Abstract
Rationale: Bronchopulmonary dysplasia (BPD) is a heterogenous condition with poorly characterized disease subgroups.
Objectives: To define the frequency of three disease components: moderate–severe parenchymal disease, pulmonary hypertension (PH), or large airway disease, in a referral cohort of preterm infants with severe BPD. The association between each component and a primary composite outcome of death before hospital discharge, tracheostomy, or home pulmonary vasodilator therapy was assessed.
Methods: This was a retrospective, single-center cohort study of infants born at <32 weeks’ gestation with severe BPD who underwent both chest computed tomography with angiography (CTA) and echocardiography between 40 and 50 weeks postmenstrual age between 2011 and 2015. Moderate–severe parenchymal lung disease was defined as an Ochiai score ≥8 on CTA. PH was diagnosed by echocardiogram using standard criteria. Large airway disease was defined as tracheomalacia or bronchomalacia on bronchoscopy and/or tracheoscopy or CTA.
Measurements and Main Results: Of 76 evaluated infants, 73 (96%) were classifiable into phenotypic subgroups: 57 with moderate–severe parenchymal disease, 48 with PH, and 44 with large airway disease. The presence of all three disease components was most common (n = 23). Individually, PH and large airway disease, but not moderate–severe parenchymal disease, were associated with increased risk for the primary study outcome. Having more disease components was associated with an incremental increase in the risk for the primary outcome (2 vs. 1: odds ratio, 4.9; 95% confidence interval, 1.4–17.2 and 3 vs. 1: odds ratio, 12.8; 95% confidence interval, 2.4–70.0).
Conclusions: Infants with severe BPD are variable in their predominant pathophysiology. Disease phenotyping may enable better risk stratification and targeted therapeutic intervention.
Keywords: bronchopulmonary dysplasia, pulmonary hypertension, parenchymal, alveolar, tracheobronchomalacia
At a Glance Commentary
Scientific Knowledge on the Subject
Bronchopulmonary dysplasia (BPD) is a heterogenous condition with poorly characterized disease subgroups.
What This Study Adds to the Field
1) This study demonstrates that there is variability and frequent overlap in the predominant clinical pathophysiology in infants with severe BPD. 2) The study suggests that patients with severe BPD can be classified into phenotypic subgroups, and this classification may help provide important prognostic information.
Bronchopulmonary dysplasia (BPD) is one of the most common, serious complications of prematurity (1). Based on current diagnostic criteria, approximately 40% of very preterm infants develop BPD (2). In 2001, an NIH consensus conference proposed the first widely used severity-based definition of BPD (3). This classifies BPD among very preterm infants as mild, moderate, or severe, according to the amount of supplemental oxygen and the level of respiratory support administered at 36 weeks postmenstrual age (PMA) (3). Although these criteria improved stratification of BPD severity, using respiratory support alone does not adequately inform about the underlying pathophysiology in an individual infant. Whether portioning infants with severe BPD into subgroups based on the predominant disease endotypes will enable “phenotype” descriptions to assist disease prevention, treatment, and outcome prognostication is unknown.
Developmental arrest of the preterm lung, injury from intrauterine and postnatal insults, and abnormal repair contribute to the pathophysiology of BPD (4). Each process can affect any of the three primary compartments of the newborn lung: the airways, the alveolar spaces and adjoining lung parenchyma, and the pulmonary vasculature (5).
Administration of positive pressure may deform the immature airway, leading to tracheomalacia or bronchomalacia (6–9). Parenchymal lung disease in BPD can result from disrupted lung growth, impaired alveolarization, and exposure to various proinflammatory triggers (5, 10, 11). Decreased and dysmorphic growth plus abnormal remodeling of the pulmonary microvasculature may follow hyperoxia, hypoxemia, pulmonary overcirculation, and inflammation (3, 12–14). Clinically, this presents as pulmonary vascular disease, typically pulmonary hypertension (PH). These individual pathologic processes are well described and there is growing interest in the potential association between these disease endotypes in BPD (15–19). However, their frequency and rate of cooccurrence in infants with severe BPD is not yet well established. We assessed whether very preterm infants with severe BPD could be clinically categorized into phenotypic subgroups based on the presence or absence of moderate–severe parenchymal lung disease, PH, and large airway disease. We also explored associations of such characterization with clinical and prognostic outcomes measured at hospital discharge.
Some of the results of this study have been previously reported in the form of an abstract (20).
Methods
Study Infants
This retrospective study was performed at a single tertiary referral neonatal ICU (NICU) from 2011 to 2015. Infants were eligible if born at <32 weeks’ gestation and 1) fulfilled criteria of severe BPD (use of supplemental oxygen for at least 28 d plus treatment with ≥30% FiO2 or positive pressure ventilation at 36 wk PMA) (3), 2) were free of severe congenital anomalies or genetic syndromes, and 3) underwent chest computed tomography with angiography (CTA) and echocardiography between 40 and 50 weeks PMA. This PMA range ensured a consistent time point of diagnostic testing in the study cohort. Infants receiving nasal cannula flow >2 L/min at 36 weeks PMA were considered as receiving positive pressure ventiation and therefore fulfilled the criteria for severe BPD.
Demographic data was captured by a prospective database maintained by the Newborn and Infant Chronic Lung Disease Program. Additional review extracted imaging results. The Institutional Review Board at the Children’s Hospital of Philadelphia approved this study.
Classification of Parenchymal Lung Disease
The severity of parenchymal lung disease was classified by CTA using the Ochiai criteria (21). These criteria score the presence and severity of 10 separate lung radiographic features on a scale ranging from 0 to 2 (hyperexpansion, mosaic pattern of attenuation, intercostal bulging, air cysts, bullae, blebs, cyst size, triangular subpleural opacities, distortion and thickening of bronchovascular bundle, consolidation, and subjective interpretation of overall lung disease severity). The maximum possible Ochiai score is 18 (21). For this analysis, moderate–severe parenchymal lung disease was defined as an Ochiai score ≥8. This cutoff approximates the upper 75% of scores received by infants who were discharged breathing in room air in the original publication (21). A post hoc analysis was also performed using an Ochiai score ≥10 to define severe parenchymal lung disease. All imaging from CTAs was scored independently by two pediatric radiologists (A.M.W. and D.M.B.) masked to other infant data. To mediate interobserver variation in Ochiai scores, any initially discrepant results were resolved by consensus.
Classification of PH
The presence of PH was assessed by review of the echocardiogram performed closest in time to each infant’s CTA. To ensure consistent reporting, each echocardiogram was reviewed by a single pediatric cardiologist masked to the infants’ clinical data. PH was defined by one or more of the following: 1) systolic pulmonary artery pressure ≥40 mm Hg by tricuspid regurgitant jet velocity calculated using the modified Bernoulli equation (4 × velocity2) with 5 mm Hg used as the estimated right atrial pressure (22), 2) a bidirectional or right-to-left shunt through a patent ductus arteriosus, and 3) a flattened or bowing interventricular septum at the end of systole (23–25). To ensure we had acceptable interrater reliability in the diagnosis of PH, 10 echocardiograms were reanalyzed by a second pediatric cardiologist.
Classification of Large Airway Disease
Reports from all bronchoscopy and tracheoscopy studies performed before NICU discharge were reviewed. Large airway disease was defined as the presence of tracheomalacia and/or bronchomalacia documented on bronchoscopy and/or tracheoscopy by a pediatric otolaryngologist or pulmonologist. In addition, all chest CTAs reviewed in this study were analyzed for evidence of airway malacia. Large airway disease on chest CTA was defined as a >50% decrease in the cross-sectional area of the trachea or main bronchi from inspiration to expiration. Infants who did not undergo bronchoscopy and/or tracheoscopy, and who did not have CTA evidence of airway collapse were assumed not to have large airway disease.
Clinical Outcomes
We assessed the association between the observed phenotypic subgroups and a primary, composite study outcome of death before NICU discharge, tracheostomy, or the use of a systemic pulmonary vasodilator at discharge. The individual components of the primary outcome and the use of home oxygen therapy, diuretic therapy, and inhaled pulmonary medications were assessed as secondary outcomes. The nonmortality outcomes were chosen because they are well-established clinical measures of disease severity in BPD that are strongly associated with adverse childhood outcomes; significant emotional, financial, and psychological stress in families; and substantial resource use and cost to the healthcare system (26, 27).
Data Analysis
Demographic and clinical data were summarized with standard descriptive statistics and the individual frequencies and rates of cooccurrence for each BPD phenotype subgroup were calculated. Interrater agreements between the two study radiologists and the two cardiologists were quantified using kappa statistics. The dual review of echocardiograms was limited to 10 studies after identifying full agreement between the cardiologists. The association between the evaluated BPD phenotypes and the study outcomes was assessed using logistic regression. First, we determined the odds for each study outcome based on the presence or absence of each of the three subgroups: moderate–severe parenchyma lung disease, PH, and large airway disease. Second, we assessed the odds for the study outcomes associated with the number (one, two, or three) of identified disease components. All odds ratios (ORs) are reported as unadjusted values because the recorded demographic and clinical variables may contribute to the pathophysiology of the evaluated disease phenotypes and study endpoints. Statistical testing was performed using STATASE 15.1 (StataCorp, LLC).
Results
Of the 204 infants with severe BPD admitted to our institution between 2011 and 2015, 76 (37%) satisfied all study inclusion criteria (see the patient flow diagram in Figure E1 in the online supplement). Table 1 compares the characteristics and outcomes of the included and excluded infants.
Table 1.
Characteristics of the Included and Excluded Infants
| Characteristic/Outcome | Included (n = 76) | Excluded (n = 128) | P Value |
|---|---|---|---|
| Gestational age, wk, median (IQR) | 25.8 (24.9–27.4) | 25.8 (24.5–27.5) | 0.68 |
| Birth weight, g, median (IQR) | 645 (575–763) | 691 (594–858) | 0.12 |
| Sex, M, n (%) | 44 (58) | 71 (55) | 0.74 |
| Singleton, n (%) | 58 (76) | 100 (78) | 0.77 |
| Cesarean delivery, n (%) | 64 (84) | 92 (72) | 0.05 |
| Outborn, n (%) | 73 (96) | 128 (100) | 0.05 |
| Antenatal steroids, n (%) | 64 (84) | 102 (80) | 0.42 |
| Surfactant, n (%) | 70 (92) | 117 (91) | 0.86 |
| PMA at transfer to CHOP, wk, median (IQR) | 41.6 (38.7–44.2) | 38.4 (31.7–44.4) | 0.02 |
| Invasive mechanical ventilation at transfer, n (%) | 44 (58) | 75 (59) | 0.92 |
| Treatment for PH during NICU stay, n (%) | 51 (67) | 45 (35) | <0.001 |
| PDA ligation, n (%) | 24 (32) | 37 (29) | 0.69 |
| Tracheostomy, n (%) | 43 (57) | 29 (23) | <0.001 |
| Died before discharge, n (%) | 10 (13) | 16 (13) | 0.89 |
| Home oxygen therapy, n (%)* | 60 (91) | 80 (71) | 0.002 |
| Medication prescribed for home use, n (%)* | |||
| Pulmonary vasodilator | 25 (38) | 9 (8) | <0.001 |
| Diuretic | 41 (62) | 56 (50) | 0.12 |
| Inhaled respiratory medication | 38 (58) | 42 (38) | 0.01 |
Definition of abbreviations: CHOP = Children’s Hospital of Philadelphia; IQR = interquartile range; NICU = neonatal ICU; PDA = patent ductus arteriosus; PH = pulmonary hypertension; PMA = postmenstrual age.
Calculated for survivors to discharge: 66 included infants and 112 excluded infants.
Timing of Imaging Studies and Interrater Agreement
The chest CTAs and echocardiograms used in this analysis were performed at a mean PMA of 44.6 ± 4.0 weeks and 44.0 ± 4.0 weeks, respectively. The initial interrater reliability for diagnosing the presence or absence of moderate–severe parenchymal disease was high (kappa = 0.75) (28). The level of interrater agreement for each component of the Ochiai criteria are listed in Table E1. All differences between the two radiologists were resolved by consensus and the final agreed upon results are present hereafter. There was full agreement between the two cardiologists for the diagnosis of PH using a randomly selected subset of 10 study echocardiograms. In total, 50 of the included study infants (66%) underwent tracheoscopy and/or bronchoscopy at a mean PMA of 54.0 ± 15.2 weeks.
Distribution of BPD Phenotype Subgroups
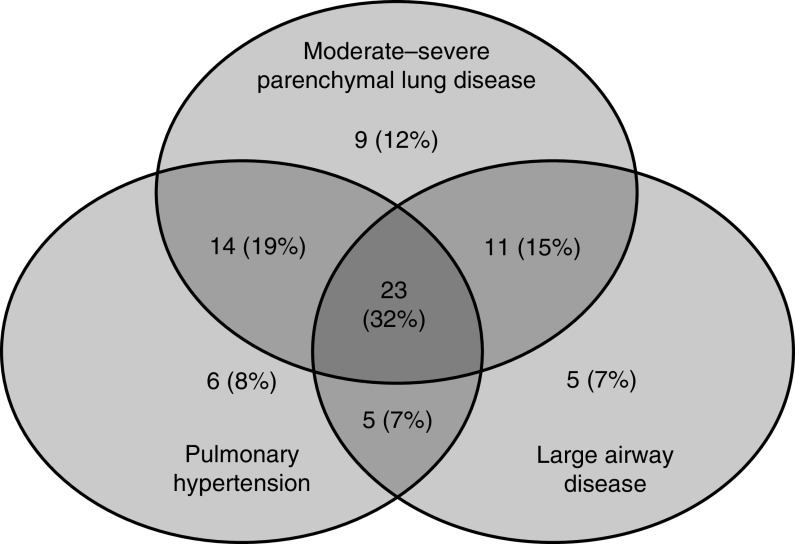
Of the 76 study infants, 73 (96%) could be classified into one or more of the phenotype subgroups (Figure 1) using a Venn diagram. The three unclassified infants had Ochiai scores below 8 (4, 6, and 7) and no apparent evidence of PH or large airway disease. Subglottic stenosis was diagnosed in one of these infants, and this infant was discharged home with a tracheostomy and a heat and moisture exchanger. The remaining two were discharged on low-flow nasal cannula; one was treated with home sildenafil therapy owing to a later diagnosis of PH.
Figure 1.
Venn diagram showing categorization of 73 classifiable infants (using Ochiai score cutoff ≥ 8) according to the presence or absence of the evaluated disease components. Of these, 57 (78%) had moderate–severe parenchymal lung disease, 48 (66%) had pulmonary hypertension, and 44 (60%) had large airway disease. Of these, 23 study infants (32%) had all three disease components, which was the most common phenotype observed.
Among the 73 classified infants, 57 (78%) had moderate–severe parenchymal lung disease, 48 (66%) had PH, and 44 (60%) had large airway disease. The presence of all three disease components was the most common phenotype, observed in 23 study infants (32%) (see Figure 1). Large airway disease alone (n = 5; 7%) and large airway disease with PH (n = 5; 7%) were the least common phenotypic subgroups (see Figure 1). A post hoc analysis using an Ochiai score cutoff of ≥10 enabled classification of 69 infants, of whom 39 (57%) were diagnosed with severe parenchymal lung disease. The cooccurrence of all three disease subtypes remained the most common phenotype (n = 15; 22%) (see Figure E2).
Association between BPD Phenotype and Clinical Outcomes
Table 2 shows the rates of the study outcomes, stratified by the presence or absence of the three evaluated disease components. The presence of PH (OR, 5.4; 95% confidence interval [CI], 1.8–16.6) or large airway disease (OR, 5.1; 95% CI, 1.7–15.9) was associated with an increased risk for the composite outcome of death before NICU discharge, tracheostomy, or home pulmonary vasodilator use. Moderate–severe parenchymal lung disease alone was not associated with an increased risk of the primary outcome (OR, 0.59; 95% CI, 0.15–2.4). When the Ochiai score was treated as a continuous variable, there was no clear association between Ochiai score and primary outcome (see Figure E3). When the individual components of the primary outcome were separately assessed, the diagnosis of PH was associated with discharge home on a pulmonary vasodilator and large airway disease with increased risk for tracheostomy (see Table 2). PH was the only individual disease component associated with increased rate of mortality (21% vs. 0%; P = 0.01).
Table 2.
Clinical Outcomes Stratified by the Presence or Absence of the Evaluated Disease Components
| Outcome | Moderate–Severe Parenchymal Lung Disease |
Pulmonary Hypertension |
Large Airway Disease |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Present | Absent | OR (95% CI) | Present | Absent | OR (95% CI) | Present | Absent | OR (95% CI) | |
| Primary composite | |||||||||
| Death, tracheostomy, or pulmonary vasodilator use at discharge, n/N (%) | 41/57 (72) | 13/16 (81) | 0.59 (0.15–2.4) | 41/48 (85) | 13/25 (52) | 5.4 (1.8–16.6) | 38/44 (86) | 16/29 (55) | 5.1 (1.7–15.9) |
| Individual components | |||||||||
| Death before NICU discharge, n/N (%) | 9/57 (16) | 1/16 (6) | 2.8 (0.33–24.0) | 10/48 (21) | 0/25 (0) | Not estimated* | 5/44 (11) | 5/29 (17) | 0.62 (0.16–2.3) |
| Tracheostomy, n/N (%) | 33/57 (58) | 9/16 (56) | 1.1 (0.35–3.3) | 30/48 (63) | 12/25 (48) | 1.8 (0.68–4.8) | 34/44 (77) | 8/29 (28) | 8.9 (3.0–26.2) |
| Pulmonary vasodilator at discharge, n/N (%)† | 17/48 (35) | 7/15 (47) | 0.63 (0.19–2.0) | 21/38 (55) | 3/25 (12) | 9.1 (2.3–35.5) | 16/39 (41) | 8/24 (33) | 1.4 (0.48–4.0) |
| Additional secondary outcomes | |||||||||
| Home oxygen therapy, n/N (%)† | 45/48 (94) | 13/15 (87) | 2.3 (0.35–15.3) | 36/38 (95) | 22/25 (88) | 2.5 (0.38–15.9) | 35/39 (90) | 23/24 (96) | 0.38 (0.04–3.6) |
| Daily diuretic use at discharge, n/N (%)† | 29/48 (60) | 11/15 (73) | 0.56 (0.15–2.0) | 23/38 (61) | 17/25 (68) | 0.72 (0.25–2.1) | 23/39 (59) | 17/24 (71) | 0.59 (0.20–1.8) |
| Daily inhaled medication use at discharge, n/N (%)† | 26/48 (54) | 11/15 (73) | 0.43 (0.12–1.5) | 23/38 (61) | 14/25 (56) | 1.2 (0.43–3.4) | 22/39 (56) | 15/24 (63) | 0.78 (0.27–2.2) |
Definition of abbreviations: CI = confidence interval; NICU = neonatal ICU; OR = odds ratio.
n indicates the number of infants with the outcome and N indicates the total number of infants in each group.
Comparison of mortality rates using Fisher’s exact test: 10/48 (21%) versus 0/25 (0%); P = 0.013.
Calculated for survivors to discharge.
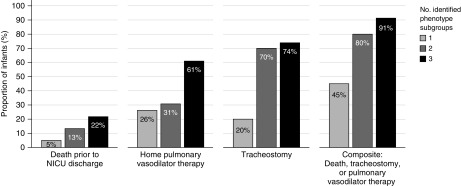
Rates of the primary composite outcome and the individual components increased with greater count of disease components (Figure 2). In total, 91% of infants with all three disease components developed the primary outcome compared with 45% of the infants with only one disease component (OR, 12.8; 95% CI, 1.4–17.2). Among the individual outcomes, an increasing number of phenotype subgroups was associated with greater risk of tracheostomy and pulmonary vasodilator use at discharge but not in-hospital mortality (Table 3).
Figure 2.
Rates of the primary composite and individual study outcomes based on the number of identified disease components. Rates of home pulmonary vasodilator therapy were calculated among 63 survivors; all other outcomes were calculated among 73 classifiable infants. Differences in outcome rates were statistically significant for the primary composite outcome (P = 0.002) and rates of tracheostomy (P < 0.001) by Fisher’s exact test. Rates were not significantly different for death (P = 0.33) or home pulmonary vasodilator therapy (P = 0.06). NICU = neonatal ICU.
Table 3.
Outcomes Stratified by the Number of Identified Disease Components
| Outcome | Count of Identified Disease Components |
|||||
|---|---|---|---|---|---|---|
| 1 |
2 |
3 |
||||
| n/N (%) | OR (95% CI) | n/N (%) | OR (95% CI) | n/N (%) | OR (95% CI) | |
| Death, tracheostomy, or pulmonary vasodilator use at discharge | 9/20 (45) | Ref. | 24/30 (80) | 4.9 (1.4–17.2) | 21/23 (91) | 12.8 (2.4–70.0) |
| Individual components | ||||||
| Death before NICU discharge | 1/20 (5) | Ref. | 4/30 (13) | 2.9 (0.30–28.3) | 5/23 (22) | 5.3 (0.56–49.7) |
| Tracheostomy | 4/20 (20) | Ref. | 21/30 (70) | 9.3 (2.4–35.8) | 17/23 (74) | 11.3 (2.7–47.7) |
| Pulmonary vasodilator at NICU discharge* | 5/19 (26) | Ref. | 8/26 (31) | 1.2 (0.33–4.7) | 11/18 (61) | 4.4 (1.1–17.7) |
| Additional secondary outcomes | ||||||
| Home oxygen therapy* | 17/19 (89) | Ref. | 24/26 (92) | 1.4 (0.18–11.0) | 17/18 (94) | 2.0 (0.17–24.2) |
| Daily diuretic use at discharge* | 15/19 (79) | Ref. | 15/26 (58) | 0.36 (0.09–1.4) | 10/18 (56) | 0.33 (0.08–1.4) |
| Daily inhaled medication use at discharge* | 13/19 (68) | Ref. | 14/26 (54) | 0.54 (0.16–1.9) | 10/18 (56) | 0.58 (0.15–2.2) |
Definition of abbreviations: CI = confidence interval; NICU = neonatal ICU; OR = odds ratio; Ref. = reference.
n indicates the number of infants with the outcome and N indicates the total number of infants in each group.
Calculated for survivors to discharge.
Discussion
Characterization of BPD severity according to the level of respiratory support administered to very preterm infants at 36 weeks PMA provides important prognostic information (29, 30). However, these standard diagnostic criteria do not distinguish between the possible causes of an infant’s respiratory support requirements. This study demonstrates that the predominant cardiorespiratory pathophysiology differs between very preterm infants diagnosed with severe BPD according to the 2001 NIH consensus definition (3). In this cohort, we evaluated the presence or absence of three disease components: moderate–severe parenchymal lung disease, PH, and malacia of the large airways. All possible combinations of one or more disease component were identified in the study population. The presence of all three disease components was the most common phenotype, occurring in approximately one-third of this referral-based cohort. Both the individual disease components and component count showed important associations with measures of adverse cardiorespiratory health at NICU discharge. The diagnosis of PH was strongly associated with increased risk for in-hospital mortality and prescription of home pulmonary vasodilator therapy. The diagnosis of large airway disease was associated with higher rates of tracheostomy. Finally, compared with infants with only one identified disease component, those with two and three disease components experienced a stepwise increase in the odds of the primary study outcome of death before NICU discharge, tracheostomy, or home pulmonary vasodilator therapy.
Previous studies have characterized various pathophysiologic disease components in BPD and suggested the presence of distinct phenotypes (31, 32). Reported rates of PH among preterm infants with severe BPD range from 15% to 53% (17, 33–37). The frequency of tracheomalacia among BPD patients undergoing bronchoscopy varies from 10% to 46% (9, 38–40). Although the incidence of parenchymal lung disease in BPD is uncertain, upwards of 85% of very preterm infants with BPD demonstrate structural abnormalities of the lung on CT imaging (41). Moreover, greater severity of BPD correlates with higher frequency and severity of radiographic abnormalities (42). Collectively, these reports provide important data on the variability in disease presentation in severe BPD. However, our data suggest that disease components in isolation may not fully convey the burden of the BPD. We focused on understanding the potential interaction of three predominant clinical components of BPD. In this tertiary referral cohort of infants with severe BPD, less than one-third were found to have only one predominant pathophysiology of moderate–severe parenchymal lung disease, PH, or large airway disease. Almost three-quarters were diagnosed with at least two of these disease components. These data suggest that the presence of a single predominant pathophysiology in infants with severe BPD may be true for only a minority of patients.
Both the number and type of identified disease components were associated with differences in the measured outcomes. Infants with tracheobronchomalacia underwent tracheostomy at nearly three times the rate of those without known large airway disease. Not surprisingly, PH was associated with a fourfold higher rate of pulmonary vasodilator use at hospital discharge. PH was also the only individual disease component associated with increased rates of in-hospital mortality. Several other studies have reported a similar association between PH and higher mortality risk in very preterm infants (34, 36, 37). In a longitudinal study of infants with BPD and PH, Khemani and colleagues showed a progressive decline in survival during extended follow-up (24). At 6 months after the diagnosis of PH, 64% of study infants were alive (24). By 3 years, only 52% survived (24).
Surprisingly, moderate–severe parenchymal disease was not associated with increased risk of the primary composite outcome, despite being the most common of the three evaluated lung abnormalities. It may be that parenchymal injury is more amenable to remodeling and recovery during the first year of life than the vascular lesions and large airway malacia. A larger sample size of infants with more heterogenous severity of parenchymal lung disease is needed to evaluate this hypothesis. Alternatively, a modified scoring system designed for infants with the most severe forms of BPD may help to further differentiate the degree of abnormal parenchymal lung findings in this population. The use of lung magnetic resonance imaging (MRI) may better describe the parenchymal disease and allow for further evaluation for association with the primary composite outcome (19). These speculations can only be resolved by further detailed descriptions of the radiographic abnormalities observed in severe BPD. In addition, we only examined the short-term outcome at hospital discharge. The advance in respiratory support technologies in recent years may have helped to provide enough support to decrease mortality or the need for tracheostomy. However, longer term data will be needed to examine the association of different disease phenotypes and long-term outcomes.
Increasing count of disease components was associated with an incremental increase in the risk of the primary composite outcome and the two individual outcomes: tracheostomy and pulmonary vasodilator use at hospital discharge. Mortality rates also increased in a stepwise manner with greater disease component count but these differences were not statistically significant. None of the individual disease components or component counts were associated with differences in the risks for home supplemental oxygen use, discharge on diuretic therapy, or discharge on an inhaled pulmonary medication.
Three infants could not be classified into any of the evaluated phenotypic subgroups. One was diagnosed with PH on a subsequent echocardiogram and treated with sildenafil at hospital discharge. This finding underscores that PH can evolve as a late diagnosis in infants with BPD (43). A single, reassuring echocardiogram performed near term-corrected gestation does not rule out later emergence of pulmonary vascular disease in very preterm infants with ongoing respiratory support needs (43). Another infant was diagnosed with subglottic stenosis, underwent tracheostomy, and was discharged without supplemental oxygen therapy. CTA revealed mild evidence of parenchymal lung disease in this infant. Isolated upper airway abnormalities are an uncommon indication for tracheostomy among preterm infants (26). More commonly, they are acquired following prolonged endotracheal intubation for severe lung disease (26). However, in select very preterm infants who meet diagnostic criteria for BPD, the prolonged need for mechanical ventilation may result from congenital abnormalities of the upper airway or airway injury following brief exposure to invasive respiratory support rather than chronic, severe respiratory insufficiency.
The primary limitation of this study is the retrospective design. We relied on diagnostic tests obtained for clinical purposes and excluded infants who did not undergo the necessary testing or were evaluated outside of the prespecified PMA range. This may favor inclusion of a cohort with higher disease acuity than is present in the full population of infants with severe BPD at our center. Moreover, our study represents infants who are cared for in a tertiary referral hospital. As such, our study results may be less generalizable to cohorts of very preterm infants receiving care at predominately inborn centers. Nonetheless, this study demonstrates one approach for exploring phenotypes in the severe BPD population. Future multicenter studies with larger sample sizes and prospective diagnostic testing performed at prespecified intervals will be essential to understand this multifaceted disease.
To increase the validity of our study results, we reevaluated all CTAs and echocardiograms using a standardized protocol and established disease definitions. The reviewers of all the imaging studies were masked to all other clinical characteristics of the study infants. To overcome initial interrater differences in the Ochiai scoring, consensus scores agreed upon by the two study radiologists were used to establish the presence or absence of moderate–severe parenchymal lung disease. However, our study depended on retrospective chart review to diagnose airway malacia by bronchoscopy and/or tracheoscopy because there were no video recordings of these studies. Approximately one-third of the study infants did not undergo tracheoscopy and/or bronchoscopy, which may further limit our ability to accurately assess this disease component. Nine of the 29 infants without large airway disease were assumed not to have airway disease given the absence of bronchoscopy and/or tracheoscopy in addition to inability to evaluate for malacia by CTA owing to lack of inspiratory or expiratory images. Lastly, data from other diagnostic procedures such as pulmonary function testing, microbiological analyses, assessments of pulmonary aspiration, measurement of lung inflammation, and MRI were not available but may enable further disease phenotyping and correlation with clinical outcomes (19, 44–48). Evaluation of lung parenchyma by MRI in particular is an emerging technique that is advantageous due to lack of ionizing radiation, and has been shown to correlate with BPD disease severity and short-term clinical outcomes (19, 48). In addition, neonatal cardiac MRI provides detailed analysis of cardiac morphology that may help inform the diagnosis and treatment of PH in BPD (14).
Conclusions
Infants with severe BPD demonstrate variability in the predominant clinical pathophysiology. Our results indicate that very preterm infants with severe BPD can be classified into phenotypic subgroups based on the presence of parenchymal, vascular, and large airway disease. The most common phenotype observed in this referral cohort was the cooccurrence of all three disease components. Both the total number and type of disease component were associated with important differences in resource use and in-hospital morbidity. Together, these results indicate frequent overlap between disease pathologies in severe BPD and suggest that different phenotypes likely carry distinct prognostic implications.
Supplementary Material
Footnotes
E.A.J. received grant support from the NHLBI (K23HL136843), and L.M.-R. received grant support from the NHLBI (K01HL125521) and from the Pulmonary Hypertension Association Supplement (K01HL125521).
Author Contributions: K.Y.W. participated in the conceptualization of and designed the study, collaborated with the investigators to collect the data, performed and reviewed the statistical analyses, and drafted the initial and final manuscripts. E.A.J. participated in the conceptualization of and designed the study, performed the statistical analyses, provided interpretation of data, critically reviewed and revised the manuscript for important intellectual content, and approved the final manuscript as submitted. H.Z. and H.K. conceptualized and designed the study, provided interpretation of data, critically reviewed and revised the manuscript for important intellectual content, and approved the final manuscript as submitted. A.M.W., Y.W., D.M.B., K.N., M.V.F., and L.M.-R. participated in the design of the study, aided with data collection and review of study imaging, revised the article for important intellectual content, and approved the final manuscript as submitted.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201907-1342OC on January 29, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Maitre NL, Ballard RA, Ellenberg JH, Davis SD, Greenberg JM, Hamvas A, et al. Prematurity and Respiratory Outcomes Program. Respiratory consequences of prematurity: evolution of a diagnosis and development of a comprehensive approach. J Perinatol. 2015;35:313–321. doi: 10.1038/jp.2015.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stoll BJ, Hansen NI, Bell EF, Walsh MC, Carlo WA, Shankaran S, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993-2012. JAMA. 2015;314:1039–1051. doi: 10.1001/jama.2015.10244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163:1723–1729. doi: 10.1164/ajrccm.163.7.2011060. [DOI] [PubMed] [Google Scholar]
- 4.Jobe AH. Mechanisms of lung injury and bronchopulmonary dysplasia. Am J Perinatol. 2016;33:1076–1078. doi: 10.1055/s-0036-1586107. [DOI] [PubMed] [Google Scholar]
- 5.Baraldi E, Filippone M. Chronic lung disease after premature birth. N Engl J Med. 2007;357:1946–1955. doi: 10.1056/NEJMra067279. [DOI] [PubMed] [Google Scholar]
- 6.Amin RS, Rutter MJ. Airway disease and management in bronchopulmonary dysplasia. Clin Perinatol. 2015;42:857–870. doi: 10.1016/j.clp.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 7.Penn RB, Wolfson MR, Shaffer TH. Effect of ventilation on mechanical properties and pressure-flow relationships of immature airways. Pediatr Res. 1988;23:519–524. doi: 10.1203/00006450-198805000-00017. [DOI] [PubMed] [Google Scholar]
- 8.Bhutani VK, Rubenstein D, Shaffer TH. Pressure-induced deformation in immature airways. Pediatr Res. 1981;15:829–832. [PubMed] [Google Scholar]
- 9.McCubbin M, Frey EE, Wagener JS, Tribby R, Smith WL. Large airway collapse in bronchopulmonary dysplasia. J Pediatr. 1989;114:304–307. doi: 10.1016/s0022-3476(89)80802-9. [DOI] [PubMed] [Google Scholar]
- 10.Coalson JJ. Pathology of bronchopulmonary dysplasia. Semin Perinatol. 2006;30:179–184. doi: 10.1053/j.semperi.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 11.Husain AN, Siddiqui NH, Stocker JT. Pathology of arrested acinar development in postsurfactant bronchopulmonary dysplasia. Hum Pathol. 1998;29:710–717. doi: 10.1016/s0046-8177(98)90280-5. [DOI] [PubMed] [Google Scholar]
- 12.Stenmark KR, Abman SH. Lung vascular development: implications for the pathogenesis of bronchopulmonary dysplasia. Annu Rev Physiol. 2005;67:623–661. doi: 10.1146/annurev.physiol.67.040403.102229. [DOI] [PubMed] [Google Scholar]
- 13.Mourani PM, Ivy DD, Gao D, Abman SH. Pulmonary vascular effects of inhaled nitric oxide and oxygen tension in bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2004;170:1006–1013. doi: 10.1164/rccm.200310-1483OC. [DOI] [PubMed] [Google Scholar]
- 14.Critser PJ, Higano NS, Tkach JA, Olson ES, Spielberg DR, Kingma PS, et al. Cardiac magnetic resonance imaging evaluation of neonatal bronchopulmonary dysplasia–associated pulmonary hypertension. Am J Respir Crit Care Med. 2020;201:73–82. doi: 10.1164/rccm.201904-0826OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hysinger EB, Friedman NL, Padula MA, Shinohara RT, Zhang H, Panitch HB, et al. Children’s Hospitals Neonatal Consortium Tracheobronchomalacia is associated with increased morbidity in bronchopulmonary dysplasia Ann Am Thorac Soc 2017141428–1435.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ibrahim J, Bhandari V. The definition of bronchopulmonary dysplasia: an evolving dilemma. Pediatr Res. 2018;84:586–588. doi: 10.1038/s41390-018-0167-9. [DOI] [PubMed] [Google Scholar]
- 17.Lagatta JM, Hysinger EB, Zaniletti I, Wymore EM, Vyas-Read S, Yallapragada S, et al. Children’s Hospital Neonatal Consortium Severe BPD Focus Group. The impact of pulmonary hypertension in preterm infants with severe bronchopulmonary dysplasia through 1 year. J Pediatr. 2018;203:218–224, e3. doi: 10.1016/j.jpeds.2018.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Logan JW, Lynch SK, Curtiss J, Shepherd EG. Clinical phenotypes and management concepts for severe, established bronchopulmonary dysplasia. Paediatr Respir Rev. 2019;31:58–63. doi: 10.1016/j.prrv.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 19.Higano NS, Spielberg DR, Fleck RJ, Schapiro AH, Walkup LL, Hahn AD, et al. Neonatal pulmonary magnetic resonance imaging of bronchopulmonary dysplasia predicts short-term clinical outcomes. Am J Respir Crit Care Med. 2018;198:1302–1311. doi: 10.1164/rccm.201711-2287OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu K, Jensen E, White A, Wang Y, Biko D, Zhang H, et al. Phenotypes of severe bronchopulmonary dysplasia. Presented at the Pediatric Academic Societies Meeting; May 5–8, 2018, Toronto, ON, Canada. Abstract 2901.847. [Google Scholar]
- 21.Ochiai M, Hikino S, Yabuuchi H, Nakayama H, Sato K, Ohga S, et al. A new scoring system for computed tomography of the chest for assessing the clinical status of bronchopulmonary dysplasia. J Pediatr. 2008;152:90–95, e3. doi: 10.1016/j.jpeds.2007.05.043. [DOI] [PubMed] [Google Scholar]
- 22.Parasuraman S, Walker S, Loudon BL, Gollop ND, Wilson AM, Lowery C, et al. Assessment of pulmonary artery pressure by echocardiography-A comprehensive review. Int J Cardiol Heart Vasc. 2016;12:45–51. doi: 10.1016/j.ijcha.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abman SH, Hansmann G, Archer SL, Ivy DD, Adatia I, Chung WK, et al. American Heart Association Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation; Council on Clinical Cardiology; Council on Cardiovascular Disease in the Young; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Surgery and Anesthesia; and the American Thoracic Society Pediatric pulmonary hypertension: guidelines from the American Heart Association and American Thoracic Society Circulation 20151322037–2099.[Published erratum appears in Circulation 133:e368.] [DOI] [PubMed] [Google Scholar]
- 24.Khemani E, McElhinney DB, Rhein L, Andrade O, Lacro RV, Thomas KC, et al. Pulmonary artery hypertension in formerly premature infants with bronchopulmonary dysplasia: clinical features and outcomes in the surfactant era. Pediatrics. 2007;120:1260–1269. doi: 10.1542/peds.2007-0971. [DOI] [PubMed] [Google Scholar]
- 25.Mourani PM, Abman SH. Pulmonary vascular disease in bronchopulmonary dysplasia: pulmonary hypertension and beyond. Curr Opin Pediatr. 2013;25:329–337. doi: 10.1097/MOP.0b013e328360a3f6. [DOI] [PubMed] [Google Scholar]
- 26.DeMauro SB, Wei JL, Lin RJ. Perspectives on neonatal and infant tracheostomy. Semin Fetal Neonatal Med. 2016;21:285–291. doi: 10.1016/j.siny.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 27.Álvarez-Fuente M, Arruza L, Muro M, Zozaya C, Avila A, López-Ortego P, et al. The economic impact of prematurity and bronchopulmonary dysplasia. Eur J Pediatr. 2017;176:1587–1593. doi: 10.1007/s00431-017-3009-6. [DOI] [PubMed] [Google Scholar]
- 28.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 29.Jensen EA, Dysart K, Gantz MG, McDonald S, Bamat NA, Keszler M, et al. The diagnosis of bronchopulmonary dysplasia in very preterm infants: an evidence-based approach. Am J Respir Crit Care Med. 2019;200:751–759. doi: 10.1164/rccm.201812-2348OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ehrenkranz RA, Walsh MC, Vohr BR, Jobe AH, Wright LL, Fanaroff AA, et al. National Institutes of Child Health and Human Development Neonatal Research Network. Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics. 2005;116:1353–1360. doi: 10.1542/peds.2005-0249. [DOI] [PubMed] [Google Scholar]
- 31.Collaco JM, McGrath-Morrow SA. Respiratory phenotypes for preterm infants, children, and adults: bronchopulmonary dysplasia and more. Ann Am Thorac Soc. 2018;15:530–538. doi: 10.1513/AnnalsATS.201709-756FR. [DOI] [PubMed] [Google Scholar]
- 32.Day CL, Ryan RM. Bronchopulmonary dysplasia: new becomes old again! Pediatr Res. 2017;81:210–213. doi: 10.1038/pr.2016.201. [DOI] [PubMed] [Google Scholar]
- 33.Kim DH, Kim HS, Choi CW, Kim EK, Kim BI, Choi JH. Risk factors for pulmonary artery hypertension in preterm infants with moderate or severe bronchopulmonary dysplasia. Neonatology. 2012;101:40–46. doi: 10.1159/000327891. [DOI] [PubMed] [Google Scholar]
- 34.Bhat R, Salas AA, Foster C, Carlo WA, Ambalavanan N. Prospective analysis of pulmonary hypertension in extremely low birth weight infants. Pediatrics. 2012;129:e682–e689. doi: 10.1542/peds.2011-1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mirza H, Ziegler J, Ford S, Padbury J, Tucker R, Laptook A. Pulmonary hypertension in preterm infants: prevalence and association with bronchopulmonary dysplasia. J Pediatr. 2014;165:909–914, e1. doi: 10.1016/j.jpeds.2014.07.040. [DOI] [PubMed] [Google Scholar]
- 36.Mourani PM, Sontag MK, Younoszai A, Miller JI, Kinsella JP, Baker CD, et al. Early pulmonary vascular disease in preterm infants at risk for bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2015;191:87–95. doi: 10.1164/rccm.201409-1594OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weismann CG, Asnes JD, Bazzy-Asaad A, Tolomeo C, Ehrenkranz RA, Bizzarro MJ. Pulmonary hypertension in preterm infants: results of a prospective screening program. J Perinatol. 2017;37:572–577. doi: 10.1038/jp.2016.255. [DOI] [PubMed] [Google Scholar]
- 38.Downing GJKH, Kilbride HW. Evaluation of airway complications in high-risk preterm infants: application of flexible fiberoptic airway endoscopy. Pediatrics. 1995;95:567–572. [PubMed] [Google Scholar]
- 39.Miller RW, Woo P, Kellman RK, Slagle TS. Tracheobronchial abnormalities in infants with bronchopulmonary dysplasia. J Pediatr. 1987;111:779–782. doi: 10.1016/s0022-3476(87)80267-6. [DOI] [PubMed] [Google Scholar]
- 40.Cohn RC, Kercsmar C, Dearborn D. Safety and efficacy of flexible endoscopy in children with bronchopulmonary dysplasia. Am J Dis Child. 1988;142:1225–1228. doi: 10.1001/archpedi.1988.02150110103030. [DOI] [PubMed] [Google Scholar]
- 41.Semple T, Akhtar MR, Owens CM. Imaging bronchopulmonary dysplasia-a multimodality update. Front Med (Lausanne) 2017;4:88. doi: 10.3389/fmed.2017.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Mastrigt E, Logie K, Ciet P, Reiss IK, Duijts L, Pijnenburg MW, et al. Lung CT imaging in patients with bronchopulmonary dysplasia: a systematic review. Pediatr Pulmonol. 2016;51:975–986. doi: 10.1002/ppul.23446. [DOI] [PubMed] [Google Scholar]
- 43.Behere S, Alapati D, McCulloch MA. Screening echocardiography and brain natriuretic peptide levels predict late pulmonary hypertension in infants with bronchopulmonary dysplasia. Pediatr Cardiol. 2019;40:973–979. doi: 10.1007/s00246-019-02100-8. [DOI] [PubMed] [Google Scholar]
- 44.Shepherd EG, Clouse BJ, Hasenstab KA, Sitaram S, Malleske DT, Nelin LD, et al. Infant pulmonary function testing and phenotypes in severe bronchopulmonary dysplasia. Pediatrics. 2018;141:e20173350. doi: 10.1542/peds.2017-3350. [DOI] [PubMed] [Google Scholar]
- 45.Tramper J, Zhang H, Foglia EE, Dysart KC, Padula MA, Sullivan KV, et al. The association between positive tracheal aspirate cultures and adverse pulmonary outcomes in preterm infants with severe bronchopulmonary dysplasia. Am J Perinatol. 2017;34:96–104. doi: 10.1055/s-0036-1584541. [DOI] [PubMed] [Google Scholar]
- 46.Farhath S, He Z, Nakhla T, Saslow J, Soundar S, Camacho J, et al. Pepsin, a marker of gastric contents, is increased in tracheal aspirates from preterm infants who develop bronchopulmonary dysplasia. Pediatrics. 2008;121:e253–e259. doi: 10.1542/peds.2007-0056. [DOI] [PubMed] [Google Scholar]
- 47.Speer CP. Chorioamnionitis, postnatal factors and proinflammatory response in the pathogenetic sequence of bronchopulmonary dysplasia. Neonatology. 2009;95:353–361. doi: 10.1159/000209301. [DOI] [PubMed] [Google Scholar]
- 48.Yoder LM, Higano NS, Schapiro AH, Fleck RJ, Hysinger EB, Bates AJ, et al. Elevated lung volumes in neonates with bronchopulmonary dysplasia measured via MRI. Pediatr Pulmonol. 2019;54:1311–1318. doi: 10.1002/ppul.24378. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.