To the Editor:
Systemic corticosteroids (SCS) have an important role in the management of acute asthma, but their use can be associated with side effects. Central serous retinopathy (CSR) is a rare ophthalmic complication associated with partial or complete visual loss that precludes further SCS (1). Here we report, for the first time, the use of benralizumab, a monoclonal antibody against anti-IL5ra (IL-5 receptor α subunit), as a treatment for an acute asthma attack when SCS is contraindicated.
Background
Mr. C is a 52-year-old never smoker who received a diagnosis of asthma at age 36. Of note, his medical history included CSR, with complete visual loss, after a trial of oral dexamethasone (dose equivalent to prednisolone 30 mg/d for 12 d) for nasal polyposis in 2016. Near-complete recovery of visual acuity took 4 months, and he was advised to never use SCS again for fear of risking recurrent CSR (2) and potential irrecoverable visual loss (3). His asthma had been well controlled with budesonide/formoterol 200/6 μg two puffs twice a day, with a best peak expiratory flow rate (PEFR) of 450 L/min, at 87% predicted PEFR (520 L/min).
On the 24th of November 2019, Mr. C developed a virus-induced asthma attack and presented to his local emergency department. Features were consistent with a near-fatal asthma attack, including a PEFR of 80 L/min (<20% predicted), tachycardia (heart rate 115 beats/min), and hypercapnia (Pco2 6.7 kPa/50.4 mm Hg). Blood tests showed a normal C-reactive protein level, with a peripheral blood eosinophil count (PBEC) of 530 cells/μl. The initial treatment included hourly short-acting β2-agonists, short-acting muscarinic receptor antagonists, and supplemental oxygen therapy. Immediate advice was sought regarding the use of SCS, and after discussion with the ophthalmologist and consultation with the patient, it was decided that SCS should not be given. Intravenous magnesium (Mg2+), intravenous aminophylline and oral montelukast, and carbocysteine were then started. Despite these treatments, minimal symptomatic improvement occurred and the patient was transferred to the tertiary respiratory center on the eighth day of his hospital admission. On the 14th day, intravenous aminophylline was reduced, owing to an apparent improvement of PEFR to 270 L/min (60% best PEFR) but was restarted once again when the PEFR deteriorated. Throughout the admission, he continued to have an elevated PBEC, with a peak at 840 cells/μl on the 16th day when his sputum eosinophil count was 44% and exhaled nitric oxide was 28 ppb. We postulated that the persistent eosinophilic inflammation was the likely driver for his incomplete recovery and proposed an alternative method of eosinophil suppression. On the 17th day of admission, Mr. C was offered benralizumab, an anti-IL5ra monoclonal antibody that is licensed for the use of preventing asthma attacks in individuals with stable disease (4). This was authorized and approved by the local Hospital Medicines Board following an individual patient request.
Progress
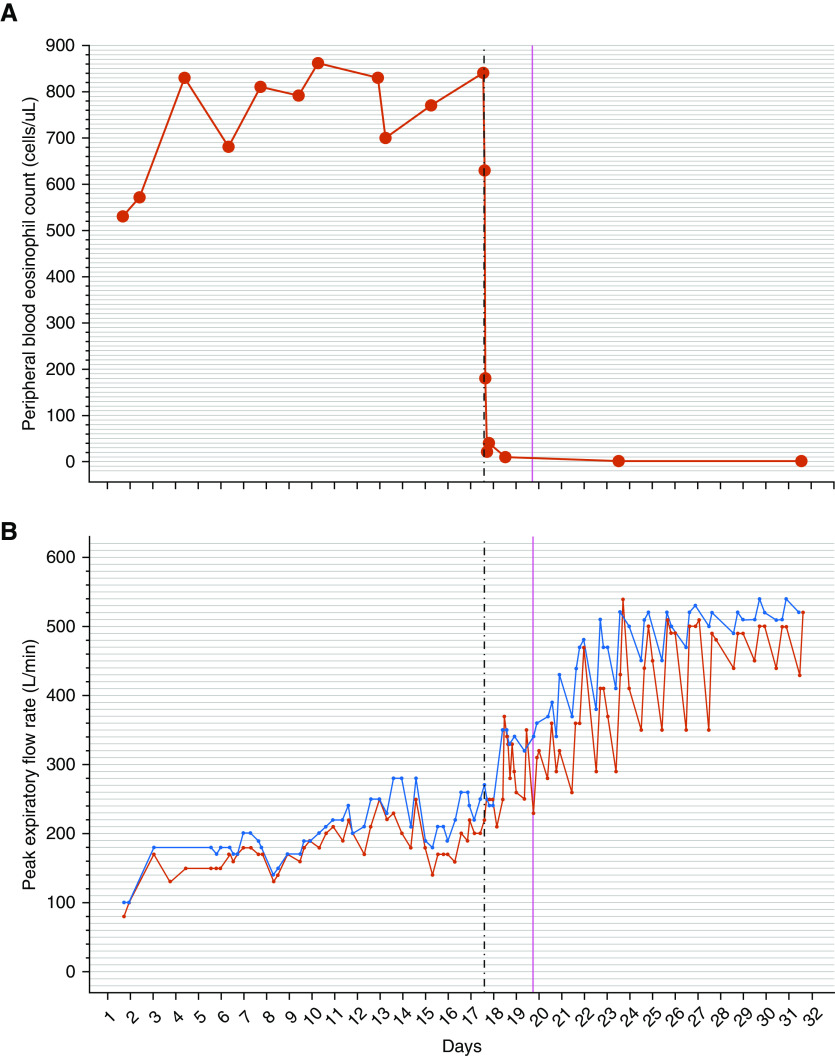
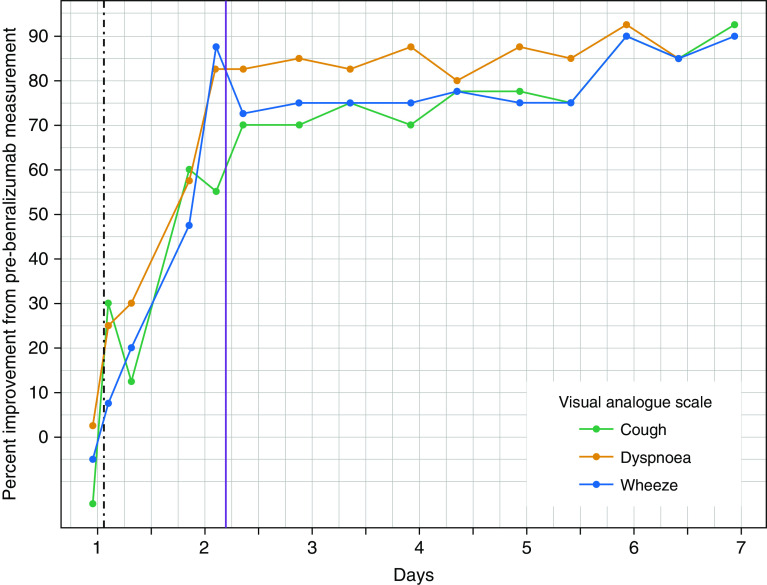
Serial physiological, symptomatic, and inflammatory data monitoring was performed. At 1, 2, 4, and 6 hours after 30 mg of subcutaneous benralizumab, the PBEC was 630, 180, 20, and 40 cells/μl, respectively (see top panel, Figure 1). Within 6 hours of treatment, he achieved a clinically significant improvement of his visual analog scale scores for breathlessness, wheeze, and cough (see Figure 2). Within 19 hours of benralizumab administration, there was marked improvement of post-bronchodilator lung function (see bottom panel, Figure 1) with a PEFR increase from 270 L/min to 370 L/min (37% improvement) and an increase in FEV1 from 1.34 L to 2.26 L (69% improvement). Within 24 hours of benralizumab administration, supplemental oxygen was ceased and he was discharged from hospital on the 19th day of hospital admission, 43 hours after benralizumab administration, with a PEFR of 380 L/min (84% best predicted), PBEC of 10 cells/μl, and sputum eosinophil count of 0%.
Figure 1.
(A and B) Serial measurements of peripheral blood eosinophil count (A) and peak expiratory flow rate (B, prebronchodilator [red] and post-bronchodilator [blue]). The vertical dashed line indicates the time of benralizumab administration. The vertical solid line indicates the time of hospital discharge.
Figure 2.
Percentage improvement in the visual analog scale for symptoms of cough, dyspnea, and wheeze after benralizumab administration. The vertical dashed line indicates the time of benralizumab administration. The vertical solid line indicates the time of hospital discharge.
After discharge, on the sixth day after benralizumab administration, Mr. C continued to improve in all his indices of physiology and symptoms. His FEV1 reached 2.91 L (92% predicted) with a PEFR of 520 L/min, which was better than his best in the preceding 5 years. He also noticed significant improvement in his sense of smell and taste. He continued to improve and reached an FEV1 of 3.31 L (104% predicted) at 2 weeks after benralizumab administration. Mr. C volunteered the following perspective to the clinical research team: “My asthma has always been well controlled with my inhalers. I took tablet steroids once to help with nasal polyps, which led to almost complete loss of vision. I was told to avoid steroids at all costs to prevent permanent eye damage. After a virus brought on my first ever asthma attack, I was petrified about possibly needing steroid tablets again. I felt stuck and I was not improving after 2 weeks in hospital. I felt better within 12 hours of the injection and was walking around the hospital within 24 hours. When I got home, I was surprised to be able to smell coffee again. In the month since coming out of hospital, my breathing is the best it has ever been. I am very reassured that medicines have improved such that I may now have another option if this happens again.”
Discussion
To the best of our knowledge, this is the first report of the use of benralizumab as an alternative to SCS at the time of an acute asthma attack. Nowak and colleagues previously showed that benralizumab is a potential adjunct to SCS in the acute setting (5). CSR is a rare complication, and in this clinical situation, both the ophthalmology specialist’s opinion and the patient’s choice precluded the use of SCS as part of routine clinical care. We found that benralizumab suppressed PBEC by 90% within 4 hours, an effect comparable to that achieved with SCS (6). The prompt clinical improvement supports our hypothesis that T-helper cell type 2 eosinophil–mediated inflammation was the primary driver of symptoms, and this raises the possibility that rapid-onset biological treatments targeting this pathway may provide a noncorticosteroid alternative for eosinophilic acute attacks (ClinicalTrials.gov ID NCT04098718).
Supplementary Material
Footnotes
Supported by the National Institute for Health Research Oxford Biomedical Research Centre. The views expressed are those of the authors and not necessarily those of the National Health Service, the National Institute for Health Research, or the Department of Health.
Author Contributions: All coauthors made substantial contributions to the conception or design of the work; the acquisition, analysis, or interpretation of data; drafting of the manuscript or critical revision of the manuscript for important intellectual content; and final approval of the version to be published. S.R. and M.B. agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Originally Published in Press as DOI: 10.1164/rccm.202001-0093LE on February 5, 2020
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Haimovici R, Koh S, Gagnon DR, Lehrfeld T, Wellik S Central Serous Chorioretinopathy Case-Control Study Group. Risk factors for central serous chorioretinopathy: a case-control study. Ophthalmology. 2004;111:244–249. doi: 10.1016/j.ophtha.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 2.Ricketti PA, Unkle DW, Cleri DJ, Prenner JL, Coluccielo M, Ricketti AJ. Central serous chorioretinopathy secondary to corticosteroids in patients with atopic disease. Allergy Asthma Proc. 2015;36:123–129. doi: 10.2500/aap.2015.36.3827. [DOI] [PubMed] [Google Scholar]
- 3.Mrejen S, Balaratnasingam C, Kaden TR, Bottini A, Dansingani K, Bhavsar KV, et al. Long-term visual outcomes and causes of vision loss in chronic central serous chorioretinopathy. Ophthalmology. 2019;126:576–588. doi: 10.1016/j.ophtha.2018.12.048. [DOI] [PubMed] [Google Scholar]
- 4.Bleecker ER, FitzGerald JM, Chanez P, Papi A, Weinstein SF, Barker P, et al. SIROCCO study investigators. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting β2-agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trial. Lancet. 2016;388:2115–2127. doi: 10.1016/S0140-6736(16)31324-1. [DOI] [PubMed] [Google Scholar]
- 5.Nowak RM, Parker JM, Silverman RA, Rowe BH, Smithline H, Khan F, et al. A randomized trial of benralizumab, an antiinterleukin 5 receptor α monoclonal antibody, after acute asthma. Am J Emerg Med. 2015;33:14–20. doi: 10.1016/j.ajem.2014.09.036. [DOI] [PubMed] [Google Scholar]
- 6.Saunders RH, Jr, Adams E. Changes in circulating leukocytes following the administration of adrenal cortex extract (ACE) and adrenocorticotropic hormone (ACTH) in infectious mononucleosis and chronic lymphatic leukemia. Blood. 1950;5:732–741. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.