Abstract
Obstructive sleep apnea (OSA) and asthma are highly prevalent chronic respiratory disorders. Beyond their frequent coexistence arising from their high prevalence and shared risk factors, these disorders feature a reciprocal interaction whereby each disease impacts the severity of the other. Emerging evidence implicates airway and systemic inflammation, neuroimmune interactions, and effects of asthma-controlling medications (corticosteroids) as factors that predispose patients with asthma to OSA. Conversely, undiagnosed or inadequately treated OSA adversely affects asthma control, partly via effects of intermittent hypoxia on airway inflammation and tissue remodeling. In this article, we review multiple lines of recently published evidence supporting this interaction. We provide a set of recommendations for clinicians involved in the care of adults with asthma, and identify critical gaps in our knowledge about this overlap.
Keywords: obstructive sleep apnea, asthma, pathophysiology, overlap, continuous positive airway pressure
Obstructive sleep apnea (OSA) and asthma are closely related. This could be due to mere coexistence, shared risk factors, or distinct interactive mechanisms between these upper- and lower-airway pathologies. Although both disorders are highly prevalent (1) and OSA in particular is on the rise (2), multiple studies have consistently reported higher OSA burdens among individuals with asthma (3–5) in relation to asthma severity (3, 6). Recent data indicate that asthma is a risk factor for incident OSA (7). Conversely, OSA has been linked to poor asthma outcomes (6, 8). Thus, a deleterious vicious cycle is established and, if OSA is not addressed, perpetuated, emphasizing the fact that we need to understand the underlying pathophysiology before we can effectively intervene. In addition to shared risk factors, such as rhinitis, gastroesophageal reflux (GER), and obesity, hypothetical mechanisms have been postulated to explain the frequent co-occurrence of OSA and asthma (9–11). However, more recently, experimental studies have suggested that asthma’s pathognomonic features, such as airway and systemic inflammation, have an impact on the risk of OSA. Conversely, the effects of OSA’s hallmark features (namely, chronic intermittent hypoxia [CIH], increased work of breathing, and sleep fragmentation) on the expression of asthma are just beginning to be elucidated. The purpose of this review is to summarize recent epidemiological, clinical, and translational evidence regarding the reciprocal mechanisms and consequences of asthma and OSA overlap in adults, articulate their implications for clinical practice, and suggest future research directions to advance the field.
Asthma as a Risk Factor for OSA
Epidemiological and Clinical Studies
Cross-sectional and longitudinal studies in individuals with asthma have described the occurrence of OSA based on self-reported snoring, questionnaires, and sleep studies (Table 1). The majority of these studies and a recent meta-analysis showed a two to three times higher prevalence of OSA among people with asthma (4, 12–14). Larsson and colleagues performed a cross-sectional analysis of more than 5,000 adults and showed that self-reported snoring (odds ratio [OR], 1.62; 95% confidence interval [CI], 1.16–2.27) and apneas (OR, 2.36; 95% CI, 1.60–3.48) were more common among individuals with asthma, adjusting for relevant covariates (4). In an analysis of 2008–2009 National Ambulatory Medical Care Surveys and National Hospital Ambulatory Medical Care Surveys, asthma diagnosis was associated with 2.7-fold increased odds of an OSA diagnosis, adjusting for age, sex, obesity, race, and socioeconomic status (adjusted OR, 2.7; 95% CI, 1.6–4.6) (15). However, these cross-sectional studies are generally limited by a lack of objective diagnostic data or a small sample size.
Table 1.
Obstructive Sleep Apnea Burden in Asthma: Epidemiologic and Clinical Studies
| Reference | Sample | Assessment and Exclusion of Treated Patients with OSA | Results |
|---|---|---|---|
| Cross-sectional studies | |||
| Community cohorts | |||
| Larsson et al., 2001 (4) | n = 4,648 | OSA*: questionnaire; OSA treatment not specified | • ↑ Prevalence of snoring (OR, 1.6†) and apnea (OR, 2.3†) in asthma |
| Asthma: physician diagnosis | |||
| Clinical populations | |||
| Yigla et al., 2003 (5) | n = 22 | OSA*: PSG; OSA treatment not specified | • 95% (21/22) prevalence of OSA |
| Pulmonary clinic | Asthma: PFT diagnosis on long-term oral steroids | • ↑ RDI in continuous OCS vs. intermittent OCS group (21.4 ± 3.4 vs. 11.1 ± 1.6) | |
| Karachaliou et al., 2007 (89) | n = 1,501 | OSA*: self-reported symptoms | • Asthma diagnosis not associated with OSA symptoms |
| Primary care | Asthma: physician diagnosis + spirometry | ||
| Auckley et al., 2008 (90) | n = 177, asthma clinic | OSA*: Berlin questionnaire | • ↑ OSA risk in asthma (39% vs. 27%) |
| n = 328, internal medicine clinic | Asthma: physician diagnosis + spirometry | • No association between asthma severity and OSA risk | |
| Julien et al., 2009 (3) | n = 52, asthma clinic | OSA*: PSG; treated OSA excluded | • ↑ OSA prevalence: severe asthma 88%, moderate asthma 58%, control 31% (P < 0.001) |
| n = 26, community control group | Asthma: physician diagnosis. Severity by spirometry, ACQ, and steroid use | • ↑ AHI in asthma | |
| Teodorescu et al., 2009 (27) | n = 244 | OSA: OSA risk (SA-SDQ); treated OSA excluded | • Use of ICS ↑ risk of habitual snoring; OR, 1.6† |
| Pulmonary and asthma clinic | Asthma: NAEPP classification severity | • OSA risk positively associated with asthma severity and ICS use | |
| Teodorescu et al., 2010 (61) | n = 472 | OSA: OSA risk (SA-SDQ); treated OSA excluded | • ↑ OSA risk in uncontrolled asthma; OR, 2.9† |
| Pulmonary and allergy clinic | Asthma: physician diagnosis and ACQ | ||
| Williams et al., 2011 (91) | Asthma, n = 200 | OSA*: habitual snoring | • ↑ Habitual snoring before (OR, 2.1†) and during (OR, 1.8†) pregnancy in asthma |
| No asthma, n = 1,135 | |||
| Prenatal clinic | Asthma: self-report of physician diagnosis | ||
| Teodorescu et al., 2012 (62) | n = 752 | OSA: SA-SDQ and medical records (diagnosis with PSG); treated OSA excluded | • ↑ OSA risk in asthma with persistent day and night symptoms; OR, 1.9† |
| Pulmonary and allergy clinic | Asthma: physician diagnosis | • ↑ Risk of PSG-diagnosed OSA in asthma with day symptoms; OR, 2.1† | |
| Braido et al., 2014 (92) | Asthma, n = 740 | OSA*: STOP-BANG questionnaire | • ↑ OSA risk in asthma with rhinitis vs. asthma without rhinitis; OR, 1.4† |
| Asthma and allergic rhinitis, n = 1,201 | |||
| Primary care | Asthma: physician diagnosis and allergic rhinitis questionnaire | ||
| Teodorescu et al., 2015 (6) | Nonsevere asthma, n = 161 | OSA: OSA risk (SA-SDQ); treated OSA excluded | • ↑ SA-SDQ scores in poorly controlled asthma |
| Severe asthma, n = 94 | |||
| Control, n = 146 | Asthma: physician diagnosis, severity by spirometry and inflammatory markers | • ↑ Sputum neutrophils associated with higher SA-SDQ | |
| Multicenter study | |||
| Longitudinal studies, population-based cohorts | |||
| Knuiman et al., 2006 (16) | n = 967 | Incident OSA*: self-reported habitual snoring | • ↑ Risk of habitual snoring in new-onset asthma; OR, 2.8† |
| Prospective | Asthma: questionnaire | ||
| Teodorescu et al., 2015 (17) | No asthma, n = 547 | No OSA or PAP use at baseline | • Adjusted RR of incident OSA, 1.4† in asthma vs. nonasthma |
| Asthma, n = 81 | Incident OSA: PSG AHI>5 or starting CPAP treatment for OSA | • ↑ Asthma duration (>10 yr) related to increased risk for incident OSA (RR, 1.71†) and for clinically significant OSA (OSA + excessive sleepiness; RR, 2.94†) | |
| Prospective | Asthma: physician diagnosis | ||
| Shen et al., 2015 (12) | n = 38,840 | Incident OSA*: ICD-9 | • ↑ OSA incidence in asthma vs. nonasthma HR 12.1 vs. 4.8 per 1,000 person-years |
| Retrospective, insurance database | Asthma: ICD-9 | • ↑ Incidence of OSA with >1 ER visit/yr (HR, 23.8†) and with ICS use (HR, 1.3†) |
Definition of abbreviations: ↑ = increased; ACQ = Asthma Control Questionnaire; AHI = apnea–hypopnea index; CPAP = continuous positive airway pressure; ER = emergency room; HR = hazard ratio; ICD = International Classification of Diseases; ICS = inhaled corticosteroid; NAEPP = National Asthma Education and Prevention Program; OCS = oral corticosteroid; OR = odds ratio; OSA = obstructive sleep apnea; PFT = pulmonary function test; PSG = polysomnography; RDI = respiratory disturbance index; RR = relative risk; SA-SDQ = sleep apnea scale of the Sleep Disorders Questionnaire; STOP-BANG = snoring, tiredness, observed apnea, blood pressure, body mass index, age, neck circumference, and gender.
History of OSA or treatment not specified.
Statistically significant OR, HR, or RR.
Data from prospective cohort studies strengthen this relationship. In the Australian Busselton Health Study, after 14 years of observation, participants with asthma were found to be nearly three times more likely to develop habitual snoring, after adjustment for potential confounders, including body mass index (BMI) at baseline and BMI change during this long time interval (16). A recent study using laboratory-based polysomnography (PSG) reported an increased 4-year incidence of OSA (relative risk, 1.58; 95% CI, 1.20–2.09) and symptomatic OSA (relative risk, 2.72; 95% CI, 1.26–5.89) among subjects with asthma compared with those with no asthma, after adjustment for multiple covariates, including baseline BMI and percent change in BMI in the time interval (17).
The few studies that collected asthma severity metrics reported associations of such measures with OSA risk. In the Wisconsin Sleep Cohort, asthma duration increased the incident OSA risk in a dose-dependent manner: each 5-year increment in duration was associated with a 7% and 18% higher risk for incident OSA (on PSG) and symptomatic OSA (with habitual sleepiness), respectively (17). In a retrospective longitudinal study, the frequency of annual emergency room visits and use of inhaled corticosteroids (ICS), were positively associated with an OSA diagnosis (12). A small number of studies reported on the prevalence of OSA, diagnosed by objective testing, in clinical asthma populations (3, 5, 18). Similar trends of increasing OSA prevalence in a dose-dependent manner with asthma severity (3) were noted, such that in individuals with difficult-to-control asthma, PSG-diagnosed OSA is almost universally present (prevalence of 88–95%) (3, 5). Interestingly, regardless of asthma severity, the vast majority of respiratory events were obstructive hypopneas with arousals (3). This may explain why another study using respiratory polygraphy (lacking electroencephalography) in subjects with severe asthma reported a lower prevalence (49%) (18) than the aforementioned studies. These discrepant findings regarding the prevalence of OSA determined by PSG versus respiratory polygraphy raise two important clinical considerations: 1) respiratory polygraphy, which is increasingly used to diagnose OSA in clinical practice, may underestimate the severity of OSA in asthma populations, and 2) asthma may influence the phenotypic expression of OSA by reducing the arousal threshold. To our knowledge, an association between the severity of asthma and severity of OSA, as measured by its standard metric, the apnea–hypopnea index (AHI), has not been reported. This is likely because of a lack of sufficiently powered prospective studies with detailed phenotyping of both asthma and OSA.
The relationship between sex and expression of OSA in individuals with asthma is not clear. On one hand, in the Wisconsin Sleep Cohort, male sex was associated with an increased incidence of OSA in individuals with asthma, whereas menopausal status did not impact the development of OSA (12). On the other hand, females with fixed airway obstruction (postbronchodilator FEV1/FVC ≤ 0.7) have been identified as having a higher risk of developing OSA (19). Further studies are needed to determine the full impact of sex on the development of OSA in patients with asthma.
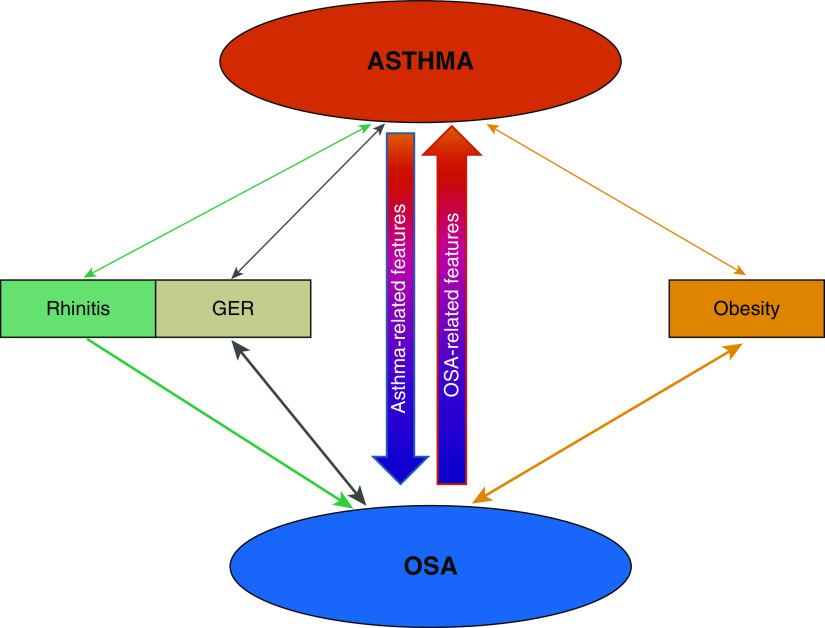
Apart from shared risk factors, such as obesity, rhinitis, and GER, asthma’s unique features appear to underlie the increased OSA risk in asthma (Figure 1).
Figure 1.
Schematic of mechanisms bidirectionally linking asthma and obstructive sleep apnea (OSA), as suggested by currently available data. In each direction, shared risk factors and specific features of each disease (asthma or OSA) may also underlie the interaction. See text for details. GER = gastroesophageal reflux.
Shared Risk Factors
Rhinitis
The nose is the principal route of breathing during sleep, and nasal factors are important determinants of pharyngeal upper-airway (pUAW) patency. According to the Starling resistor model of OSA, the collapsible segment of the tube (the pUAW) is characterized by an intraluminal (critical closing) pressure and surrounding tissues (pharyngeal muscles, pharyngeal and submucosal fat, mucosal edema, etc.) pressure. The tube is also bound by an upstream (nose) and downstream (trachea) segment, with their corresponding upstream and downstream pressures. pUAW collapse occurs in several scenarios, such as when the upstream nasal pressure falls below the critical closing pressure, resulting in symptoms of OSA such as snoring and apnea (20).
Rhinitis and asthma are common comorbidities. The prevalence of rhinitis (allergic and nonallergic) in asthma is as high as 80–90%, and rhinitis is a risk factor for developing asthma (21, 22). Rhinitis causes inflammation of the nasal passages, which leads to nasal obstruction. In the general population, both allergic rhinitis and nonallergic rhinitis are risk factors for a high AHI (23). Although the degree of nasal obstruction during wakefulness does not correlate with OSA severity (24), peak nasal resistance occurs in the early morning (due to circadian effects), possibly potentiating the expression of OSA during that time (25). Chronic sinusitis and nasal polyposis are closely associated with both asthma and inflammation of the pUAW, reducing its patency (26). Thus, upper- and lower-airway inflammation in rhinitis and asthma perpetuate each other and could promote the development of OSA (26). Indeed, a cross-sectional study of patients with asthma reported a significant independent association of diagnosed rhinitis with OSA risk, suggesting that rhinitis may be a risk factor for OSA in patients with asthma (27).
GER
Epidemiological studies reported an association between GER and OSA that was independent of other confounders (9, 28, 29). New symptoms of OSA and higher Epworth Sleepiness Scale scores were observed in subjects with new or persistent nocturnal GER, but not in those without nocturnal GER (30). GER was found to be independently predictive of habitual snoring and high OSA risk in a cohort of patients with asthma (27). In patients with poorly controlled asthma and obese patients with asthma, omental fat weight, loss of angulation of the gastroesophageal junction, and changes in the transdiaphragmatic pressure gradient can cause GER (30). GER, in turn, can cause spasm of the pUAW, edema, and neurogenic mucosal inflammation, promoting OSA (31, 32), which could worsen asthma and perpetuate a vicious cycle.
Obesity
Obesity is a major risk factor linking asthma with OSA. Epidemiologic and observational studies have shown that obesity is a significant risk factor for incident asthma and asthma severity (33, 34). Patients with asthma may also be at risk for developing obesity, as they are less physically active and receive oral corticosteroid therapy more often, which promotes weight gain (9, 35). In addition, daytime fatigue caused by sleep loss due to suboptimally controlled asthma would favor a sedentary lifestyle and promote obesity in individuals with asthma (36). On the other hand, obesity is a dominant risk factor for OSA (37), and is often used as an OSA surrogate in adult populations. In patients with obstructive pulmonary disease, neck circumference, BMI, and waist/hip ratio are all associated with symptoms of OSA (19, 38, 39). Obesity changes the structure and function (collapsibility) of the pUAW, reduces FRC, increases oxygen demand, and changes the respiratory drive and load compensation relationships (40), favoring pUAW collapse.
Unique Links of Asthma with OSA: Insights from Human and Animal Studies
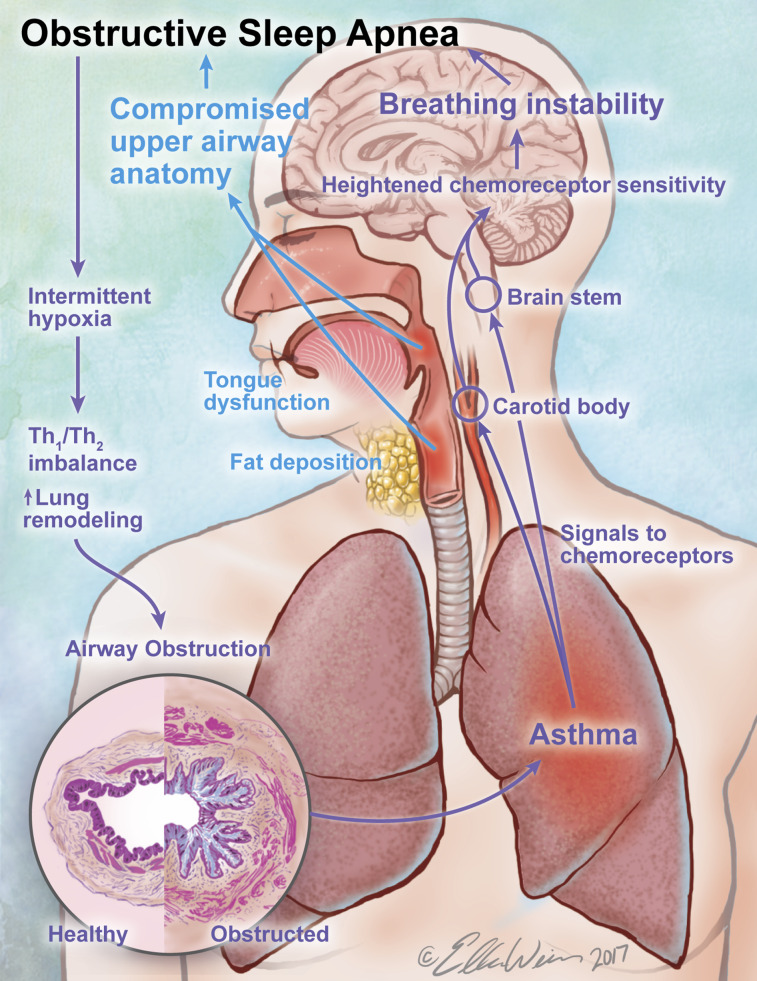
Apart from shared risk factors, asthma could predispose to OSA via its unique features (Figure 2). Asthma is an inflammatory condition whereby chronic inflammation is intermittently exacerbated by a variety of allergic, infectious, and other triggers.
Figure 2.
Disease-specific features known to contribute to the asthma/obstructive sleep apnea (OSA) overlap. Asthma commences early in life, traditionally as an eosinophilic airway disease, and has been shown to lead to incident OSA, with a new set of symptoms overlapping those of asthma. Asthma’s pathognomonic features, notably airway and systemic inflammation, could destabilize peripheral and central breathing and upper-airway control mechanisms. In conjunction with anatomical effects of long-term inhaled corticosteroid (ICS) therapy on the pharyngeal airway, it sets the stage for upper-airway collapse during sleep and OSA. Once established, OSA, through its features, notably chronic intermittent hypoxia, has been shown to shift the airway inflammatory profile away from T-helper cell type 2 (Th2) pathways, which leads to lung remodeling and airway dysfunction, in a pattern that is less responsive to ICS therapy. Without addressing OSA, achieving asthma control would likely require a step-up in ICS dose and repeated steroid bursts, further raising the risk for or the severity of OSA with its consequences for asthma, accelerating this vicious cycle and translating into irreversible airway dysfunction. See text for additional details.
In OSA, breathing control instability acts in concert with compromised pUAW anatomy to set the stage for pUAW closure. The concept that inflammatory processes originating in the lung destabilize breathing control is gaining more attention (41), and asthma may be no exception. Studies in animal models provide some mechanistic insights into how inflammation affects neural breathing control and chemoreception. For example, after bleomycin-induced lung injury in rats, Jacono and colleagues uncovered a T-helper cell type 1 (Th1) type of inflammation in brainstem regions responsible for breathing control, which mirrored that in the lungs (42). The authors proposed several immune-to-brain communication pathways: 1) active transport of cytokines across the blood–brain barrier, 2) neural transport of inflammatory mediators from the periphery through afferent fibers of the vagi, and 3) cytokine production within the central nervous system by the activated resident brain macrophage cells (microglia). In addition, the peripheral chemoreceptor appears to be sensitized in response to Th1-type lung inflammation as well (43). Five days after bleomycin instillation in rats, before lung fibrosis and arterial hypoxemia ensued, Jacono and colleagues found heightened hypoxic and hyperoxic ventilatory responses in anesthetized, spontaneously breathing bleomycin-treated rats relative to control animals (43). The enhanced hypoxic response persisted after bilateral vagotomy, but was eliminated after bilateral carotid sinus nerve sectioning.
Although it remains to be tested whether carotid body and brainstem inflammation occurs in response to Th2-predominant inflammatory lung processes, early data suggest that may be the case. In a recent study, Broytman and colleagues tested ventilatory responses to graded hypoxia (12% and 9% FiO2) in awake rats 24 hours after challenge with ovalbumin (OVA) (44). Compared with saline-challenged animals, the OVA-challenged rats demonstrated an exaggerated hypoxic ventilatory response, which, interestingly, was more apparent when airflow obstruction was relieved with the long-acting bronchodilator formoterol. These observations suggest mediation in greater part by cytokine signaling in the chemoreceptors rather than by mechanoreceptor feedback from the lungs.
Other phenomenons related to asthma, particularly when they occur at night, may help set the stage for ventilatory instability and OSA. It is known that approximately 90% of severe asthma exacerbations are associated with hypoxemia on arterial blood gas analysis, owing to a ventilation–perfusion mismatch, which persists for several days (45). In addition, nocturnal asthma, independently of OSA, leads to frequent arousals and poor sleep quality (46). These observations raise the question as to whether these additional asthma features could potentiate carotid body sensitization and/or alter other phenotypic traits (e.g., the arousal threshold and pUAW collapsibility) that are known to contribute to OSA pathogenesis (47). Further studies are needed to investigate these putative mechanisms.
Inflammatory pathways related to asthma pathology may also undermine protective mechanisms of pUAW patency. The site of obstruction in OSA is at the level of the pharyngeal airway, an area extending from the posterior end of the nasal septum to the epiglottis. This region lacks rigid support and is vulnerable to collapse during sleep. Its patency is maintained by a fine balance of two opposing forces: those promoting collapse and those maintaining patency. The genioglossus is the most important pUAW dilator, but neural inputs from the hypoglossal pool are needed to activate it. One characteristic of the respiratory system is its plasticity, which allows it to adapt to various physiological conditions. One form of this plasticity is long-term facilitation (LTF), which consists of a prolonged augmentation of neural activity (including, but not limited to, activity of the hypoglossal and phrenic nerves), lasting for at least 60 minutes after termination of a stimulus. Various stimuli, such as acute intermittent hypoxia (48) and pUAW negative pressure (49), give rise to LTF, whereas intermittent hypercarbia depresses LTF (50). Hypoglossal and ventilatory LTF to acute intermittent hypoxia occurs in healthy sleeping humans (48). Although the role of LTF in breathing stability remains to be understood, it appears to behave as a “double-edged sword” that may stabilize pUAW but may also precipitate breathing instability (51). Derangements in gas exchange occur during episodes of lower-airways obstruction, and depending on the severity of obstruction, could manifest as concurrent hypoxia with or without hypercarbia. These gas exchange abnormalities have opposite effects on LTF, as above. This is due to different signaling pathways, which have been suggested to feature a mutual inhibition, with one preventing initiating the other (52). Depending on the initial stimulus (hypoxia or hypercapnia), the effects of one will govern the other, determining the net LTF magnitude (52). In addition, other factors may depress or eliminate LFT. Preclinical studies showed that Th1 inflammatory responses to systemic LPS abolished phrenic LTF, whereas inhibition of inflammation with ketoprofen restored aspects of phrenic LTF (53). Although the effects of Th2 on various forms of respiratory LTF remain to be tested, in the chronic allergic rat model, concurrent CIH creates a shift in airway inflammation toward a more Th1 pattern (54), which may behave in the manner discussed above.
The observation of a dose-dependent relationship between asthma duration and incident OSA in humans (17) lends itself to the hypothesis that the effects of asthma on breathing control mechanisms start early in life, possibly in utero. Hypoxemia during severe asthma exacerbations (45) may affect the propensity for breathing instability in newborns. Interestingly, CIH exposure in animal models, both during pregnancy and early postpartum, attenuated the hypoxic ventilatory responses and phrenic LTF in the offspring and newborns (55, 56). In the exposed newborns, alterations outlasted the exposures and persisted for up to 30 days beyond it. Collectively, these data imply that in utero and early-life challenges may impact breathing control mechanisms during development and lead to a vulnerability to OSA later in life.
Finally, anatomical compromise of the pUAW in OSA may be the result of a standard asthma therapy, ICS (Figure 2). Myopathy and weight gain/centripetal fat redistribution to the neck area resulting from systemic absorption are well-known side-effects of ICS, particularly at higher doses (57, 58). Interestingly, in a survey of patients with asthma in specialty clinics, the use of ICS was dose-dependently associated with an increased risk for habitual snoring and OSA, independently of asthma severity and other known risk factors, including excess weight (27). Furthermore, 4-month treatment with high-dose fluticasone propionate in patients with overall mild asthma increased wakefulness tongue strength but also decreased endurance (59), in a pattern much like that observed in untreated patients with OSA (60). The treatment affected pUAW collapsibility during sleep in a manner that depended on baseline characteristics—that is, in a subset of older, male, heavier patients with less-controlled asthma and higher AHI at baseline, the fluticasone treatment increased collapsibility. In addition, in another subset of participants whose BMI and pUAW collapsibility during sleep did not change during treatment, the fat content in the neck increased by ∼21% from baseline (59). These findings suggest that ICS alter the structure of the pUAW and ultimately, in the case of susceptible pUAWs, tilt the balance in favor of collapse. Because a 4-month treatment represents a snapshot in the life of a patient with asthma on chronic ICS therapy, these are critical findings that should prompt clinical investigations of their implications for breathing during sleep and other relevant functions, such as swallowing.
Effects of OSA on Expression of Asthma
Epidemiological and Experimental Studies
Several cross-sectional and retrospective studies have shown associations of OSA with multiple asthma outcomes, across the healthcare continuum (Table 2). Relationships between OSA risk or diagnosis and worse daytime and nighttime asthma symptoms, bronchodilator use, FEV1 decline in time, increased exacerbations, and reduced quality of life have been documented in patients with asthma (6–8, 61–64), and especially in older individuals (7). Conversely, continuous positive airway pressure (CPAP) for OSA attenuated risk for worse asthma outcomes and FEV1 decline (7, 62, 64) in older subjects much more than in younger ones (7). Moreover, in patients hospitalized for asthma exacerbations, OSA diagnosis was related to poorer outcomes, such as the need for invasive respiratory therapy, increased lengths of stay, and costs (65). The burden of OSA on health resource utilization was higher than that imposed by obesity alone, and the two comorbidities together had a multiplicative adverse impact on health resource utilization, length of stay, and routine disposition to home at discharge (65).
Table 2.
Impact of Obstructive Sleep Apnea on Asthma: Epidemiologic and Clinical Studies
| Reference | Sample | Assessment and Exclusion of Treated Patients with OSA | Results |
|---|---|---|---|
| Cross-sectional studies | |||
| ten Brinke et al., 2005 (8) | n = 136 | OSA: symptoms or PSG; OSA treatment not reported | • ↑ Asthma exacerbation in OSA; adjusted OR, 3.4* |
| Pulmonary clinic | Asthma: severe, one or more exacerbations/yr or OCS use | ||
| Teodorescu et al., 2013 (7) | Age 18–59 yr, n = 659 | OSA: PSG (medical records); treated OSA excluded | • ↑ Severe asthma in the older age group with OSA; OR, 6.7* |
| Age 60–75 yr, n = 154 | Asthma: NAEPP classification of asthma severity | ||
| Pulmonary and allergy clinics | |||
| Kim et al., 2013 (93) | n = 217 | OSA: Berlin Questionnaire; treated OSA excluded | • ↓ Asthma disease-specific quality of life in high-risk OSA |
| Specialty clinic | Asthma: physician diagnosis and airway reversibility on spirometry | ||
| Tay et al., 2016 (94) | n = 90 | OSA: Berlin or PSG; OSA treatment not specified | • OSA not associated with asthma exacerbations or quality of life after adjustment for obesity |
| Asthma clinic | Asthma: referred for difficult asthma | ||
| Longitudinal studies | |||
| Jordan et al., 2015 (95) | n = 2,445 | OSA: self-reported physician diagnosis; OSA treatment not specified | • ↑ Risk of poorly controlled asthma in OSA; OR, 1.4–1.5* |
| Prospective cohort study | Asthma: physician-diagnosed incident asthma after 9/11, modified NAEPP classification of asthma severity | ||
| World Trade Center Health Registry | |||
| Wang et al., 2016 (64) | Asthma, n = 146 | OSA: PSG; treated OSA excluded | • AHI associated with ↑ risk of severe asthma exacerbation; OR, 1.3* |
| No asthma, n = 157 | Asthma: spirometry or MCT | ||
| Prospective case control | |||
| Yii et al., 2017 (96) | n = 177 | OSA: PSG; OSA treatment not specified | • No significant ↑ risk of severe asthma exacerbations in OSA |
| Prospective clinical cohort, 5-yr follow-up | Asthma: step 4 of GINA treatment |
Definition of abbreviations: ↓ = decreased; ↑ = increased; AHI = apnea–hypopnea index; CPAP = continuous positive airway pressure; GINA = Global Initiative for Asthma; MCT = methacholine challenge test; NAEPP = National Asthma Education and Prevention Program; OCS = oral corticosteroid; OR = odds ratio; OSA = obstructive sleep apnea; PSG = polysomnography.
Statistically significant OR.
A more robust (causal) relationship between OSA and asthma can be inferred from prospective interventional studies of OSA treatment in patients with asthma (Table 3). In quasi-experimental designs, CPAP improved daytime and nighttime symptoms, rescue bronchodilator use, exacerbations, quality of life, and a.m. and p.m. peak inspiratory flow rates (66–69). In addition, some of these effects occurred in a dose-dependent fashion, with the largest improvements in asthma control and asthma-related quality of life noted in patients with moderate–severe persistent asthma or severe OSA diagnosed by respiratory polygraphy (respiratory disturbance index > 30) (69).
Table 3.
Effects of Treatment for Obstructive Sleep Apnea on Asthma Outcomes
| Reference | Design/Sample | Assessment | Results | Limitations |
|---|---|---|---|---|
| CPAP treatment | ||||
| Teodorescu et al., 2012 (62) | Cross-sectional | OSA: SA-SDQ and PSG | • ↓ Daytime asthma symptoms (*OR, 0.46) | Objective CPAP adherence not available |
| n = 132 with OSA, 75 on CPAP | Asthma: physician diagnosis | |||
| Teodorescu et al., 2013 (7) | Cross-sectional | OSA: PSG | • ↓ Risk of severe asthma (more in older vs. younger patients, by 91% vs. 57%) | PSG-derived OSA severity or objective CPAP adherence not available |
| Age 18–59 yr vs. 60–75 yr | Asthma: physician diagnosis | |||
| n = 140 | ||||
| Kauppi et al., 2016 (97) | Longitudinal, retrospective study | OSA: physician diagnosis and CPAP ≥3 mo | • ↓ Asthma severity (ACT and VAS) | Generalizability limited with high CPAP adherence (6.3 h daily) |
| n = 152 | Asthma: on asthma medication | |||
| Wang et al., 2017 (63) | Longitudinal, retrospective study | OSA: PSG | • ↓ The annual decline in FEV1 in severe OSA | No control group |
| n = 77 | Asthma: spirometry | |||
| Lafond et al., 2007 (68) | Interventional: CPAP × 6 wk | OSA: PSG (AHI ≥ 15/h) and CPAP titration | • No difference in methacholine challenge test | Per-protocol analysis of 13 patients with ≥4 h daily CPAP use |
| n = 20 | Asthma: ATS criteria | • ↑ Asthma-specific quality of life | ||
| Serrano-Pariente et al., 2017 (69) | Interventional: CPAP × 6 mo | OSA: AHI ≥20/h | • ↑ Asthma control, disease-specific quality of life | No control group |
| n = 99 | Asthma: physician diagnosis | • ↓ Bronchial reactivity (reduced proportion of patients with positive bronchodilator response), exhaled NO | ||
| Other OSA treatments | ||||
| Bachour et al., 2016 (71) | Cross-sectional survey, n = 303 | OSA: referred for oral appliance treatment | • ↑ ACT with oral appliance treatment | No control group |
| Asthma, n = 18 | Asthma: physician diagnosis and asthma medication use | |||
| Omana et al., 2010 (73) | Retrospective bariatric cohort | OSA: CPAP use | • Self-reported improvement in symptoms or cessation of treatment; in asthma 21/31, in OSA 14/32 | Lack of validated questionnaire |
| Follow-up 17 mo, n = 123 | ||||
| Asthma, n = 31 | Asthma: use of asthma medications | |||
| OSA, n = 32 | ||||
| Simard et al., 2004 (72) | Prospective bariatric cohort | OSA: self-reported | • Self-reported improvement in asthma control in 23/34 | Lack of validated questionnaire |
| Follow-up 2 yr, n = 398 | Asthma: self-reported | |||
| Asthma, n = 34 | ||||
| OSA, n = 47 | ||||
| OSA and asthma, n = 18 |
Definition of abbreviations: ↓ = decreased; ↑ = increased; ACT = asthma control test; AHI = apnea–hypopnea index; ATS = American Thoracic Society; CPAP = continuous positive airway pressure; OR = odds ratio; OSA = obstructive sleep apnea; PSG = polysomnography; SA-SDQ = sleep apnea scale of the Sleep Disorders Questionnaire; VAS = visual analog scale.
Statistically significant OR.
Data regarding the effects of CPAP on bronchial reactivity, assessed by bronchodilator response or bronchial provocation, are mixed. Studies have reported a significant reduction in the proportion of patients with a positive bronchodilator response (69) and no change in the provocative concentration of methacholine causing a 20% fall in FEV1 (PC20) (68). No effects of CPAP on FEV1 have been found in prospective studies (69), suggesting potential remodeling effects of OSA on the lower airway (54). There are limited human data on changes in inflammatory markers with CPAP treatment for OSA in asthma (69). A reduction in the fraction of exhaled nitric oxide was reported in one study (69), but no other biomarkers were collected for a more detailed characterization of lower-airway inflammatory phenotypes. Overall, these data show improvements in subjective (symptoms and quality of life) and objective (rescue bronchodilator use and morning peak expiratory flow rates) asthma characteristics with CPAP treatment for OSA, but mixed and generally negative results with respect to physiologic measures of lung function (FEV1) during wakefulness. These discrepancies in physiologic responses may be due to irreversible airway remodeling (18) that could occur with delayed OSA recognition, a focus on wakefulness (instead of nocturnal) airway measures that are known to be more affected during sleep (70), lack of uniformity with regard to asthma treatment regimens within each study, and variable durations of CPAP therapy and nightly adherence, which were not reported in some studies. In this regard, it is important to note that the minimal duration of nightly CPAP use for effects on the aforementioned asthma outcomes remains unknown. Another factor that may contribute to the discrepancies in physiologic results relates to intrinsic characteristics, such as the phenotype, of the populations studied. For example, Lafond and colleagues assessed PC20 three times serially (2–3 d apart) at baseline and after CPAP (68). They excluded six patients with >2 dilutions variability in PC20 on serial baseline testing, which is actually a marker of unstable asthma. Presumably, these patients would have been the most reactive and the ones most likely to experience benefit from CPAP. Thus, the issue of CPAP’s effects on physiologic measures of airway functions needs to be studied in thorough experiments and in well-defined populations. The effects of alternative treatments for OSA, such as oral appliances and surgical treatments, on asthma remain mostly unexplored. In an observational study, oral appliances were associated with improvements in Asthma Control Test scores (71). Although a few studies have examined the effects of different bariatric approaches and reported improvements in OSA and asthma severity, they did not specifically evaluate asthma/OSA overlap (72, 73). To our knowledge, no studies have examined the role of other surgical interventions or hypoglossal nerve stimulation in asthma/OSA overlap.
A modulation of the effects of OSA on asthma by sex appears to exist, with women more likely to be affected. Women with asthma and OSA who are hospitalized for an asthma exacerbation have poorer outcomes than their male counterparts (65). Conversely, female sex was identified as an independent predictor for improvement in asthma-related quality of life after CPAP treatment for OSA (69).
Aside from shared risk factors that may be at play in this relationship, such as obesity, GER, and rhinitis (Figure 1), we are beginning to learn how OSA’s features could impact the asthmatic airway.
Shared Risk Factors
Obesity, GER, and rhinitis have all been proposed to modulate OSA’s effects on asthma (Figure 1) (10). Obesity is related to both asthma incidence and severity (34), and a meta-analysis found the incidence of asthma to be two times higher in overweight/obese individuals compared with healthy control subjects (33). Alterations in lung mechanics, airway hyperresponsiveness, a sustained proinflammatory state, and increased production of adipokines are believed to underlie these effects (74). Patients with GER are approximately two times more likely to have asthma or asthma exacerbations (31). The acid exposure increases vagal tone, and subsequently respiratory resistance and bronchial reactivity (31). Chronic nasal disease is commonly associated with asthma, where it increases inflammatory cells and bronchial responsiveness (26). A report by Serrano-Pariente and colleagues provides some insights into these relationships (69). A 6-month CPAP treatment for OSA did not impact BMI but it significantly reduced the proportion of subjects who reported nasal symptoms, heartburn, and regurgitation. These findings cast doubt on the significant effects of obesity on asthma, independent of OSA.
Pathophysiological Links of OSA with Asthma: Human and Animal Studies
Underlying asthma is a complex cellular milieu that leads to its clinical expression, consisting of mucus hypersecretion, bronchial reactivity, and remodeling of airway walls and surrounding parenchyma. Although traditionally an eosinophilic, Th2 type of inflammation has been principally implicated in asthma pathobiology, more recently, it is increasingly recognized that non-Th2 pathways are also involved, leading to accumulation of other cellular orchestrators, such as neutrophils, monocytes, macrophages, and even fibrocytes. This inflammatory phenotype appears to affect >50% of patients with persistent asthma, where it is associated with more severe disease expression, remodeling, poor response to corticosteroids, and fatal events (75–77). Thus, asthma is a heterogeneous disease, with several clusters, phenotypes, and a multitude of endotypes being described (78). However, very little is known about what underlies this heterogeneity and, consequently, variability in response to therapies.
OSA may be a contributor to Th1 pathways in asthma, and to the heterogeneity of the disease. The observation that OSA impacts asthma control around the clock gives credence to the idea that nocturnal sleep-breathing disorders have carryover effects on daytime asthma, just as they do on daytime systemic blood pressures. Indeed, recent data converge in showing that OSA changes the expression of the asthmatic inflamed airway. For instance, in a study of 139 individuals with asthma, higher OSA risk was associated with higher sputum neutrophils, and even more so after adjustment for other potential contributors, such as obesity (6). No relationship between OSA risk and any eosinophilic marker of airway inflammation was observed. Moreover, in a study of 55 patients with difficult-to-treat asthma studied with bronchoscopy and mucosal biopsy, sputum induction, and respiratory polygraphy, the proportion of sputum neutrophils was higher in patients with OSA than in subjects without OSA, paralleled by higher levels of IL-8 and matrix metalloproteinase-9 (18). In addition, the bronchial reticular basement membrane was significantly thinner in patients with OSA than in subjects without OSA. Altogether, these findings show the potential of OSA to shift the traditional eosinophilic, Th2 lower airway inflammation of asthma toward a more noneosinophilic, Th1 phenotype leading to remodeling. More importantly, all these processes are known to be poorly responsive to current standard therapies, such as corticosteroids.
All of OSA’s hallmark features (recurrent increases in work of breathing, chronic sleep fragmentation, and CIH) have the potential to detrimentally impact the distal airway. For example, the large intrathoracic pressure swings during obstructive sleep-related events are accompanied by significant changes in lung mechanics, such as increased total resistance and elastance (79), reduced lung volumes (80, 81), and specific airway conductance (82), which are abolished with the cessation of events. These changes indicate that closure of distal respiratory units occurs during obstructive events. Their reversal at the end of the obstruction suggests that pUAW collapse is responsible for their occurrence (79). Moreover, the expiratory phase seems to be more impacted, as in the few cycles preceding collapse of the pUAW, increases in its expiratory resistance occurred earlier than during inspiration (83). Such premature “pUAW expiratory closure” would be expected to adversely impact the expiratory flow limitation that is characteristic of asthma. In addition, the repetitive strenuous cycles of closure and opening, as opposed to tidal breathing, could impose “cyclic mechanical stress” on the lower airway structures, which in similar lung models (84) and in the pUAW (85) is known to induce significant Th1, neutrophilic–predominant responses. Also, in one study, 10 days of experimental sleep fragmentation in otherwise healthy rats led to a doubling of lung myeloperoxidase activity relative to control animals, which was associated with increased hemeoxygenase-1, a marker of cellular stress and tissue injury (86). The granulocytes had already migrated into the extravascular tissue, and the augmented myeloperoxidase activity normalized with recovery sleep.
A robust body of literature implicates CIH in the pathogenesis of fibrosis and remodeling leading to dysfunction in several organ systems (systemic vessels, liver, and kidney), including in the airway. In one study, in uninjured mice, CIH induced lung injury, a Th1 type of inflammation (tumor necrosis factor-α, IL-6, and IL-8), and oxidative stress (malondialdehyde content and nicotinamide adenine dinucleotide phosphate oxidase 2 expression), and reduced antioxidant defenses (superoxide dismutase activity) (87). In another study, a 4-week exposure of OVA-sensitized and -challenged rats to CIH decreased baseline eosinophils, amplified the effect of OVA on monocyte numbers, and altered the protease/antiprotease balance compared with rats exposed to normoxia (54). This shift from the traditional eosinophilic, Th2 profile of this model to a Th1 pattern of inflammation led to lung tissue remodeling, consisting of proximal airway wall fibrosis, distal airway basement membrane thinning, and “emphysema-like” formations in the lung periphery. These processes culminated in the physiologic deficits of expiratory flow limitation. Collectively, these data imply that OSA-related features have the potential to lead to structural airway and parenchymal changes with physiologic deficits, in a manner that casts doubt about their response to current standard therapies for asthma. These observations may explain the failure of CPAP to improve airway physiology measures in the aforementioned clinical studies, indicating irreversible airway remodeling.
Conclusions and Clinical Implications
Despite years of costly research in asthma, in the last decade, the morbidity and mortality of this disease have remained stagnant (88), to say the least, while a myriad of clusters, phenotypes, and endotypes have been described. This leads one to conclude that there are basic knowledge gaps and approaches that need to be consistently considered and exploited.
At present, the recognition of sleep-disordered breathing and OSA offers investigators a unique opportunity. Accumulating epidemiologic, physiologic, and biologic data converge to support a bidirectional interaction between asthma and OSA aside from shared factors, such that the nasal, pharyngeal, and lower airways are indeed “united” (Figure 2)—that is, the severity and duration of asthma impact the predisposition to OSA. Underlying pathways include 1) “spillover” systemic inflammation or neuroimmune cross-talk to alter breathing control mechanisms, and 2) the effects of ICS on upper-airway muscle and fat content, to alter the anatomy. The relationship between asthma duration and OSA incidence suggests that this interaction commences early in life, potentially in utero. However, once established, OSA is not an innocent bystander. All of its features have the potential to impact the asthmatic airway, perhaps each in a different way, and their interacting effects ultimately influence the expression of asthma and its heterogeneity. We know that at least CIH modulates airway inflammation and leads to remodeling and dysfunction in a manner that is unlikely to respond to standard therapies (18, 54) (Figure 2). This may explain in part the association of OSA with worse clinical outcomes across the healthcare continuum. Thus, failure to address OSA could lead to a step-up in ICS therapy, which in turn would accelerate this vicious cycle in the “unified airway” and translate into irreversible lower-airway dysfunction (18, 54). In addition, although the role of sex in OSA pathogenesis in asthma remains to be determined, females with asthma (who represent the majority of patients with asthma) seem to be more impacted by this interaction.
This review emphasizes the importance of thoroughly assessing asthma control around the clock, as part of a multidisciplinary care program. Clinicians should periodically screen their patients with asthma for OSA, particularly those who have had asthma for longer durations, have uncontrolled disease, or are on higher doses of ICS. They should keep in mind that home sleep studies lacking EEG recordings are likely going to underestimate clinically significant OSA in patients with asthma. CPAP treatment should be offered and adherence emphasized, as it holds the potential to reduce asthma morbidity and improve quality of life.
Future Directions
The field of OSA in asthma is in its infancy. However, the current evidence and its limitations bring forth multiple key questions (Table 4). These questions highlight the need for expedited, larger, and carefully designed studies in well-characterized populations, encompassing objective assessments, to elucidate the pathogenesis of OSA in asthma and the role of OSA in asthma heterogeneity, sex differences, and response to medications, and facilitate a thorough, personalized approach to patient care.
Table 4.
Key Research Questions Emerging from Current Evidence
| Direction of the Interaction | Key Questions |
|---|---|
| Asthma→OSA | • Natural history of the interaction, starting early in life (e.g., during pregnancy in birth cohorts), and the role of sex in this relationship |
| • Relationship of asthma clinical/inflammatory phenotypes with OSA incidence | |
| • Mechanistic studies of effects of asthma and related features on OSA phenotypic traits (loop gain, arousal threshold, pharyngeal upper-airway collapsibility) | |
| • Role of inhaled corticosteroids in upper-airway patency during sleep and other relevant functions (swallowing and speech) | |
| • Comparative effectiveness of screening algorithms and diagnostic tools for OSA in patients with asthma | |
| OSA→asthma | • Role of other OSA features (i.e., “mechanical stress” and sleep fragmentation) in modulating asthmatic airways |
| • Reliability of home sleep apnea tests in asthma clinical/inflammatory phenotypes | |
| • Whether asthma should be an indication for CPAP treatment in mild OSA | |
| • Role of OSA in responsiveness of asthma to various therapies | |
| • Role of other OSA treatment modalities in asthma burden | |
| • Randomized controlled trials on effects of PAP or other therapies for OSA on patient-centric asthma outcomes and determinants (sex and other variables) of response | |
| • Role of OSA in the management algorithms for asthma |
Definition of abbreviations: CPAP = continuous positive airway pressure; OSA = obstructive sleep apnea; PAP = positive airway pressure.
Supplementary Material
Footnotes
B.P. receives research support from Clinical Science Research and Development, U.S. Department of Veterans Affairs; S.M.N. receives research support from the NIH; and M.T. receives research support from Biomedical Laboratory Research and Development, U.S. Department of Veterans Affairs. The content of this article is solely the responsibility of the authors and does not represent the views of the Department of Veterans Affairs, the NIH, or the United States Government.
Author Contributions: I.I. and A.S. conducted the literature review. B.P., S.M.N., and M.T. synthesized the literature and wrote the manuscript.
Originally Published in Press as DOI: 10.1164/rccm.201810-1838TR on December 16, 2019
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 2.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177:1006–1014. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Julien JY, Martin JG, Ernst P, Olivenstein R, Hamid Q, Lemière C, et al. Prevalence of obstructive sleep apnea-hypopnea in severe versus moderate asthma. J Allergy Clin Immunol. 2009;124:371–376. doi: 10.1016/j.jaci.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 4.Larsson LG, Lindberg A, Franklin KA, Lundbäck B. Symptoms related to obstructive sleep apnoea are common in subjects with asthma, chronic bronchitis and rhinitis in a general population. Respir Med. 2001;95:423–429. doi: 10.1053/rmed.2001.1054. [DOI] [PubMed] [Google Scholar]
- 5.Yigla M, Tov N, Solomonov A, Rubin AH, Harlev D. Difficult-to-control asthma and obstructive sleep apnea. J Asthma. 2003;40:865–871. doi: 10.1081/jas-120023577. [DOI] [PubMed] [Google Scholar]
- 6.Teodorescu M, Broytman O, Curran-Everett D, Sorkness RL, Crisafi G, Bleecker ER, et al. National Institutes of Health, National Heart, Lung and Blood Institute Severe Asthma Research Program (SARP) Investigators. Obstructive sleep apnea risk, asthma burden, and lower airway inflammation in adults in the Severe Asthma Research Program (SARP) II. J Allergy Clin Immunol Pract. 2015;3:566–575.e1. doi: 10.1016/j.jaip.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teodorescu M, Polomis DA, Gangnon RE, Fedie JE, Consens FB, Chervin RD, et al. Asthma control and its relationship with obstructive sleep apnea (OSA) in older adults. Sleep Disord. 2013;2013:251567. doi: 10.1155/2013/251567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.ten Brinke A, Sterk PJ, Masclee AA, Spinhoven P, Schmidt JT, Zwinderman AH, et al. Risk factors of frequent exacerbations in difficult-to-treat asthma. Eur Respir J. 2005;26:812–818. doi: 10.1183/09031936.05.00037905. [DOI] [PubMed] [Google Scholar]
- 9.Kasasbeh A, Kasasbeh E, Krishnaswamy G. Potential mechanisms connecting asthma, esophageal reflux, and obesity/sleep apnea complex: a hypothetical review. Sleep Med Rev. 2007;11:47–58. doi: 10.1016/j.smrv.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Alkhalil M, Schulman E, Getsy J. Obstructive sleep apnea syndrome and asthma: what are the links? J Clin Sleep Med. 2009;5:71–78. [PMC free article] [PubMed] [Google Scholar]
- 11.Ioachimescu OC, Teodorescu M. Integrating the overlap of obstructive lung disease and obstructive sleep apnoea: OLDOSA syndrome. Respirology. 2013;18:421–431. doi: 10.1111/resp.12062. [DOI] [PubMed] [Google Scholar]
- 12.Shen TC, Lin CL, Wei CC, Chen CH, Tu CY, Hsia TC, et al. Risk of obstructive sleep apnea in adult patients with asthma: a population-based cohort study in Taiwan. PLoS One. 2015;10:e0128461. doi: 10.1371/journal.pone.0128461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sweeney J, Patterson CC, Menzies-Gow A, Niven RM, Mansur AH, Bucknall C, et al. British Thoracic Society Difficult Asthma Network. Comorbidity in severe asthma requiring systemic corticosteroid therapy: cross-sectional data from the optimum patient care research database and the British Thoracic Difficult Asthma Registry. Thorax. 2016;71:339–346. doi: 10.1136/thoraxjnl-2015-207630. [DOI] [PubMed] [Google Scholar]
- 14.Kong DL, Qin Z, Shen H, Jin HY, Wang W, Wang ZF. Association of obstructive sleep apnea with asthma: a meta-analysis. Sci Rep. 2017;7:4088. doi: 10.1038/s41598-017-04446-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhattacharyya N, Kepnes LJ. Ambulatory office visits and medical comorbidities associated with obstructive sleep apnea. Otolaryngol Head Neck Surg. 2012;147:1154–1157. doi: 10.1177/0194599812459850. [DOI] [PubMed] [Google Scholar]
- 16.Knuiman M, James A, Divitini M, Bartholomew H. Longitudinal study of risk factors for habitual snoring in a general adult population: the Busselton Health Study. Chest. 2006;130:1779–1783. doi: 10.1378/chest.130.6.1779. [DOI] [PubMed] [Google Scholar]
- 17.Teodorescu M, Barnet JH, Hagen EW, Palta M, Young TB, Peppard PE. Association between asthma and risk of developing obstructive sleep apnea. JAMA. 2015;313:156–164. doi: 10.1001/jama.2014.17822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taillé C, Rouvel-Tallec A, Stoica M, Danel C, Dehoux M, Marin-Esteban V, et al. Obstructive sleep apnoea modulates airway inflammation and remodelling in severe asthma. PLoS One. 2016;11:e0150042. doi: 10.1371/journal.pone.0150042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jonassen TM, Eagan TM, Bjorvatn B, Lehmann S. Associations between obstructive lung disease and symptoms of obstructive sleep apnoea in a general population. Clin Respir J. 2018;12:31–39. doi: 10.1111/crj.12472. [DOI] [PubMed] [Google Scholar]
- 20.Dempsey JA, Veasey SC, Morgan BJ, O’Donnell CP. Pathophysiology of sleep apnea. Physiol Rev. 2010;90:47–112. doi: 10.1152/physrev.00043.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Togias A. Rhinitis and asthma: evidence for respiratory system integration. J Allergy Clin Immunol. 2003;111:1171–1183. doi: 10.1067/mai.2003.1592. quiz 1184. [DOI] [PubMed] [Google Scholar]
- 22.Leynaert B, Neukirch F, Demoly P, Bousquet J. Epidemiologic evidence for asthma and rhinitis comorbidity. J Allergy Clin Immunol. 2000;106(Suppl):S201–S205. doi: 10.1067/mai.2000.110151. [DOI] [PubMed] [Google Scholar]
- 23.Kalpaklioğlu AF, Kavut AB, Ekici M. Allergic and nonallergic rhinitis: the threat for obstructive sleep apnea. Ann Allergy Asthma Immunol. 2009;103:20–25. doi: 10.1016/S1081-1206(10)60138-X. [DOI] [PubMed] [Google Scholar]
- 24.Olsen KD, Kern EB. Nasal influences on snoring and obstructive sleep apnea. Mayo Clin Proc. 1990;65:1095–1105. doi: 10.1016/s0025-6196(12)62722-0. [DOI] [PubMed] [Google Scholar]
- 25.Koinis-Mitchell D, Craig T, Esteban CA, Klein RB. Sleep and allergic disease: a summary of the literature and future directions for research. J Allergy Clin Immunol. 2012;130:1275–1281. doi: 10.1016/j.jaci.2012.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Braunstahl GJ. Chronic rhinosinusitis, nasal polyposis and asthma: the united airways concept reconsidered? Clin Exp Allergy. 2011;41:1341–1343. doi: 10.1111/j.1365-2222.2011.03796.x. [DOI] [PubMed] [Google Scholar]
- 27.Teodorescu M, Consens FB, Bria WF, Coffey MJ, McMorris MS, Weatherwax KJ, et al. Predictors of habitual snoring and obstructive sleep apnea risk in patients with asthma. Chest. 2009;135:1125–1132. doi: 10.1378/chest.08-1273. [DOI] [PubMed] [Google Scholar]
- 28.Janson C, Gislason T, De Backer W, Plaschke P, Björnsson E, Hetta J, et al. Prevalence of sleep disturbances among young adults in three European countries. Sleep. 1995;18:589–597. [PubMed] [Google Scholar]
- 29.Gislason T, Janson C, Vermeire P, Plaschke P, Björnsson E, Gislason D, et al. Respiratory symptoms and nocturnal gastroesophageal reflux: a population-based study of young adults in three European countries. Chest. 2002;121:158–163. doi: 10.1378/chest.121.1.158. [DOI] [PubMed] [Google Scholar]
- 30.Emilsson OI, Bengtsson A, Franklin KA, Torén K, Benediktsdóttir B, Farkhooy A, et al. Nocturnal gastro-oesophageal reflux, asthma and symptoms of OSA: a longitudinal, general population study. Eur Respir J. 2013;41:1347–1354. doi: 10.1183/09031936.00052512. [DOI] [PubMed] [Google Scholar]
- 31.Havemann BD, Henderson CA, El-Serag HB. The association between gastro-oesophageal reflux disease and asthma: a systematic review. Gut. 2007;56:1654–1664. doi: 10.1136/gut.2007.122465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Orr WC, Robert JJ, Houck JR, Giddens CL, Tawk MM. The effect of acid suppression on upper airway anatomy and obstruction in patients with sleep apnea and gastroesophageal reflux disease. J Clin Sleep Med. 2009;5:330–334. [PMC free article] [PubMed] [Google Scholar]
- 33.Beuther DA, Sutherland ER. Overweight, obesity, and incident asthma: a meta-analysis of prospective epidemiologic studies. Am J Respir Crit Care Med. 2007;175:661–666. doi: 10.1164/rccm.200611-1717OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dixon AE, Holguin F, Sood A, Salome CM, Pratley RE, Beuther DA, et al. American Thoracic Society Ad Hoc Subcommittee on Obesity and Lung Disease. An official American Thoracic Society Workshop report: obesity and asthma. Proc Am Thorac Soc. 2010;7:325–335. doi: 10.1513/pats.200903-013ST. [DOI] [PubMed] [Google Scholar]
- 35.Avallone KM, McLeish AC. Asthma and aerobic exercise: a review of the empirical literature. J Asthma. 2013;50:109–116. doi: 10.3109/02770903.2012.759963. [DOI] [PubMed] [Google Scholar]
- 36.Teodorescu M, Polomis DA, Gangnon RE, Consens FB, Chervin RD, Teodorescu MC. Sleep duration, asthma and obesity. J Asthma. 2013;50:945–953. doi: 10.3109/02770903.2013.831871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–1239. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 38.Maltz L, Matz EL, Gordish-Dressman H, Pillai DK, Teach SJ, Camargo CA, Jr, et al. Sex differences in the association between neck circumference and asthma. Pediatr Pulmonol. 2016;51:893–900. doi: 10.1002/ppul.23381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fadzil Abdullah AA, Jamalludin AR, Norrashidah AW, Norzila MZ, Asiah Kassim K, Rus Anida A, et al. Prevalence of sleep disordered breathing symptoms among Malay school children in a primary school in Malaysia. Med J Malaysia. 2012;67:181–185. [PubMed] [Google Scholar]
- 40.Isono S. Obesity and obstructive sleep apnoea: mechanisms for increased collapsibility of the passive pharyngeal airway. Respirology. 2012;17:32–42. doi: 10.1111/j.1440-1843.2011.02093.x. [DOI] [PubMed] [Google Scholar]
- 41.Jacono FJ. Control of ventilation in COPD and lung injury. Respir Physiol Neurobiol. 2013;189:371–376. doi: 10.1016/j.resp.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 42.Jacono FJ, Mayer CA, Hsieh YH, Wilson CG, Dick TE. Lung and brainstem cytokine levels are associated with breathing pattern changes in a rodent model of acute lung injury. Respir Physiol Neurobiol. 2011;178:429–438. doi: 10.1016/j.resp.2011.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jacono FJ, Peng YJ, Nethery D, Faress JA, Lee Z, Kern JA, et al. Acute lung injury augments hypoxic ventilatory response in the absence of systemic hypoxemia. J Appl Physiol (1985) 2006;101:1795–1802. doi: 10.1152/japplphysiol.00100.2006. [DOI] [PubMed] [Google Scholar]
- 44.Broytman O, Brinkman J, Pegelow D, Morgan M, Teodorescu M. Ovalbumin: induced airway inflammation enhances hypoxic ventilatory response in rats. FASEB J. 2017;31:728.9. [Google Scholar]
- 45.McFadden ER, Jr, Lyons HA. Arterial-blood gas tension in asthma. N Engl J Med. 1968;278:1027–1032. doi: 10.1056/NEJM196805092781901. [DOI] [PubMed] [Google Scholar]
- 46.Luyster FS, Teodorescu M, Bleecker E, Busse W, Calhoun W, Castro M, et al. Sleep quality and asthma control and quality of life in non-severe and severe asthma. Sleep Breath. 2012;16:1129–1137. doi: 10.1007/s11325-011-0616-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wellman A, Eckert DJ, Jordan AS, Edwards BA, Passaglia CL, Jackson AC, et al. A method for measuring and modeling the physiological traits causing obstructive sleep apnea. J Appl Physiol (1985) 2011;110:1627–1637. doi: 10.1152/japplphysiol.00972.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mateika JH, Syed Z. Intermittent hypoxia, respiratory plasticity and sleep apnea in humans: present knowledge and future investigations. Respir Physiol Neurobiol. 2013;188:289–300. doi: 10.1016/j.resp.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ryan S, Nolan P. Long-term facilitation of upper airway muscle activity induced by episodic upper airway negative pressure and hypoxia in spontaneously breathing anaesthetized rats. J Physiol. 2009;587:3343–3353. doi: 10.1113/jphysiol.2009.169698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bach KB, Mitchell GS. Hypercapnia-induced long-term depression of respiratory activity requires alpha2-adrenergic receptors. J Appl Physiol (1985) 1998;84:2099–2105. doi: 10.1152/jappl.1998.84.6.2099. [DOI] [PubMed] [Google Scholar]
- 51.Mateika JH, Narwani G. Intermittent hypoxia and respiratory plasticity in humans and other animals: does exposure to intermittent hypoxia promote or mitigate sleep apnoea? Exp Physiol. 2009;94:279–296. doi: 10.1113/expphysiol.2008.045153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mateika JH, Panza G, Alex R, El-Chami M. The impact of intermittent or sustained carbon dioxide on intermittent hypoxia initiated respiratory plasticity: what is the effect of these combined stimuli on apnea severity? Respir Physiol Neurobiol. 2018;256:58–66. doi: 10.1016/j.resp.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 53.Huxtable AG, Vinit S, Windelborn JA, Crader SM, Guenther CH, Watters JJ, et al. Systemic inflammation impairs respiratory chemoreflexes and plasticity. Respir Physiol Neurobiol. 2011;178:482–489. doi: 10.1016/j.resp.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Broytman O, Braun RK, Morgan BJ, Pegelow DF, Hsu PN, Mei LS, et al. Effects of chronic intermittent hypoxia on allergen-induced airway inflammation in rats. Am J Respir Cell Mol Biol. 2015;52:162–170. doi: 10.1165/rcmb.2014-0213OC. [DOI] [PubMed] [Google Scholar]
- 55.Gozal D, Reeves SR, Row BW, Neville JJ, Guo SZ, Lipton AJ. Respiratory effects of gestational intermittent hypoxia in the developing rat. Am J Respir Crit Care Med. 2003;167:1540–1547. doi: 10.1164/rccm.200208-963OC. [DOI] [PubMed] [Google Scholar]
- 56.Reeves SR, Mitchell GS, Gozal D. Early postnatal chronic intermittent hypoxia modifies hypoxic respiratory responses and long-term phrenic facilitation in adult rats. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1664–R1671. doi: 10.1152/ajpregu.00851.2005. [DOI] [PubMed] [Google Scholar]
- 57.Martin RJ, Szefler SJ, Chinchilli VM, Kraft M, Dolovich M, Boushey HA, et al. Systemic effect comparisons of six inhaled corticosteroid preparations. Am J Respir Crit Care Med. 2002;165:1377–1383. doi: 10.1164/rccm.2105013. [DOI] [PubMed] [Google Scholar]
- 58.Williams AJ, Baghat MS, Stableforth DE, Cayton RM, Shenoi PM, Skinner C. Dysphonia caused by inhaled steroids: recognition of a characteristic laryngeal abnormality. Thorax. 1983;38:813–821. doi: 10.1136/thx.38.11.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Teodorescu M, Xie A, Sorkness CA, Robbins J, Reeder S, Gong Y, et al. Effects of inhaled fluticasone on upper airway during sleep and wakefulness in asthma: a pilot study. J Clin Sleep Med. 2014;10:183–193. doi: 10.5664/jcsm.3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Eckert DJ, Lo YL, Saboisky JP, Jordan AS, White DP, Malhotra A. Sensorimotor function of the upper-airway muscles and respiratory sensory processing in untreated obstructive sleep apnea. J Appl Physiol (1985) 2011;111:1644–1653. doi: 10.1152/japplphysiol.00653.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Teodorescu M, Polomis DA, Hall SV, Teodorescu MC, Gangnon RE, Peterson AG, et al. Association of obstructive sleep apnea risk with asthma control in adults. Chest. 2010;138:543–550. doi: 10.1378/chest.09-3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Teodorescu M, Polomis DA, Teodorescu MC, Gangnon RE, Peterson AG, Consens FB, et al. Association of obstructive sleep apnea risk or diagnosis with daytime asthma in adults. J Asthma. 2012;49:620–628. doi: 10.3109/02770903.2012.689408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang TY, Lo YL, Lin SM, Huang CD, Chung FT, Lin HC, et al. Obstructive sleep apnoea accelerates FEV1 decline in asthmatic patients. BMC Pulm Med. 2017;17:55. doi: 10.1186/s12890-017-0398-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang Y, Liu K, Hu K, Yang J, Li Z, Nie M, et al. Impact of obstructive sleep apnea on severe asthma exacerbations. Sleep Med. 2016;26:1–5. doi: 10.1016/j.sleep.2016.06.013. [DOI] [PubMed] [Google Scholar]
- 65.Becerra MB, Becerra BJ, Teodorescu M. Healthcare burden of obstructive sleep apnea and obesity among asthma hospitalizations: results from the U.S.-based nationwide inpatient sample. Respir Med. 2016;117:230–236. doi: 10.1016/j.rmed.2016.06.020. [DOI] [PubMed] [Google Scholar]
- 66.Chan CS, Woolcock AJ, Sullivan CE. Nocturnal asthma: role of snoring and obstructive sleep apnea. Am Rev Respir Dis. 1988;137:1502–1504. doi: 10.1164/ajrccm/137.6.1502. [DOI] [PubMed] [Google Scholar]
- 67.Guilleminault C, Quera-Salva MA, Powell N, Riley R, Romaker A, Partinen M, et al. Nocturnal asthma: snoring, small pharynx and nasal CPAP. Eur Respir J. 1988;1:902–907. [PubMed] [Google Scholar]
- 68.Lafond C, Sériès F, Lemière C. Impact of CPAP on asthmatic patients with obstructive sleep apnoea. Eur Respir J. 2007;29:307–311. doi: 10.1183/09031936.00059706. [DOI] [PubMed] [Google Scholar]
- 69.Serrano-Pariente J, Plaza V, Soriano JB, Mayos M, López-Viña A, Picado C, et al. CPASMA Trial Group. Asthma outcomes improve with continuous positive airway pressure for obstructive sleep apnea. Allergy. 2017;72:802–812. doi: 10.1111/all.13070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Irvin CG, Pak J, Martin RJ. Airway-parenchyma uncoupling in nocturnal asthma. Am J Respir Crit Care Med. 2000;161:50–56. doi: 10.1164/ajrccm.161.1.9804053. [DOI] [PubMed] [Google Scholar]
- 71.Bachour P, Bachour A, Kauppi P, Maasilta P, Makitie A, Palotie T. Oral appliance in sleep apnea treatment: respiratory and clinical effects and long-term adherence. Sleep Breath. 2016;20:805–812. doi: 10.1007/s11325-015-1301-0. [DOI] [PubMed] [Google Scholar]
- 72.Simard B, Turcotte H, Marceau P, Biron S, Hould FS, Lebel S, et al. Asthma and sleep apnea in patients with morbid obesity: outcome after bariatric surgery. Obes Surg. 2004;14:1381–1388. doi: 10.1381/0960892042584021. [DOI] [PubMed] [Google Scholar]
- 73.Omana JJ, Nguyen SQ, Herron D, Kini S. Comparison of comorbidity resolution and improvement between laparoscopic sleeve gastrectomy and laparoscopic adjustable gastric banding. Surg Endosc. 2010;24:2513–2517. doi: 10.1007/s00464-010-0995-0. [DOI] [PubMed] [Google Scholar]
- 74.Shore SA, Johnston RA. Obesity and asthma. Pharmacol Ther. 2006;110:83–102. doi: 10.1016/j.pharmthera.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 75.Hung CH, Wang CC, Suen JL, Sheu CC, Kuo CH, Liao WT, et al. Altered pattern of monocyte differentiation and monocyte-derived TGF-β1 in severe asthma. Sci Rep. 2018;8:919. doi: 10.1038/s41598-017-19105-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Israel E, Reddel HK. Severe and difficult-to-treat asthma in adults. N Engl J Med. 2017;377:965–976. doi: 10.1056/NEJMra1608969. [DOI] [PubMed] [Google Scholar]
- 77.Schmidt M, Sun G, Stacey MA, Mori L, Mattoli S. Identification of circulating fibrocytes as precursors of bronchial myofibroblasts in asthma. J Immunol. 2003;171:380–389. doi: 10.4049/jimmunol.171.1.380. [DOI] [PubMed] [Google Scholar]
- 78.Desai M, Oppenheimer J. Elucidating asthma phenotypes and endotypes: progress towards personalized medicine. Ann Allergy Asthma Immunol. 2016;116:394–401. doi: 10.1016/j.anai.2015.12.024. [DOI] [PubMed] [Google Scholar]
- 79.Bijaoui EL, Champagne V, Baconnier PF, Kimoff RJ, Bates JH. Mechanical properties of the lung and upper airways in patients with sleep-disordered breathing. Am J Respir Crit Care Med. 2002;165:1055–1061. doi: 10.1164/ajrccm.165.8.2107144. [DOI] [PubMed] [Google Scholar]
- 80.Appelberg J, Nordahl G, Janson C. Lung volume and its correlation to nocturnal apnoea and desaturation. Respir Med. 2000;94:233–239. doi: 10.1053/rmed.1999.0730. [DOI] [PubMed] [Google Scholar]
- 81.Sériès F, Cormier Y, La Forge J. Role of lung volumes in sleep apnoea-related oxygen desaturation. Eur Respir J. 1989;2:26–30. [PubMed] [Google Scholar]
- 82.Zerah-Lancner F, Lofaso F, Coste A, Ricolfi F, Goldenberg F, Harf A. Pulmonary function in obese snorers with or without sleep apnea syndrome. Am J Respir Crit Care Med. 1997;156:522–527. doi: 10.1164/ajrccm.156.2.9609015. [DOI] [PubMed] [Google Scholar]
- 83.Tamisier R, Pepin JL, Wuyam B, Deschaux C, Levy P. Expiratory changes in pressure: flow ratio during sleep in patients with sleep-disordered breathing. Sleep. 2004;27:240–248. doi: 10.1093/sleep/27.2.240. [DOI] [PubMed] [Google Scholar]
- 84.Waters CM, Sporn PHS, Liu M, Fredberg JJ. Cellular biomechanics in the lung. Am J Physiol Lung Cell Mol Physiol. 2002;283:L503–L509. doi: 10.1152/ajplung.00141.2002. [DOI] [PubMed] [Google Scholar]
- 85.Almendros I, Acerbi I, Puig F, Montserrat JM, Navajas D, Farré R. Upper-airway inflammation triggered by vibration in a rat model of snoring. Sleep. 2007;30:225–227. doi: 10.1093/sleep/30.2.225. [DOI] [PubMed] [Google Scholar]
- 86.Everson CA, Thalacker CD, Hogg N. Phagocyte migration and cellular stress induced in liver, lung, and intestine during sleep loss and sleep recovery. Am J Physiol Regul Integr Comp Physiol. 2008;295:R2067–R2074. doi: 10.1152/ajpregu.90623.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang X, Rui L, Wang M, Lian H, Cai L. Sinomenine attenuates chronic intermittent hypoxia-induced lung injury by inhibiting inflammation and oxidative stress. Med Sci Monit. 2018;24:1574–1580. doi: 10.12659/MSM.906577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Holbrook JT, Sugar EA, Brown RH, Drye LT, Irvin CG, Schwartz AR, et al. American Lung Association Airways Clinical Research Centers. Effect of continuous positive airway pressure on airway reactivity in asthma: a randomized, sham-controlled clinical trial. Ann Am Thorac Soc. 2016;13:1940–1950. doi: 10.1513/AnnalsATS.201601-043OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Karachaliou F, Kostikas K, Pastaka C, Bagiatis V, Gourgoulianis KI. Prevalence of sleep-related symptoms in a primary care population—their relation to asthma and COPD. Prim Care Respir J. 2007;16:222–228. doi: 10.3132/pcrj.2007.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Auckley D, Moallem M, Shaman Z, Mustafa M. Findings of a Berlin questionnaire survey: comparison between patients seen in an asthma clinic versus internal medicine clinic. Sleep Med. 2008;9:494–499. doi: 10.1016/j.sleep.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 91.Williams MA, Gelaye B, Qiu C, Fida N, May Cripe S. Habitual snoring and asthma comorbidity among pregnant women. J Asthma. 2011;48:91–97. doi: 10.3109/02770903.2010.535882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Braido F, Baiardini I, Lacedonia D, Facchini FM, Fanfulla F, Molinengo G, et al. Italian Society of Respiratory Medicine (SIMeR) Sleep apnea risk in subjects with asthma with or without comorbid rhinitis. Respir Care. 2014;59:1851–1856. doi: 10.4187/respcare.03084. [DOI] [PubMed] [Google Scholar]
- 93.Kim MY, Jo EJ, Kang SY, Chang YS, Yoon IY, Cho SH, et al. Obstructive sleep apnea is associated with reduced quality of life in adult patients with asthma. Ann Allergy Asthma Immunol. 2013;110:253–257, 257.e1. doi: 10.1016/j.anai.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 94.Tay TR, Radhakrishna N, Hore-Lacy F, Smith C, Hoy R, Dabscheck E, et al. Comorbidities in difficult asthma are independent risk factors for frequent exacerbations, poor control and diminished quality of life. Respirology. 2016;21:1384–1390. doi: 10.1111/resp.12838. [DOI] [PubMed] [Google Scholar]
- 95.Jordan HT, Stellman SD, Reibman J, Farfel MR, Brackbill RM, Friedman SM, et al. Factors associated with poor control of 9/11-related asthma 10-11 years after the 2001 World Trade Center terrorist attacks. J Asthma. 2015;52:630–637. doi: 10.3109/02770903.2014.999083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yii ACA, Tan JHY, Lapperre TS, Chan AKW, Low SY, Ong TH, et al. Long-term future risk of severe exacerbations: distinct 5-year trajectories of problematic asthma. Allergy. 2017;72:1398–1405. doi: 10.1111/all.13159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kauppi P, Bachour P, Maasilta P, Bachour A. Long-term CPAP treatment improves asthma control in patients with asthma and obstructive sleep apnoea. Sleep Breath. 2016;20:1217–1224. doi: 10.1007/s11325-016-1340-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.