To the Editor:
An outbreak caused by a newly identified coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was first reported in Wuhan, China, in December 2019 (1) and has since spread across mainland China and to other countries. The clinical spectrum of coronavirus disease (COVID-19) ranges from asymptomatic to severe disease, and studies in China showed that 5.0% of patients had been admitted to the ICU (2, 3). Real-time RT-PCR assays are recommended for the diagnosis of SARS-CoV-2 infection (4). A previous study reported SARS-CoV-2 viral loads in upper-respiratory specimens from patients with COVID-19 (5). Here, we investigated the viral load in specimens from multiple sites and the duration of viral shedding in respiratory-tract samples from laboratory-confirmed critically ill patients with COVID-19 requiring ICU admission.
Methods
We conducted a retrospective, descriptive study that included 16 consecutive critically ill patients with COVID-19 who had been admitted to the ICU of the First Affiliated Hospital of Guangzhou Medical University. The study was approved by the Ethics Committee of the First Affiliated Hospital of Guangzhou Medical University. The requirement for informed consent was waived for the retrospective collection of data. A protocol was developed for sample collection when the first patient was admitted to the ICU, as follows: serial samples from the upper respiratory tract (throat and nasal swabs) and lower respiratory tract (sputum or endotracheal aspirate [ETA]) were collected daily during the first week after admission and every 2–3 days after the first week, until two sequential negative results were obtained or the patient was discharged from the ICU. Plasma, serum, conjunctival swabs, and urine samples were also collected in the first week after ICU admission. Fifteen patients tested negative in these samples, and in the remaining patient, sample collection was discontinued when two sequential negative results were obtained. Fecal samples were collected when available, and if unavailable, anal swabs were collected instead. Gastric fluid samples were collected only in patients with an indwelling gastric tube. Most sampling was done according to the designed protocol (see the online supplement). Swab samples were immediately placed into sterile tubes containing 3 ml of viral transport medium. The specimens were sent to the virology laboratory of our hospital for sample processing and viral RNA extraction. We used 0.25 ml of liquid samples (viral transport medium or directly from biological specimens) for RNA extraction. Viral RNA of SARS-CoV-2 was detected according to the recommendations of the Chinese Center for Disease Control and Prevention (6). Two target genes, ORF1ab (open reading frame 1ab) and N (nucleocapsid protein), were simultaneously amplified and tested using a real-time RT-PCR assay. The viral load was indicated as the cycle threshold (Ct) value of the N gene of SARS-CoV-2. Positive and negative controls were included in the assay according to the manufacturer’s protocol. A Ct value of <40 was defined as positive for SARS-CoV-2 RNA, and >40 was defined as negative. Samples with a Ct value between 37 and 40 were retested at least twice. The Ct values of all samples collected and tested are shown in the online supplement.
Results
A total of 16 patients (13 men and 3 women; median age, 59.5 yr; range, 26–79 yr) who were admitted to our ICU from January 26 through February 25, 2020, were included in this study. Twelve patients were imported cases who had recently returned from Hubei Province, and four had been exposed to patients with confirmed SARS-CoV-2 infection. Most patients (75%) had at least one preexisting chronic condition. All of the patients showed evidence of pneumonia in chest radiographs, and 15 patients were diagnosed with acute respiratory distress syndrome (ARDS) (eight with moderate ARDS and seven with severe ARDS) upon admission. Four patients (25%) were supported with noninvasive positive-pressure ventilation, and 12 (75%) were supported with invasive mechanical ventilation. Extracorporeal membrane oxygenation was applied in five patients (31%) (Table 1). As of March 20, nine patients had been discharged from the ICU and all 16 patients were alive.
Table 1.
Baseline and Clinical Characteristics, Main Interventions, and Detection of SARS-CoV-2 in Specimens from Patients with SARS-CoV-2 Infection Admitted to the ICU
| Variables | All Patients (N = 16) |
|---|---|
| Age, yr, median (range) | 59.5 (26–79) |
| Male sex, n (%) | 13 (81%) |
| Body mass index, kg/m2, median (Q1–Q3) | 24.1 (22.0–27.5) |
| Chronic conditions, n (%) | 12 (75%) |
| Diabetes | 6 (37%) |
| Chronic cardiac disease | 10 (63%) |
| Chronic pulmonary disease | 5 (31%) |
| Chronic neurologic disease | 2 (13%) |
| Any malignancy | 1 (6%) |
| Liver disease | 2 (13%) |
| Smoker (including ex-smoker), n (%) | 9 (56%) |
| Exposure, n (%) | |
| Exposure to Hubei | 12 (75%) |
| Exposure to confirmed patients | 4 (25%) |
| Days from onset of symptoms to ICU admission, median (Q1–Q3) | 12.0 (9.0–16.5) |
| Ratio of PaO2 to FiO2 on Day 1, mm Hg, mean ± SD | 120.7 ± 60.8 |
| APACHE II score on Day 1, mean ± SD | 16.4 ± 7.8 |
| SOFA score on Day 1, mean ± SD | 6.9 ± 3.8 |
| ARDS, n (%) | 15 (94%) |
| Mild ARDS | 0 |
| Moderate ARDS | 8 (50%) |
| Severe ARDS | 7 (44%) |
| Mechanical ventilation during ICU stay, n (%) | |
| Noninvasive | 4 (25%) |
| Invasive | 12 (75%) |
| Extracorporeal membrane oxygenation during ICU stay, n (%) | 5 (31%) |
| Positive for SARS-CoV-2 during ICU stay, n/patients tested | |
| Nasal swab | 13/16 |
| Throat swab | 10/16 |
| Sputum/ETA | 16/16 |
| Conjunctival swab | 1/15 |
| Blood | 1/16 |
| Urine | 1/16 |
| Gastric fluid | 6/13 |
| Feces | 11/16 |
| Anal swab | 4/15 |
Definition of abbreviations: APACHE II = Acute Physiology and Chronic Health Evaluation II; ARDS = acute respiratory distress syndrome; ETA = endotracheal aspirate; Q1 = quartile 1; Q3 = quartile 3; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2; SOFA = Sequential Organ Failure Assessment.
The median number of days from the onset of symptoms to ICU admission was 12.0 days (quartile 1–quartile 3: 9.0–16.5). During the ICU stay, nasal swab samples from 13 patients (81%) and throat swab samples from 10 patients (63%) tested positive for SARS-CoV-2, but lower-respiratory specimens (sputum or ETA) were positive in all 16 patients (100%). Viral RNA was also detected in urine (1 patient), conjunctival swab (1 of 15 patients; 1 patient refused to provide a conjunctival swab), and gastric fluid (6 of 13 patients). SARS-CoV-2 viral RNA was also detected in fecal samples from 11 patients (69%) and anal swabs (4 patients). In one patient, viral RNA was present in all types of specimens taken, suggesting that infection in this patient may have been systemic (Table 1).
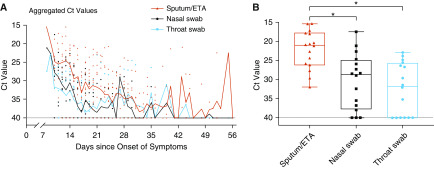
We analyzed the viral load and duration of virus shedding in nasal and throat swabs and lower-respiratory specimens in relation to the day of symptom onset (Figure 1A). Surprisingly, 11 patients (69%) showed prolonged viral shedding in lower-respiratory specimens, beyond 28 days after the onset of symptoms. As of March 20, the longest observed period of viral shedding in lower-respiratory-tract specimens was 55 days (patient #4; online supplement). In addition, lower-respiratory-tract specimens (sputum or ETA) had significantly higher SARS-CoV-2 viral RNA levels (inversely related to the Ct value) than nasal and throat swab specimens (Figure 1B). Our results indicated that samples from the lower respiratory tract had the highest viral load but slowest resolution of viral shedding in comparison with throat and nasal swab samples.
Figure 1.
Viral load detected in respiratory specimens obtained from critically ill patients infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). (A) Aggregated cycle threshold (Ct) values of the nucleocapsid protein gene of SARS-CoV-2 in serial throat swabs, nasal swabs, and sputum/endotracheal aspirate (ETA) samples from 16 patients, according to days after symptom onset. (B) Box plot of the lowest Ct values in throat swabs, nasal swabs, and sputum/ETA samples during the entire ICU stay among patients with coronavirus disease (COVID-19). Box-and-whiskers plot features are as follows: the central line in the box is the median, the bottom line of the box is the first quartile, and the top line of the box is the third quartile. Bottom of whiskers: maximum Ct value; top of whiskers: minimum Ct value. Groups were compared using the Kruskal-Wallis test with Dunn’s multiple comparison test. *P < 0.01.
Discussion
It is generally believed that the lung is the major target organ of SARS-CoV-2; however, we detected viral RNA in numerous different clinical samples, including conjunctival swabs, blood samples, gastric juices, feces, anal swabs, and urine from critically ill patients. Wang and colleagues tested 1,070 specimens collected from 205 patients with COVID-19 and found that the virus could be detected in different types of clinical specimens, including respiratory-tract samples, feces, and blood (7). However, the detection of viral RNA does not always equate with the presence of infectious virus, and viral RNA shedding of SARS-CoV-2 does not equate with infectivity. Our colleagues (Sun and colleagues, manuscript under review) had previously succeeded in isolating infectious virus from a urine sample from one of our patients. This suggests that the SARS-CoV-2 virus can replicate in extrapulmonary sites, as has been observed in some patients with severe viral pneumonia, such as that caused by the highly pathogenic avian influenza (8). However, the role of SARS-CoV-2 transmission via extrarespiratory routes (e.g., fecal–oral transmission) in the spread of COVID-19 must be further investigated. Our findings are in accord with reports showing that ACE2 (angiotensin converting enzyme II), the putative cell entry receptor of SARS-CoV-2, is widely expressed in a variety of epithelial cells in multiple organs (9). It is still unclear whether replication of SARS-CoV-2 in extrapulmonary organs contributes to organ injury and dysfunction, considering that secondary organ injury owing to hypoxia, tissue hypoperfusion, and inflammation is common in critically ill patients.
Zou and colleagues found that SARS-CoV-2 viral RNA could be weakly detected in nasal and throat swabs after 14 days from symptom onset (5). Pan and colleagues reported viral loads from different types of clinical specimens collected from 82 infected individuals within a maximum of 15 days after symptom onset (10). Zhou and colleagues found that the median duration of viral shedding in throat swabs was 20.0 days in COVID-19 survivors (11). Here, we found that SARS-CoV-2 viral RNA could be detected in sputum or ETA beyond 28 days from symptom onset in 11 patients (69%), as well as in extrapulmonary samples from these critically ill patients. These findings have important implications for assessing transmission risk and protecting ICU staff, and highlight the importance of effective antiviral treatment for critically ill patients with COVID-19.
This study is limited by the small number of critically ill patients and the lack of nonsurvivor data (there were no deaths in our ICU during the study period). In addition, we were not able to sample consistently according to the designed protocol, and sampling was discontinued after patients were discharged to the hospital’s isolation ward. Longitudinal studies in a larger cohort would enhance our understanding of viral load and shedding in patients with COVID-19.
In conclusion, critically ill patients infected with SARS-CoV-2 demonstrated higher viral loads and prolonged shedding in lower-respiratory-tract specimens than in upper-respiratory-tract specimens. Sampling from the lower respiratory tract may be required to assess the true viral clearance in such patients.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank Drs. Sook-San Wong and Mark Zanin for language assistance and insightful suggestions.
Footnotes
Funded by the National Science and Technology Major Project (2017ZX10204401), National Natural Science Foundation of China (81870069), the Special Project for Emergency of the Ministry of Science and Technology (2020YFC0841300), and the Special Project of Guangdong Science and Technology Department (2020B111105001).
This letter has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.202003-0572LE on April 15, 2020
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. China Novel Coronavirus Investigating and Research Team. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. China Medical Treatment Expert Group for COVID-19. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. [online ahead of print] 28 Feb 2020; DOI: 10.1056/NEJMoa2002032. [Google Scholar]
- 3.Bai Y, Yao L, Wei T, Tian F, Jin DY, Chen L, et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA. doi: 10.1001/jama.2020.2565. [online ahead of print] 21 Feb 2020; DOI: 10.1001/jama.2020.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. Laboratory testing for 2019 novel coronavirus (2019-nCoV) in suspected human cases [accessed 2020 Mar 8]. Available from: https://www.who.int/publications-detail/laboratory-testing-for-2019-novel-coronavirus-in-suspected-human-cases-20200117.
- 5.Zou L, Ruan F, Huang M, Liang L, Huang H, Hong Z, et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382:1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chinese Center for Disease Control and Prevention. Laboratory guideline for novel coronavirus pneumonia [accessed 2020 Mar 8]. Available from: http://www.chinacdc.cn/jkzt/crb/zl/szkb_11803/jszl_11815/202001/t20200123_211378.html.
- 7.Wang W, Xu Y, Gao R, Lu R, Han K, Wu G, et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. doi: 10.1001/jama.2020.3786. [online ahead of print] 11 Mar 2020; DOI: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uiprasertkul M, Puthavathana P, Sangsiriwut K, Pooruk P, Srisook K, Peiris M, et al. Influenza A H5N1 replication sites in humans. Emerg Infect Dis. 2005;11:1036–1041. doi: 10.3201/eid1107.041313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zou X, Chen K, Zou J, Han P, Hao J, Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. doi: 10.1007/s11684-020-0754-0. [online ahead of print] 12 Mar 2020; DOI: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pan Y, Zhang D, Yang P, Poon LLM, Wang Q. Viral load of SARS-CoV-2 in clinical samples. Lancet Infect Dis. 2020;20:411–412. doi: 10.1016/S1473-3099(20)30113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.