Abstract
Rationale: High-dose isoniazid is recommended in short-course regimens for multidrug-resistant tuberculosis (TB). The optimal dose of isoniazid and its individual contribution to efficacy against TB strains with inhA or katG mutations are unknown.
Objectives: To define the optimal dose of isoniazid for patients with isoniazid-resistant TB mediated by inhA mutations.
Methods: AIDS Clinical Trials Group A5312 is a phase 2A, open-label trial in which individuals with smear-positive pulmonary TB with isoniazid resistance mediated by an inhA mutation were randomized to receive isoniazid 5, 10, or 15 mg/kg daily for 7 days (inhA group), and control subjects with drug-sensitive TB received the standard dose (5 mg/kg/d). Overnight sputum cultures were collected daily. The 7-day early bactericidal activity (EBA) of isoniazid was estimated as the average daily change in log10 cfu on solid media (EBAcfu0–7) or as time to positivity (TTP) in liquid media in hours (EBATTP0–7) using nonlinear mixed-effects models.
Measurements and Main Results: Fifty-nine participants (88% with cavitary disease, 20% HIV-positive, 16 with isoniazid-sensitive TB, and 43 with isoniazid-monoresistant or multidrug-resistant TB) were enrolled at one site in South Africa. The mean EBAcfu0–7 at doses of 5, 10, and 15 mg/kg in the inhA group was 0.07, 0.17, and 0.22 log10 cfu/ml/d, respectively, and 0.16 log10 cfu/ml/d in control subjects. EBATTP0–7 patterns were similar. There were no drug-related grade ≥3 adverse events.
Conclusions: Isoniazid 10–15 mg/kg daily had activity against TB strains with inhA mutations similar to that of 5 mg/kg against drug-sensitive strains. The activity of high-dose isoniazid against strains with katG mutations will be explored next.
Clinical trial registered with www.clinicaltrials.gov (NCT01936831).
Keywords: tuberculosis, isoniazid resistance, early bactericidal activity, inhA mutation, phase 2 clinical trial
At a Glance Commentary
Scientific Knowledge on the Subject
Several observations suggest that isoniazid retains activity against multidrug-resistant tuberculosis (TB) (TB resistant to rifampicin and isoniazid), but the independent effects of isoniazid against drug-resistant tuberculosis strains, wherein isoniazid resistance is mediated by inhA or katG mutations, has never been demonstrated, nor has the right dose of isoniazid in these situations been established.
What This Study Adds to the Field
We demonstrate that isoniazid, given at doses of 10–15 mg/kg provides similar early bactericidal activity against strains with inhA mutations as isoniazid 5 mg/kg against drug-sensitive strains in patients with pulmonary TB. This provides an important option for clinicians and programs treating patients with isoniazid-monoresistant or multidrug-resistant tuberculosis.
About 10% of the strains of Mycobacterium tuberculosis (M.tb) that have been isolated globally are resistant to isoniazid, and multidrug-resistant tuberculosis (MDR-TB; TB resistant to at least isoniazid and rifampicin) is increasingly a global health threat (1). Data to inform treatment guidelines for isoniazid-monoresistant TB are limited (2, 3). For MDR-TB, in 2016 the World Health Organization introduced a “short-course” treatment regimen based on favorable results from cohort studies conducted in Bangladesh and several West African countries (4, 5). This regimen involves seven medications—high-dose isoniazid, ethionamide, amikacin, moxifloxacin, ethambutol, pyrazinamide, and clofazimine—and a treatment duration of 9–12 months. It is the only short-course regimen currently endorsed for MDR-TB. The standard-duration (18–24 mo) MDR-TB treatment regimen recommendations were updated in 2019 after the publication of individual-patient meta-analyses (4, 6), and now include drugs that are strongly recommended (group A: bedaquiline, linezolid, levofloxacin, or moxifloxacin) and drugs that are conditionally recommended (groups B and C). There were insufficient data to include high-dose isoniazid in the meta-analyses. It remains unclear what role isoniazid can and should play.
Isoniazid is an essential first-line drug for the treatment of drug-sensitive TB. It is orally available, low-cost, widely approved, better tolerated than many second-line anti-TB agents, and generally free of drug–drug interactions. Isoniazid is a prodrug that is converted to its active form by the M.tb catalase-peroxidase encoded by katG (7). The active moiety then forms a covalent adduct with nicotinamide adenine dinucleotide in the active site of its target enzyme, inhA, to inhibit mycolic acid synthesis (8). Isoniazid has potent early bactericidal activity (EBA). At the standard dose (5 mg/kg) against drug-susceptible TB, isoniazid monotherapy reduces bacterial burden by 90–95% within the first 2 days of treatment, and bactericidal activity can be observed even at doses 10–20 times lower (9). The potent EBA of isoniazid suggests that doses higher than 5 mg/kg may largely overcome low-level resistance caused by mutations in the inhA gene or its promoter, which typically confer only four- to eightfold increases in the isoniazid minimum inhibitory concentration (MIC), whereas katG mutations are associated with higher-level resistance (10). Both bacterial mutations are easily detected using rapid molecular technologies (11).
Several observations suggest that high-dose isoniazid is efficacious against MDR-TB (12–17). To test the hypothesis that high-dose isoniazid exerts substantial EBA in a subset of MDR-TB cases with low-level isoniazid resistance, we designed a trial to study the dose-ranging EBA of isoniazid among individuals with pulmonary MDR-TB caused by inhA mutant strains. Individuals with isoniazid-sensitive TB receiving standard-dose isoniazid were enrolled for comparison. Some of the results of this study have been previously reported in the form of an abstract (18).
Methods
Study Design and Participants
AIDS Clinical Trials Group A5312, or INHindsight (www.clinicaltrials.gov identifier: NCT01936831), is a phase 2A, single-site, randomized open-label study in adults with isoniazid-sensitive or isoniazid-resistant pulmonary TB, with or without HIV, conducted at a single site and laboratory (TASK Applied Science, Cape Town, South Africa). The trial was approved by the local ethics committee and the South African Health Products Regulatory Authority. All participants gave written informed consent.
Potential participants with drug-sensitive or drug-resistant TB were identified among patients who had received a diagnosis of TB from the National Health Laboratory Service in their community clinics. Individuals with isoniazid-resistant TB were largely identified from among patients with rifampicin-resistant TB (detected via Gene Xpert MTB/RIF). Rifampicin resistance is highly predictive of additional isoniazid resistance. Because of this recruitment algorithm, most of these participants were expected to have TB strains with resistance to at least rifampicin and isoniazid. Control subjects were selected from those with negative tests for rifampicin resistance by Gene Xpert MTB/RIF. After informed consent was obtained, an extra sputum sample was collected to confirm the presence and type of isoniazid resistance at the study laboratory, and MIC testing was performed. Adults (age ≥18 and ≤65 yr) with sputum smear-positive pulmonary TB (at least grade 1+ on the International Union Against Tuberculosis and Lung Disease scale) with a strain shown to have 1) inhA promoter or gene mutations but not katG mutations (inhA group) or 2) neither inhA nor katG mutations (control group) (via Hain GenoType MTBDRplus) were eligible for participation in the treatment trial. Other inclusion criteria were weight 40–90 kg, absolute neutrophil count >750 cells/mm3, Hb >7.4 g/dl, platelet count >50,000/mm3, aspartate aminotransferase and alanine aminotransferase ≤3 times the upper limit of normal, and total bilirubin ≤2.5 × upper limit of normal. Individuals with HIV and a CD4 count of ≥50 cells/mm3 could participate. Participants needed to demonstrate an ability to produce an overnight sputum sample of ≥10 ml over a 16-hour period. Participants with central nervous system TB, grade 2 or greater peripheral neuropathy, or receipt of more than 7 days of second-line TB drugs were excluded. Chest radiographs were performed to determine cavitary disease status.
Procedures
Participants in the inhA group were randomized 1:1:1 to receive isoniazid at a dose of 5, 10, or 15 mg/kg daily for 7 days. Control subjects received standard-dose isoniazid at 5 mg/kg daily. Participants were hospitalized during study treatment. Overnight sputum collections for 16 hours were performed at preentry (Day −1), Day 0 (pretreatment), and then daily while receiving isoniazid (Days 1–7). Sputum samples were transported to the laboratory in refrigerated containers. Samples were homogenized and then cultured on selective Middlebrook 7H11S agar plates for colony-forming unit (cfu) determination in quadruplicate in 10-fold dilutions, on selective Middlebrook 7H11S agar plates. Liquid culture media were inoculated in duplicate for time to positivity (TTP, in h) after standard NaOH-based decontamination and using the Bactec Mycobacteria Growth Indicator Tube 960 system. Genotypic drug susceptibility for isoniazid, rifampicin, fluoroquinolones, and aminoglycosides was assessed on baseline sputum samples using the Hain GenoType MTBDRplus and MTBDRsl assays. The MIC using the 1% agar proportion method was determined for available strains from EBA study participants and participants who failed screening, in order to obtain a comparable number of nonmutated inhA- or katG-mutated strains. Pharmacokinetic (PK) sampling was performed on Day 6. Blood samples for routine safety testing were collected at entry, Day 7, and Day 21. After completion of the study treatment, the participants’ care was transitioned to the local TB program for full TB treatment.
Outcomes
Primary outcomes included the following: 1) daily change in log10 cfu/ml sputum from baseline to Day 7 of study treatment, with individual-based parameter estimates from nonlinear mixed-effect models (EBAcfu0–7); 2) daily change in TTP over 7 days (EBATTP0–7); and 3) grade ≥2 adverse clinical or laboratory events. Early-phase (EBA0–2) and late-phase (EBA2–7) EBA estimates were also determined.
Statistical Considerations
The standard sample size in EBA studies is 12–15 participants per arm (19). The planned sample size in INHindsight was 16 participants per treatment cohort. This sample size was selected to achieve a two-sided 95% confidence interval (CI) with a precision (half-width) of ≤0.064 with SDs between 0.06 and 0.12 for the primary cfu analyses. Safety analyses included all participants who took at least one dose of isoniazid. PK analyses were performed using noncompartmental analysis in Phoenix WinNonLin version 8.0. The efficacy population included all participants with at least one cfu or TTP measurement. Nonlinear mixed-effects models using NLMIXED in SAS version 9.4 were used to estimate the decrease of log10 cfu counts per day and the increase of TTP per day. The pretreatment measures at preentry and Day 0 were combined into one baseline measure by taking the mean of the two measures. All available measures at every time point were included in the NLMIXED models separately for cfu and TTP measurements. Details regarding the statistical modeling can be found in the online supplement.
Results
Enrollment and Baseline Characteristics
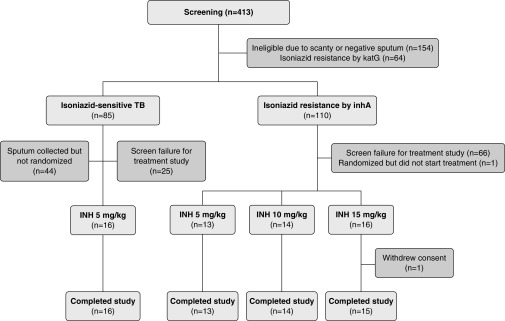
From August 2014 to November 2017, 413 participants were screened, and 154 of these participants failed screening for sputum criteria, mostly owing to negative or scanty sputum smears. Among those who met the sputum criteria, common reasons for ineligibility were high severity of illness (too ill to participate), participant declining hospitalization, or previous or current TB treatment (online supplement). Fifty-nine participants (43 in the inhA group and 16 in the control group) were enrolled in the treatment trial (Figure 1). The median age was 32 years, 27% were women, 20% were HIV-positive, the median weight was 51 kg, and 88% had cavitary disease (Table 1). The baseline sputum bacillary load, as measured by cfu counts on solid culture medium, ranged from a median of 5.41 to 7.04 log10 cfu and was generally higher in participants in the inhA group than in the control group. However, the baseline TTP in liquid cultures was overall shorter in control subjects (median 97 h) than in participants in the inhA group (median 124–142 h).
Figure 1.
CONSORT (Consolidated Standards of Reporting Trials) diagram showing study participant flow. Participants were selected in a two-step procedure. Only participants with a positive sputum smear of at least grade 1+ and with isolate having no katG mutation were screened for participation in the treatment study. INH = isoniazid; TB = tuberculosis.
Table 1.
Baseline Demographic and Tuberculosis Disease Characteristics of Participants with Isoniazid-Resistant Tuberculosis (Wherein Isoniazid Resistance Is Mediated by inhA Mutation) or Isoniazid-Sensitive Tuberculosis (Control Subjects)
| Type of TB | Isoniazid-Resistant TB |
Control Subjects | All | ||
|---|---|---|---|---|---|
| 5 mg/kg (n = 13) | 10 mg/kg (n = 14) | 15 mg/kg (n = 16) | 5 mg/kg (n = 16) | Total (N = 59) | |
| Age, yr | |||||
| Median (IQR) | 31 (26–44) | 32 (23–41) | 34 (22–44) | 30 (22–41) | 32 (23–41) |
| Sex, n (%) | |||||
| Male | 10 (77) | 11 (79) | 10 (63) | 12 (75) | 43 (73) |
| Female | 3 (23) | 3 (21) | 6 (38) | 4 (25) | 16 (27) |
| Race, n (%) | |||||
| White | 0 | 0 | 0 | 1 (6) | 1 (2) |
| Black African | 12 (92) | 14 (100) | 16 (100) | 15 (94) | 57 (97) |
| Other | 1 (8) | 0 | 0 | 0 | 1 (2) |
| HIV-1 positive, n (%) | |||||
| Yes | 2 (15) | 3 (21) | 4 (25) | 3 (19) | 12 (20) |
| No | 11 (85) | 11 (79) | 12 (75) | 13 (81) | 47 (80) |
| Weight, kg | |||||
| Median (IQR) | 50 (47–55) | 50 (46–59) | 50 (45–52) | 52 (48–57) | 51 (47–56) |
| Cavitary disease, n (%) | |||||
| Yes | 11 (85) | 14 (100) | 15 (94) | 12 (75) | 52 (88) |
| No | 2 (15) | 0 | 1 (6) | 4 (25) | 7 (12) |
| Rifampicin resistance, n (%) | |||||
| Yes | 9 (69) | 9 (64) | 13 (81) | 3 (19) | 34 (58) |
| No | 4 (31) | 5 (36) | 3 (19) | 13 (81) | 25 (42) |
| Bacillary load* | |||||
| Median (IQR) | 5.65 (5.34–7.02) | 6.93 (6.56–7.43) | 7.04 (6.18–7.33) | 5.41 (4.81–6.68) | 6.56 (5.39–7.24) |
| Time to positivity† | |||||
| Median (IQR) | 142 (110–173) | 127 (9104–143) | 124 (100–152) | 97 (92–120) | 118 (97–151) |
Definition of abbreviations: IQR = interquartile range; TB = tuberculosis.
Baseline bacillary load on solid culture medium, in log10 cfu.
Baseline culture time to positivity in liquid culture, in hours.
Pharmacokinetics and Safety
Overall, isoniazid displayed dose-proportional pharmacokinetics (Table 2). All but one participant (98%) completed study treatment, and one participant in group 1 (15 mg/kg arm) withdrew consent for family reasons on Day 5. Overall, there were nine grade 3 adverse events. All were unrelated or unlikely to be related to study treatments (one each of fever, pain, and dyspnea; two episodes of pneumothorax; and four cases of anemia). There were no grade 4 events or deaths. Three participants, all in the inhA group (one in the 5 mg/kg arm and two in the 10 mg/kg arm), developed grade 2 liver enzyme elevations.
Table 2.
Mean Isoniazid Pharmacokinetic Data, by Dose and Arm
| Isoniazid-Resistant Tuberculosis |
Control Subjects | |||
|---|---|---|---|---|
| 5 mg/kg | 10 mg/kg | 15 mg/kg | 5 mg/kg | |
| Cmax, μg/ml | 5.44 | 9.41 | 17.1 | 5.13 |
| AUC0–24, μg · h/ml | 19.3 | 45.7 | 67.7 | 15.1 |
| CL/F, L/h | 24.3 | 18.0 | 14.2 | 26.5 |
Definition of abbreviations: AUC0–24 = area under the concentration–time curve over 24 hours; CL/F = oral clearance; Cmax = maximum concentration.
Activity
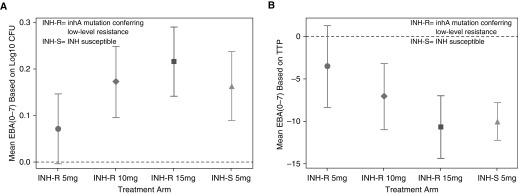
Nonlinear models were used to estimate EBAcfu0–7 and EBATTP0–7 because the log-transformed daily bactericidal activity over 7 days was not linear for most arms. For the EBA estimation based on the cfu counts, model-based fitted curves could not be produced for two participants in the 10 mg/kg arm, one in the 15 mg/kg arm, and one in the control group because of missing cfu data or failure of the nonlinear mixed models to converge, and for the estimation based on TTP, one participant in the 10 mg/kg arm was missing because of the convergence issue. Figure 2 shows the model-based means of EBAcfu0–7 and EBATTP0–7 with 95% CI, by arm. Overall, the standard 5 mg/kg dose in the control group and doses of 10 and 15 mg/kg in the inhA group had activity with a 95% CI that did not include zero. The 5 mg/kg dose in the inhA group had measurable activity, but the 95% CI included zero. Table 3 shows the median EBAcfu0–7 and EBATTP0–7, as well as early-phase (EBAcfu0–2 and EBATTP0–2) and late-phase (EBAcfu2–7 and EBATTP2–7) EBA estimates with interquartile ranges.
Figure 2.
Seven-day early bactericidal activity (EBA) of isoniazid (INH). (A) Mean (95% confidence interval) EBA by decrease in colony-forming units (cfu) on solid culture medium, averaged over 7 days, based on log10 cfu count, by arm. (B) Mean (95% confidence interval) EBA based on decrease in time to positivity (TTP) in liquid medium, in hours, averaged over 7 days.
Table 3.
Model-based Estimates of EBA on Solid Culture Media and Liquid Culture Media in Participants with Isoniazid-Resistant (inhA Mutation) and Isoniazid-Susceptible Tuberculosis
| Type of TB | Isoniazid-Resistant TB |
Control Subjects | ||
|---|---|---|---|---|
| 5 mg/kg (n = 13) | 10 mg/kg (n = 14) | 15 mg/kg (n = 16) | 5 mg/kg (n = 16) | |
| EBAcfu0–7* | ||||
| n | 13 | 12 | 15 | 15 |
| Median | 0.08 | 0.12 | 0.20 | 0.15 |
| IQR | 0.01 to 0.14 | 0.10 to 0.23 | 0.11 to 0.28 | 0.11 to 0.25 |
| EBAcfu0–2 | ||||
| Median | 0.09 | 0.06 | −0.09 | 0.40 |
| IQR | −0.01 to 0.38 | −0.13 to 0.39 | −0.55 to 0.48 | 0.21 to 0.67 |
| EBAcfu2–7 | |
|||
| Median | −0.04 | 0.15 | 0.25 | 0.09 |
| IQR | −0.16 to 0.02 | 0.04 to 0.29 | 0.04 to 0.48 | −0.43 to 0.18 |
| EBATTP0–7† | |
|||
| n | 13 | 13 | 16 | 16 |
| Median | −2 | −4 | −10 | −9 |
| IQR | −8 to 3 | −7 to −3 | −12 to −6 | −12 to −8 |
| EBATTP0–2 | ||||
| Median | −2 | −10 | −4 | −23 |
| IQR | −15 to 2 | −12 to −7 | −15 to 1 | −31 to −15 |
| EBATTP2–7 | ||||
| Median | −4 | −3 | −10 | −5 |
| IQR | −9 to 4 | −9 to 0 | −17 to −6 | −9 to −2 |
Definition of abbreviations: EBA = early bactericidal activity; IQR = interquartile range; TB = tuberculosis; TTP = time to positivity.
EBAcfu and EBATTP estimates were obtained on liquid culture media and solid culture media, respectively.
Change in log10 cfu/ml/d, averaged over 7 days.
Change in TTP in liquid culture per day, averaged over 7 days.
Microbiology
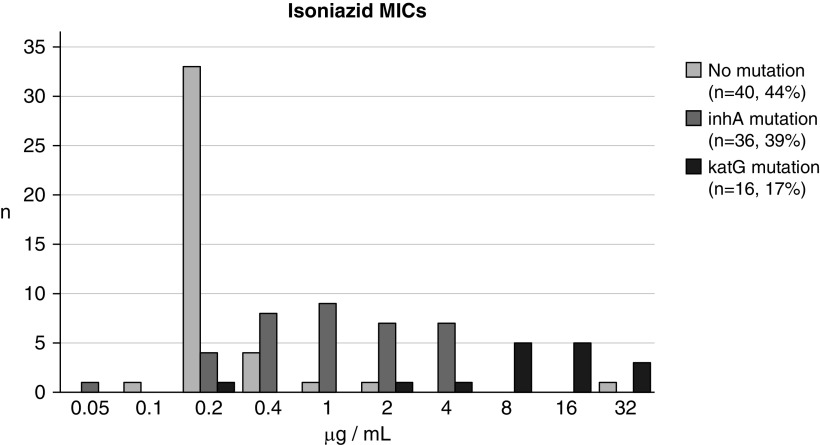
Among the screened participants, the M.tb median isoniazid MIC was 0.2 μg/ml (range 0.1–32 μg/ml) among those with strains with no mutations, 1 μg/ml (range 0.05–4 μg/ml) among those with inhA mutations, and 12 μg/ml (range 0.2–32 μg/ml) among those with katG mutations (Figure 3). Among the EBA study participants, the MIC was 0.2 μg/ml (range 0.2–1 μg/ml) and 1 μg/ml (range 0.05–4 μg/ml), respectively, for control subjects and participants with strains with inhA mutations. Resistance characterization of the isolates from the 59 EBA study participants was based on available genotypic data, with all assessed for isoniazid and rifampicin resistance, and 36 (61%) additionally assessed for fluoroquinolone and aminoglycoside resistance. In the inhA group, 31 of 43 (72%) had MDR-TB, 12 (28%) had isoniazid-monoresistant TB (Table 1), and 4 (9%) were classified as preextensively drug resistant (with 3 [7%] being fluoroquinolone resistant and 1 [2%] being aminoglycoside resistant). Among the 16 control subjects, 13 (81%) had no detectable resistance, and 3 (19%) had rifampicin monoresistance.
Figure 3.
Distribution of minimum inhibitory concentrations (MICs) of isoniazid against strains with no inhA or katG mutations, with inhA mutations, or with katG mutations. Strains are from participants who provided sputum specimens only or who provided specimens and also participated in the treatment trial.
Discussion
The role of isoniazid in the treatment of TB with isoniazid resistance, either isolated or combined with resistance to other drugs, is highly debated. In our trial, which is the first EBA study to be conducted among participants with drug-resistant TB, we demonstrated that isoniazid, provided as a single drug, has measurable bactericidal activity against TB strains that have inhA mutations, provided it is given at a dose of 10–15 mg/kg daily. Furthermore, these doses produce EBA similar to that observed with isoniazid 5 mg/kg (the standard dose) in participants with isoniazid-sensitive TB. To our knowledge, this is the first trial to demonstrate the independent activity of high-dose isoniazid in the treatment of TB caused by inhA-mutated strains.
Our study adds to the emerging literature on TB treatment by demonstrating the independent bactericidal activity of isoniazid, without companion drugs, against strains harboring inhA mutations and at least additional rifampicin resistance. Several lines of evidence suggest that high-dose isoniazid provides a treatment benefit to patients with MDR-TB. Katiyar and colleagues randomized patients in India with MDR-TB to receive isoniazid (5 mg/kg or 16–18 mg/kg) or placebo, in addition to multidrug background therapy (13). Patients in the 16–18 mg/kg arm had 74% 6-month culture conversion, compared with 45–49% in the other arms, using liquid culture. In the cohort study that provided the basis for the short-course MDR-TB regimen now recommended by the World Health Organization, the inclusion of high-dose isoniazid in the regimen increased treatment success from approximately 55% to 70% (12, 14, 20, 21). In an independent patient data meta-analysis of 975 children with MDR-TB, the use of high-dose isoniazid increased the odds of treatment success nearly sixfold (adjusted odds ratio, 5.9; 95% CI, 1.7–20.5; P = 0.007). Lastly, patients in Haiti with MDR-TB between 2011 and 2014 received high-dose isoniazid (16–18 mg/kg), whereas those with MDR-TB in 2009–2011 and 2014–2015 received para-aminosalicylic acid because of local treatment guideline changes. Companion drugs were consistent from 2009 to 2015. Those who received high-dose isoniazid had 2.5-fold higher odds (adjusted odds ratio, 2.53; 95% CI, 1.08–6.28; P = 0.036) of treatment success than those who did not (15). Common to all these studies is the lack of knowledge about the type of isoniazid resistance. However, global surveillance data indicate that katG mutations are more frequent than inhA mutations (22), which, given the magnitude of the improvements with high-dose isoniazid, raises the question as to whether there is not some measurable effect of high-dose isoniazid on katG-mutated strains as well. Our trial has recently expanded to include patients with MDR-TB caused by strains with katG mutations. Participants are randomized to receive isoniazid at 15 or 20 mg/kg, and the independent effect of isoniazid in this population will be characterized and quantified.
EBA studies have a storied history (23). They are commonly used early in clinical drug development of novel compounds for proof of concept that a new drug candidate is active in humans at the anticipated doses (24–26). Their role in predicting drug efficacy and interpretation of the results are debated (27) because such studies are unable to quantify the potential benefits of drugs without measurable activity against actively dividing, culturable bacilli when assessed over short treatment durations. The EBA of isoniazid, however, has been well studied. Doses as low as 18.75 mg have measurable EBA, and doses higher than 300 mg (the standard dose) do not increase bactericidal activity (9). The EBA of isoniazid, measured in most trials over just 2 days (EBAcfu0–2), depends on the mean baseline bacillary load, with higher baseline loads resulting in larger effects. The control arm in our study was similar to that in other studies (mean baseline bacillary load 5.71 log10 cfu, mean EBAcfu0–2 0.39 ∆log10 cfu/ml/d) (28).
EBA studies over longer periods (e.g., 14 d) have been proposed as one way to assess the potential sterilizing activity of a drug or regimen (29–32). In addition, given the challenges of counting cfu on solid culture media, the use of TTP in Mycobacteria Growth Indicator Tube liquid medium, which is easier to standardize and more widely available, has been explored (33, 34). Although the cfu count is a direct measure of the number of bacteria that are able to grow on solid culture medium, the TTP reflects both the number of viable bacilli present and their growth rate. Although these measures largely correlate well, dose effects may differ in magnitude between the two methods (35). Furthermore, the extent to which these measures correlate for drug-resistant bacilli, which may have fitness defects, remains unknown. Interestingly, in the arms with resistance-conferring mutations, although the baseline cfu counts were higher than in the drug-sensitive arms, the baseline TTP was longer than that observed for drug-sensitive strains. This suggests that fitness was impaired in the mutated strains and is consistent with other studies (36). Furthermore, bactericidal activity in the inhA arms was somewhat delayed, with average daily killing higher in Days 2–7 than in Days 0–2, whereas the opposite was true in the drug-sensitive arm. Rapid bactericidal activity in the first 2 days followed by little measurable activity after Day 2 is typical in studies of isoniazid against drug-sensitive strains (32), perhaps because most of the actively multiplying organisms that are killed readily by isoniazid are eliminated in the first 2 days of therapy. In our study, in strains in which the target (inhA) was mutated, this rapid initial killing in the first 2 days was not seen; instead, the drug activity was delayed and more prolonged in nature. The relative utility of cfu versus TTP measurements for assessing EBA against drug-resistant strains requires more study.
For patients with MDR-TB, treatment options are improving with the introduction of new drugs, but composing regimens containing four to five active drugs remains challenging. This is because of possible side effects (skin discoloration, ichthyosis, deafness, severe nausea, gastrointestinal distress, blindness, bone marrow toxicity, neuropathy, and central nervous system side effects), poor oral bioavailability, drug–drug interactions (with HIV or other TB drugs), poor or unproven efficacy, and economic factors. High-dose isoniazid represents a useful additional treatment option for clinicians and patients because it is orally administered, low-cost, and readily available, and the available data suggest it is well tolerated. Our results conclusively demonstrate that it is also highly efficacious against MDR-TB strains with isolated inhA mutations. Fortunately, inhA and katG mutations are easily detected by rapid molecular technologies, thus enabling rapid identification of MDR-TB patients with isolated inhA mutations. Next-generation molecular tests that simultaneously detect the presence of M.tb and resistance to both isoniazid and rifampicin are coming soon (37), so the inhA and katG mutation status will soon be available at the time of diagnosis. The safety of isoniazid doses of 10–15 mg/kg is not as well established as that of the 5 mg/kg dose. Although the data we have suggest that these doses are tolerable, provided pyridoxine is coadministered, more systematically collected safety data (especially for doses of >10 mg/kg) are needed to confirm this (12, 13, 15, 20, 38). Evidence of the activity of isoniazid against more resistant strains with katG mutations is currently indirect (12, 13, 15, 20), as noted above, but there is a putative mechanism for host activation of the prodrug (39), and its independent effects will be tested prospectively in our trial.
Our study has several limitations. First, MIC data were not available for all participants, owing to difficulties in regrowing strains after a long period of storage. However, MIC data were available for 76 participants with nonmutated or inhA-mutated strains. The median MIC values (1 μg/ml for inhA mutants and 0.2 μg/ml for drug-sensitive strains) were similar to those seen in other settings (40). It is interesting that only a two- to threefold increase in dose was required to have similar effects for isoniazid-resistant TB compared with isoniazid-sensitive TB, despite a fivefold difference in MIC. Second, the current results do not allow for individualization of dose selection for treatment with high-dose isoniazid. However, modeling work is underway that incorporates PK data, available MIC information, NAT2 (n-acetyl transferase 2) genotype, and cfu and TTP data, with the goal of providing more nuanced information for providers to help them decide whether or not to use high-dose isoniazid in a given patient and to select the optimal dose. Lastly, there were higher bacillary loads in terms of cfu in participants with isoniazid-resistant TB than in those with isoniazid-sensitive TB. This could have amplified the effect of isoniazid in the drug-resistant arms. However, in the field, bacterial loads are commonly higher at the time of a diagnosis of isoniazid-resistant TB than they are in patients with drug-sensitive TB, and thus our populations appear to reflect patients with TB generally. In some resource-limited settings where isoniazid resistance testing is not readily available, patients with a high bacterial load are prioritized for drug susceptibility testing, as this is considered a potential marker of drug-resistant disease (41, 42).
In conclusion, this randomized clinical trial demonstrated that isoniazid at doses of 10–15 mg/kg daily had measurable EBA against MDR-TB with inhA mutation–mediated isoniazid resistance. The EBA was similar to that seen with a standard 5 mg/kg dose against drug-susceptible TB. Modeling work to assess pharmacogenomic–PK–pharmacodynamic relationships and testing of high-dose isoniazid against strains with katG mutations will follow. The safety and tolerability of these higher isoniazid doses must be determined in longer-term studies.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank the study participants and their families. They also acknowledge the INHindsight Study Team members (listed below) who contributed to the trial.
INHindsight (A5312) Study Team members: Rachel Issa, Christopher Lane, Mark Lojacono, Rachel Mahachi, William Murtaugh, Lynette Purdue, Akbar Shahkolahi, and Ronald Ssenyonga.
Footnotes
Supported by the Division of AIDS, NIH. Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases, NIH, under award numbers UM1 AI068634, UM1 AI068636, and UM1 AI106701. This work was supported in part by TASK Clinical Research Site grant UM1AI069521. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Author Contributions: K.E.D., S.M., R.H., S.L.R., E.L.N., L.M., S.S., and A.H.D. designed the study. F.v.G.-B., N.V., and A.H.D. conducted the study. S.M., X.S., and E.H.I. performed the analyses. K.E.D., S.M., X.S., R.H., S.L.R., L.M., S.S., N.V., and A.H.D. drafted the manuscript. K.D. managed the data. All authors provided input and reviewed and approved the final manuscript.
A complete list of A5312 Study Team members may be found before the beginning of the References.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201910-1960OC on January 16, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
Contributor Information
Collaborators: on behalf of the A5312 Study Team, Rachel Issa, Christopher Lane, Mark Lojacono, Rachel Mahachi, William Murtaugh, Lynette Purdue, Akbar Shahkolahi, and Ronald Ssenyonga
References
- 1.World Health Organization. Global tuberculosis report 2019. WHO/CDS/TB/2019.15. 2018. [accessed 2019 Nov 27]. Available from: https://apps.who.int/iris/bitstream/handle/10665/329368/9789241565714-eng.pdf?ua=1.
- 2.World Health Organization. WHO treatment guidelines for isoniazid-resistant tuberculosis: supplement to the WHO treatment guidelines for drug-resistant tuberculosis. 2018. [accessed 2019 Nov 27]. Available from: https://www.who.int/tb/publications/2018/WHO_guidelines_isoniazid_resistant_TB/en/ [PubMed]
- 3.Nahid P, Mase SR, Migliori GB, Sotgiu G, Bothamley GH, Brozek JL, et al. Treatment of drug-resistant tuberculosis: an official ATS/CDC/ERS/IDSA clinical practice guideline. Am J Respir Crit Care Med. 2019;200:e93–e142. doi: 10.1164/rccm.201909-1874ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. Consolidated guidelines on the treatment of drug resistant tuberculosis. 2019. [accessed 2019 Nov 27]. Available from: https://www.who.int/tb/publications/2019/consolidated-guidelines-drug-resistant-TB-treatment/en/ [PubMed]
- 5.Nunn AJ, Phillips PPJ, Meredith SK, Chiang CY, Conradie F, Dalai D, et al. STREAM Study Collaborators. A trial of a shorter regimen for rifampin-resistant tuberculosis. N Engl J Med. 2019;380:1201–1213. doi: 10.1056/NEJMoa1811867. [DOI] [PubMed] [Google Scholar]
- 6.Ahmad N, Ahuja SD, Akkerman OW, Alffenaar JC, Anderson LF, Baghaei P, et al. Collaborative Group for the Meta-Analysis of Individual Patient Data in MDR-TB treatment-2017. Treatment correlates of successful outcomes in pulmonary multidrug-resistant tuberculosis: an individual patient data meta-analysis. Lancet. 2018;392:821–834. doi: 10.1016/S0140-6736(18)31644-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Warren RM, Streicher EM, Gey van Pittius NC, Marais BJ, van der Spuy GD, Victor TC, et al. The clinical relevance of mycobacterial pharmacogenetics. Tuberculosis (Edinb) 2009;89:199–202. doi: 10.1016/j.tube.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 8.Vilchèze C, Jacobs WR., Jr The mechanism of isoniazid killing: clarity through the scope of genetics. Annu Rev Microbiol. 2007;61:35–50. doi: 10.1146/annurev.micro.61.111606.122346. [DOI] [PubMed] [Google Scholar]
- 9.Donald PR, Sirgel FA, Botha FJ, Seifart HI, Parkin DP, Vandenplas ML, et al. The early bactericidal activity of isoniazid related to its dose size in pulmonary tuberculosis. Am J Respir Crit Care Med. 1997;156:895–900. doi: 10.1164/ajrccm.156.3.9609132. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization. Technical report on critical concentrations for drug susceptibility testing of medicines used in the treatment of drug-resistant tuberculosis. WHO/CDS/TB/2018.5 [accessed 2019 Nov 27]. Available from: https://www.who.int/tb/publications/2018/WHO_technical_report_concentrations_TB_drug_susceptibility/en/
- 11.Drobniewski F, Cooke M, Jordan J, Casali N, Mugwagwa T, Broda A, et al. Systematic review, meta-analysis and economic modelling of molecular diagnostic tests for antibiotic resistance in tuberculosis. Health Technol Assess. 2015;19:1–188, vii–viii. doi: 10.3310/hta19340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Piubello A, Harouna SH, Souleymane MB, Boukary I, Morou S, Daouda M, et al. High cure rate with standardised short-course multidrug-resistant tuberculosis treatment in Niger: no relapses. Int J Tuberc Lung Dis. 2014;18:1188–1194. doi: 10.5588/ijtld.13.0075. [DOI] [PubMed] [Google Scholar]
- 13.Katiyar SK, Bihari S, Prakash S, Mamtani M, Kulkarni H. A randomised controlled trial of high-dose isoniazid adjuvant therapy for multidrug-resistant tuberculosis. Int J Tuberc Lung Dis. 2008;12:139–145. [PubMed] [Google Scholar]
- 14.Van Deun A, Salim MA, Das AP, Bastian I, Portaels F. Results of a standardised regimen for multidrug-resistant tuberculosis in Bangladesh. Int J Tuberc Lung Dis. 2004;8:560–567. [PubMed] [Google Scholar]
- 15.Walsh KF, Vilbrun SC, Souroutzidis A, Delva S, Joissaint G, Mathurin L, et al. Improved outcomes with high-dose isoniazid in multidrug-resistant tuberculosis treatment in Haiti. Clin Infect Dis. 2019 doi: 10.1093/cid/ciz039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harausz EP, Garcia-Prats AJ, Law S, Schaaf HS, Kredo T, Seddon JA, et al. Collaborative Group for Meta-Analysis of Paediatric Individual Patient Data in MDR-TB. Treatment and outcomes in children with multidrug-resistant tuberculosis: a systematic review and individual patient data meta-analysis. PLoS Med. 2018;15:e1002591. doi: 10.1371/journal.pmed.1002591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petty TL, Mitchell RS. Successful treatment of advanced isoniazid- and streptomycin-resistant pulmonary tuberculosis with ethionamide, pyrazinamide, and isoniazid. Am Rev Respir Dis. 1962;86:503–512. doi: 10.1164/arrd.1962.86.4.503. [DOI] [PubMed] [Google Scholar]
- 18.Dooley KE, Miyahara S, von Groote-Bidlingmaier F, Sun X, Hafner R, Rosenkranz SL, et al. Early bactericidal activity of high-dose isoniazid against multidrug-resistant TB. Presented at the Conference on Retroviruses and Opportunistic Infections. March 4–7, 2019, Seattle, WA. [Google Scholar]
- 19.Hafner R, Cohn JA, Wright DJ, Dunlap NE, Egorin MJ, Enama ME, et al. Early bactericidal activity of isoniazid in pulmonary tuberculosis. Optimization of methodology: the DATRI 008 Study Group. Am J Respir Crit Care Med. 1997;156:918–923. doi: 10.1164/ajrccm.156.3.9612016. [DOI] [PubMed] [Google Scholar]
- 20.Van Deun A, Maug AK, Salim MA, Das PK, Sarker MR, Daru P, et al. Short, highly effective, and inexpensive standardized treatment of multidrug-resistant tuberculosis. Am J Respir Crit Care Med. 2010;182:684–692. doi: 10.1164/rccm.201001-0077OC. [DOI] [PubMed] [Google Scholar]
- 21.Aung KJ, Van Deun A, Declercq E, Sarker MR, Das PK, Hossain MA, et al. Successful ‘9-month Bangladesh regimen’ for multidrug-resistant tuberculosis among over 500 consecutive patients. Int J Tuberc Lung Dis. 2014;18:1180–1187. doi: 10.5588/ijtld.14.0100. [DOI] [PubMed] [Google Scholar]
- 22.Zignol M, Cabibbe AM, Dean AS, Glaziou P, Alikhanova N, Ama C, et al. Genetic sequencing for surveillance of drug resistance in tuberculosis in highly endemic countries: a multi-country population-based surveillance study. Lancet Infect Dis. 2018;18:675–683. doi: 10.1016/S1473-3099(18)30073-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jindani A, Aber VR, Edwards EA, Mitchison DA. The early bactericidal activity of drugs in patients with pulmonary tuberculosis. Am Rev Respir Dis. 1980;121:939–949. doi: 10.1164/arrd.1980.121.6.939. [DOI] [PubMed] [Google Scholar]
- 24.Diacon AH, Dawson R, Hanekom M, Narunsky K, Maritz SJ, Venter A, et al. Early bactericidal activity and pharmacokinetics of PA-824 in smear-positive tuberculosis patients. Antimicrob Agents Chemother. 2010;54:3402–3407. doi: 10.1128/AAC.01354-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diacon AH, Dawson R, Hanekom M, Narunsky K, Venter A, Hittel N, et al. Early bactericidal activity of delamanid (OPC-67683) in smear-positive pulmonary tuberculosis patients. Int J Tuberc Lung Dis. 2011;15:949–954. doi: 10.5588/ijtld.10.0616. [DOI] [PubMed] [Google Scholar]
- 26.Rustomjee R, Diacon AH, Allen J, Venter A, Reddy C, Patientia RF, et al. Early bactericidal activity and pharmacokinetics of the diarylquinoline TMC207 in treatment of pulmonary tuberculosis. Antimicrob Agents Chemother. 2008;52:2831–2835. doi: 10.1128/AAC.01204-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davies G, Boeree M, Hermann D, Hoelscher M. Accelerating the transition of new tuberculosis drug combinations from Phase II to Phase III trials: new technologies and innovative designs. PLoS Med. 2019;16:e1002851. doi: 10.1371/journal.pmed.1002851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Jager V, van der Merwe L, Venter A, Donald PR, Diacon AH. Time trends in sputum mycobacterial load and two-day bactericidal activity of isoniazid-containing antituberculosis therapies. Antimicrob Agents Chemother. 2017;61:e02088-16. doi: 10.1128/AAC.02088-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson JL, Hadad DJ, Boom WH, Daley CL, Peloquin CA, Eisenach KD, et al. Early and extended early bactericidal activity of levofloxacin, gatifloxacin and moxifloxacin in pulmonary tuberculosis. Int J Tuberc Lung Dis. 2006;10:605–612. [PubMed] [Google Scholar]
- 30.Dietze R, Hadad DJ, McGee B, Molino LP, Maciel EL, Peloquin CA, et al. Early and extended early bactericidal activity of linezolid in pulmonary tuberculosis. Am J Respir Crit Care Med. 2008;178:1180–1185. doi: 10.1164/rccm.200806-892OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sirgel FA, Fourie PB, Donald PR, Padayatchi N, Rustomjee R, Levin J, et al. The early bactericidal activities of rifampin and rifapentine in pulmonary tuberculosis. Am J Respir Crit Care Med. 2005;172:128–135. doi: 10.1164/rccm.200411-1557OC. [DOI] [PubMed] [Google Scholar]
- 32.Jindani A, Doré CJ, Mitchison DA. Bactericidal and sterilizing activities of antituberculosis drugs during the first 14 days. Am J Respir Crit Care Med. 2003;167:1348–1354. doi: 10.1164/rccm.200210-1125OC. [DOI] [PubMed] [Google Scholar]
- 33.Bark CM, Okwera A, Joloba ML, Thiel BA, Nakibali JG, Debanne SM, et al. Time to detection of Mycobacterium tuberculosis as an alternative to quantitative cultures. Tuberculosis (Edinb) 2011;91:257–259. doi: 10.1016/j.tube.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bark CM, Gitta P, Ogwang S, Nsereko M, Thiel BA, Boom WH, et al. Comparison of time to positive and colony counting in an early bactericidal activity study of anti-tuberculosis treatment. Int J Tuberc Lung Dis. 2013;17:1448–1451. doi: 10.5588/ijtld.13.0063. [DOI] [PubMed] [Google Scholar]
- 35.Diacon AH, Maritz JS, Venter A, van Helden PD, Andries K, McNeeley DF, et al. Time to detection of the growth of Mycobacterium tuberculosis in MGIT 960 for determining the early bactericidal activity of antituberculosis agents. Eur J Clin Microbiol Infect Dis. 2010;29:1561–1565. doi: 10.1007/s10096-010-1043-7. [DOI] [PubMed] [Google Scholar]
- 36.Svensson EM, Karlsson MO. Modelling of mycobacterial load reveals bedaquiline’s exposure-response relationship in patients with drug-resistant TB. J Antimicrob Chemother. 2017;72:3398–3405. doi: 10.1093/jac/dkx317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gupta A, Nema V. Imminent weapons in the field of tuberculosis diagnostics and management. J Gen Pract Med Diagn. 2017;2:5. [Google Scholar]
- 38.Trébucq A, Schwoebel V, Kashongwe Z, Bakayoko A, Kuaban C, Noeske J, et al. Treatment outcome with a short multidrug-resistant tuberculosis regimen in nine African countries. Int J Tuberc Lung Dis. 2018;22:17–25. doi: 10.5588/ijtld.17.0498. [DOI] [PubMed] [Google Scholar]
- 39.Mahapatra S, Woolhiser LK, Lenaerts AJ, Johnson JL, Eisenach KD, Joloba ML, et al. A novel metabolite of antituberculosis therapy demonstrates host activation of isoniazid and formation of the isoniazid-NAD+ adduct. Antimicrob Agents Chemother. 2012;56:28–35. doi: 10.1128/AAC.05486-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ghodousi A, Tagliani E, Karunaratne E, Niemann S, Perera J, Koser CU, et al. Isoniazid resistance in Mycobacterium tuberculosis is a heterogeneous phenotype composed of overlapping MIC distributions with different underlying resistance mechanisms. Antimicrob Agents Chemother. 2019;63:e00092-19. doi: 10.1128/AAC.00092-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sander MS, Vuchas CY, Numfor HN, Nsimen AN, Abena JL, Noeske J, et al. Sputum bacterial load predicts multidrug-resistant tuberculosis in retreatment patients: a case-control study. Int J Tuberc Lung Dis. 2016;20:793–799. doi: 10.5588/ijtld.15.0259. [DOI] [PubMed] [Google Scholar]
- 42.Baya B, Achenbach CJ, Kone B, Toloba Y, Dabitao DK, Diarra B, et al. Clinical risk factors associated with multidrug-resistant tuberculosis (MDR-TB) in Mali. Int J Infect Dis. 2019;81:149–155. doi: 10.1016/j.ijid.2019.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.