Abstract
Rationale: Limited information is available on racial/ethnic differences in pulmonary arterial hypertension (PAH).
Objectives: Determine effects of race/ethnicity and ancestry on mortality and disease outcomes in diverse patients with PAH.
Methods: Patients with Group 1 PAH were included from two national registries with genome-wide data and two local cohorts, and further incorporated in a global meta-analysis. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated for transplant-free, all-cause mortality in Hispanic patients with non-Hispanic white (NHW) patients as the reference group. Odds ratios (ORs) for inpatient-specific mortality in patients with PAH were also calculated for race/ethnic groups from an additional National Inpatient Sample dataset not included in the meta-analysis.
Measurements and Main Results: After covariate adjustment, self-reported Hispanic patients (n = 290) exhibited significantly reduced mortality versus NHW patients (n = 1,970) after global meta-analysis (HR, 0.60 [95% CI, 0.41–0.87]; P = 0.008). Although not significant, increasing Native American genetic ancestry appeared to account for part of the observed mortality benefit (HR, 0.48 [95% CI, 0.23–1.01]; P = 0.053) in the two national registries. Finally, in the National Inpatient Sample, an inpatient mortality benefit was also observed for Hispanic patients (n = 1,524) versus NHW patients (n = 8,829; OR, 0.65 [95% CI, 0.50–0.84]; P = 0.001). An inpatient mortality benefit was observed for Native American patients (n = 185; OR, 0.38 [95% CI, 0.15–0.93]; P = 0.034).
Conclusions: This study demonstrates a reproducible survival benefit for Hispanic patients with Group 1 PAH in multiple clinical settings. Our results implicate contributions of genetic ancestry to differential survival in PAH.
Keywords: pulmonary arterial hypertension, Hispanic American, Native American, survival, health disparities
At a Glance Commentary
Scientific Knowledge on the Subject
Limited information is available on racial/ethnic differences in disease presentation and outcomes in pulmonary arterial hypertension (PAH), particularly in Hispanic patients. Despite comprehensive investigations of the influence of genetic variation in European ancestry samples, the impact of race/ethnicity and global genetic ancestry on mortality, functional status, and disease severity in Group 1 PAH cases is not well studied.
What This Study Adds to the Field
This study reports a comprehensive evaluation of race, ethnicity, and admixture in Group 1 PAH, made possible by national repositories of patients with PAH with genome-wide data. We detail influences of self-reported race/ethnicity, genetically defined race/ethnicity, and ancestry on all-cause mortality in Hispanic and African American populations across five cohorts. This study demonstrates a reproducible survival benefit for Hispanic patients with group 1 PAH in multiple clinical settings. Our results suggest that survival benefit is influenced by ancestral proportions. Our observation of improved survival in Hispanic patients emphasizes the need for well-powered studies evaluating the influence of ancestry and admixture on clinical outcomes. Our results reinforce the presence of racial/ethnic disparities in PAH, suggesting that these disparities are due in part to genetic differences between race/ethnic groups.
Pulmonary arterial hypertension (PAH) is a rare disease characterized by pathological obliterative remodeling of the pulmonary vasculature resulting in increased pulmonary vascular resistance (PVR), right heart failure, and death (1). Despite a low prevalence (2, 3), World Symposium of Pulmonary Hypertension (WSPH) Group 1 PAH disorders are considered fatal with a 40% to 60% 5-year survival rate when untreated (4, 5). Genetic studies in PAH have also shown varying survival associations with rare variant or common genetic variant carriers (6). Preliminary observations from a paucity of studies have shown greater potential disease severity and mortality rates in African American (AA) patients (7–9). However, a more recent study failed to show similar trends (5). Racial differences in treatment responses have also been described, with reduced benefits observed for AA patients (10). It is unclear whether these initial observations in PAH based on race/ethnicity may reflect pathophysiologic differences; distinct disease phenotypes; genetic differences; or racial disparities in environment, socioeconomic status, and access to care.
Although conflicting data are available for AA populations, little is known about Hispanic populations, the largest minority group in the United States (11). Hispanic patients are routinely unaccounted for or underrepresented in national registries (12), raising significant challenges in studying disease predisposition and severity in this population. Despite several investigations of the influence of genetic variation in European ancestry samples, such investigations are not available in patients with PAH who are admixed or non-European (6, 13, 14). Based on small sample sizes of minority representation in individual institutional registries, we determined the impact of race/ethnicity and global genetic ancestry on mortality and functional status in a relatively homogenous population of idiopathic or heritable (I/H) PAH cases from a national sample repository, the PAH Biobank. We then performed these analyses across all Group 1 PAH cases, combining three additional and independent cohorts, including another smaller national sample repository (Allegheny Health Network [AHN] cohort) and two Hispanic-enriched institutional registries (University of Arizona [UA] cohort and Stanford University Cohort). We next conducted a meta-analysis of all four cohorts in PAH and also evaluated the impact of race/ethnicity on inpatient mortality in idiopathic PAH (IPAH) cases from the National Inpatient Samples (NIS) database.
Methods
All studies were Institutional Review Board–approved at participating institutions and were conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all patients, excluding those in the NIS database. Detailed descriptions of cohorts, genotyping, quality control, genetic analyses, and statistical methodology are available in eMethods in the online supplement.
Participants
PAH biobank
Clinical data for participants represented WSPH Group 1 PAH cases defined as I/H PAH and were extracted from the National Biological Sample and Data Repository for PAH (www.pahbiobank.org) (6, 15). In this cohort, patients were recruited prospectively from 36 enrolling centers and only adults older than 18 years of age were included for this study from 2012 to 2018.
AHN cohort
Patients with WSPH Group 1 PAH treated with an endothelin receptor antagonist were enrolled prospectively between 2007 and 2010 from 45 U.S. and Canadian pulmonary hypertension centers or retrospectively from global sites participating in the STRIDE (Sitaxsentan To Relieve Impaired Exercise) trials (16, 17). Sites enrolling patients prospectively were instructed to conform to the standard definition of PAH described at that time by the 2007 Venice classification clinical guidelines. Detailed descriptions of this cohort are available (see eMethods) and have been previously described (18).
UA cohort
Patients in this registry included all WSPH Group 1 PAH cases enrolled at the UA pulmonary hypertension clinic from 2011 to 2016.
Stanford cohort
Adults with newly diagnosed WSPH Group 1 PAH were prospectively enrolled at Stanford University from 2008 to 2017. Incident Group 1 PAH cases were treatment-naive with no formal PAH diagnosis preceding referral. All incident PAH cases were then hemodynamically confirmed. Detailed descriptions of this cohort are available (see eMethods).
NIS database
The NIS database is a Healthcare Utilization Project database, representing a random, 20% stratified sample of all inpatient hospitalizations from approximately 1,000 nonfederal hospitals in 46 states (representing >97% of the total U.S. population) and includes approximately 7 to 8 million hospitalizations per year. We identified all patients from January 1, 2007, to December 31, 2016, with a primary discharge diagnosis of IPAH using an International Classification of Diseases, Ninth Revision (ICD-9) primary diagnosis code of 416.0 and ICD-10 primary diagnosis code of I27.0 (19). For each discharge record with a primary diagnosis of PAH, we obtained age, sex, race/ethnicity, inpatient length of hospitalization, inpatient mortality, Elixhauser comorbidities, and the Elixhauser comorbidity index previously validated in administrative databases (20).
Definitions of Race/Ethnicity and Genetic Admixture
Primary analyses related to PAH outcomes and indices of severity used self-reported race/ethnicity. Participants reporting race as white with no Hispanic ethnicity or AA with no Hispanic ethnicity were considered to be non-Hispanic white (NHW) patients or non-Hispanic AA patients, respectively. Patients reporting Hispanic ethnicity were considered Hispanic regardless of race.
In sensitivity analyses, principal components analysis (PCA) was used to determine genetically defined race/ethnicity in samples in which genomic data were available. Genome-wide array data were acquired for the PAH Biobank using the Illumina HumanOmni5-QUAD BeadChip and the AHN cohort data were acquired using the Illumina Omni Express array. Individuals belonging to African, European, admixed (Hispanic), and Asian ancestry groups were manually identified using the first two principal components alongside 1000 Genomes Project reference samples (21). Because Hispanic ethnicity represents a combination of genetic, environmental, and sociocultural factors, individuals were only categorized as Hispanic using PCA if they also self-reported as Hispanic. Proportions of African, European, and Native American or East Asian ancestry were generated using ancestry-informative markers input into STRUCTURE with three subpopulations (k = 3) using reference samples from the 1000 Genomes Project (21, 22). Estimation of global ancestral proportions did not have sufficient resolution to distinguish between Native American and East Asian ancestry. Asian ancestral proportions were assumed to represent Native American ancestry based on prior observations in Hispanic populations (23–25). Identity-by-descent analysis was performed on combined PAH Biobank and AHN cohort data to exclude patients enrolled in both biorepositories (n = 45) from the analysis.
Statistical Analysis
All-cause, transplant-free mortality was used as the primary endpoint in survival analyses in all four registries (see eMethods and Table E1). Adjusted Cox proportional hazards regressions, Kaplan-Meier estimates, and logrank tests were used to estimate effects of self-reported race/ethnicity, PCA-based race/ethnicity, and ancestry proportions on all-cause, transplant-free mortality. Multivariable linear regressions were also performed for association of self-reported race/ethnicity and ancestry proportions with PVR and 6-minute-walk distance. All Cox and linear regressions were adjusted for age, sex, use of prostacyclin analogs, and log (PVR). PVR was transformed to its natural logarithm and used in all models (except models in which PVR was the dependent variable) to adjust for hemodynamics at the time of enrollment, and to account for its significant and consistent difference across race/ethnic groups in our cohorts (26, 27). We adjusted for prostacyclin analog use based on differences in use across race/ethnic groups in our cohorts and its significant effects on PAH severity and mortality (15, 28–31). The Stanford cohort analyses did not adjust for prostacyclin use because these newly diagnosed patients were treatment-naive. In all analyses of race/ethnicity, Hispanic and AA patients were compared with NHW patients as the reference group. Sensitivity analyses with survival time from diagnostic right heart catheterization to enrollment were performed to account for left truncation of survival data. A sensitivity analysis was also performed excluding patients with BMPR2 (bone morphogenetic protein receptor type 2) mutations to account for increased mortality in familial PAH (32–34). Meta-analysis of Cox proportional hazards regression was performed using the rmeta package in R (version 2.16). A validation meta-analysis was performed combining three of the cohorts (AHN, UA, and Stanford) to validate observations in the PAH Biobank. A global meta-analysis was also performed that combined all four cohorts (PAH Biobank, AHN, UA, and Stanford). Fixed effects models were used when the test of heterogeneity among studies was nonsignificant and inverse SE was used as a weight for each study. NIS data were not incorporated into meta-analysis because the analysis of NIS data used logistic regression rather than survival analysis and because of differences in the registry-based and inpatient-specific mortality outcomes. An α-level of 0.05 was used for statistical significance.
NIS database
National estimates for total number of discharges with primary diagnosis of IPAH from 2007 to 2016 were generated from the NIS database using prespecified weights and published Healthcare Utilization Project methods (35). Nationally weighted PAH population data were used to compare racewise distribution of patients in terms of their age, sex, frequency of comorbidities, length of hospital stay, and all-cause inpatient mortality. Multivariable logistic regression analysis was performed to determine association between race/ethnicity and inpatient mortality after adjustment for age, sex, and Elixhauser comorbidity index.
Results
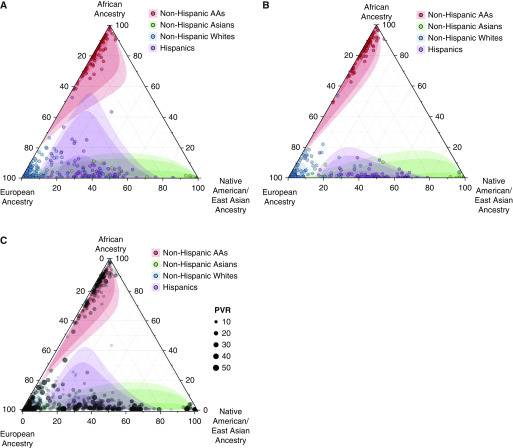
A total of 2,515 Group 1 PAH cases were identified from the PAH Biobank (n = 1,326), the AHN cohort (n = 822), the UA cohort (n = 164), and the Stanford cohort (n = 203). This total included 1,970 NHW patients, 255 AA patients, and 290 Hispanic patients. Characteristics of the PAH Biobank and AHN cohorts are presented in Table 1. In all four cohorts, the majority of patients were female and patients with Hispanic ethnicity were younger than other race/ethnic groups (see Tables E2–E5). Prostacyclin use was lowest among Hispanic patients. Hispanic patients had the highest mean pulmonary arterial pressure (mPAP) and PVR on average, and AA patients had the lowest average mPAP and PVR. AA patients had the lowest average 6-minute-walk distance. Estimated ancestral proportions were consistent with proportions expected for self-reported race/ethnicity categories (Figure 1). Detailed descriptions of estimated proportions of ancestry are available (see eResults).
Table 1.
Demographic, Clinical, and Hemodynamic Characteristics by Self-reported Race/Ethnicity
| Variable | PAH Biobank | AHN Cohort | |||||
|---|---|---|---|---|---|---|---|
| Non-Hispanic White (n = 1,039) | Non-Hispanic AA (n = 158) | Hispanic (n = 129) | Non-Hispanic White (n = 656) | AA (n = 97) | Hispanic (n = 69) | ||
| Age, yr | 53 ± 18 | 52 ± 17 | 42 ± 16 | 52 ± 16 | 51 ± 14 | 36 ± 11 | |
| Sex, F | 786 (76) | 134 (85) | 111 (86) | 523 (79) | 90 (93) | 60 (87) | |
| Prostacyclin analogs | 284 (27) | 34 (22) | 24 (19) | 87 (13) | 19 (20) | 3 (4) | |
| Ancestry estimates*, % | |||||||
| African | 0.01 | 0.82 | 0.07 | 0.02 | 0.82 | 0.02 | |
| European | 0.97 | 0.16 | 0.58 | 0.97 | 0.17 | 0.50 | |
| NA | 0.02 | 0.02 | 0.36 | 0.02 | 0.01 | 0.47 | |
| mRAP, mm Hg | 9.27 ± 5.65 | 10.2 ± 5.91 | 9.87 ± 7.97 | 8.66 ± 5.29 | 10.41 ± 8.04 | 10.09 ± 12.34 | |
| mPAP, mm Hg | 52.2 ± 14.1 | 47.7 ± 13.6 | 56.1 ± 15.8 | 51.2 ± 16.9 | 45.8 ± 15.2 | 66.0 ± 20.0 | |
| PCWP, mm Hg | 10.1 ± 3.99 | 10.3 ± 3.79 | 9.34 ± 3.34 | 10.52 ± 5.22 | 10.91 ± 4.48 | 8.94 ± 4.16 | |
| Cardiac index, L/min/m2 | 2.52 ± 1.26 | 2.34 ± 0.93 | 2.12 ± 0.72 | 2.61 ± 1.34 | 2.39 ± 0.69 | 3.17 ± 1.41 | |
| PVR, WU | 10.93 ± 6.54 | 10.2 ± 6.40 | 13.6 ± 8.12 | 10.91 ± 6.80 | 8.58 ± 5.25 | 14.32 ± 9.55 | |
| 6MWD, m | 356 ± 138 | 331 ± 114 | 378 ± 99 | 353 ± 128 | 297 ± 107 | 311 ± 96 | |
Definition of abbreviations: 6MWD = 6-minute-walk distance; AA = African American; AHN = Allegheny Health Network; AIMs = ancestry-informative markers; mPAP = mean pulmonary arterial pressure; mRAP = mean right atrial pressure; NA = Native American; PAH = pulmonary arterial hypertension; PCWP = pulmonary capillary wedge pressure; PVR = pulmonary vascular resistance; WU = Wood units.
Continuous data are presented as mean ± SD, and categorical variables are presented as n (%) unless otherwise specified.
Ancestry estimates were generated using AIMs input into STRUCTURE using HapMap reference populations.
Figure 1.
Proportions of African, European, and Native American ancestry were generated in the (A) Pulmonary Arterial Hypertension Biobank and the (B) Allegheny Health Network cohort using ancestry informative markers input into STRUCTURE with three subpopulations (k = 3) using 1000 Genomes reference populations. Each observation is positioned within the triangle, reflecting the combined estimate for percentage of African, European, and Native American ancestry. Color for each dot indicates self-reported race/ethnicity. Confidence intervals (50%, 95%, and 99%) for percentage of ancestry by self-reported race/ethnicity were calculated using the Mahalanobis distance in R. (C) Combined Pulmonary Arterial Hypertension Biobank and Allegheny Health Network cohorts are overlaid with magnitude of pulmonary vascular resistance (PVR). In adjusted regressions, increased PVR was associated with Native American ancestry and decreased PVR was associated with African ancestry. AAs = African American patients.
Influence of Race/Ethnicity on Mortality in PAH Registries
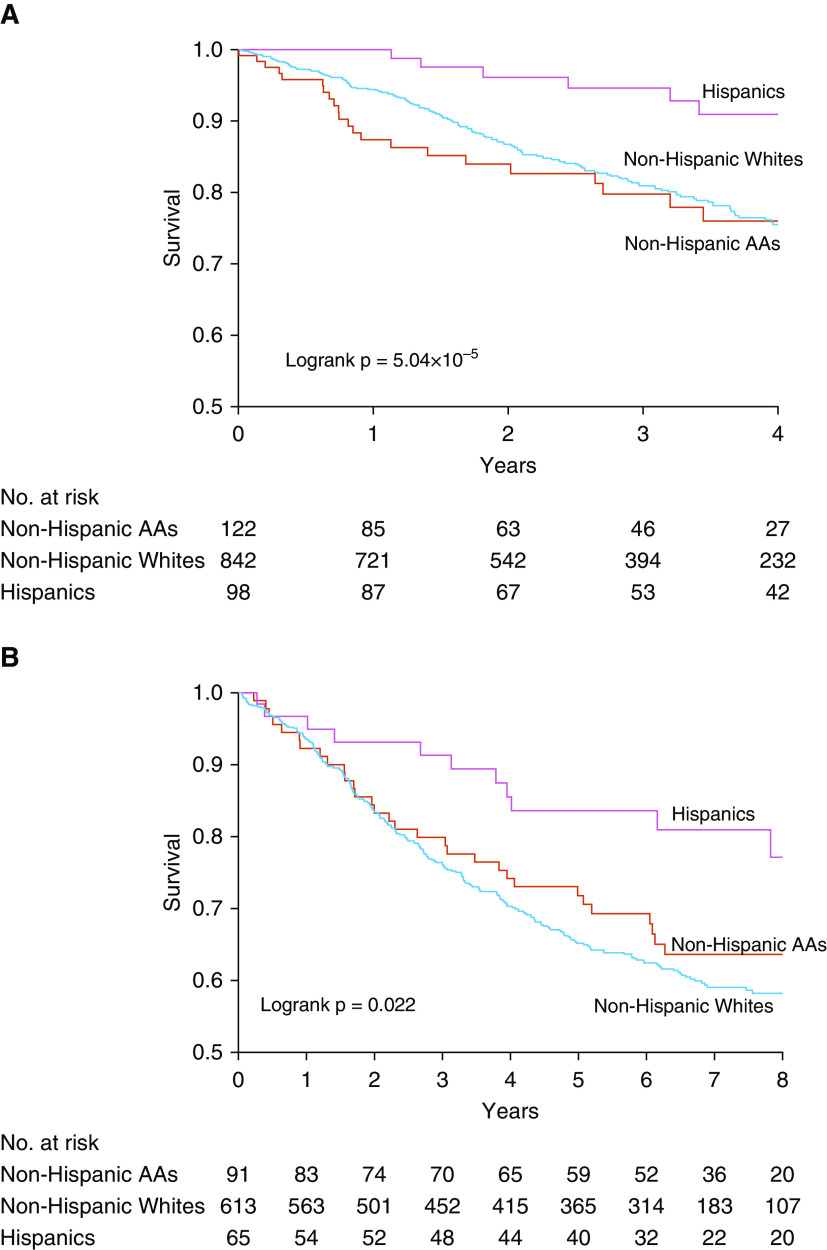
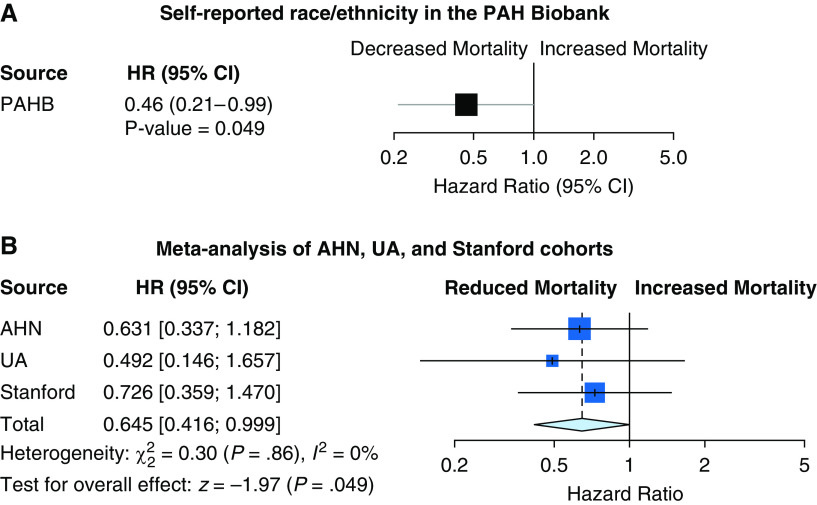
Significant logrank tests for survival by race/ethnicity were observed for the PAH Biobank and the AHN cohort (Figure 2). Although individual cohorts lack sufficient numbers of minority subjects for survival analyses, we observed a significant mortality benefit with self-reported Hispanic ethnicity in a homogenous cohort of patients with I/H PAH from the PAH Biobank (hazard ratio [HR], 0.46 [0.21–0.99]; P = 0.049) (Figure 3A). This mortality benefit for Hispanic patients extended to broader Group 1 PAH cases and was validated in a validation meta-analysis of three independent cohorts from the AHN, UA, and Stanford cohorts (HR, 0.65 [0.42–0.99]; P = 0.049) (see Figure 3B). Finally, a global meta-analysis of all four cohorts (PAH Biobank, AHN, UA, and Stanford) indicated a strong mortality benefit for self-reported Hispanic patients (HR, 0.60 [0.41–0.87]; P = 0.008) (see Figure E1).
Figure 2.
Kaplan-Meier curves for all-cause mortality by race/ethnicity in the (A) Pulmonary Arterial Hypertension Biobank and the (B) Allegheny Health Network cohort. Survival was calculated from enrollment up to date of death or censoring. In the Pulmonary Arterial Hypertension Biobank, January 1, 2018, was used as the date of censoring. In the Allegheny Health Network cohort, if a specific date for last contact was not recorded, the date December 19, 2014, was used as the censoring date. Logrank tests were performed for differential survival between race/ethnic groups. Kaplan-Meier curves were stopped when 10% of the original number at risk remained. AAs = African American patients; no. = number.
Figure 3.
All-cause mortality hazard ratios for self-reported race/ethnicity in the (A) Pulmonary Arterial Hypertension (PAH) Biobank and in (B) validation meta-analysis of the AHN, UA, and Stanford cohorts. Cox proportional hazards regression for all-cause mortality was performed in idiopathic PAH (PAH Biobank) and patients with Group 1 PAH (AHN, UA, and Stanford cohorts), adjusted for age, sex, prostacyclin use, and log (pulmonary vascular resistance). The Stanford cohort analyses did not adjust for prostacyclin use because these newly diagnosed patients were treatment naive. Patients with Hispanic ethnicity were compared with non-Hispanic white patients. Meta-analysis of Cox proportional hazards regression used a fixed effects model, and inverse SE was used as a weight for each study. AHN = Allegheny Health Network; CI = confidence interval; HR = hazard ratio; PAHB = Pulmonary Arterial Hypertension Biobank; UA = University of Arizona.
Significant results were consistently observed in sensitivity analyses, including analyses accounting for left truncation, excluding BMPR2 mutation carriers, and using PCA-defined race/ethnicity (see eResults and Figure E2). AA patients did not have significantly different survival versus NHW patients in the PAH Biobank or the AHN cohort. Results from the ancestry meta-analysis suggested that the observed mortality benefit in Hispanic patients may partly be due to decreased mortality with increasing Native American ancestry (HR, 0.48 [0.23–1.01]; P = 0.053), although this did not reach statistical significance. No significant associations were observed between European or African ancestry and mortality. Detailed descriptions of sensitivity analyses, survival analyses for proportions of ancestry, and the influence of race/ethnicity and admixture on hemodynamics and functional outcomes are available (see Tables E6 and E7, and eResults).
Influence of Race/Ethnicity on Mortality in the NIS Database
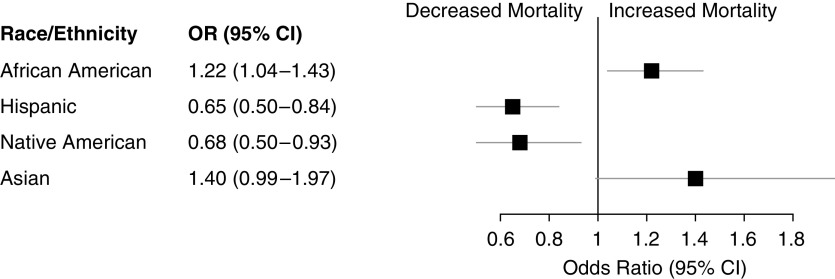
In the NIS database, which was not included in meta-analyses, 8,829 NHW, 2,628 AA, 1,524 Hispanic, 403 Asian, and 185 Native American patients with Group 1 PAH were identified. Consistent with the registry databases, the majority of patients were female and patients with Hispanic ethnicity were younger than other race/ethnic groups (see Table E8). In the NIS database, Hispanic patients showed a marked mortality benefit versus NHW patients (odds ratio [OR], 0.65 [0.50–0.84]; P = 0.001) during inpatient stay (Figure 4). AA patients had increased mortality versus NHW patients (OR, 1.22 [1.04–1.43]; P = 0.014) and a similar trend was observed with Asian patients (OR, 1.40 [1.00–1.97]; P = 0.051). A significant protective effect was also observed in Native American patients versus NHW patients (OR, 0.38 [0.15–0.93]; P = 0.034).
Figure 4.
Odds ratios for inpatient mortality in race/ethnic groups versus non-Hispanic white patients. Logistic regression for all-cause inpatient mortality was performed in patients with pulmonary arterial hypertension (adjusted for age, sex, and Elixhauser comorbidity index). Non-Hispanic white patients served as the reference group and were compared with non-Hispanic African American, Hispanic, non-Hispanic Asian, and Native American patients. CI = confidence interval; OR = odds ratio.
Discussion
We report the first comprehensive characterization and meta-analysis of Group 1 PAH, including ancestral contributions and outcomes across racial/ethnic populations in the United States. Our major finding was a reduction in mortality in Hispanic patients in a meta-analysis of four independent cohorts comprising two national and two Hispanic-enriched institutional registries across both outpatient and inpatient settings. This survival advantage was observed in both homogenous I/H PAH cases, as well as in a mixed Group 1 PAH population. This mortality benefit persisted when heritable PAH cases were excluded and was also consistent across self-reported and genetically defined (PCA-based) race/ethnicity. Hispanic patients demonstrate this survival advantage despite exhibiting increased PVR. This conflicting observation may partly arise from a high contribution of Native American race/ethnicity in Hispanic patients relative to other race/ethnic groups, although the ancestry analysis did not reach statistical significance. Consistent with a possible survival advantage with Native American ancestry in the national PAH registries, an inpatient mortality benefit was also observed for Native American patients.
Hispanic patients experienced a reproducible survival benefit in the current study. Differences in mortality are observed in the context of the younger Hispanic population, which may partly explain the natural ability to better tolerate increased hemodynamic burden. However, our survival analyses remained significant after adjustment for age, underscoring age-independent observation and effects. Although socioeconomic differences between race/ethnic groups, including access to both health care and PAH medications, might also contribute to differences in outcomes in Hispanic patients (15), the current study observed a significant mortality benefit in Hispanic patients despite reduced prostacyclin use. Notably, prostacyclin use was assessed at baseline in all cohorts in the current study and the effects of time-varying covariates related to drug use or hemodynamics remain unknown. These data are consistent with previous reports of the “Hispanic paradox,” referring to longer Hispanic survival despite a high prevalence of cardiovascular disease risk factors and lower average socioeconomic status. (36, 37) The paradox has previously been observed in a variety of cardiovascular diseases and has been suggested to arise from racial differences in access to care, adherence, adequacy of follow-up, dietary habits, and social support; or from the theory of acculturation. Our results suggest that the mortality benefit for Hispanic patients in PAH may be partly attributed to genetic admixture, including increased Native American ancestry.
Hispanic individuals are generally considered to be genetically admixed, being made up genetically of varying proportions of European, Native American, and African ancestry (23, 38, 39). Genetic admixture is the result of interbreeding among genetically distinct populations. In our analysis of PCA-based race/ethnicity and estimated ancestral proportions, we evaluated the effect of genetically defined ancestry and proportions of ancestry on mortality in Group 1 PAH. Although PCA-based race is an objective measure of genetic diversity, categorizations are not always accurate, meaningful, or even possible, especially for admixed groups. Self-reported Hispanic ethnicity represents a combination of genetic, environmental, and sociocultural factors. Although self-reported race/ethnicity might introduce misclassification from a genetic standpoint, it has the potential to capture a host of sociocultural factors such as diet, lifestyle choices, and healthcare practices. In our analyses of genetic admixture, increasing Native American ancestry seemed to account for a portion of the survival benefit in Hispanic patients and this was further supported by the analysis in the NIS database. An important limitation of the NIS database is the potential for misclassification associated with use of diagnostic codes. However, we used published ICD definitions designed to reduce misclassification (19, 40). Although genetic variability specific to Native American populations may provide a survival advantage, our observations might also have been due to the various sociocultural factors captured by self-report of Hispanic ethnicity.
A prior study in a large prospective cohort of healthy Hispanic patients without pulmonary hypertension and non-Hispanic populations revealed significantly increased right ventricular (RV) mass measured by cardiac magnetic resonance imaging in Hispanic patients compared with non-Hispanic patients (41). Whether these differences reflect adaptive versus maladaptive remodeling is unknown, but structural adaptations of the right ventricle in PAH are known to have prognostic significance, including RV mass (41–47). Although the current study did not evaluate RV mass, we speculate that the baseline RV mass differences reflect an ability to generate more efficient RV power and function during PAH development and contribute to mortality benefits seen in Hispanic patients with PAH.
Although we consider the use of a discovery PAH Biobank cohort followed by validation with three independent cohorts in our meta-analysis as a strength, the current study remains limited in terms of samples size and highlights the need to evaluate long-term outcomes and survival in larger cohorts of minority populations with PAH. Because of small numbers, for example, we were unable to investigate associations with Asian race. We also recognize that self-reported race/ethnicity categories are based on ancestral genomic backgrounds, as well as social/cultural factors, and both factors may have influenced PAH severity. Although these data were adjusted for hemodynamics, data for socioeconomic differences, clinical practice and treatment patterns, and referral patterns were not available for the current study. Because a majority of patients exhibited longer lead-in times (from diagnosis to enrollment) than survival times, we performed a sensitivity analysis to account for left truncation time. In addition, results for inpatient mortality, which is less susceptible to left truncation, were consistent with the primary analysis.
Conclusion
We report a comprehensive evaluation of race, ethnicity, and admixture in Group 1 PAH, made possible by large national repositories and minority-enriched centers of patients with PAH. We detail influences of self-reported race/ethnicity, PCA-based race/ethnicity, and genetic ancestry across all-cause mortality, right heart catheterization measures, and functional status in Hispanic and AA populations across four cohorts. For patients with Group 1 PAH, this study demonstrates a reproducible survival benefit for Hispanic patients in multiple clinical settings. Our results suggest that survival benefits are influenced by Native American race/ethnicity, although this finding did not reach statistical significance in the ancestry analysis. Our observation of improved survival in Hispanic patients emphasizes the need for well-powered studies evaluating the influence of ancestry and admixture on clinical outcomes. Our results reinforce the presence of racial/ethnic disparities in PAH and suggest that these disparities are due in part to genetic differences between race/ethnic groups.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank Edward Bedrick, Ph.D., Prakash Suryanarayana, M.D., Parinita Dherange, M.D., Morgan Whitaker, M.D., Hayley Patterson, B.S., and Vivek Yarlagadda, M.D., for their general assistance. The authors also thank contributors, including the Pulmonary Hypertension Centers who collected samples used in this study, as well as patients and their families, whose help and participation made this work possible.
Footnotes
Supported by NIH–NHLBI, including HL136603 (A.A.D.), HL478946 (R.L.B.), and HL125208 (J.X.-J.Y. and F.R.). Data are from the National Biological Sample and Data Repository for Pulmonary Arterial Hypertension, which receives government support under an investigator-initiated grant, R24 HL105333 (W.C.N.), awarded by the NHLBI. Research was supported by NHLBI Award Number K01HL143137 (J.H.K.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. None of the external funders or sponsors had any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Author Contributions: Conception and design of the work, the analysis and interpretation of data for the work, the drafting and revision of the manuscript, and approval of final version to be published: J.H.K., T.-H.S.-A., and A.A.D. Conception and design of the work, the analysis and interpretation of data for the work, critical revision of key intellectual content, and approval of final version to be published: H.W.W., B.N., A.J.S., A.A., K.B., M.W.P., J.G.N.G., J.X.-J.Y., R.K., V.d.J.P., R.T.Z., H.K.T., W.C.N., and R.L.B. Collection of data and assistance with processing and manuscript revision: A.C., V.N., H.E.S., J.B.G., J.Y., M.H., K.A.L., A.W.C., J.F., R.V., H.T., and F.R.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201907-1447OC on January 9, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Runo JR, Loyd JE. Primary pulmonary hypertension. Lancet. 2003;361:1533–1544. doi: 10.1016/S0140-6736(03)13167-4. [DOI] [PubMed] [Google Scholar]
- 2.Lau EMT, Giannoulatou E, Celermajer DS, Humbert M. Epidemiology and treatment of pulmonary arterial hypertension. Nat Rev Cardiol. 2017;14:603–614. doi: 10.1038/nrcardio.2017.84. [DOI] [PubMed] [Google Scholar]
- 3.Hoeper MM, Simon R Gibbs J. The changing landscape of pulmonary arterial hypertension and implications for patient care. Eur Respir Rev. 2014;23:450–457. doi: 10.1183/09059180.00007814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thenappan T, Ryan JJ, Archer SL. Evolving epidemiology of pulmonary arterial hypertension. Am J Respir Crit Care Med. 2012;186:707–709. doi: 10.1164/rccm.201207-1266ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farber HW, Miller DP, Poms AD, Badesch DB, Frost AE, Muros-Le Rouzic E, et al. Five-year outcomes of patients enrolled in the REVEAL Registry. Chest. 2015;148:1043–1054. doi: 10.1378/chest.15-0300. [DOI] [PubMed] [Google Scholar]
- 6.Rhodes CJ, Batai K, Bleda M, Haimel M, Southgate L, Germain M, et al. UK NIHR BioResource Rare Diseases Consortium; UK PAH Cohort Study Consortium; US PAH Biobank Consortium. Genetic determinants of risk in pulmonary arterial hypertension: international genome-wide association studies and meta-analysis. Lancet Respir Med. 2019;7:227–238. doi: 10.1016/S2213-2600(18)30409-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lilienfeld DE, Rubin LJ. Mortality from primary pulmonary hypertension in the United States, 1979-1996. Chest. 2000;117:796–800. doi: 10.1378/chest.117.3.796. [DOI] [PubMed] [Google Scholar]
- 8.George MG, Schieb LJ, Ayala C, Talwalkar A, Levant S. Pulmonary hypertension surveillance: United States, 2001 to 2010. Chest. 2014;146:476–495. doi: 10.1378/chest.14-0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blanco I, Mathai S, Shafiq M, Boyce D, Kolb TM, Chami H, et al. Severity of systemic sclerosis-associated pulmonary arterial hypertension in African Americans. Medicine (Baltimore) 2014;93:177–185. doi: 10.1097/MD.0000000000000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gabler NB, French B, Strom BL, Liu Z, Palevsky HI, Taichman DB, et al. Race and sex differences in response to endothelin receptor antagonists for pulmonary arterial hypertension. Chest. 2012;141:20–26. doi: 10.1378/chest.11-0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wain-Hobson J, Sabatier R, Koné M, Legallois D, Lognoné T, Dahdouh Z, et al. Increase of pulmonary artery wedge pressure above 15 mm Hg in patients with pre-capillary pulmonary hypertension. IJC Heart & Vessels. 2014;4:161–169. [Google Scholar]
- 12.Medrek SK, Sahay S. Ethnicity in pulmonary arterial hypertension: possibilities for novel phenotypes in the age of personalized medicine. Chest. 2018;153:310–320. doi: 10.1016/j.chest.2017.08.1159. [DOI] [PubMed] [Google Scholar]
- 13.Germain M, Eyries M, Montani D, Poirier O, Girerd B, Dorfmüller P, et al. Genome-wide association analysis identifies a susceptibility locus for pulmonary arterial hypertension. Nat Genet. 2013;45:518–521. doi: 10.1038/ng.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gräf S, Haimel M, Bleda M, Hadinnapola C, Southgate L, Li W, et al. Identification of rare sequence variation underlying heritable pulmonary arterial hypertension. Nat Commun. 2018;9:1416. doi: 10.1038/s41467-018-03672-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al-Naamani N, Paulus JK, Roberts KE, Pauciulo MW, Lutz K, Nichols WC, et al. Racial and ethnic differences in pulmonary arterial hypertension. Pulm Circ. 2017;7:793–796. doi: 10.1177/2045893217732213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sandoval J, Torbicki A, Souza R, Ramírez A, Kurzyna M, Jardim C, et al. STRIDE-4 investigators. Safety and efficacy of sitaxsentan 50 and 100 mg in patients with pulmonary arterial hypertension. Pulm Pharmacol Ther. 2012;25:33–39. doi: 10.1016/j.pupt.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 17.Benza RL, Barst RJ, Galie N, Frost A, Girgis RE, Highland KB, et al. Sitaxsentan for the treatment of pulmonary arterial hypertension: a 1-year, prospective, open-label observation of outcome and survival. Chest. 2008;134:775–782. doi: 10.1378/chest.07-0767. [DOI] [PubMed] [Google Scholar]
- 18.Benza RL, Gomberg-Maitland M, Demarco T, Frost AE, Torbicki A, Langleben D, et al. Endothelin-1 pathway polymorphisms and outcomes in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2015;192:1345–1354. doi: 10.1164/rccm.201501-0196OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anand V, Roy SS, Archer SL, Weir EK, Garg SK, Duval S, et al. Trends and outcomes of pulmonary arterial hypertension-related hospitalizations in the United States: analysis of the nationwide inpatient sample database from 2001 through 2012. JAMA Cardiol. 2016;1:1021–1029. doi: 10.1001/jamacardio.2016.3591. [DOI] [PubMed] [Google Scholar]
- 20.Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 21.Abecasis GR, Altshuler D, Auton A, Brooks LD, Durbin RM, Gibbs RA, et al. 1000 Genomes Project Consortium. A map of human genome variation from population-scale sequencing. Nature. 2010;467:1061–1073. doi: 10.1038/nature09534. [Published erratum appears in Nature 473:544.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Galanter JM, Fernandez-Lopez JC, Gignoux CR, Barnholtz-Sloan J, Fernandez-Rozadilla C, Via M, et al. LACE Consortium. Development of a panel of genome-wide ancestry informative markers to study admixture throughout the Americas. PLoS Genet. 2012;8:e1002554. doi: 10.1371/journal.pgen.1002554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Homburger JR, Moreno-Estrada A, Gignoux CR, Nelson D, Sanchez E, Ortiz-Tello P, et al. Genomic insights into the ancestry and demographic history of South America. PLoS Genet. 2015;11:e1005602. doi: 10.1371/journal.pgen.1005602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Banda Y, Kvale MN, Hoffmann TJ, Hesselson SE, Ranatunga D, Tang H, et al. Characterizing race/ethnicity and genetic ancestry for 100,000 subjects in the genetic epidemiology research on adult health and aging (GERA) cohort. Genetics. 2015;200:1285–1295. doi: 10.1534/genetics.115.178616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Swiston JR, Johnson SR, Granton JT. Factors that prognosticate mortality in idiopathic pulmonary arterial hypertension: a systematic review of the literature. Respir Med. 2010;104:1588–1607. doi: 10.1016/j.rmed.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 27.Benza RL, Gomberg-Maitland M, Elliott CG, Farber HW, Foreman AJ, Frost AE, et al. Predicting survival in patients with pulmonary arterial hypertension: the REVEAL risk score calculator 2.0 and comparison with ESC/ERS-based risk assessment strategies. Chest. 2019;156:323–337. doi: 10.1016/j.chest.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 28.Barst RJ, Rubin LJ, Long WA, McGoon MD, Rich S, Badesch DB, et al. Primary Pulmonary Hypertension Study Group. A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. N Engl J Med. 1996;334:296–301. doi: 10.1056/NEJM199602013340504. [DOI] [PubMed] [Google Scholar]
- 29.McLaughlin VV, Shillington A, Rich S. Survival in primary pulmonary hypertension: the impact of epoprostenol therapy. Circulation. 2002;106:1477–1482. doi: 10.1161/01.cir.0000029100.82385.58. [DOI] [PubMed] [Google Scholar]
- 30.Sitbon O, Vonk Noordegraaf A. Epoprostenol and pulmonary arterial hypertension: 20 years of clinical experience. Eur Respir Rev. 2017;26:160055. doi: 10.1183/16000617.0055-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barnes H, Yeoh HL, Fothergill T, Burns A, Humbert M, Williams T. Prostacyclin for pulmonary arterial hypertension. Cochrane Database Syst Rev. 2019;5:CD012785. doi: 10.1002/14651858.CD012785.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Evans JD, Girerd B, Montani D, Wang XJ, Galiè N, Austin ED, et al. BMPR2 mutations and survival in pulmonary arterial hypertension: an individual participant data meta-analysis. Lancet Respir Med. 2016;4:129–137. doi: 10.1016/S2213-2600(15)00544-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sztrymf B, Coulet F, Girerd B, Yaici A, Jais X, Sitbon O, et al. Clinical outcomes of pulmonary arterial hypertension in carriers of BMPR2 mutation. Am J Respir Crit Care Med. 2008;177:1377–1383. doi: 10.1164/rccm.200712-1807OC. [DOI] [PubMed] [Google Scholar]
- 34.Sankelo M, Flanagan JA, Machado R, Harrison R, Rudarakanchana N, Morrell N, et al. BMPR2 mutations have short lifetime expectancy in primary pulmonary hypertension. Hum Mutat. 2005;26:119–124. doi: 10.1002/humu.20200. [DOI] [PubMed] [Google Scholar]
- 35.Healthcare Utilization Project, US Department of Health and Human Services. HCUP method series. 2016 [accessed 2019 Mar 9]. Available from: https://www.hcup-us.ahrq.gov/reports/methods/2006_05_NISTrendsReport_1988-2004.pdf.
- 36.Medina-Inojosa J, Jean N, Cortes-Bergoderi M, Lopez-Jimenez F. The Hispanic paradox in cardiovascular disease and total mortality. Prog Cardiovasc Dis. 2014;57:286–292. doi: 10.1016/j.pcad.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 37.Shaw PM, Chandra V, Escobar GA, Robbins N, Rowe V, Macsata R. Controversies and evidence for cardiovascular disease in the diverse Hispanic population. J Vasc Surg. 2018;67:960–969. doi: 10.1016/j.jvs.2017.06.111. [DOI] [PubMed] [Google Scholar]
- 38.Norris ET, Wang L, Conley AB, Rishishwar L, Mariño-Ramírez L, Valderrama-Aguirre A, et al. Genetic ancestry, admixture and health determinants in Latin America. BMC Genomics. 2018;19:861. doi: 10.1186/s12864-018-5195-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ruiz-Linares A, Adhikari K, Acuña-Alonzo V, Quinto-Sanchez M, Jaramillo C, Arias W, et al. Admixture in Latin America: geographic structure, phenotypic diversity and self-perception of ancestry based on 7,342 individuals. PLoS Genet. 2014;10:e1004572. doi: 10.1371/journal.pgen.1004572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mathai SC, Hemnes AR, Manaker S, Anguiano RH, Dean BB, Saundankar V, et al. Identifying patients with pulmonary arterial hypertension using administrative claims algorithms. Ann Am Thorac Soc. 2019;16:797–806. doi: 10.1513/AnnalsATS.201810-672CME. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kawut SM, Lima JA, Barr RG, Chahal H, Jain A, Tandri H, et al. Sex and race differences in right ventricular structure and function: the multi-ethnic study of atherosclerosis-right ventricle study. Circulation. 2011;123:2542–2551. doi: 10.1161/CIRCULATIONAHA.110.985515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Austin C, Alassas K, Burger C, Safford R, Pagan R, Duello K, et al. Echocardiographic assessment of estimated right atrial pressure and size predicts mortality in pulmonary arterial hypertension. Chest. 2015;147:198–208. doi: 10.1378/chest.13-3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Benza RL, Miller DP, Gomberg-Maitland M, Frantz RP, Foreman AJ, Coffey CS, et al. Predicting survival in pulmonary arterial hypertension: insights from the registry to evaluate early and long-term pulmonary arterial hypertension disease management (REVEAL) Circulation. 2010;122:164–172. doi: 10.1161/CIRCULATIONAHA.109.898122. [DOI] [PubMed] [Google Scholar]
- 44.Ghio S, Klersy C, Magrini G, D’Armini AM, Scelsi L, Raineri C, et al. Prognostic relevance of the echocardiographic assessment of right ventricular function in patients with idiopathic pulmonary arterial hypertension. Int J Cardiol. 2010;140:272–278. doi: 10.1016/j.ijcard.2008.11.051. [DOI] [PubMed] [Google Scholar]
- 45.Raymond RJ, Hinderliter AL, Willis PW, Ralph D, Caldwell EJ, Williams W, et al. Echocardiographic predictors of adverse outcomes in primary pulmonary hypertension. J Am Coll Cardiol. 2002;39:1214–1219. doi: 10.1016/s0735-1097(02)01744-8. [DOI] [PubMed] [Google Scholar]
- 46.van Wolferen SA, Marcus JT, Boonstra A, Marques KM, Bronzwaer JG, Spreeuwenberg MD, et al. Prognostic value of right ventricular mass, volume, and function in idiopathic pulmonary arterial hypertension. Eur Heart J. 2007;28:1250–1257. doi: 10.1093/eurheartj/ehl477. [DOI] [PubMed] [Google Scholar]
- 47.Sano H, Tanaka H, Motoji Y, Fukuda Y, Mochizuki Y, Hatani Y, et al. Right ventricular relative wall thickness as a predictor of outcomes and of right ventricular reverse remodeling for patients with pulmonary hypertension. Int J Cardiovasc Imaging. 2017;33:313–321. doi: 10.1007/s10554-016-1004-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.