the degree to which participants felt “tempted” to smoke in different situations and confident in their ability to abstain in those situations. Both scales have 20 items rated with 5-point Likert scales. Scale scores are computed separately for four subscales (Negative Affect, Social/Positive Influences, Physical and Other Concerns, and Withdrawal and Urges). Psycho-metric properties of these scales are strong (DiClemente et al., 1985). In addition, the SHF included two variables assessing confidence to quit smoking now and in the future.
Procedures
This study was approved by the institutional review boards at the University of Maryland School of Medicine and the Washington, DC Veterans Affairs Medical Center and the VA Maryland Healthcare System Research and Development Committee. Participants were recruited through medical records screening, clinician referrals, and posted flyers. Following referral and confirmation of inclusion criteria via review of the medical record and/or discussion with the individuals’ treatment teams, a study staff member met with potential participants, described the study, and, for those who were interested, completed written informed consent. The study was conducted in accordance with the Declaration of Helsinki, and the informed consent document included the name of the institutional review board that approved and monitored the study, outlined the study aims, what participants were being asked to do, that participation was voluntary and would not affect the mental health or medical care received at any VA medical center, and that participants could end their participation at any time. Participants completed the baseline assessment and were randomized to study condition using a permuted-block randomization with varying block sizes. Randomization was stratified by site (3 sites) and diagnosis (schizophrenia spectrum versus any other diagnosis). Randomization information was provided to the research assistant after the baseline assessment was completed. Post-treatment assessments were scheduled 12 weeks later. Assessments were administered by trained masters or doctoral level interviewers and took approximately 2.5 hours. Assessment training included rating videotaped interviews to establish reliability, co-rating of interviews administered by a trained assessor, and completion of interviews while being observed. Interviewers were supervised biweekly by a doctoral level researcher via review of videotaped interviews, making consensus ratings, and receiving corrective feedback. Interventionists were masters-level clinicians with substantial experience working with individuals with serious mental illness. They received weekly training for three months that included discussion of intervention materials, watching demonstrations, and conducting mock meetings and then participated in twice-monthly supervision that involved watching and discussing videotaped meetings and receiving corrections as needed.
Analysis Plan
Descriptive statistics were used to examine demographic and clinical characteristics, smoking history, and use of intervention components. We examined the number of meetings attended for each participant and calculated the percentage of the total number of meetings (24 meetings). Means were compared with t-tests. Comparisons of conditions on abstinence and reduction outcomes were performed on the randomized sample (n = 178) including all observations at the baseline and post-treatment assessments using routine mixed-effects models. The mixed models specified below generally assume missing values are “missing at random” (Little & Rubin, 2002). All analyses were performed using SAS version 9.4.
Abstinence and reduction outcomes were examined using baseline and post-treatment assessments (baseline-post data) and data from intervention meetings (meeting data). Baseline-post data were used to examine four variables (n = 178): abstinence (self-reported no smoking in the last 7 days + expired carbon monoxide ≤ 10 ppm), number of cigarettes smoked per day during the last 7 days, nicotine dependence (FTND total score), and whether the participant made at least one quit attempt between baseline and post-treatment (yes, no). Number of cigarettes smoked per day was skewed, so a natural log transformation was applied before analysis. To assess difference in change between conditions, we tested the significance (F-test) of the condition-by-time interaction using mixed models with a random participant effect. Time was binary: post-treatment versus baseline. We used a logistic model for binary outcomes and a linear model for continuous outcomes. A simple chi-square test was used for the quit attempt variable.
Meeting data (n = 152) were used to examine two outcomes. First, we compared treatment groups on abstinence since last meeting (defined as self-reported no smoking since the last group meeting + expired carbon monoxide ≤ 10 ppm), analyzed as a binary outcome (each participant assessed at each meeting) with a logistic repeated measures mixed model allowing comparison of aggregate rates between treatment conditions (F-test of the treatment group main effect). Second, we compared treatment groups on abstinence for 1-, 2-, and 4-week blocks (defined as self-reported no smoking since the last group meeting + expired carbon monoxide ≤ 10 ppm for 2, 4, or 8 consecutive meetings), Comparisons were made using Fisher’s exact tests. To achieve 1, 2, or 4 weeks of abstinence, participants were required to attend all meetings during the block.
Meeting data were used in exploratory analyses examining time-to-event outcomes in a subsample of participants who engaged in either treatment condition, defined as attending three or more meetings (n = 127): (1) time to 50% reduction in the number of cigarettes smoked (defined as the time until a participant self-reported smoking 50% fewer cigarettes compared to the number reported smoked during the last 7 days at baseline); and (2) time to first quit attempt (defined as the time until a participant first self-reported no smoking since baseline + expired carbon monoxide ≤ 10 ppm). We estimated survival curves for these variables for each treatment group with Kaplan-Meier curves and tested whether there was a difference between the two with log-rank tests. We also estimated the median event time with a 95% confidence interval. “Time” was measured as meeting number (meeting 1 to 24; time period ≈ 12 weeks). Since the two events are positive events (not negative, like death) we display 1 minus the survival probability over time, that is, the probability of achieving the event by a certain number of meetings for each treatment group. Participants who never met the definition for quitting or 50% reduction, whether they missed intervention meetings, were assigned an event time equal to 24 with the event-time designated as censored (censor indicator = 1 “yes” versus 0 “no”). Participants who did not attend were assumed not to have quit nor achieved a 50% reduction at that meeting.
Changes in self-efficacy, temptation, and confidence were examined in secondary analyses of the engaged sample using linear mixed models. F-tests of the group-by-time interaction were conducted first. If not significant, an F-test of the overall pre/post change was performed (after removing the interaction term).
RESULTS
Demographic and Clinical Characteristics of the Sample
Table 1 provides baseline characteristics for the total sample and by treatment group. The sample was predominantly male and African American, with a mean age in the mid-50s and a mean of 13 years of education. Overall, 43% (n = 77) of the sample had a schizophrenia spectrum diagnosis and 27.5% (n = 49) had a diagnosis of bipolar disorder; smaller percentages had diagnoses of depression with psychotic features (5%, n = 9), psychosis not otherwise specified (2.2%, n = 4), or PTSD (21.9%, n = 39). There was one difference by treatment group: The behavioral treatment had more women than the supportive program (χ2 = 6.59, df = 1, p = .010).
TABLE 1.
Demographic and Clinical Characteristics of the Sample at Baseline
| Variable | Total (N = 178) | BTSCS (n = 91) | SSCP (n = 87) |
|---|---|---|---|
| M(SD) | M(SD) | M(SD) | |
| Age | 54.8 (7.2) | 55.4 (7.4) | 54.1 (7.0) |
| Highest grade completed | 13.0(1.7) | 13.0(1.8) | 12.9(1.7) |
| BPRS scores (20 items) | 34.2 (8.2) | 34.3 (8.1) | 34.1 (8.3) |
| n (%) | n (%) | n (%) | |
| Gender* | |||
| Male | 159 (89.3%) | 76 (83.5%) | 83 (95.4%) |
| Female | 19(10.7%) | 15 (16.5%) | 4 (4.6%) |
| Race | |||
| Black | 126 (70.8%) | 64 (70.3%) | 62(71.3%) |
| White | 40 (22.5%) | 23 (25.3%) | 17 (19.5%) |
| Other | 12 (6.7%) | 4 (4.4%) | 8 (9.2%) |
| Marital status | |||
| Never married | 59(33.1%) | 23 (25.3%) | 36(41.4%) |
| Married | 35 (19.7%) | 19 (20.9%) | 16(18.4%) |
| Widowed, divorced, or separated | 84 (47.2%) | 49 (53.8%) | 35 (40.2%) |
| Employed | |||
| Yes | 22(12.4%) | 9 (10.0%) | 13 (14.9%) |
| No | 155 (87.6%) | 81 (90.0%) | 74(85.1%) |
| Diagnosis | |||
| Schizophrenia spectrum | 77 (43.3%) | 40 (44.0%) | 37 (42.5%) |
| Bipolar disorder | 49 (27.5%) | 22 (24.2%) | 27(31.0%) |
| Depression with psychotic features | 9(5.1%) | 6 (6.6%) | 3 (3.4%) |
| Psychosis not otherwise specified | 4 (2.2%) | 3 (3.3%) | 1 (1.1%) |
| Posttraumatic stress disorder | 39(21.9%) | 20 (22.0%) | 19(21.8%) |
Note. BTSCS = Behavioral Treatment for Smoking Cessation in Serious Mental Illness; SSCP = Supportive Smoking Cessation Program; BPRS = Brief Psychiatric Rating Scale.
There was a significant difference between groups for gender (χ2 = 6.46, df = 1, p = .011).
Descriptive Data on Smoking and Quitting
Table 2 lists descriptive data on smoking and quitting at baseline. On average, participants had been smoking since age 16, endorsed a moderate level of nicotine dependence, and smoked a mean of 15.2 cigarettes per day. Participants reported a mean of 4.9 lifetime “serious” quit attempts and 2.4 occasions of quitting for at least 24 hours. Almost all participants reported that they were considering quitting in the next 6 months (n = 164, 93%) and most (n = 114, 64.4%) were considering quitting in the next 30 days. There were no significant differences between treatment groups on any of these variables.
TABLE 2.
Descriptive Data on Smoking and Quitting at Baseline
| Variable | Total (N = 178) | BTSCS (n = 91) | SSCP (n = 87) |
|---|---|---|---|
| M(SD) | M(SD) | M(SD) | |
| Age began smoking | 16.5 (6.5) | 16.5 (7.0) | 16A (6.0) |
| Cigarettes smoked per day | 15.2 (9.8) | 15.6 (9.7) | 14.9 (10.0) |
| Fagerström total score | 7.3 (2.0) | 7.4(1.9) | 7.3 (2.1) |
| Times quit for 24 hours (last year)1 | 2.4 (2.8) | 2.3 (2.9) | 2.5 (2.8) |
| Serious quit attempts (lifetime) | 4.9 (7.8) | 5.1 (7.6) | 4.8 (8.0) |
| n (%) | n (%) | n (%) | |
| Ever quit for 24 hours | |||
| Yes | 165 (92.7%) | 85 (93.4%) | 80 (92.0%) |
| No | 13 (7.3%) | 6 (6.6%) | 7 (8.0%) |
| Considering quitting (6 mos) | |||
| Yes | 164 (92.7%) | 85 (94.4%) | 79 (90.8%) |
| No | 13 (7.3%) | 5 (5.6%) | 8 (9.2%) |
| Considering quitting (30 days) | |||
| Yes | 114(64.4%) | 61 (67.8%) | 53 (60.9%) |
| No | 63 (35.6%) | 29 (32.2%) | 34(39.1%) |
Note. There were no significant differences between treatment conditions. BTSCS = Behavioral Treatment for Smoking Cessation in Serious Mental Illness; SSCP Supportive Smoking Cessation Program.
More than=9 quit attempts coded as 9.
Use of Intervention Components
The mean number of group meetings attended for the total sample was 9.3 (SD = 7.8, range: 0 to 24); there was no difference between conditions (behavioral treatment = 9.9, SD = 7.9; supportive program = 8.7, SD = 7.7; t = 1.06, ns) Overall, 127 participants (71%) engaged in study interventions; there was no difference between conditions (behavioral treatment = 74.7%; supportive program = 67.8%; χ2 = 1.04, df = 1, ns). Participants received a mean of 3.7 outreach contacts (SD = 3.0), with those in supportive program receiving more outreach contacts (M = 4.7, SD = 3.4)than those in the behavioral treatment (M = 3.2, SD = 2.7); t(102) = −2.27, p = .027. The most frequently provided form of outreach was a phone call to remind participants to attend intervention meetings. Participants received a mean of 1.8 bupropion/nicotine replacement therapy education contacts (SD =1.2), with those in behavioral treatment receiving significantly more (M = 2.2, SD = 1.4) than those in the supportive program (M = 1.4, SD = 0.8); t(84) = 3.87, p < .0001. This difference was expected because extended bupropion/nicotine replacement therapy support was a component of behavioral treatment and not the supportive program.
In the total sample, 72 participants (47.4% of the total sample) tried bupropion/nicotine replacement therapy at some point during the study period. Overall, 32 participants (21% of the sample) used the nicotine patch at least once, 19 participants (12.4% of the sample) used the nicotine lozenge at least once, 16 participants (10.5% of the sample) used nicotine gum at least once, and 14 participants (9.2% of the sample) used bupropion at least once; 11 participants (7.2% of the sample) tried some other form of nicotine replacement therapy or other medication at least once during the study period. More participants in the behavioral treatment tried bupropion/nicotine replacement therapy at some point during the study period (n = 48, 62.3%) than those in the supportive program (n = 24, 32%); χ2 = 14.03, df = 1, p < .0001. Those in behavioral treatment were more likely to use the nicotine patch during the study period (n = 26, 33.3%) than those in the supportive program (n = 2, 8.0%); χ2 = 14.84, df = 1, p < .0001. More participants in behavioral treatment used bupropion/nicotine replacement therapy three or more times during the study period (n = 40; 51.9%) than those in the supportive program (n = 21; 28%); χ2 = 9.07, df = 1, p = .003.
Abstinence and Reduction Outcomes Using Baseline-Post Data
Outcomes using baseline-post data (n = 178) are listed in Table 3. Overall, 16 (11.8%) participants met abstinence criteria at post-treatment. The number of cigarettes smoked per day decreased from a mean of 15.2 (SD = 9.8) at baseline to a mean of 7.5 (SD = 7.7) at post-treatment; F(1,135) = 104.08, p < .0001. FTND total scores decreased from a mean of 7.3 (SD = 2.0) at baseline to a mean of 5.2 (SD = 2.7) at post-treatment; F(1,126) = 70.43, p < .0001. When analyzed using only participants who did not quit (i.e., excluding the 16 participants who met the definition of quit at post-treatment, n = 162), changes in the number of cigarettes smoked per day from baseline to post-treatment remained significant, although there was slightly less reduction at post-treatment. Overall, 88 participants (72.7%) made a quit attempt during the study intervention period. There were no differences between conditions on these variables.
TABLE 3.
Smoking Cessation Outcomes Using Baseline-Post Data
| Baseline Assessment | Post-Treatment Assessment | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Total (n = 178) | BTSCS (n = 91) | SSCP (n = 87) | Total (n = 136) | BTSCS (n = 71) | SSCP (n = 65) | Test | df | Value | P |
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |||||
| Smoking abstinence1 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 16(11.8) | 10(14.1) | 6 (9.2) | F | 1/134 | .78 | .379 |
| Made a quit attempt during treatment | 88 (72.7) | 47 (73.4) | 41 (71.9) | χ2 | 1 | .03 | .853 | |||
| M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | |||||
| Number cigarettes smoked/day2 | 15.2(9.8) | 15.6 (9.7) | 14.9 (10.0) | 7.5 (7.7) | 7.6 (8.6) | 7.5 (6.7) | F | 2/133 | .75 | .473 |
| Fagerström total | 7.3 (2.0) | 7.4(1.9) | 7.3(2.1) | 5.2 (2.7) | 5.1 (3.0) | 5.3 (2.3) | F | 2/124 | .46 | .633 |
Note. Comparisons were made using a logistic mixed effects model for the “No smoking last 7 days” and a linear mixed model for both number of cigarettes smoked (after natural log transformation) and Fagerström total. Chi-square test was used for “Made a quit attempt during treatment.”
BTSCS = Behavioral Treatment for Smoking Cessation in Serious Mental Illness; SSCP = Supportive Smoking Cessation Program.
Defined as self-reported no smoking last 7 days + PPM ≤ 10.
Defined as self-reported number of cigarettes smoked per day in the last 7 days.
Abstinence and Reduction Outcomes Using Meeting Data
Outcomes using meeting data (n = 152) are presented in Table 4. Participants attended meetings and met the definition for being abstinent for a mean of 1.3 (SD = 3.4) meetings in behavioral treatment and a mean of 1.0 (SD = 2.4) meetings in the supportive program (t = .62, df = 133.3, p = .54). Overall, 11%, 6.6%, and 2% of the total sample had at least one 1-, 2-, and 4-week block of abstinence, respectively. There were no differences by condition on these variables. There was also no difference between conditions in the aggregate proportion of attended sessions with breath carbon monoxide < 10 ppm (.71 in behavioral treatment and .77 in the supportive program); F(1,1430) = .33, p = .56.
TABLE 4.
Smoking Cessation Outcomes Using Meeting Data (n = 152)
| Variable | Total (n = 152) | BTSCS (n = 78) | SSCP (n = 74) | Test | df | Value | P |
|---|---|---|---|---|---|---|---|
| Quit since last meeting1 | .018 | .017 | .019 | F | 1,1373 | .04 | .84 |
| 1-week block [n(%)] | 17(11.2) | 9(11.5) | 8 (10.8) | Fisher’s Exact | — | — | > .99 |
| 2-week block [n(%)] | 10(6.6) | 7 (9.0) | 3 (4.1) | Fisher’s Exact | — | — | .33 |
| 4-week block [n(%)] | 3 (2.0) | 2 (2.6) | 1 (1.4) | Fisher’s Exact | — | — | > .99 |
Note. BTSCS = Behavioral Treatment for Smoking Cessation in Serious Mental Illness; SSCP = Supportive Smoking Cessation Program.
Defined as self-reported no smoking since the last group meeting + expired carbon monoxide ≤ 10 ppm. Value is the proportion of the total number of meetings attended by all participants in each group (estimated using a logistic mixed-effects model).
Survival Analyses
Survival curves for two variables (time to 50% reduction in smoking and time to first quit) are shown in Figure 2. There were no differences between treatment groups on either variable (log-rank p’s = .50 and .39, respectively). For time to 50% reduction in the number of cigarettes smoked, 87% of the sample reported smoking 50% fewer cigarettes as compared to baseline at some point during the study period. The median time to achieve this reduction was equal to 4 intervention group meetings (95% confidence interval: 4 to 6 meetings). For “time to first quit,” 31% of the sample met the definition of quitting at least once during the study period. Median time until first quit attempt could not be calculated since fewer than 50% of the sample made a quit attempt.
FIGURE 2.
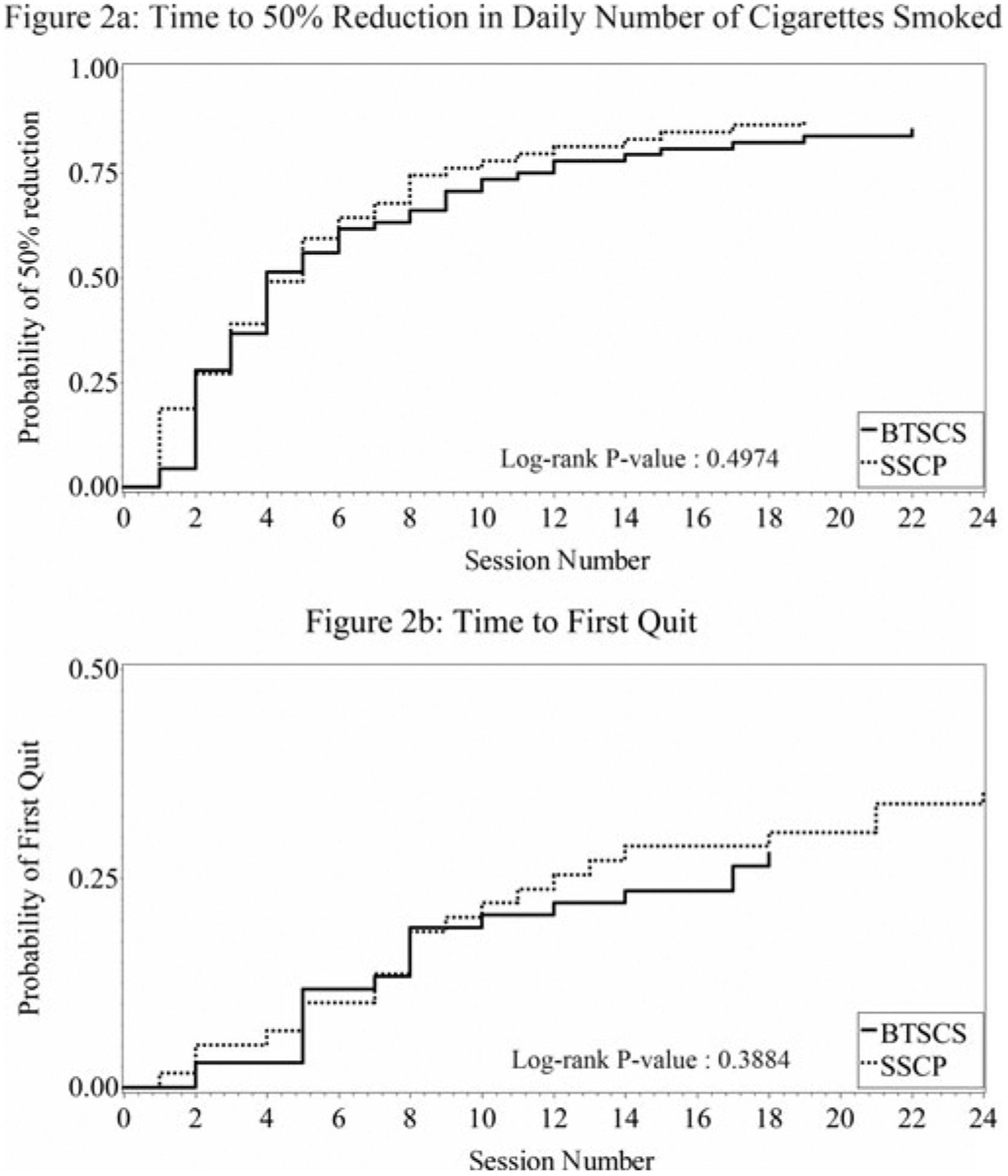
Cumulative probability of achieving 2 positive events based on Kaplan-Meier curves. 2a: Time to 50% Reduction in Daily Number of Cigarettes Smoked. 2b: Time to First Quit
Changes in Temptation and Self-Efficacy
Table 5 presents changes in temptation to smoke and self-efficacy for nonsmoking in the engaged sample. Participants reported decreased temptation to smoke, increased self-efficacy for nonsmoking, and increased confidence in their ability to quit smoking in the future. There were no differences by condition. In exploratory analyses, greater attendance was associated with greater increases in self-efficacy for nonsmoking when the interaction between time (i.e., post-treatment vs. baseline) and attendance was added to the main model (t = 2.28, df = 131, p = .0241). Causality for this association cannot be determined given the design of the study.
TABLE 5.
Changes in Temptation and Self-Efficacy From Baseline to Post-Treatment in the Engaged Sample (n = 127)1
| Variable | Baseline (n = 127) | Post-treatment (n = 104) | df | F | P | Effect size2 |
|---|---|---|---|---|---|---|
| Temptation to smoke, total score | 3.6 (0.6) | 3.2 (0.8) | 1,102 | 34.91 | < .001 | .70 |
| Abstinence self-efficacy, total score | 2.4 (0.7) | 2.8 (0.9) | 1,100 | 19.74 | < .001 | .57 |
| Confidence in ability to quit smoking now | 3.0 (1.1) | 3.2 (1.2) | 1,90 | 3.72 | .06 | .24 |
| Confidence in ability to quit smoking in the future | 6.9 (2.7) | 7.6 (2.9) | 1,102 | 6.02 | .016 | .26 |
Note. There were no differences by treatment condition.
Pre/post comparisons made using linear mixed models.
Effect size equals model-based mean change divided by pooled standard deviation at baseline.
DISCUSSION
This study examined 1-week smoking abstinence and reduction outcomes in individuals with serious mental illness who attended psychosocial smoking cessation programs embedded within VA mental health services. Overall, 178 individuals agreed to participate, with most engaging in the program to which they were assigned. Participants had a long history of smoking and reported many quit attempts, in line with other work (Lucksted et al., 2000; Dickerson et al., 2011). A small percentage of the sample (11.7%) achieved abstinence, smoking quantity was significantly reduced (from 15 to 7 cigarettes per day), nicotine dependence scores decreased, 11% had at least one 1-week block of abstinence, and 72% reported making a quit attempt while they were engaged in either the behavioral or supportive program. There were no instances of symptom exacerbations due to smoking reduction or cessation. As participants were not required to use medications or nicotine replacement therapy and were not selected based on stated readiness to quit, it is encouraging that many reduced their smoking and sampled quitting during their involvement in the study.
Contrary to our hypotheses, there were no differences by condition on any abstinence or reduction outcomes. Both interventions were active treatments delivered by experienced clinicians who received ongoing supervision. Given the known standard of care for smoking cessation (Fiore et al., 2008), both conditions shared important features, including education about the negative effects of smoking and the benefits of quitting, discussion about coping with cravings and preparing to quit, and encouragement from interventionists and peers. Our findings suggest that programs that include these strategies can help participants get started on quitting via marked reductions in the number of cigarettes smoked. Implementation of smoking cessation services within mental health services is feasible and, if delivered in line with best practices, either a behavioral or a supportive approach is likely to be helpful.
These findings are similar to other psychosocial intervention studies. Addington (1998) offered smokers with serious mental illness seven weeks of psychosocial intervention plus nicotine replacement therapy and found that 42% quit smoking for at least four weeks. Ziedonis and George (1997) provided smokers with serious mental illness a behavioral group intervention plus nicotine replacement therapy and found that 40% decreased cigarette use by half and 17% had at least one episode of weekly abstinence. George et al. (2000) assigned smokers with serious mental illness to one of two psychosocial group interventions plus nicotine replacement therapy and found that 35.6% achieved abstinence; there were no differences between interventions. Baker et al. (2006) assigned smokers with serious mental illness to either routine care or an 8-session individual intervention that included nicotine replacement therapy, motivational interviewing, and cognitive behavioral strategies and found that 15% of those in the experimental intervention were abstinent at post-treatment. Williams et al. (2010) assigned individuals with schizophrenia spectrum disorders to 26 weeks of nicotine replacement therapy plus either a group intervention that included motivational interviewing, skills training, and relapse prevention or medication management; 21% of participants reported abstinence at follow-up, with no differences between conditions.
Neither of our interventions required use of nicotine replacement therapy or medications such as bupropion, the intervention with the strongest evidence base in serious mental illness (Buchanan et al., 2010). There is evidence that nicotine replacement therapy is associated with better outcomes when used in conjunction with a smoking cessation program in general population smokers (Moore et al., 2009) and in smokers with serious mental illness (Bennett et al., 2013, Currie et al., 2008). All of the psychosocial trials described above included use of nicotine replacement therapy as a central component of their programs. In the Addington trial (1998), nicotine patches were offered to all participants and 20 of 21 successful quitters used them. Ziedonis and George (1997) offered nicotine replacement therapy to all participants and more than 80% tried it in some form. George et al. (2000) required that participants set a quit date and use a nicotine patch. In the Baker et al. trial (2006), discussion and distribution of nicotine replacement therapy were part of every meeting, and in the Williams et al. trial (2010), all participants received nicotine replacement therapy for 16 weeks with good compliance. Importantly, use of nicotine replacement therapy was associated with quitting in these trials. In our study, use of nicotine replacement therapy was not required. Those in behavioral treatment received enhanced medication education services and tried medications or nicotine replacement therapy at higher rates than those in the supportive program and more often used these agents three or more times. Taken together, these findings suggest that use of nicotine replacement therapy by individuals with serious mental illness engaged in psychosocial smoking cessation programs is to be encouraged. While there are many reasons that use of medications and nicotine replacement therapy should not be required, it could be that we need to find a middle ground by which to assertively yet collaboratively communicate the ways that use of nicotine replacement therapy could enhance success. While we provided assistance and repeatedly asked about using bupropion or nicotine replacement therapy, it could be that an additional meeting or set of meetings, using principles of shared decision making (Deegan & Drake, 2006; Kreyenbuhl, Nossel, & Dixon, 2009) focused specifically on use of bupropion or nicotine replacement therapy, may help individuals make the decision to try these agents. In addition, there has been growing evidence for the safety and efficacy of varenicline in individuals with serious mental illness (Evins, Cather, & Laffer, 2015; Evins et al., 2014; Williams et al., 2012); moving forward, this medication can be offered more widely to those engaged in integrated smoking cessation services.
The survival analyses in the engaged sample indicated that participants reduced the number of cigarettes smoked within the first two weeks of programming. However, these early reductions did not translate into quitting, and we did not have enough participants who met abstinence criteria to allow us to examine when initial quitting was most likely to occur. Those in the engaged sample also showed increased self-efficacy for quitting over time. These findings illustrate different points in smoking cessation in serious mental illness: transitioning from reduction to attempting to quit and then using improved self-efficacy to build these attempts into sustained cessation. Facilitating the transition from reduction to attempting to quit might require a change in intervention. While our programs continued regardless of where a participant was in the change process, it could be that some skills building or support is needed to get people to reduce their smoking but that, once this reduction has been made, continued skills building or support may not be helpful in moving an individual toward initial quitting. A switch from group to individual meetings or from skills/support to motivational interviewing or shared decision making approaches in which personal reasons for change and an individual’s own ambivalence can be explored may encourage individuals to use skills they have learned in support of an initial quit attempt. There is growing evidence for these approaches in smokers with serious mental illness (Brunette et al., 2011); these results suggest that there may be particular times within the treatment process to deploy these approaches for optimal effect. Or, individuals with serious mental illness may be more open to using medications or nicotine replacement therapy once they have been successful at cutting down on their smoking, such that providing education about these agents in weeks 5 or 6 may be more effective. It could be that experiencing some initial success with smoking reduction may make individuals more willing to try medication or nicotine replacement therapy in order to convert reductions in smoking to quitting and abstinence, especially if use of medication is described as time-limited. Another possibility is that those who make early reductions in smoking may best be served by simultaneous use of multiple treatment strategies. There is evidence that use of multiple strategies such as behavioral interventions, bupropion, and nicotine replacement therapy, in combination, may yield higher short- and longer-term quit rates (Evins et al., 2014). Time to relapse in the population remains high (Cather et al., 2013), and further work needs to address ways to change or extend interventions over time to reduce relapse rates.
This study had several limitations. The sample was mostly male, African American, and recruited at VA sites; it is unclear whether these findings would generalize to community mental health settings. The VA has implemented large-scale screening for smoking (Duffy et al., 2012), many of which may have simplified the process of establishing our programs and recruiting participants. In addition, many individuals were approached for participation but could not be found or declined, suggesting that our sample is not representative of all smokers with serious mental illness but rather of a subsample that is interested in learning strategies for quitting. Finally, chart diagnoses were used to determine eligibility rather than administering structured diagnostic interviews.
While participants generally had documented histories of significant psychiatric symptoms and hospitalizations, use of a structured interview would have provided greater certainty that participants met DSM-5 criteria for serious mental illness diagnoses.
These findings suggest that psychosocial smoking cessation programs can be implemented within mental health treatment settings and that individuals with serious mental illness will engage in these programs and reduce their smoking. Initial reductions do not take a long time and are not associated with increased symptoms of mental illness. We need to understand how to convert reductions in smoking into quit attempts. Thinking flexibly and creatively about how to structure interventions so that strategies can change as individuals change may yield a more successful approach.
ACKNOWLEDGMENTS
We gratefully acknowledge the veterans who participated in this research. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
FUNDING
Support for this research was provided by grant NURA-003-09S from the Clinical Science Research and Development Service of the US Department of Veteran Affairs (VA) and the VA Capitol Health Care Network VISN5 Mental Illness Research, Education and Clinical Center.
Footnotes
DISCLOSURES
Drs. Bennett, Dixon, Brown, Himelhoch, and Bellack and Ms. Li report no financial relationships with commercial interests.
REFERENCES
- Addington J (1998). Group treatment for smoking cessation among persons with schizophrenia. Psychiatric Services, 49, 925–928. [DOI] [PubMed] [Google Scholar]
- Auquier P, Lancon C, Rouillon F, Lader M, & Holmes C (2006). Mortality in schizophrenia. Pharmacoepidemiology and Drug Safety, 15(12), 873–879. [DOI] [PubMed] [Google Scholar]
- Baker A, Richmond R, Haile M, Lewin TJ, Carr VJ, Taylor RL, …& Wilhelm K (2006). A randomized controlled trial of a smoking cessation intervention among people with a psychotic disorder. American Journal of Psychiatry, 163, 1934–1942. [DOI] [PubMed] [Google Scholar]
- Bedfont Scientific U. (n.d.). EC50 Micro III Smokerlyzer Breath Carbon Monoxide Monitor. Medford: Bedfont Scientific Ltd Ed. [Google Scholar]
- Bellack AS, Bennett ME, Gearon JS, Brown CH, & Yang Y (2006). A randomized clinical trial of a new behavioral intervention for drug abuse in people with severe and persistent mental illness. Archives of General Psychiatry, 63, 426–432. [DOI] [PubMed] [Google Scholar]
- Bennett ME, Bellack AS, & Gearon JS (2001). Treating substance abuse in schizophrenia: An initial report. Journal of Substance Abuse Treatment, 20, 163–175. [DOI] [PubMed] [Google Scholar]
- Bennett ME, Dixon L, & Dickerson F (2008, November). Behavioral treatment for smoking cessation in serious and persistent mental illness. Presented at the 42nd annual meeting of the Association for the Advancement of Behavioral and Cognitive Therapies, Orlando, Florida. [Google Scholar]
- Bennett ME, Wilson AL, Genderson M, & Saperstein AM (2013). Smoking cessation in people with schizophrenia. Current Drug Abuse Reviews, 6(3), 180–190. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Amass L, Higgins ST, Badger GJ, & Esch RA (1997). Effects of adding behavioral treatment to opioid detoxification with buprenorphine. Journal of Consulting and Clinical Psychology, 65, 803–810. [DOI] [PubMed] [Google Scholar]
- Brunette MF, Ferron JC, McHugo GJ, Davis KE, Devitt TS, Wilkness SM, & Drake RE (2011). An electronic decision support system to motivate people with severe mental illnesses to quit smoking. Psychiatric Services, 62(4), 360–366. doi: 10.1176/appi.ps.62.4.360 [DOI] [PubMed] [Google Scholar]
- Buchanan RW, Kreyenbuhl J, Kelly DL, Noel JM, Boggs DL, Fischer BA, …Keller W (2010). The 2009 schizophrenia PORT psychopharmacological treatment recommendations and summary statements. Schizophrenia Bulletin, 36(1), 71–93. doi: 10.1093/schbul/sbp116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushe CJ, Taylor M, & Haukka J (2010). Mortality in schizophrenia: A measurable clinical endpoint. Journal of Psychopharmacology, 24(4 supplement), 17–25. doi: 10.1177/1359786810382468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney CP, & Jones LE (2006). Medical comorbidity in women and men with bipolar disorders: A population-based controlled study. Psychosomatic Medicine, 68(5), 684–691. [DOI] [PubMed] [Google Scholar]
- Carney CP, Jones L, & Woolson RF (2006). Medical comorbidity in women and men with schizophrenia: A population-based controlled study. Journal of General Internal Medicine, 21(11), 1133–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cather C, Dyer MA, Burrell HA, Hoeppner B, Goff DC, & Evins EA (2013). An open trial of relapse prevention therapy for smokers with schizophrenia. Journal of Dual Diagnosis, 9(1), 87–93. doi: 10.1080/15504263.2012.749559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (2014). Best Practices for Comprehensive Tobacco Control Programs–2014. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health. [Google Scholar]
- Compton MT, Daumit GL, & Druss BG (2006). Cigarette smoking and overweight/obesity among individuals with serious mental illnesses: A preventive perspective. Harvard Review of Psychiatry, 14(4), 212–222. [DOI] [PubMed] [Google Scholar]
- Currie SR, Karltyn J, Lussier D, de Denus E, Brown D, & El-Guebaly N (2008). Outcome from a community-based smoking cessation program for persons with serious mental illness. Community Mental Health Journal, 44(3), 187–194. [DOI] [PubMed] [Google Scholar]
- Deegan PE, & Drake RE (2006). Shared decision making and medication management in the recovery process. Psychiatric Services, 57, 1636–1639. [DOI] [PubMed] [Google Scholar]
- Department of Health and Human Services, National Center for Health Statistics. (1998). The Third National Health and Nutrition Examination Survey 1988–94 (NHANES III). Hyattsville, MD: Center for Disease Control and Prevention. [Google Scholar]
- Department of Veterans Affairs, Veterans Health Administration. (2012). VHA DIRECTIVE 2012–002: Re-Engaging Veterans with Serious Mental Illness in Treatment. Washington, DC. [Google Scholar]
- Dickerson F, Bennett M, Dixon L, Burke E, Vaughan C, Delahanty J, & DiClemente C (2011). Smoking cessation in persons with serious mental illness: The experience of successful quitters. Psychiatric Rehabilitation Journal, 34(4), 311–316. doi: 10.2975/34.4.2011.311.316 [DOI] [PubMed] [Google Scholar]
- Dickerson F, Stallings C, Origoni A, Schroeder J, Khushalani S, & Yolken R (2013). Mortality in schizophrenia: Clinical and serological predictors. Schizophrenia Bulletin, 40(4), 796–803. doi: 10.1093/schbul/sbt113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson F, Stallings CR, Origoni AE, Vaughan C, Khushalani S, Schroeder J, & Yolk RH (2013). Cigarette smoking among persons with schizophrenia or bipolar disorder in routine clinical settings, 1999–2011. Psychiatric Services, 64(1), 44–50. doi: 10.1176/appi.ps.201200143 [DOI] [PubMed] [Google Scholar]
- DiClemente CC, Prochaska JO, & Gibertinin M (1985). Self-efficacy and the stages of self-change of smoking. Cognitive Therapy and Research, 9, 181–200. [Google Scholar]
- Dixon L, Dickerson F, Bellack A, Bennett M, Dickinson D, Goldberg R, …Kreyenbuhl J (2010). The 2009 PORT psychosocial treatment recommendations and summary statement. Schizophrenia Bulletin, 36(1), 48–70. doi: 10.1093/schbul/sbp115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon L, Medoff DR, Wohlheiter K, DiClemente C, Goldberg R, Kreyenbuhl J, …Davin C (2007). Correlates of severity of smoking among persons with severe mental illness. American Journal on Addictions, 16(2), 101–110. [DOI] [PubMed] [Google Scholar]
- Drake RE, Mueser KT, & Brunette MF (2007). Management of persons with co-occurring severe mental illness and substance use disorder: Program implications. World Psychiatry, 6(3), 131–136. [PMC free article] [PubMed] [Google Scholar]
- Duffy SA, Kilbourne AM, Austin KL, Dalack GW, Woltmann EM, Waxmonsky J, & Noonan D (2012). Risk of smoking and receipt of cessation services among veterans with mental disorders. Psychiatric Services, 63(4), 325–332. doi: 10.1176/appi.ps.201100097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein DH, Hawkins WE, Covi L, Umbricht A, & Preston KL (2003). Cognitive-behavioral therapy plus contingency management for cocaine use: Findings during treatment and across 12-month follow-up. Psychology of Addictive Behaviors, 17, 73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterberg ML, & Compton MT (2005). Smoking behavior in persons with a schizophrenia-spectrum disorder: A qualitative investigation of the transtheoretical model. Social Science and Medicine, 61(2), 293–303. [DOI] [PubMed] [Google Scholar]
- Evins AE, Cather C, & Laffer A (2015). Treatment of tobacco use disorder in smokers with serious mental illness: Toward clinical best practices. Harvard Review of Psychiatry, 23(2), 90–98. doi: 10.1097/HRP.0000000000000063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evins AE, Cather C, Pratt SA, Pachas GN, Hoeppner SS, Goff DC, …Schoenfeld DA (2014). Maintenance treatment with varenicline for smoking cessation in patients with schizophrenia and bipolar disorder: A randomized clinical trial. JAMA: The Journal of the American Medical Association, 311(2), 145–154. doi: 10.1001/jama.2013.285113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferron JC, Alterman A, McHugo GJ, Brunette MF, & Drake RE (2009). A review of research on smoking cessation interventions for adults with schizophrenia spectrum disorders. Mental Health and Substance Abuse: Dual Diagnosis, 2(1), 64–79. doi: 10.1080/17523280802593327 [DOI] [Google Scholar]
- Fiore MC, Jaen CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ, …Wewers ME (2008). Treating tobacco use and dependence: 2008 update. Rockville, MD: U.S. Department of Health and Human Services. Public Health Service; Retrieved from http://www.ahrq.gov/professionals/clinicians-providers/guidelines-recommendations/tobacco/clinicians/update/treatingtobaccouse08.pdf [Google Scholar]
- George TP, Ziedonis DM, Feingold A, Pepper WT, Satterburg CA, Winkel J, …Kosten TR (2000). Nicotine transdermal patch and atypical antipsychotic medications for smoking cessation in schizophrenia. American Journal of Psychiatry, 157, 1835–1842. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO (1991) The Fagerström Test for Nicotine Dependence: A revision of the Fagerström tolerance questionnaire. British Journal of Addictions, 86, 1119–1127. [DOI] [PubMed] [Google Scholar]
- Hennekens CH, Hennekens AR, Hollar D, & Casey DE (2005). Schizophrenia and increased risks of cardiovascular disease. American Heart Journal, 150(6), 1115–1121. [DOI] [PubMed] [Google Scholar]
- Himelhoch S, & Daumit G (2003). To whom do psychiatrists offer smoking-cessation counseling?. American Journal of Psychiatry, 160, 2228–2230. [DOI] [PubMed] [Google Scholar]
- Himelhoch S, Lehman A, Kreyenbuhl J, Daumit G, Brown C, & Dixon L (2004). Prevalence of chronic obstructive pulmonary disease among those with serious mental illness. American Journal of Psychiatry, 161, 2317–2319. [DOI] [PubMed] [Google Scholar]
- Himelhoch S, Riddle J, & Goldman HH (2014). Barriers to implementing evidence-based smoking cessation practices in nine community mental health sites. Psychiatric Services, 65(1), 75–80. doi: 10.1176/appi.ps.201200247 [DOI] [PubMed] [Google Scholar]
- Jeste DV, Gladsjo JA, Lindamer LA, & Lacro JP (1996). Medical comorbidity in schizophrenia. Schizophrenia Bulletin, 22(3), 413–430. [DOI] [PubMed] [Google Scholar]
- Kelly DL, McMahon RP, Liu F, Love RC, Wehring HJ, Shim JC, …Conley RR (2010). Cardiovascular disease mortality in patients with chronic schizophrenia treated with clozapine: A retrospective cohort study. Journal of Clinical Psychiatry, 71, 304–311. doi: 10.4088/JCP.08m04718yel [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly DL, Raley H, Lo S, Wright K, Moolchan E, Feldman S, … Heishman S (2012). Perception of smoking risks and motivation to quit among nontreatment-seeking smokers with and without schizophrenia. Schizophrenia Bulletin, 38, 543–551. doi: 10.1093/schbul/sbq124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly DL, Wehring HJ, Linthicum J, Feldman S, McMahon RP, Love RC, …Fowler DR (2009). Cardiac-related findings at autopsy in people with severe mental illness treated with clozapine or risperidone. Schizophrenia Research, 107, 134–138. doi: 10.1016/j.schres.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreyenbuhl J, Nossel IR, & Dixon LB (2009). Disengagement from mental health treatment among individuals with schizophrenia and strategies for facilitating connections to care: A review of the literature. Schizophrenia Bulletin, 35(4), 696–703. doi: 10.1093/schbul/sbp046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawn S, & Campion J (2013). Achieving smoke-free mental health services: Lessons from the past decade of implementation research. International Journal of Environmental Research and Public Health, 10, 4224–4244. doi: 10.3390/ijerph10094224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard S, & Adams CE (2006). Smoking cessation and schizophrenia. American Journal of Psychiatry, 163 (11), 1877. [DOI] [PubMed] [Google Scholar]
- Little RJA, & Rubin DB (2002). Statistical analysis with missing data, 2nd ed Hoboken, NJ: Wiley. [Google Scholar]
- Lucksted A, Dixon LB, & Sembly JB (2000). A focus group pilot study of tobacco smoking among psychosocial rehabilitation clients. Psychiatric Services, 51, 1544–1548. [DOI] [PubMed] [Google Scholar]
- Mann-Wrobel MC, Bennett ME, Weiner EE, Buchanan RW, & Ball MP (2010). Smoking history and motivation to quit in smokers with schizophrenia in a smoking cessation program. Schizophrenia Research, 126(1–3), 277–283. doi: 10.1016/j.schres.2010.10.030 [DOI] [PubMed] [Google Scholar]
- Miller BJ, Paschall CB, & Svendsen DP (2006). Mortality and medical comorbidity among patients with serious mental illness. Psychiatric Services, 57(1), 1482–1487. [DOI] [PubMed] [Google Scholar]
- Moore D, Aveyard P, Connock M, Wang D, Fry-Smith A, & Barton P (2009). Effectiveness and safety of nicotine replacement therapy assisted reduction to stop smoking: Systematic review and meta-analysis. British Medical Journal, 338, 1024. doi: 10.1136/bmj.b1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir-Cochrane E (2006). Medical comorbidity risk factors and barriers to care for people with schizophrenia. Journal of Psychiatric Mental Health Nursing, 13(4), 447–452. [DOI] [PubMed] [Google Scholar]
- Newcomer JW (2006). Medical risk in patients with bipolar disorder and schizophrenia. Journal of Clinical Psychiatry, 67(11), e16. [PubMed] [Google Scholar]
- Overall JE, & Gorham DR (1962). The brief psychiatric rating scale. Psychological Reports, 10, 799–812. [Google Scholar]
- Prochaska JJ (2010a). Failure to treat tobacco use in mental health and addiction treatment settings: A form of harm reduction? Drug and Alcohol Dependence, 110(3), 177–182. doi:0.1016/j.drugalcdep.2010.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochaska JJ (2010b). Integrating tobacco treatment into mental health settings. JAMA: The Journal of the American Medical Association, 304(22), 2534–2535. doi: 10.1001/jama.2010.1759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratschen E, Britton J, & McNeill A (2011). The smoking culture in psychiatry: Time for change. British Journal of Psychiatry, 198, 6–7. doi: 10.1192/bjp.bp.110.081372 [DOI] [PubMed] [Google Scholar]
- Selzer ML (1971). The Michigan Alcoholism Screening Test: The quest for a new diagnostic instrument. American Journal of Psychiatry, 127, 1653–1658. [DOI] [PubMed] [Google Scholar]
- Shim JC, Jung DU, Jung SS, Seo YS, Cho DM, Lee JH, … Kelly DL (2012). Adjunctive varenicline treatment with antipsychotic medications for cognitive impairments in people with schizophrenia: A randomized double-blind placebo-controlled trial. Neuropsychopharmacology, 37(3), 660–668. doi: 10.1038/npp.2011.238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigmon SC, & Patrick ME (2012). The use of financial incentives in promoting smoking cessation. Preventative Medicine, 55(Suppl), S24–S32. doi: 10.1016/j.ypmed.2012.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner H (1982). The Drug Abuse Screening Test. Addictive Behaviors, 7, 363–371. [DOI] [PubMed] [Google Scholar]
- Smith PH, Mazure CM, & McKee SA (2014). Smoking and mental illness in the U.S. population. Tobacco Control, 23, 147–153. doi: 10.1136/tobaccocontrol-2013-051466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokal J, Messias E, Dickeron FB, Kreyenbuhl J, Brown CH, Goldberg RW, & Dixon LB (2004). Comorbidity of medical illnesses among adults with serious mental illness who are receiving community psychiatric services. Journal of Nervous and Mental Disease, 192, 421–427. [DOI] [PubMed] [Google Scholar]
- Stitzer ML (1999). Combined behavioral and pharmacological treatments for SC. Nicotine and Tobacco Research, 1(Suppl 2), S181–187. [DOI] [PubMed] [Google Scholar]
- Tsoi DT, Porwal M, & Webster AC (2013a). Interventions for smoking cessation and reduction in individuals with schizophrenia. Cochrane Database of Systematic Reviews, 2, CD007253. doi: 10.1002/14651858.CD007253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsoi DT, Porwal M, & Webster AC (2013b). Efficacy and safety of bupropion for smoking cessation and reduction in schizophrenia: Systematic review and meta-analysis. British Journal of Psychiatry, 196(5), 346–353. doi: 10.1192/bjp.bp.109.066019 [DOI] [PubMed] [Google Scholar]
- Weiner E, Ball MP, Summerfelt A, Gold J, & Buchanan RW (2001). Effects of sustained-release bupropion and supportive group therapy on cigarette smoking in patients with schizophrenia. American Journal of Psychiatry, 158(4), 635–637. [DOI] [PubMed] [Google Scholar]
- Williams JM, Anthenelli RM, Morris CD, Treadow J, Thompson JR, Yunis C, & George TP (2012). A randomized, double-blind, placebo-controlled study evaluating the safety and efficacy of varenicline for smoking cessation in patients with schizophrenia or schizoaffective disorder. Journal of Clinical Psychiatry, 73(5), 654–660. doi: 10.4088/JCP.11m07522 [DOI] [PubMed] [Google Scholar]
- Williams JM, Steinberg ML, Griffiths KG, & Cooperman N (2013). Smokers with behavioral health comorbidity should be designated a Tobacco Use Disparity Group. American Journal of Public Health, 103, 1549–1555. doi: 10.2105/AJPH.2013.301232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JM, Steinberg ML, Zimmermann MH, Gandhi KK, Stipelman B, Budsock PD, & Ziedonis DM (2010). Comparison of two intensities of tobacco dependence counseling in schizophrenia and schizoaffective disorder. Journal of Substance Abuse Treatment, 38(4), 384–393. doi: 10.1016/j.jsat.2010.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziedonis DM, & George TP (1997). Schizophrenia and nicotine use: Report of a pilot smoking cessation program and review of neurobiological and clinical issues. Schizophrenia Bulletin, 23, 247–254. [DOI] [PubMed] [Google Scholar]
- Ziedonis D, Hitsman B, Beckham JC, Zvolensky M, Adler LE, Audrain-McGovern J, …Riley WT (2008). Tobacco use and cessation in psychiatric disorders: National Institute of Mental Health report. Nicotine and Tobacco Research, 10(12), 1691–1715. doi: 10.1080/14622200802443569 [DOI] [PubMed] [Google Scholar]


