Abstract
Rationale: Neuroendocrine cell hyperplasia of infancy (NEHI) is an important form of children’s interstitial and diffuse lung disease for which the diagnostic strategy has evolved. The prevalence of comorbidities in NEHI that may influence treatment has not been previously assessed.
Objectives: To evaluate a previously unpublished NEHI clinical score for assistance in diagnosis of NEHI and to assess comorbidities in NEHI.
Methods: We performed a retrospective chart review of 199 deidentified patients with NEHI from 11 centers. Data were collected in a centralized Research Electronic Data Capture registry and we performed descriptive statistics.
Results: The majority of patients with NEHI were male (66%). The sensitivity of the NEHI Clinical Score was 87% (95% confidence interval [CI], 0.82–0.91) for all patients from included centers and 93% (95% CI, 0.86–0.97) for those with complete scores (e.g., no missing data). Findings were similar when we limited the population to the 75 patients diagnosed by lung biopsy (87%; 95% CI, 0.77–0.93). Of those patients evaluated for comorbidities, 51% had gastroesophageal reflux, 35% had aspiration or were at risk for aspiration, and 17% had evidence of immune system abnormalities.
Conclusions: The NEHI Clinical Score is a sensitive tool for clinically evaluating NEHI; however, its specificity has not yet been addressed. Clinicians should consider evaluating patients with NEHI for comorbidities, including gastroesophageal reflux, aspiration, and immune system abnormalities, because these can contribute to the child’s clinical picture and may influence clinical course and treatment.
Keywords: infant, neuroendocrine cells, interstitial lung disease
Neuroendocrine cell hyperplasia of infancy (NEHI) is a form of children’s interstitial and diffuse lung disease (chILD). It was initially named persistent tachypnea of infancy (1) and subsequently NEHI, after biopsy findings of increased numbers of neuroendocrine cells in the airways were described (2). NEHI is a distinct entity with characteristic clinical features, infant pulmonary function testing, and high-resolution chest computed tomography (HRCT) (inspiratory and expiratory) findings. The pathophysiology of NEHI is poorly understood. Patients with NEHI exhibit tachypnea, retractions, crackles, and hypoxemia. Clubbing is absent, as are cough or wheeze at well baseline (2). Nonpulmonary manifestations of NEHI include failure to thrive and developmental delays (3). Infant pulmonary function tests reveal tachypnea, air trapping, and variable degrees of airflow obstruction (4). Characteristic HRCT findings include air trapping and ground glass opacities, predominantly in the right middle lobe, lingula, and/or perihilar regions (5). NEHI can be diagnosed clinically by HRCT scan in the appropriate clinical context (6). Pathologic findings of NEHI are notable for an increased percentage of airway neuroendocrine cells (2). Currently, lung biopsies are typically performed in children with atypical presentations and/or at centers less familiar with chILD. Anecdotally, some patients with NEHI have been found to have a variety of comorbidities, including immunologic abnormalities and aspiration/gastroesophageal reflux, but this has not been formally evaluated or reported.
Leland Fan, M.D., initially proposed a clinical score for NEHI that includes 10 prototypical clinical features. We used the largest prospective multicenter cohort of clinician-defined NEHI cases to date to test the hypothesis that the NEHI Clinical Score is a sensitive test for NEHI. We also evaluated the frequency of comorbidities in this large cohort of patients with NEHI.
Methods
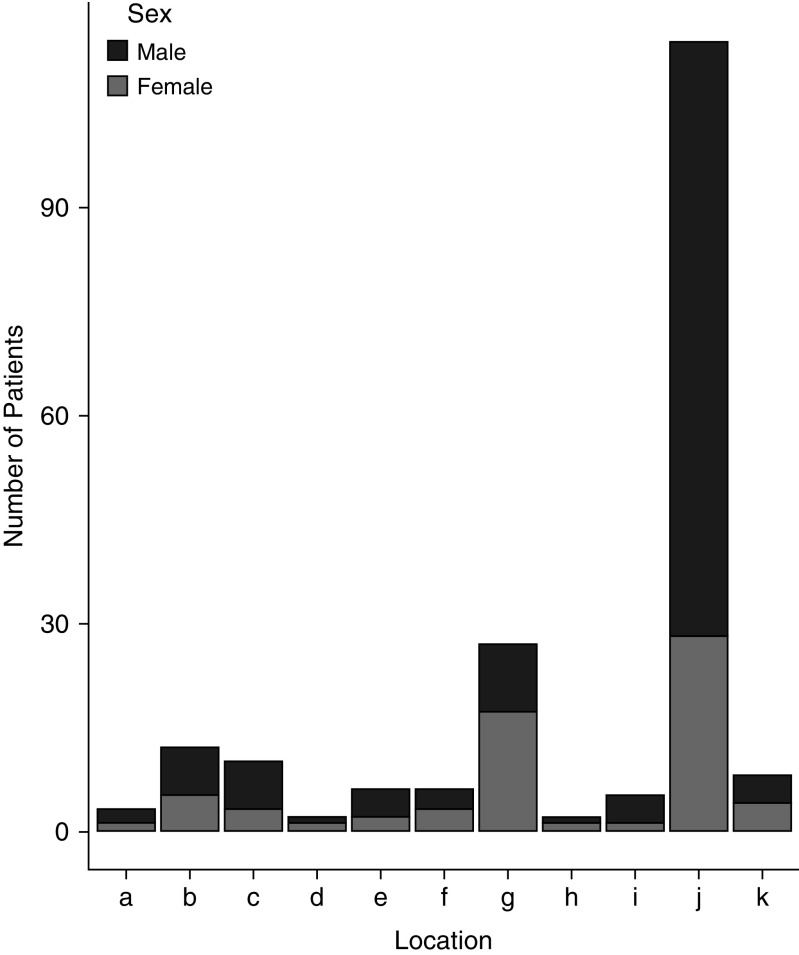
Patients were enrolled through 11 North American centers within the chILD Research Network: Arkansas Children’s Hospital, University of Arkansas Medical School; Boston Children’s Hospital, Harvard Medical School; Children’s Hospital Colorado, University of Colorado School of Medicine; Children’s Hospital of Pittsburgh of University of Pittsburgh Medical Center, Children’s Mercy Kansas City, University of Missouri-Kansas City; Cincinnati Children’s Hospital Medical Center, St. Louis Children’s Hospital, Washington University School of Medicine; Rady Children’s Hospital, University of California San Diego; Children’s Hospital of Michigan, Wayne State University; Vanderbilt University Medical Center, and Hospital for Sick Children. Centers entered as few as 2 patients to as many as 114 patients (Figure 1). The institutional review board of each participating institution approved this study and informed consent was waived.
Figure 1.
Distribution of patients by center and by sex.
The NEHI Clinical Score consists of the following 10 items: symptom onset before 12 months of age, failure to thrive, absence of clubbing, absence of cough when well, absence of wheeze when well, abnormal chest wall (barrel chest or pectus excavatum), crackles, hypoxemia, tachypnea, and retractions. Each of the 10 items are assigned a value of 0 (no/not present) or 1 (yes/present), and the score is the sum of these values, with a maximum score of 10 (e.g., patient with symptom onset at 5 months of age, failure to thrive, no clubbing, no cough when well, no wheeze when well, barrel chest, crackles, hypoxemia, tachypnea, and retractions). A score of 7 or higher is considered consistent with NEHI based on our clinical experience.
Data were entered retrospectively by the site clinician into a standardized data collection form in Research Electronic Data Capture, a secure, web-based application designed to support data capture for research studies (7). Identification of patients was unique to each center: there was no unifying electronic health record search for patients. Diagnosis of NEHI was determined by the entering clinician who noted if the patient was diagnosed by lung biopsy or by HRCT and consistent clinical course. Data collection included information on chILD center location, sex, race, ethnicity, mode of diagnosis (lung biopsy or by HRCT and consistent clinical course), age at symptom onset, clinical findings, and comorbidities. Failure to thrive was defined as body mass index or weight for length less than the 25th percentile, or if failure to thrive was documented in the medical record. Hypoxemia, tachypnea, chest wall abnormalities, and retractions were determined at the discretion of the clinician entering data. Database fields for comorbidities included reflux testing: abnormal/normal/not done, swallow study: frank aspiration/abnormal without frank aspiration/normal/not done, and immune testing: abnormal/normal/not done.
Data elements that were not entered were considered missing data. Descriptive statistics were calculated and summarized. Not all data were available for each data element. The available number is presented in the tables with the variable. Sensitivity was calculated for a cutoff NEHI Clinical Score of 7 or greater compared with clinician diagnosis of NEHI based on lung biopsy and/or diagnosed clinically. Proportions were presented with 95% Wilson confidence intervals (CIs).
Results
Demographic data are presented in Table 1. The patients were predominantly male (66%), white (69%), non-Hispanic (68%), and born at full term (88%). The rate of preterm births (<37 wk) was 11% (95% CI, 7.55–16.73). Median age at symptom onset was 4 months (interquartile range = 2–6 mo). A majority (62%) of patients were diagnosed by clinical and radiographic features rather than by lung biopsy (38%).
Table 1.
Demographics of subjects diagnosed with neuroendocrine cell hyperplasia of infancy (N = 199)
| N (%) or Median [Inner Quartile–Outer Quartile] | |
|---|---|
| Sex (n = 195) | |
| M | 129 (66) |
| F | 66 (34) |
| Gestational age at birth (n = 185) | |
| Full term (>37 wk) | 164 (89) |
| >32-36 wk | 17 (9) |
| 28–32 wk | 4 (2) |
| <28 wk | N/A |
| Race (n = 173) | |
| White | 120 (69) |
| African American | 4 (2) |
| American Indian | N/A (N/A) |
| Hawaiian | N/A (N/A) |
| Pacific Islander | N/A (N/A) |
| Asian | 2 (1) |
| Other | 21 (12) |
| Ethnicity (n = 164) | |
| Hispanic | 20 (12) |
| Non-Hispanic | 112 (68) |
| Refused or unknown | 32 (20) |
| Age of symptom onset (in months) | 4 [2–6] |
| Mode of diagnosis (n = 198) | |
| Lung biopsy | 75 (38) |
| No biopsy | 123 (62) |
Definition of abbreviation: N/A = not available.
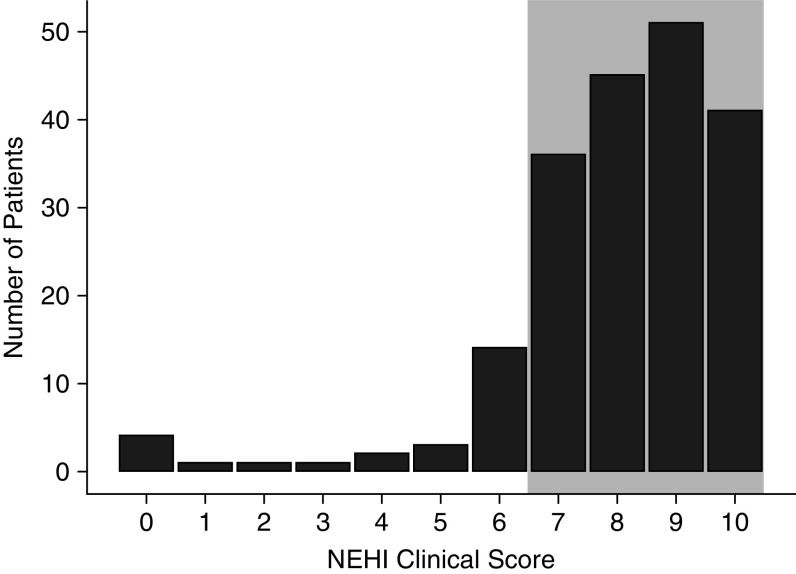
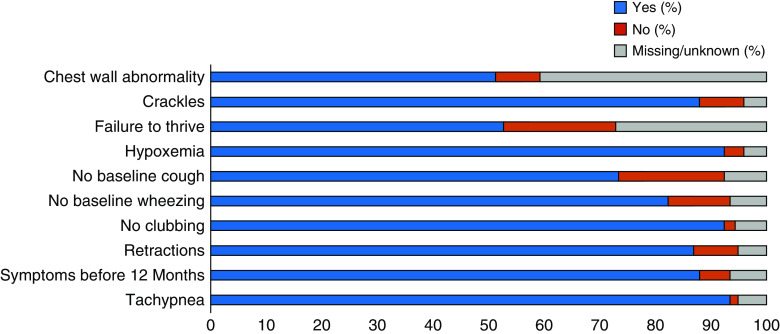
Figure 2 demonstrates the NEHI Clinical Score for the population of 199 patients. The sensitivity of the NEHI Clinical Score was 87% (95% CI, 0.82–0.91) for all patients from included centers. When only patients with complete data (the clinician entered yes or no for all 10 components) were included, 93% of 91 patients (95% CI, 0.86–0.97) had a NEHI Clinical Score of 7 or greater. The breakdown for each component of the NEHI Clinical Score is presented in Figure 3; the most common missing data were chest wall abnormalities (81 patients) and failure to thrive (54 patients). When only the 75 patients who were diagnosed by biopsy were included, 65 had NEHI Clinical Scores suggestive of a diagnosis of NEHI (i.e., ≥7) resulting in a sensitivity of 87% (95% CI, 0.77–0.93). When only those diagnosed by biopsy with no missing data are evaluated (24 patients), all had a NEHI Clinical Score suggestive of NEHI (i.e., ≥7), resulting in a sensitivity of 100%.
Figure 2.
Distribution of the neuroendocrine cell hyperplasia of infancy (NEHI) Clinical Score in 199 patients. The shaded area indicates those with scores above 7 and consistent with NEHI.
Figure 3.
Each component of the neuroendocrine cell hyperplasia of infancy clinical score in 199 patients with neuroendocrine cell hyperplasia of infancy. Each component of the score is yes (blue), no (red), or missing (gray). Components are presented as percentages of all included patients.
Table 2 demonstrates the comorbidities assessed. Of the 98 who were tested for gastroesophageal reflux, half had abnormal results. Of the 103 who were evaluated for aspiration, one-third had abnormal results. Sixty two percent (117/189) of patients underwent some type of immunologic evaluation and 17% of those (20/117) had abnormalities. Immune system abnormalities included seven patients with low immunoglobulin G (IgG); one with elevated IgG; two with elevated IgM; and one each with elevated IgA, elevated IgE, low IgA, low complement 3, and cyclic neutropenia of infancy. Seven patients had unspecified abnormalities of the immune system. Several patients had more than one abnormality noted. Those who had low IgG levels were evaluated from 4 months of age to 27 months of age. Several patients were tested on more than one occasion and were included as having immune system abnormalities if they were abnormally low ever. IgG ranges were adjusted by age.
Table 2.
Evaluation of comorbidities in 199 patients with neuroendocrine cell hyperplasia of infancy
| Comorbidities | N (%) |
|---|---|
| Gastroesophageal reflux testing (n = 185) | |
| Abnormal | 50 (27) |
| Normal | 48 (26) |
| Not done | 87 (47) |
| Swallow study (n = 189) | |
| Frank aspiration | 8 (4) |
| Abnormal without frank aspiration | 28 (15) |
| Normal | 67 (35) |
| Not done | 86 (46) |
| Immune system evaluation (n = 189) | |
| Abnormal | 20 (11) |
| Normal | 97 (51) |
| Not done | 72 (38) |
| Specific immune abnormalities (n = 12) | |
| Low immunoglobulin G | 7 (58) |
| Other | 5 (42) |
Discussion
Establishing the diagnosis of NEHI can be challenging if the HRCT findings are atypical. With a sensitivity of 87% (all 199 patients and all patients with biopsies) to 93% (patients with complete data) to 100% (patients with biopsies and complete data), the NEHI Clinical Score may be a helpful tool in recognition of NEHI. Some centers have been using this score informally. When evaluated serially, the score decreases with age as symptoms like failure to thrive, crackles, hypoxemia, tachypnea, and retractions improve over time in children with NEHI. Parts of the score, such as symptom onset before 12 months of age, will persist over time. Including patients who were diagnosed clinically raises the question of whether these patients truly had NEHI. The literature suggests that biopsy is unnecessary to diagnose NEHI (6). Additionally, we evaluated the subset of patients who were diagnosed by biopsy, and the NEHI Clinical Score was similarly sensitive. This study was limited by its retrospective design, missing data elements, and lack of a control group. Prospective studies such as those being performed through the chILD Research Network will permit further analysis of the NEHI Clinical Score as a clinical prediction score in combination with HRCT for diagnosis and to assess disease trajectory (8, 9).
In this cohort, we found a male predominance, which has been previously reported (2, 4, 6, 10, 11) but not consistently in all NEHI cohorts (3, 12, 13) and not consistently in all included centers (see Figure 1). The etiology of this sex difference in NEHI is poorly understood. There is a known male predominance in respiratory diseases among infants and young children in general, though this also is not well understood (14). Most NEHI patients were born full term, and the rate of preterm births was not significantly different than the national preterm birth rate of 9.62% (15).
Clinicians should consider evaluating patients with NEHI for comorbidities, including sleep-disordered breathing (16), gastroesophageal reflux, aspiration, and hypogammaglobulinemia. Except for sleep-disordered breathing (16), these comorbidities have not previously been described and may significantly influence treatment in an individual patient. It is unknown whether some of these comorbidities contribute to the clinical picture of NEHI and/or are exacerbated by NEHI.
The etiology of gastroesophageal reflux in patients with NEHI may be explained by hyperinflation leading to increased elastic recoil of the lung, which, in turn, leads to more negative intrapleural pressure. More negative intrapleural pressure leads to a pressure gradient from the stomach to the intrathoracic esophagus, leading to more reflux. Gastroesophageal reflux has been described in other forms of obstructive lung disease such as cystic fibrosis and chronic obstructive pulmonary disease (17–19). Aspiration may be more common among NEHI patients because of the tachypnea and increased work of breathing making coordination of swallowing more difficult. Aspiration in the setting of tachypnea and increased work of breathing has been described in infants with bronchiolitis (20).
Immunologic evaluation is not universally performed in patients with NEHI. The mechanism leading to hypogammaglobulinemia in the eight patients with NEHI in this cohort is unknown. It is possible that patients have transient hypogammaglobulinemia of infancy (21). We suggest evaluating for hypogammaglobulinemia among NEHI patients because this may lead to specific therapy (e.g., prophylactic antibiotic therapy and/or intravenous immunoglobulin) given the higher morbidity of respiratory infections in patients with underlying lung disease. Some patients were noted to have other immunologic abnormalities, such as elevated IgA, elevated IgE, elevated IgG, low IgA, low complement 3, and elevated IgM. Assessing for immunologic abnormalities in future prospective studies would be helpful to better define the spectrum of immunologic abnormalities in NEHI.
The pathophysiology of NEHI is not well understood, but the constellation of symptoms and laboratory findings reinforce that NEHI is a clinical entity with a unique combination of physiologic changes, HRCT findings, symptoms, and lung biopsy findings (2, 4–6). Chest wall shape was not consistently evaluated in all centers and would be useful to assess in future prospective studies. NEHI patients have significant elevated residual volumes and air trapping (4), and HRCT scans have demonstrated that patients with NEHI have altered chest wall shape with increased anterior-posterior diameter (22). Having physicians closely evaluate chest shape during the physical exam is a useful assessment that may turn out to reflect underlying physiology and anatomy. Future radiologic evaluations may allow for more objective evaluation of chest wall shape.
We propose the NEHI Clinical Score as a sensitive tool for clinicians to identify patients for whom further evaluation for NEHI should be considered (e.g., HRCT scan, infant pulmonary function tests). In future studies, we hope to compare the NEHI score in this cohort to disease control groups such as asthma or cystic fibrosis, or in other forms of chILD in the chILDRN (children’s interstitial lung disease research network) prospective database. We highlight several comorbidities that should be evaluated for and addressed in patients with NEHI because appropriate treatments may modify disease course. Prospective studies in infants with suspected NEHI could evaluate the predictive characteristics of the NEHI clinical score, and a longitudinal study could evaluate the NEHI clinical score’s variation over time.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank Leland Fan, M.D., for his contributions to the field of chILD, including the creation of the NEHI Clinical Score described in the current manuscript. They also thank the research coordinators who made the current study possible, including Pamela Morel, Emily Trefethen, Elizabeth Carpino, and Heidi Luckey Zahller.
Footnotes
Supported by grant UL1 TR001082 from the National Institutes of Health (NIH)/National Center for Research Resources Colorado Clinical and Translational Sciences Institute, U01HL13475 (J.W.), and NIH/NHLBI K24HL143281 (L.R.Y.).
Author Contributions: D.R.L. conceived and designed the study, collected, analyzed and interpreted the data, and wrote and revised the manuscript. R.R.D. designed the study, interpreted the data, revised the manuscript, and approved for submission. K.P. and J.T.B. assisted in designing the study, analyzed and interpreted the data, wrote and revised the manuscript, and approved for submission. A.A., M.P.F., A.C., C.T.T., J.B.T., G.K., J.S.H., J.W., R.S., H.A.-S., S.D.D., and L.R.Y. contributed to implementation of the study, collection and analysis of data, critically reviewed and revised the manuscript, and approved for submission.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Deterding RR, Fan LL, Morton R, Hay TC, Langston C. Persistent tachypnea of infancy (PTI)--a new entity. Pediatr Pulmonol. 2001;(Suppl 23):72–73. [PubMed] [Google Scholar]
- 2.Deterding RR, Pye C, Fan LL, Langston C. Persistent tachypnea of infancy is associated with neuroendocrine cell hyperplasia. Pediatr Pulmonol. 2005;40:157–165. doi: 10.1002/ppul.20243. [DOI] [PubMed] [Google Scholar]
- 3.Nevel RJ, Garnett ET, Schaudies DA, Young LR. Growth trajectories and oxygen use in neuroendocrine cell hyperplasia of infancy. Pediatr Pulmonol. 2018;53:656–663. doi: 10.1002/ppul.23958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kerby GS, Wagner BD, Popler J, Hay TC, Kopecky C, Wilcox SL, et al. Abnormal infant pulmonary function in young children with neuroendocrine cell hyperplasia of infancy. Pediatr Pulmonol. 2013;48:1008–1015. doi: 10.1002/ppul.22718. [DOI] [PubMed] [Google Scholar]
- 5.Brody AS, Crotty EJ. Neuroendocrine cell hyperplasia of infancy (NEHI) Pediatr Radiol. 2006;36:1328. doi: 10.1007/s00247-006-0302-3. [DOI] [PubMed] [Google Scholar]
- 6.Brody AS, Guillerman RP, Hay TC, Wagner BD, Young LR, Deutsch GH, et al. Neuroendocrine cell hyperplasia of infancy: diagnosis with high-resolution CT. AJR Am J Roentgenol. 2010;194:238–244. doi: 10.2214/AJR.09.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Young LR, Nevel RJ, Casey AMH, Fishman MP, Welsh S, Liptzin D, et al. The National Registry for Childhood Interstitial and Diffuse Lung Diseases: An initial report from the ChILD Research Network [abstract] Am J Respir Crit Care Med. 2018;197:A4191. [Google Scholar]
- 9.Young L, Nevel R, Casey A, Fishman M, Welsh S, Liptzin D, et al. A national registry for childhood interstitial and diffuse lung diseases in the United States [abstract] Eur Respir J. 2018;52:OA3786. [Google Scholar]
- 10.Lukkarinen H, Pelkonen A, Lohi J, Malmström K, Malmberg LP, Kajosaari M, et al. Neuroendocrine cell hyperplasia of infancy: a prospective follow-up of nine children. Arch Dis Child. 2013;98:141–144. doi: 10.1136/archdischild-2012-302115. [DOI] [PubMed] [Google Scholar]
- 11.Gomes VC, Silva MC, Maia Filho JH, Daltro P, Ramos SG, Brody AS, et al. Diagnostic criteria and follow-up in neuroendocrine cell hyperplasia of infancy: a case series. J Bras Pneumol. 2013;39:569–578. doi: 10.1590/S1806-37132013000500007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Popler J, Gower WA, Mogayzel PJ, Jr, Nogee LM, Langston C, Wilson AC, et al. Familial neuroendocrine cell hyperplasia of infancy. Pediatr Pulmonol. 2010;45:749–755. doi: 10.1002/ppul.21219. [DOI] [PubMed] [Google Scholar]
- 13.Young LR, Brody AS, Inge TH, Acton JD, Bokulic RE, Langston C, et al. Neuroendocrine cell distribution and frequency distinguish neuroendocrine cell hyperplasia of infancy from other pulmonary disorders. Chest. 2011;139:1060–1071. doi: 10.1378/chest.10-1304. [DOI] [PubMed] [Google Scholar]
- 14.Liptzin DR, Landau LI, Taussig LM. Sex and the lung: Observations, hypotheses, and future directions. Pediatr Pulmonol. 2015;50:1159–1169. doi: 10.1002/ppul.23178. [DOI] [PubMed] [Google Scholar]
- 15.Martin JA, Hamilton BE, Osterman MJK, Driscoll AK, Mathews TJ. Births: Final Data for 2015. 2017 [cited 2019 10/1/2019]. Available from: https://www.cdc.gov/nchs/data/nvsr/nvsr66/nvsr66_01.pdf. [PubMed]
- 16.Liptzin DR, Hawkins SMM, Wagner BD, Deterding RR. Sleeping chILD: Neuroendocrine cell hyperplasia of infancy and polysomnography. Pediatr Pulmonol. 2018;53:917–920. doi: 10.1002/ppul.24042. [DOI] [PubMed] [Google Scholar]
- 17.Casanova C, Baudet JS, del Valle Velasco M, Martin JM, Aguirre-Jaime A, de Torres JP, et al. Increased gastro-oesophageal reflux disease in patients with severe COPD. Eur Respir J. 2004;23:841–845. doi: 10.1183/09031936.04.00107004. [DOI] [PubMed] [Google Scholar]
- 18.Button BM, Roberts S, Kotsimbos TC, Levvey BJ, Williams TJ, Bailey M, et al. Gastroesophageal reflux (symptomatic and silent): a potentially significant problem in patients with cystic fibrosis before and after lung transplantation. J Heart Lung Transplant. 2005;24:1522–1529. doi: 10.1016/j.healun.2004.11.312. [DOI] [PubMed] [Google Scholar]
- 19.Scott RB, O’Loughlin EV, Gall DG. Gastroesophageal reflux in patients with cystic fibrosis. J Pediatr. 1985;106:223–227. doi: 10.1016/s0022-3476(85)80291-2. [DOI] [PubMed] [Google Scholar]
- 20.Khoshoo V, Edell D. Previously healthy infants may have increased risk of aspiration during respiratory syncytial viral bronchiolitis. Pediatrics. 1999;104:1389–1390. doi: 10.1542/peds.104.6.1389. [DOI] [PubMed] [Google Scholar]
- 21.Tiller TL, Jr, Buckley RH. Transient hypogammaglobulinemia of infancy: review of the literature, clinical and immunologic features of 11 new cases, and long-term follow-up. J Pediatr. 1978;92:347–353. doi: 10.1016/s0022-3476(78)80417-x. [DOI] [PubMed] [Google Scholar]
- 22.Mastej EJ, DeBoer EM, Humphries SM, Cook MC, Hunter KS, Liptzin DR, et al. Lung and airway shape in neuroendocrine cell hyperplasia of infancy. Pediatr Radiol. 2018;48:1745–1754. doi: 10.1007/s00247-018-4189-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.