Abstract
Aims
To estimate the prevalence of established diabetes and its association with the clinical severity and in-hospital mortality associated with COVID-19.
Data synthesis
We systematically searched PubMed, Scopus and Web of Science, from 1st January 2020 to 15th May 2020, for observational studies of patients admitted to hospital with COVID-19. Meta-analysis was performed using random-effects modeling. A total of 83 eligible studies with 78,874 hospitalized patients with laboratory-confirmed COVID-19 were included. The pooled prevalence of established diabetes was 14.34% (95% CI 12.62–16.06%). However, the prevalence of diabetes was higher in non-Asian vs. Asian countries (23.34% [95% CI 16.40–30.28] vs. 11.06% [95% CI 9.73–12.39]), and in patients aged ≥60 years vs. those aged <60 years (23.30% [95% CI 19.65–26.94] vs. 8.79% [95% CI 7.56–10.02]). Pre-existing diabetes was associated with an approximate twofold higher risk of having severe/critical COVID-19 illness (n = 22 studies; random-effects odds ratio 2.10, 95% CI 1.71–2.57; I2 = 41.5%) and ~threefold increased risk of in-hospital mortality (n = 15 studies; random-effects odds ratio 2.68, 95% CI 2.09–3.44; I2 = 46.7%). Funnel plots and Egger's tests did not reveal any significant publication bias.
Conclusions
Pre-existing diabetes is significantly associated with greater risk of severe/critical illness and in-hospital mortality in patients admitted to hospital with COVID-19.
Keywords: Diabetes, COVID-19, Coronavirus disease 2019, SARS-CoV-2, Meta-analysis
Highlights
-
•
Little is known about the association of diabetes with the clinical severity and in-hospital mortality associated with COVID-19.
-
•
We meta-analyzed 83 observational studies for a total of 78,874 in-patients with COVID-19.
-
•
Diabetes was associated with a greater risk of severe/critical illness and in-hospital mortality associated with COVID-19.
Introduction
The outbreak of coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has been recently declared a pandemic by the World Health Organization, and the disease has spread to nearly all countries worldwide [1]. It is known that people with diabetes have a higher overall risk of infection(s) resulting from multiple perturbations of innate immunity [[2], [3], [4]]. Whether people with diabetes are also at greater susceptibility to COVID-19 is currently uncertain, but there is a perception that the risk is higher; both of infection, and of greater severity of illness [5,6].
We have therefore carried out an updated and comprehensive systematic review and meta-analysis of observational studies that have estimated the global prevalence of pre-existing diabetes in patients admitted to hospital with laboratory-confirmed SARS-CoV-2 infection. We also examined whether there is an association between presence of pre-existing diabetes and severity of COVID-19 illness or risk of in-hospital mortality amongst infected patients.
Materials and methods
Data sources and searches
We conducted a literature search from 1st January 2020 to 15th May 2020 (date last searched) of PubMed, Scopus and Web of Science databases for non-randomized observational studies examining the main clinical and biochemical characteristics of hospitalized patients with laboratory-confirmed COVID-19. We also searched preprint manuscripts available at https://www.medrxiv.org/collection/endocrinology-including-diabetes-mellitus-and-metabolic-disease. The search free text terms were “coronavirus disease 2019” (OR “COVID19” OR “COVID-19 disease” OR “SARS-CoV-2”). We also searched for MeSH (Medical Subject Headings) terms. Searches were restricted to human studies. Non-English-language articles were excluded. Additionally, we reviewed references from relevant original papers and review articles for identifying further eligible studies not covered by the original database searches.
We performed a systematic review in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (http://www.prisma-statement.org). Additionally, because the included studies were observational in design, we followed the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) guidelines for the meta-analysis of these studies [7].
Study selection
Original studies were included if they met the following inclusion criteria: (1) observational studies examining the clinical and biochemical characteristics of hospitalized patients with laboratory-confirmed COVID-19; and (2) all studies that reported data on presence of established diabetes among hospitalized patients with COVID-19. Study participants included in the meta-analysis were adult individuals (aged ≥18 years) of either sex without any restriction in terms of age, race, ethnicity or comorbidities.
Criteria for exclusion of selected studies from our meta-analysis were as follows: (1) congress abstracts, case reports, review articles, practice guidelines, commentaries or editorials; (2) studies in which information on presence of pre-existing diabetes was not specifically reported; (3) pre-print manuscripts that have yet to be reviewed; and (4) studies performed in pediatric population (aged <18 years).
Two investigators (AM and GT) independently examined all titles and abstracts, and obtained full texts of potentially relevant papers. Working independently and in duplicate the papers were read by both investigators (AM and GT), and whether they met inclusion criteria was then assessed. Discrepancies were resolved by consensus, referring back to the original article, in consultation with a third author.
Quality assessment of eligible studies was also performed by two investigators (AM and GT), using the Newcastle-Ottawa Quality Assessment Scale (NOS), which is a validated scale for non-randomized observational studies in meta-analyses [8]. A NOS scale adapted for cross-sectional studies was specifically used [9]. The NOS scale uses a star system to assess the quality of a study in three domains: selection, comparability and outcome/exposure. The NOS assigns a maximum of five stars for selection, two stars for comparability, and three stars for outcome/exposure. Studies achieving a score of at least eight stars were classified as being at low risk of bias (i.e., thus reflecting the highest quality).
Data extraction and quality assessment
For all eligible studies, we extracted information on study country, study size, patients’ characteristics, including demographics and percentage of individuals with established diabetes (i.e., defined as self-reported history of diabetes and/or use of any glucose-lowering medication), and other outcome measures of interest. In the case of multiple publications, we included the most up-to-date or comprehensive information.
Data synthesis and analysis
The primary outcome measures of the meta-analysis were the proportion of established diabetes amongst patients with COVID-19 at hospital admission, as well as the risk of patients with established diabetes of having severe/critical illness or increased in-hospital mortality associated with COVID-10. The severity of COVID-19 illness was assessed during hospitalization and classified as non-severe and severe/critical [10].
The pooled prevalence of established diabetes and the odds of having severe/critical COVID-19 illness or in-hospital mortality were considered as the effect size for all eligible studies, and an overall estimate of effect size was calculated using a random-effects model, as this methodology takes into account any differences between studies even if there is no statistically significant heterogeneity [8,11]. The 95% confidence intervals for the eligible studies that were used for estimating the pooled prevalence of established diabetes amongst hospitalized patients with COVID-19 were computed by the Wilson's score method [12].
Visual inspection of the forest plots was used to examine the possibility of statistical heterogeneity. The statistical heterogeneity among studies was assessed by the I 2 -statistics, which provides an estimate of percentage of variability across studies that is due to heterogeneity rather than chance alone. According to Higgins and Thompson [13], a rough guide to interpretation is as follows: I 2 values of approximately 25% represent low heterogeneity; approximately 50% represent medium heterogeneity; and approximately 75% represent high heterogeneity.
The possibility of publication bias was evaluated using the funnel plot and the Egger's regression asymmetry test [14].
To examine the possible sources of (expected) high heterogeneity among the pooled studies and to test the robustness of the associations, we conducted some subgroup analyses. In particular, based on the data from eligible studies, the pooled prevalence of established diabetes was assessed stratifying the studies according to study country (Asian vs. non-Asian countries), age (<60 vs. ≥60 years), COVID-19 severity of illness (non-severe vs. severe/critical), or discharge vital status (dead or alive). Additionally, we tested for possible excessive influence of individual studies using a meta-analysis influence test that eliminated each of the included studies at a time. We also performed univariable meta-regression analyses in order to examine the effect of age and sex on the association between established diabetes and risk of both COVID-19 severity and in-hospital mortality in the eligible studies.
P-values for chi-square tests are reported in all forest plots. A chi-square test p-value <0.10 was used to determine statistical significance considered for heterogeneity. The proportion of heterogeneity accounted by between-study variability was also estimated using the I 2-statistics and adjudicated to be significant if I 2 value was >50%. We used STATA® 14.2 (StataCorp, College Station, Texas) for all statistical analyses. Specifically, the STATA metaprop command was used for statistical analyses.
Results
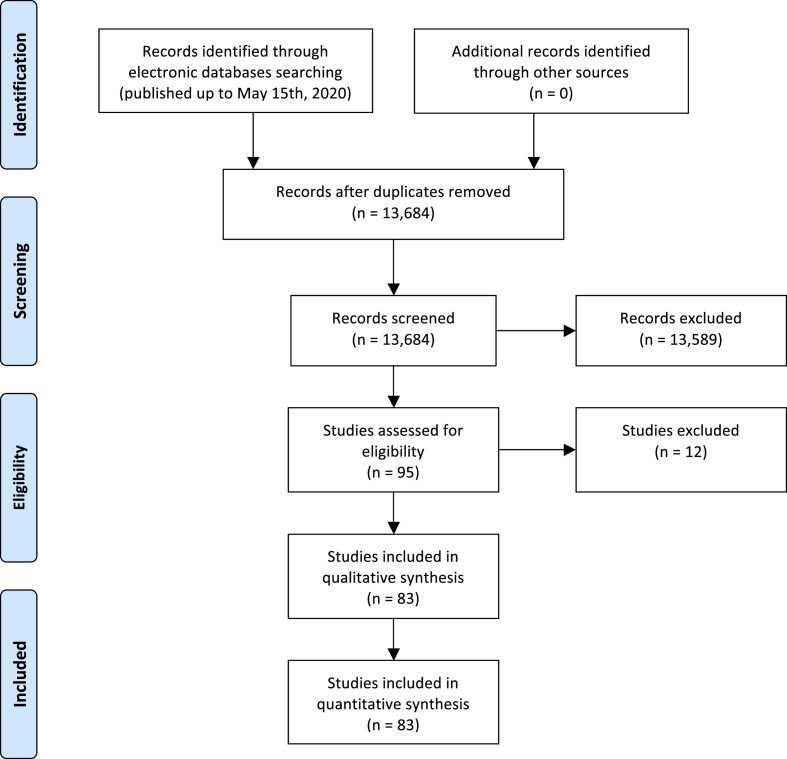
Fig. 1 summarizes the PRISMA flow diagram of the literature search and study selection. After excluding duplicates, based on titles and abstracts of 13,684 citations (in accordance with the aforementioned exclusion criteria of the meta-analysis), we initially identified 95 potentially eligible studies from PubMed, Web of Science and Scopus databases that were published until 15th May 2020 (last date searched) [[15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75], [76], [77], [78], [79], [80], [81], [82], [83], [84], [85], [86], [87], [88], [89], [90], [91], [92], [93], [94], [95], [96], [97], [98], [99], [100], [101], [102], [103], [104], [105], [106], [107], [108], [109]]. After examining the full text of these 95 articles, we further excluded 12 studies, because of unsatisfactory inclusion criteria [15] or being a pre-print manuscript that has yet to be reviewed [[16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26]], as specified in the PRISMA flow diagram.
Figure 1.
The PRISMA flow diagram of the meta-analysis.
In total, 83 observational studies were eligible for inclusion in our meta-analysis and were assessed for quality [[27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75], [76], [77], [78], [79], [80], [81], [82], [83], [84], [85], [86], [87], [88], [89], [90], [91], [92], [93], [94], [95], [96], [97], [98], [99], [100], [101], [102], [103], [104], [105], [106], [107], [108], [109]]. The main characteristics of these studies are summarized in Supplementary Table 1. Overall, in the 83 studies included in the meta-analysis there were 78,874 confirmed COVID-19 cases (52.1% men; median age 54 years [inter-quartile range: 49–62 years]). Sixty-two studies were conducted in Asian countries, mostly in China (involving a total of 65,946 COVID-19 patients with a median age of 52 years), and 21 studies were conducted in the Europe (Italy, France and United Kingdom), Australia and United States (involving a total of 12,928 COVID-19 patients with a median age of 63 years). In eligible studies, the diagnosis of diabetes was mainly based on the self-reported history of disease and/or use of glucose-lowering medications. Data on severity of COVID-19 illness at hospital admission were available for 22 eligible studies performed in China, France and United States (involving a total of 14,017 patients: 11,831 with non-severe COVID-19 and 2186 with severe/critical COVID-19). Data on total in-hospital deaths for the meta-analysis were available in 15 eligible studies, most of which were performed in China (involving a total of 56,057 COVID-19 patients with 1832 in-hospital deaths). As also shown in Supplementary Table 1, all the eligible studies received five or six stars on the NOS indicating that those studies had a high risk of bias.
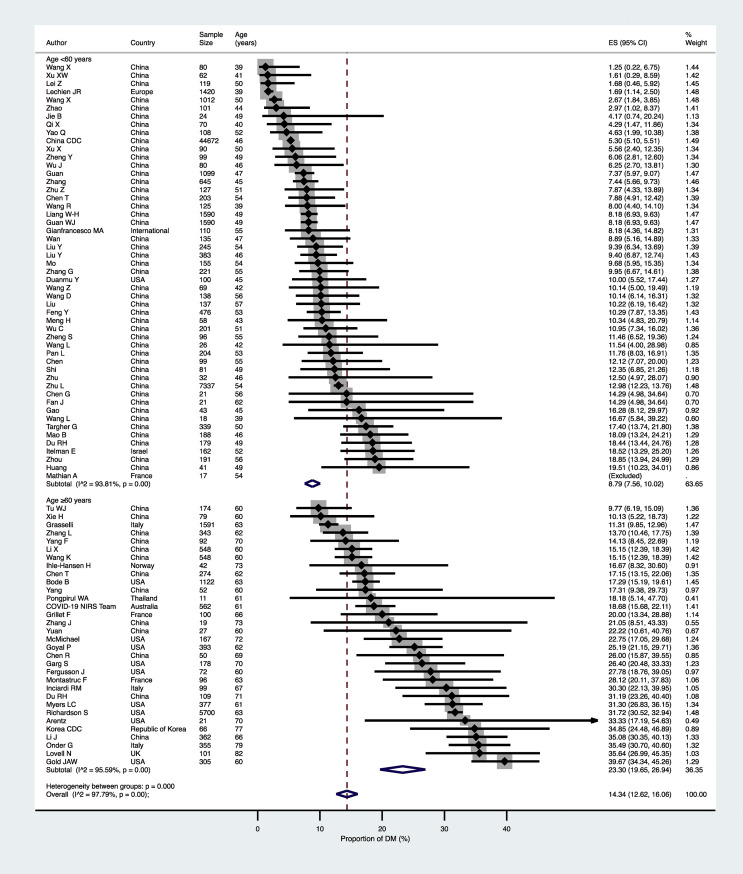
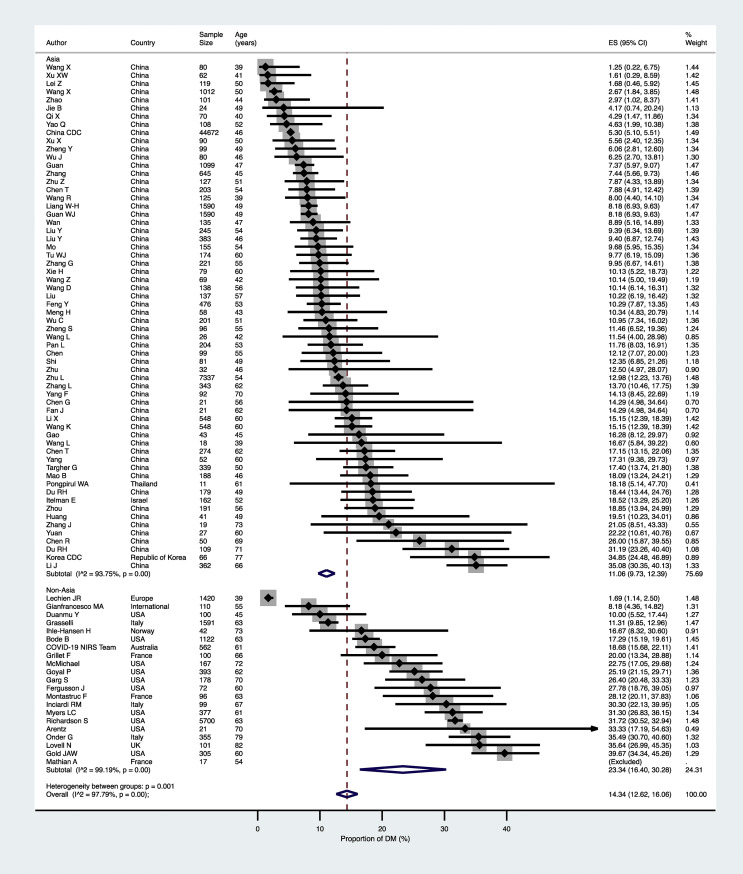
As shown in Fig. 2 , the pooled prevalence of established diabetes in the overall population of confirmed COVID-19 cases (n = 83 studies included) was 14.34% (95% confidence intervals [CI] 12.62–16.06%). The high heterogeneity observed in the overall primary analysis of these studies (I 2 = 97.8%) likely reflects differences in the characteristics of study populations (mostly age and country). Indeed, the pooled prevalence of pre-existing diabetes was remarkably greater amongst COVID-19 patients aged ≥60 years than amongst those aged <60 years (23.30% [95%CI 19.65–26.94] vs. 8.79% [95%CI 7.56–10.02]; p < 0.0001 – Fig. 2). Furthermore, the pooled prevalence of diabetes was also significantly greater in non-Asian countries than in Asian countries (23.34% [95%CI 16.40–30.28] vs. 11.06% [95%CI 9.73–12.39]; p = 0.001 - Fig. 3 ), possibly reflecting the marked differences in median age values of the study populations between the two countries.
Figure 2.
Forest plot and pooled prevalence of established diabetes among patients with laboratory-confirmed COVID-19, stratified by age (n = 83 studies included).
Figure 3.
Forest plot and pooled prevalence of established diabetes among patients with laboratory-confirmed COVID-19, stratified by study country (n = 83 studies included).
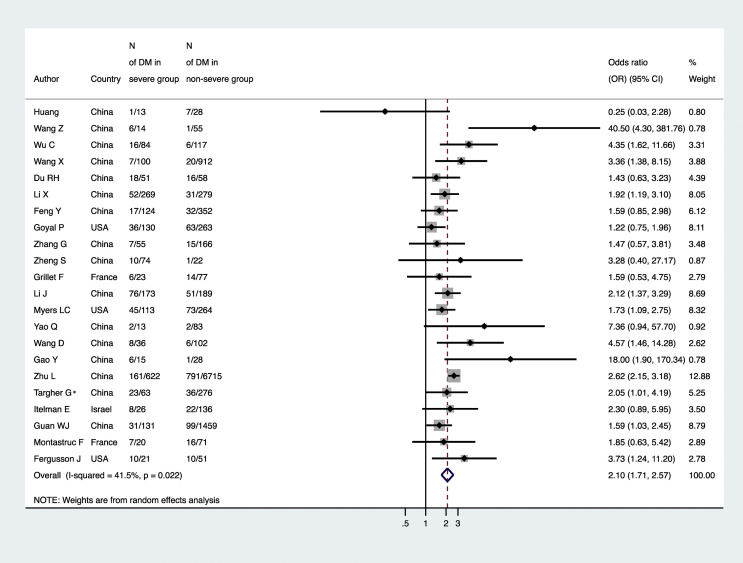
The distribution of studies by estimate of the association between diabetes and risk of having severe/critical COVID-19 illness at hospital admission is plotted in Fig. 4 . Patients with established diabetes had an approximate twofold greater risk of severe/critical COVID-19 illness compared to their counterparts without diabetes (n = 22 studies included; random-effects odds ratio 2.10, 95%CI 1.71–2.57; I 2 = 41.5%).
Figure 4.
Forest plot and pooled risk of having severe/critical COVID-19 among patients with and without established diabetes (n = 22 studies included). Note: ∗in the study of Targher et al. [96] the odds ratio for severe/critical COVID-19 was adjusted for age, sex, smoking history, obesity and hypertension.
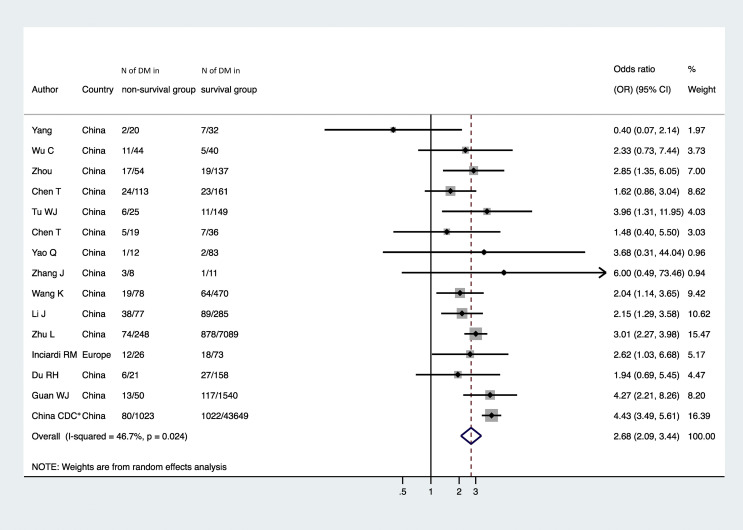
Fig. 5 summarized the distribution of studies by estimate of the association between diabetes and risk of in-hospital mortality associated with COVID-19. Pre-existing diabetes was significantly associated with a ~three-fold greater risk of in-hospital mortality associated with COVID-19 (n = 15 studies included; random-effects odds ratio 2.68, 95%CI 2.09–3.44; I 2 = 46.7%).
Figure 5.
Forest plot and pooled risk of COVID-19-related in-hospital mortality among patients with and without established diabetes (n = 15 studies included). Note: ∗in a subsequent study conducted on the same database (Crit Care. 2020 Apr 28; 24:179), Deng G et al. reported that the fatality rate of COVID-19 patients with diabetes was higher than that of patients without diabetes.
We also tested for the possibility of excessive influence of individual studies using an influence test that eliminated each of the included studies one at a time. Eliminating each of the eligible studies from the aforementioned analyses had no significant effect on the diabetes-related risk on both COVID-19 severity and in-hospital mortality (data not shown).
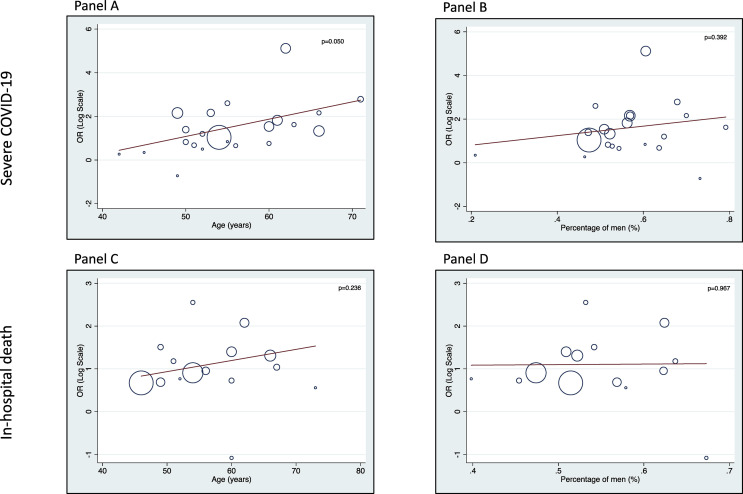
Fig. 6 shows the results of univariable meta-regression analyses showing the effect of age and sex on the association between pre-existing diabetes and risk of severity of illness and in-hospital mortality associated with COVID-19. This analysis supports an adverse effect of pre-existing diabetes on these two clinical outcomes, irrespective of sex. There was a clearer effect of increasing age (p = 0.05) on the association between pre-existing diabetes and severity of COVID-19. Conversely, age did not appear to exert any significant effect on the association between pre-existing diabetes and risk of in-hospital mortality.
Figure 6.
Univariable linear meta-regression analyses. A meta-analysis of the association of either age (panels A and C) or sex (panels B and D) with the diabetes-related risk of COVID-19 severity or in-hospital mortality.
Finally, as shown in Supplementary Fig. 1, the Egger's regression test did not show statistically significant asymmetry of the funnel plots (except for a borderline significance for the eligible studies with available data for in-hospital mortality analysis), thus suggesting that publication bias for the main clinical outcomes of interest (panels A to C) was unlikely.
Discussion
In this updated and comprehensive systematic review and meta-analysis of 83 non-randomized observational studies from Asia (mostly China), Europe and United States (involving a total of nearly 79,000 adult individuals), we found that the pooled prevalence of established diabetes at hospital admission was 14.34% (95%CI 12.62–16.06) in patients with laboratory-confirmed COVID-19. However, the prevalence of established diabetes was markedly higher in non-Asian vs. Asian countries (23.34% [95%CI 16.40–30.28] vs. 11.06% [95%CI 9.73–12.39]), as well as in patients aged ≥60 years than in those aged <60 years (23.30% [95%CI 19.65–26.94] vs. 8.79% [95%CI 7.56–10.02]). In addition and most importantly, our results show that COVID-19 patients with established diabetes had an approximate twofold higher risk of having severe/critical illness requiring Intensive Care Unit care (n = 22 studies; random-effects odds ratio 2.10, 95%CI 1.71–2.57; I 2 = 41.5%) and ~threefold increased risk of in-hospital mortality associated with COVID-19 (n = 15 studies; random-effects odds ratio 2.68, 95%CI 2.09–3.44; I 2 = 46.7%). Based on our meta-regression analyses, the association between established diabetes and risk of these two clinical outcomes (especially for in-hospital mortality) appeared to be independent of age and sex.
Our results corroborate and extend the recent findings of some smaller meta-analyses performed in Chinese patients with laboratory-confirmed COVID-19. In a meta-analysis of 12 studies including 2108 Chinese hospitalized patients with COVID-19, Fadini et al. reported that the pooled prevalence of established diabetes was 10%, and that patients with diabetes had a twofold higher risk of having severe COVID-19 (random-effects odds ratio 2.26, 95%CI 1.47–3.49) [110]. Similar results were also reported by Jang et al. in a meta-analysis of 7 studies that included a total of 1576 Chinese patients with COVID-19 [111], and by Huang et al. in a meta-analysis of 30 studies (most of which were preprint studies that have yet to be reviewed) involving 6450 Chinese patients with COVID-19 [112]. Lastly, in a meta-analysis of 43 studies (that also included pre-print manuscripts) involving 3600 Chinese patients, Fu et al. reported that the overall prevalence of pre-existing diabetes amongst patients with COVID-19 was 10.1% in the 26 studies where this information was available [113].
Overall, therefore, our findings corroborate on a much larger sample size and number of published studies (83 observational studies involving a total of 78,874 individuals) the results that have been previously reported by the aforementioned four meta-analyses in Chinese in-patients with laboratory-confirmed COVID-19, but extend these results also to patients hospitalized for COVID-19 in non-Asian countries, such as United States, Europe (Italy, France and United Kingdom) and Australia. Most importantly, our meta-analysis is the first to analyze the pooled effect of the association between pre-existing diabetes at admission and the risk of in-hospital mortality among patients with COVID-19.
To date, the pathophysiological and virologic mechanisms underpinning the strong association between pre-existing diabetes and risk of having severe/critical illness or increased in-hospital mortality with COVID-19 are poorly elucidated. It is reasonable to hypothesize that more severe COVID-19 illness in patients with established diabetes may be the consequence of underlying metabolic changes, chronic inflammation and/or attenuation of innate and adaptive immune responses (e.g., impaired phagocytosis by leukocytes, impaired neutrophil chemotaxis and bactericidal activity, and impaired innate cell-mediated immunity), thereby predisposing people with diabetes to infectious events of varying severity [2,3]. Additionally, patients with diabetes could also have an increased expression of the angiotensin-converting enzyme 2 (ACE-2), thereby facilitating viral uptake and increasing the risk of severe infection [114,115]. Finally, it is also possible to speculate that the altered microenvironment associated with diabetes might support the emergence of pathogenic SARS-CoV-2 variants capable of causing greater disease severity of COVID-19 illness.
Whilst our meta-analysis provides the most comprehensive assessment to date on the prevalence of pre-existing diabetes and its role as a risk factor for severe/critical COVID-19 illness and increased in-hospital mortality, some important limitations that are strictly inherent to the studies included in the meta-analysis should be mentioned. First, the observational design of the eligible studies does not allow for proving causality. Second, although we found a medium level of heterogeneity for the pooled primary analysis of studies examining the impact of pre-existing diabetes on severity of illness (I 2 = 41.5%) and in-hospital mortality (I 2 = 46.7%) associated with COVID-19, the overall quality of these studies was relatively low, suggesting a high risk of bias according to the Newcastle-Ottawa scale (e.g., only few of the eligible studies examining the impact of pre-existing diabetes on COVID-19 severity or in-hospital mortality have adjusted the results for age, sex, obesity and other comorbidities; so the possibility of residual confounding cannot be excluded). That said, the few eligible studies that adjusted the results for age, sex, obesity and other relevant comorbidities showed that pre-existing diabetes was independently associated with poorer in-hospital outcomes, and that diabetic patients with better controlled blood glucose had a less severe COVID-19 illness and lower mortality rate compared to those with poorly controlled blood glucose during hospitalization [95,96]. Third, the majority of patients (i.e., ~85% of total) included in the meta-analysis were of Asian ancestry (mostly Chinese population), and it was not possible to test for ethnic-specific differences in risk of COVID-19 severity and COVID-19 linked death, because of the limited number of studies in non-Asian individuals. Fourth, since the diagnosis of diabetes was not always consistent among the included studies, some inaccuracy in the estimated prevalence of diabetes and in the identification of diabetic sub-types may not be excluded, although the vast majority of diabetic cases were likely to be type 2. Fifth, none of the eligible studies did provide detailed information on hemoglobin A1c level or use of specific classes of glucose-lowering medications. Finally, although a selective reporting bias of eligible studies could be not definitely excluded, we believe that our comprehensive search has made it unlikely that any published reports were missed and visual inspection of funnel plots and formal tests demonstrated no statistical evidence of any publication bias. However, further studies, especially in European and American populations, are needed to confirm these findings, and future mechanistic studies are also required to better understand the link between diabetes and risk of severe disease and in-hospital mortality associated with COVID-19.
In conclusion, health care professionals caring for patients with COVID-19 need to be aware that pre-existing diabetes (in most cases type 2 diabetes mellitus) is significantly associated with a two to three times greater risk of severe/critical illness and in-hospital mortality associated with COVID-19. These findings highlight the urgent need of a multidisciplinary team-based approach to the management of this patient population.
Handling Editor: A. Siani
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.numecd.2020.05.014.
Contributor Information
Alessandro Mantovani, Email: alessandro.mantovani@univr.it.
Giovanni Targher, Email: giovanni.targher@univr.it.
Declaration of Competing Interest
None declared.
Sources of funding
MHZ is supported by grants from the National Natural Science Foundation of China (81500665). CDB is supported in part by the Southampton NIHR Biomedical Research Centre (IS-BRC-20004), UK. GT is supported in part by grants from the School of Medicine, University of Verona, Verona, Italy.
Authors contributions
Study concept and design: Alessandro Mantovani, Giovanni Targher.
Acquisition of data: Alessandro Mantovani, Giovanni Targher.
Analysis and interpretation of data: Alessandro Mantovani, Giovanni Targher.
Drafting of the manuscript: Giovanni Targher.
Critical revision of the manuscript for important intellectual contents: Christopher D. Byrne, Ming-Hua Zheng.
All authors contributed to the manuscript for important intellectual contents and approved the final submission.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplementary Fig. 1. Funnel plots of standard error by logit-transformed prevalence rate of established diabetes (panel A, n = 83 eligible studies); by log-odds ratio for risk of severe COVID-19 (panel B, n = 22 studies); and by log-odds ratio for risk of in-hospital mortality (panel C, n = 15 studies) among confirmed COVID-19 cases with and without established diabetes.
References
- 1.WHO characterizes COVID-19 as a pandemic. Accessed April 30, 2020; https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen.
- 2.Toniolo A., Cassani G., Puggioni A., Rossi A., Colombo A., Onodera T. The diabetes pandemic and associated infections: suggestions for clinical microbiology. Rev Med Microbiol. 2019;30:1–17. doi: 10.1097/MRM.0000000000000155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hulme K.D., Gallo L.A., Short K.R. Influenza virus and glycemic variability in diabetes: a killer combination? Front Microbiol. 2017;8:861. doi: 10.3389/fmicb.2017.00861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fuso L., Pitocco D., Antonelli-Incalzi R. Diabetic lung, an underrated complication from restrictive functional pattern to pulmonary hypertension. Diabetes Metabol Res Rev. 2019;35:e3159. doi: 10.1002/dmrr.3159. [DOI] [PubMed] [Google Scholar]
- 5.Fang L., Karakiulakis G., Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020 Mar 11;(20):30116–30118. doi: 10.1016/S2213-2600(20)30116-8. pii: S2213-2600. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muniyappa R., Gubbi S. COVID-19 pandemic, coronaviruses, and diabetes mellitus. Am J Physiol Endocrinol Metab. 2020;318(5):E736–E741. doi: 10.1152/ajpendo.00124.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stroup D.F., Berlin J.A., Morton S.C., Olkin I., Williamson G.D., Rennie D. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. J Am Med Assoc. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 8.Higgins J.P.T., Green S., editors. Cochrane handbook for systematic reviews of interventions, version 5.1.0. Cochrane Collaboration; 2011. https://handbook-5-1.cochrane.org/ [Google Scholar]
- 9.Modesti P.A., Reboldi G., Cappuccio F.P., Agyemang C., Remuzzi G., Rapi S. ESH Working Group on CV risk in low resource settings. Panethnic differences in blood pressure in Europe: a systematic review and meta-analysis. PloS One. 2016;11 doi: 10.1371/journal.pone.0147601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Medicine NHCSAoTC . WHO; 2020. Diagnosis and treatment protocol for novel coronavirus pneumonia (Trial Version 7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Higgins J.P., Thompson S.G., Spiegelhalter D.J. A re-evaluation of random-effects meta-analysis. J Roy Stat Soc Stat Soc. 2009;172(1):137-159. doi: 10.1111/j.1467-985X.2008.00552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Newcombe R.G. Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat Med. 1998;17:857–872. doi: 10.1002/(sici)1097-0258(19980430)17:8<857::aid-sim777>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 13.Higgins J.P., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 14.Egger M., Smith G.D., Phillips A.N. Meta-analysis: principles and procedures. BMJ. 1997;315:1533–1537. doi: 10.1136/bmj.315.7121.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu T., Chen C., Zhu Z., Cui M., Chen C., Dai H. Clinical features and dynamics of viral load in imported and non-imported patients with COVID-19. Int J Infect Dis. 2020 Mar 13;(20):30141–30147. doi: 10.1016/j.ijid.2020.03.022. pii: S1201-9712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qian GQ, Yang NB, Ding F, Ma AHY, Wang ZY, Shen YF, et al. Epidemiologic and clinical characteristics of 91 hospitalized patients with COVID-19 in Zhejiang, China: a retrospective, multi-centre case series. MedRxiv preprint. doi: 10.1101/2020.02.23.20026856. [DOI] [PMC free article] [PubMed]
- 17.Cao M., Zhang D., Wang Y., Lu Y., Zhu X., Li Y. Clinical features of patients infected with the 2019 novel coronavirus (COVID-19) in Shanghai, China. MedRxiv. 2020 doi: 10.1101/2020.03.04.20030395. preprint. 2020. [DOI] [Google Scholar]
- 18.Fu L., Fei J., Xiang H.-X., Xiang Y., Tan Z.-X., Li M.-D. Analysis of death risk factors among 200 COVID-19 patients in Wuhan, China: a hospital-based case-cohort study. SSRN Electron J. 2020;86 doi: 10.2139/ssrn.3551430. [DOI] [Google Scholar]
- 19.Li K., Chen D., Chen S., Feng Y., Chang C. Radiographic findings and other predictors in adults with Covid-19. MedRxiv preprint. 2020;2 doi: 10.1101/2020.03.23.20041673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Q., Ling Y., Zhang J., Li W., Zhang X., Jin Y. Clinical characteristics of SARS- CoV-2 infections involving 325 hospitalized patients outside Wuhan. Res Sq. 2020:1e15. doi: 10.21203/rs.3.rs-18699/v1. [DOI] [Google Scholar]
- 21.Liu J., Liu Y., Xiang P., Pu L., Xiong H., Li C. Neutrophil-to-Lymphocyte ratio predicts severe illness patients with 2019 novel coronavirus in the early stage. MedRxiv. 2020 doi: 10.1101/2020.02.10.20021584. preprint. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lei L., Jian-ya G., Hu W., Zhang X., Gua L., Liu C. Clinical characteristics of 51 patients discharged from hospital with COVID-19 in Chongqing, China. MedRxiv. 2020 preprint. [Google Scholar]
- 23.Liu J., Li S., Liu J., Liang B., Wang X., Wang H. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS- CoV-2 infected patients. MedRxiv preprint. 2020:2020. doi: 10.1101/2020.02.16.20023671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feng Z., Yu Q., Yao S., Luo L., Duan J., Yan Z. Early prediction of disease progression in 2019 novel coronavirus pneumonia patients outside Wuhan with CT and clinical characteristics. MedRxiv. 2020 preprint. [Google Scholar]
- 25.Wang Y., Zhou Y., Yang Z., Xia D., Geng S. Clinical characteristics of patients with severe pneumonia caused by the 2019 novel coronavirus in Wuhan, China. MedRxiv. 2020:1e15. doi: 10.1101/2020.03.02.20029306. preprint. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yuan B., Chen Y., Wang J., Wang C., Song S., Liu H.-Q. Epidemiological Characteristics of 417 patients infected with COVID-19 and 368 discharged cases among them in Shenzhen City, China. CURRENT STATUS: under review. Res Sq. 2020:1e14. doi: 10.21203/rs.3.rs-19554/v1. [DOI] [Google Scholar]
- 27.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020 Feb 15;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. Epub 2020 Jan 24. Erratum in: Lancet. 2020 Jan 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020 Feb 15;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. Epub 2020 Jan 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shi H., Han X., Jiang N., Cao Y., Alwalid O., Gu J. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20:425–434. doi: 10.1016/S1473-3099(20)30086-4. Epub 2020 Feb 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020 Feb 24 doi: 10.1016/S2213-2600(20)30079-5. Epub ahead of print. Erratum in: Lancet Respir Med. 2020 Apr;vol. 8(4):e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao W., Zhong Z., Xie X., Yu Q., Liu J. Relation between chest ct findings and clinical conditions of coronavirus disease (COVID-19) pneumonia: a multicenter study. AJR Am J Roentgenol. 2020;214:1072–1077. doi: 10.2214/AJR.20.22976. Epub 2020 Mar 3. [DOI] [PubMed] [Google Scholar]
- 32.Wu J., Liu J., Zhao X., Liu C., Wang W., Wang D. Clinical characteristics of imported cases of COVID-19 in Jiangsu province: a multicenter descriptive study. Clin Infect Dis. 2020 Feb 29:ciaa199. doi: 10.1093/cid/ciaa199. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mo P., Xing Y., Xiao Y., Deng L., Zhao Q., Wang H. Clinical characteristics of refractory COVID-19 pneumonia in Wuhan, China. Clin Infect Dis. 2020 Mar 16:ciaa270. doi: 10.1093/cid/ciaa270. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Z., Yang B., Li Q., Wen L., Zhang R. Clinical features of 69 cases with coronavirus disease 2019 in Wuhan, China. Clin Infect Dis. 2020 Mar 16:ciaa272. doi: 10.1093/cid/ciaa272. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu K., Fang Y.Y., Deng Y., Liu W., Wang M.F., Ma J.P. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J. 2020 Feb 7 doi: 10.1097/CM9.0000000000000744. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. J Am Med Assoc. 2020 Feb 7;323(11):1061–1069. doi: 10.1001/jama.2020.1585. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu W., Xie K., Lu H., Xu L., Zhou S., Fang S. Initial clinical features of suspected coronavirus disease 2019 in two emergency departments outside of Hubei, China. J Med Virol. 2020 Mar 13 doi: 10.1002/jmv.25763. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gao Y., Li T., Han M., Li X., Wu D., Xu Y. Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID-19. J Med Virol. 2020 Mar 17 doi: 10.1002/jmv.25770. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X. China medical treatment expert group for covid-19. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. Epub 2020 Feb 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020 Mar 13:e200994. doi: 10.1001/jamainternmed.2020.0994. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu X., Yu C., Qu J., Zhang L., Jiang S., Huang D. Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2. Eur J Nucl Med Mol Imag. 2020;47:1275–1280. doi: 10.1007/s00259-020-04735-9. Epub 2020 Feb 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wan S., Xiang Y., Fang W., Zheng Y., Li B., Hu Y. Clinical features and treatment of COVID-19 patients in northeast Chongqing. J Med Virol. 2020 Mar 21 doi: 10.1002/jmv.25783. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;vol. 395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. Epub 2020 Mar 11. Erratum in: Lancet. 2020 Mar 28;395(10229):1038. Erratum in: Lancet. 2020 Mar 28;395(10229):1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yuan M., Yin W., Tao Z., Tan W., Hu Y. Association of radiologic findings with mortality of patients infected with 2019 novel coronavirus in Wuhan, China. PloS One. 2020;15:e0230548. doi: 10.1371/journal.pone.0230548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arentz M., Yim E., Klaff L., Lokhandwala S., Riedo F.X., Chong M. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington state. J Am Med Assoc. 2020;323:1612–1614. doi: 10.1001/jama.2020.4326. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu X.W., Wu X.X., Jiang X.G., Xu K.J., Ying L.J., Ma C.L. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ. 2020 Feb 19;vol. 368:m606. doi: 10.1136/bmj.m606. Erratum in: BMJ. 2020 Feb 27;368:m792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang X., Cai H., Hu J., Lian J., Gu J., Zhang S. Epidemiological, clinical characteristics of cases of SARS-CoV-2 infection with abnormal imaging findings. Int J Infect Dis. 2020;94:81–87. doi: 10.1016/j.ijid.2020.03.040. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang L., Gao Y.H., Lou L.L., Zhang G.J. The clinical dynamics of 18 cases of COVID-19 outside of Wuhan, China. Eur Respir J. 2020 Apr 23;55(4):2000398. doi: 10.1183/13993003.00398-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McMichael T.M., Currie D.W., Clark S., Pogosjans S., Kay M., Schwartz N.G. Epidemiology of COVID-19 in a long-term care facility in King County, Washington. N Engl J Med. 2020 Mar 27 doi: 10.1056/NEJMoa2005412. NEJMoa2005412. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen T., Wu D., Chen H., Yan W., Yang D., Chen G. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020 Mar 26:368. doi: 10.1136/bmj.m1091. m1091. Erratum in: BMJ. 2020 Mar 31;vol. 368:m1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grasselli G., Zangrillo A., Zanella A., Antonelli M., Cabrini L., Castelli A., COVID-19 Lombardy ICU Network Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323(16):1574–1581. doi: 10.1001/jama.2020.5394. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang X., Fang J., Zhu Y., Chen L., Ding F., Zhou R. Clinical characteristics of non-critically ill patients with novel coronavirus infection (COVID-19) in a Fangcang Hospital. Clin Microbiol Infect. 2020 Apr 3;S1198–743X(20):30177–30184. doi: 10.1016/j.cmi.2020.03.032. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.The novel coronavirus pneumonia emergency response epidemiology team. China CDC Weekly. 2020;2:113–122. [PMC free article] [PubMed] [Google Scholar]
- 54.Du R.H., Liu L.M., Yin W., Wang W., Guan L.L., Yuan M.L. Hospitalization and critical care of 109 decedents with COVID-19 pneumonia in Wuhan, China. Ann Am Thorac Soc. 2020 Apr 7 doi: 10.1513/AnnalsATS.202003-225OC. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xie H., Zhao J., Lian N., Lin S., Xie Q., Zhuo H. Clinical characteristics of non-ICU hospitalized patients with coronavirus disease 2019 and liver injury: a retrospective study. Liver Int. 2020 Apr 2 doi: 10.1111/liv.14449. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.COVID-19 National Emergency Response Center Epidemiology and case management team, Korea centers for disease control and prevention. Coronavirus disease-19: the first 7,755 cases in the Republic of Korea. Osong Public Health Res Perspect. 2020 Apr;11(2):85–90. doi: 10.24171/j.phrp.2020.11.2.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tu W.J., Cao J., Yu L., Hu X., Liu Q. Clinico-laboratory study of 25 fatal cases of COVID-19 in Wuhan. Intensive Care Med. 2020 Apr 6:1–4. doi: 10.1007/s00134-020-06023-4. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pan L., Mu M., Yang P., Sun Y., Wang R., Yan J. Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China: a descriptive, cross-sectional, multicenter study. Am J Gastroenterol. 2020 Apr 14:115. doi: 10.14309/ajg.0000000000000620. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang R., Pan M., Zhang X., Fan X., Han M., Zhao F. Epidemiological and clinical features of 125 hospitalized patients with COVID-19 in Fuyang, Anhui, China. Int J Infect Dis. 2020 Apr 11;(20):S1201–S9712. doi: 10.1016/j.ijid.2020.03.070. 30203-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Garg S. Weekly/April 17, 2020/69 (15); 458-464 https://www.cdc.gov/mmwr/volumes/69/wr/mm6915e3.htm#suggestedcitation.
- 61.Liu Y., Sun W., Guo Y., Chen L., Zhang L., Zhao S. Association between platelet parameters and mortality in coronavirus disease 2019: retrospective cohort study. Platelets. 2020:1–7. doi: 10.1080/09537104.2020.1754383. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang F., Shi S., Zhu J., Shi J., Dai K., Chen X. Analysis of 92 deceased patients with COVID-19. J Med Virol. 2020 Apr 15 doi: 10.1002/jmv.25891. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen G., Wu D., Guo W., Cao Y., Huang D., Wang H. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020 Apr 13:137244. doi: 10.1172/JCI137244. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Meng H., Xiong R., He R., Lin W., Hao B., Zhang L. CT imaging and clinical course of asymptomatic cases with COVID-19 pneumonia at admission in Wuhan, China. J Infect. 2020 Apr 12;S0163–4453(20):30211–30215. doi: 10.1016/j.jinf.2020.04.004. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li X., Xu S., Yu M., Wang K., Tao Y., Zhou Y. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. 2020 Apr 12;S0091–6749(20):30495–30504. doi: 10.1016/j.jaci.2020.04.006. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen T., Dai Z., Mo P., Li X., Ma Z., Song S. Clinical characteristics and outcomes of older patients with coronavirus disease 2019 (COVID-19) in Wuhan, China (2019): a single-centered, retrospective study. J Gerontol Biol Sci Med Sci. 2020 Apr 11:glaa089. doi: 10.1093/gerona/glaa089. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu Y., Du X., Chen J., Jin Y., Peng L., Wang H.H.X. Neutrophil-to-lymphocyte ratio as an independent risk factor for mortality in hospitalized patients with COVID-19. J Infect. 2020 Apr 10;S0163–4453(20):30208–30215. doi: 10.1016/j.jinf.2020.04.002. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Feng Y., Ling Y., Bai T., Xie Y., Huang J., Li J. COVID-19 with different severity: a multi-center study of clinical features. Am J Respir Crit Care Med. 2020 Apr 10 doi: 10.1164/rccm.202002-0445OC. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lei Z., Cao H., Jie Y., Huang Z., Guo X., Chen J. A cross-sectional comparison of epidemiological and clinical features of patients with coronavirus disease (COVID-19) in Wuhan and outside Wuhan, China. Trav Med Infect Dis. 2020 Apr 9:101664. doi: 10.1016/j.tmaid.2020.101664. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liang W.H., Guan W.J., Li C.C., Li Y.M., Liang H.R., Zhao Y. Clinical characteristics and outcomes of hospitalised patients with COVID-19 treated in Hubei (epicenter) and outside Hubei (non-epicenter): a nationwide analysis of China. Eur Respir J. 2020 Apr 8:2000562. doi: 10.1183/13993003.00562-2020. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pongpirul W.A., Mott J.A., Woodring J.V., Uyeki T.M., MacArthur J.R., Vachiraphan A. Clinical characteristics of patients hospitalized with coronavirus disease, Thailand. Emerg Infect Dis. 2020 Apr 8;(7):26. doi: 10.3201/eid2607.200598. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Qi X., Liu C., Jiang Z., Gu Y., Zhang G., Shao C. Multicenter analysis of clinical characteristics and outcome of COVID-19 patients with liver injury. J Hepatol. 2020 Apr 16;S0168-S8278(20):30222–30231. doi: 10.1016/j.jhep.2020.04.010. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen R., Liang W., Jiang M., Guan W., Zhan C., Wang T., Medical Treatment Expert Group for COVID-19 Risk factors of fatal outcome in hospitalized subjects with coronavirus disease 2019 from a nationwide analysis in China. Chest. 2020 Apr 15;S0012–3692(20):30710–30718. doi: 10.1016/j.chest.2020.04.010. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zheng Y., Xu H., Yang M., Zeng Y., Chen H., Liu R. Epidemiological characteristics and clinical features of 32 critical and 67 noncritical cases of COVID-19 in Chengdu. J Clin Virol. 2020 Apr 10;127:104366. doi: 10.1016/j.jcv.2020.104366. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang X., Liu W., Zhao J., Lu Y., Wang X., Yu C. Clinical characteristics of 80 hospitalized frontline medical workers infected with COVID-19 in Wuhan, China. J Hosp Infect. 2020 Apr 14;S0195-S6701(20):30194–30198. doi: 10.1016/j.jhin.2020.04.019. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Goyal P., Choi J.J., Pinheiro L.C., Schenck E.J., Chen R., Jabri A. Clinical characteristics of covid-19 in New York city. N Engl J Med. 2020 Apr 17 doi: 10.1056/NEJMc2010419. NEJMc2010419. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang L., Yan X., Fan Q., Liu H., Liu X., Liu Z. D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19. J Thromb Haemostasis. 2020 Apr 19 doi: 10.1111/jth.14859. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W., the Northwell COVID-19 Research Consortium Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. J Am Med Assoc. 2020 Apr 22:e206775. doi: 10.1001/jama.2020.6775. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang G., Hu C., Luo L., Fang F., Chen Y., Li J. Clinical features and short-term outcomes of 221 patients with COVID-19 in Wuhan, China. J Clin Virol. 2020 Apr 9;127:104364. doi: 10.1016/j.jcv.2020.104364. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jie B., Liu X., Suo H., Qiao G., Zheng Q., Xu W. Clinical and dynamic computed tomography features of 24 patients with coronavirus disease 2019. Can Assoc Radiol J. 2020 Apr 20 doi: 10.1177/0846537120918834. 846537120918834. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 81.Wang L., Duan Y., Zhang W., Liang J., Xu J., Zhang Y. Epidemiologic and clinical characteristics of 26 cases of COVID-19 arising from patient-to-patient transmission in Liaocheng, China. Clin Epidemiol. 2020 Apr 9;12:387–391. doi: 10.2147/CLEP.S249903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gianfrancesco M.A., Hyrich K.L., Gossec L., Strangfeld A., Carmona L., Mateus E.F. COVID-19 global rheumatology alliance steering committee. Rheumatic disease and COVID-19: initial data from the COVID-19 global rheumatology alliance provider registries. Lancet Rheumatol. 2020 Apr 16 doi: 10.1016/S2665-9913(20)30095-3. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fan J., Wang H., Ye G., Cao X., Xu X., Tan W. Low-density lipoprotein is a potential predictor of poor prognosis in patients with coronavirus disease 2019. Metabolism. 2020 Apr 19:154243. doi: 10.1016/j.metabol.2020.154243. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yao Q., Wang P., Wang X., Qie G., Meng M., Tong X. Retrospective study of risk factors for severe SARS-Cov-2 infections in hospitalized adult patients. Pol Arch Intern Med. 2020 Apr 24 doi: 10.20452/pamw.15312. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 85.Zhu Z., Cai T., Fan L., Lou K., Hua X., Huang Z. Clinical value of immune-inflammatory parameters to assess the severity of coronavirus disease 2019. Int J Infect Dis. 2020 Apr 22;S1201-S9712(20):30257–30265. doi: 10.1016/j.ijid.2020.04.041. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang J., Liu P., Wang M., Wang J., Chen J., Yuan W. The clinical data from 19 critically ill patients with coronavirus disease 2019: a single-centered, retrospective, observational study. Z Gesundh Wiss. 2020 Apr 21:1–4. doi: 10.1007/s10389-020-01291-2. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang K., Zhang Z., Yu M., Tao Y., Xie M. 15-day mortality and associated risk factors for hospitalized patients with COVID-19 in Wuhan, China: an ambispective observational cohort study. Intensive Care Med. 2020 Apr 23:1–3. doi: 10.1007/s00134-020-06047-w. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zheng S., Fan J., Yu F., Feng B., Lou B., Zou Q. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January-March 2020: retrospective cohort study. BMJ. 2020 Apr 21;369:m1443. doi: 10.1136/bmj.m1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mathian A., Mahevas M., Rohmer J., Roumier M., Cohen-Aubart F., Amador-Borrero B. Clinical course of coronavirus disease 2019 (COVID-19) in a series of 17 patients with systemic lupus erythematosus under long-term treatment with hydroxychloroquine. Ann Rheum Dis. 2020 Apr 24:217566. doi: 10.1136/annrheumdis-2020-217566. annrheumdis-2020. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 90.Grillet F., Behr J., Calame P., Aubry S., Delabrousse E. Acute Pulmonary embolism associated with COVID-19 pneumonia detected by pulmonary CT angiography. Radiology. 2020 Apr 23:201544. doi: 10.1148/radiol.2020201544. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lovell N., Maddocks M., Etkind S.N., Taylor K., Carey I., Vora V. Characteristics, symptom management and outcomes of 101 patients with COVID-19 referred for hospital palliative care. J Pain Symptom Manag. 2020 Apr 20;S0885–3924(20):30211–30216. doi: 10.1016/j.jpainsymman.2020.04.015. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li J., Wang X., Chen J., Zhang H., Deng A. Association of renin-angiotensin system inhibitors with severity or risk of death in patients with hypertension hospitalized for coronavirus disease 2019 (COVID-19) infection in Wuhan, China. JAMA Cardiol. 2020 Apr 23 doi: 10.1001/jamacardio.2020.1624. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Myers L.C., Parodi S.M., Escobar G.J., Liu V.X. Characteristics of hospitalized adults with COVID-19 in an integrated health care system in California. J Am Med Assoc. 2020 Apr 24 doi: 10.1001/jama.2020.7202. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Onder G., Rezza G., Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. J Am Med Assoc. 2020 Mar 23 doi: 10.1001/jama.2020.4683. Epub ahead of print. PMID: 32203977. [DOI] [PubMed] [Google Scholar]
- 95.Zhu L., She Z.G., Cheng X., Qin J.J., Zhang X.J., Cai J. Association of blood glucose control and outcomes in patients with covid-19 and pre-existing type 2 diabetes. Cell Metabol. 2020 May 1;S1550–4131(20):30238–30242. doi: 10.1016/j.cmet.2020.04.021. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Targher G., Mantovani A., Wang X.B., Yan H.D., Sun Q.F., Pan K.H. Patients with diabetes are at higher risk for severe illness from COVID-19. Diabetes Metab. 2020 May 13;S1262–3636(20):30075–30076. doi: 10.1016/j.diabet.2020.05.001. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.COVID-19 National Incident Room Surveillance Team COVID-19, Australia: epidemiology report 14 (reporting week to 23:59 AEST 3 may 2020) Comm Dis Intell. 2018;2020:44. doi: 10.33321/cdi.2020.44.42. Erratum in: Commun Dis Intell (2018). 2020;44. [DOI] [PubMed] [Google Scholar]
- 98.Inciardi R.M., Adamo M., Lupi L., Cani D.S., Di Pasquale M., Tomasoni D. Characteristics and outcomes of patients hospitalized for COVID-19 and cardiac disease in Northern Italy. Eur Heart J. 2020;41:1821–1829. doi: 10.1093/eurheartj/ehaa388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gold J.A.W., Wong K.K., Szablewski C.M., Patel P.R., Rossow J., da Silva J. Characteristics and clinical outcomes of adult patients hospitalized with COVID-19 - Georgia, March 2020. MMWR Morb Mortal Wkly Rep. 2020;69:545–550. doi: 10.15585/mmwr.mm6918e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ihle-Hansen H., Berge T., Tveita A., Rønning E.J., Ernø P.E., Andersen E.L. COVID-19: symptoms, course of illness and use of clinical scoring systems for the first 42 patients admitted to a Norwegian local hospital. Tidsskr Nor Laegeforen. 2020 Apr 10;140(7) doi: 10.4045/tidsskr.20.0301. [DOI] [PubMed] [Google Scholar]
- 101.Itelman E., Wasserstrum Y., Segev A., Avaky C., Negru L., Cohen D. Clinical characterization of 162 COVID-19 patients in Israel: preliminary report from a large tertiary center. Isr Med Assoc J. 2020;22:271–274. [PubMed] [Google Scholar]
- 102.Du R.H., Liang L.R., Yang C.Q., Wang W., Cao T.Z., Li M. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: a prospective cohort study. Eur Respir J. 2020;55:2000524. doi: 10.1183/13993003.00524-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Guan W.J., Liang W.H., Zhao Y., Liang H.R., Chen Z.S., Li Y.M. China Medical Treatment Expert Group for COVID-19. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J. 2020;55:2000547. doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mao B., Liu Y., Chai Y.H., Jin X.Y., Lu H.W., Yang J.W. Assessing risk factors for SARS-CoV-2 infection in patients presenting with symptoms in Shanghai, China: a multicentre, observational cohort study. Lancet Digit Health. 2020 doi: 10.1016/S2589-7500(20)30109-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lechien J.R., Chiesa-Estomba C.M., Place S., Van Laethem Y., Cabaraux P., Mat Q., COVID-19 Task Force of YO-IFOS Clinical and epidemiological characteristics of 1,420 European patients with mild-to-moderate coronavirus disease 2019. J Intern Med. 2020 Apr 30 doi: 10.1111/joim.13089. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Montastruc F., Romano C., Montastruc J.L., Silva S., Seguin T., Minville V. Pharmacological characteristics of patients infected with SARS-Cov-2 admitted to intensive care unit in South of France. Therapie. 2020 May 15 doi: 10.1016/j.therap.2020.05.005. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ferguson J., Rosser J.I., Quintero O., Scott J., Subramanian A., Gumma M. Characteristics and outcomes of coronavirus disease patients under nonsurge conditions, Northern California, USA, March-April 2020. Emerg Infect Dis. 2020 May 14;(8):26. doi: 10.3201/eid2608.201776. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bode B., Garrett V., Messler J., McFarland R., Crowe J., Booth R. Glycemic Characteristics and clinical outcomes of COVID-19 patients hospitalized in the United States. J Diabetes Sci Technol. 2020 May 9 doi: 10.1177/1932296820924469. 1932296820924469. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Duanmu Y., Brown I.P., Gibb W.R., Singh J., Matheson L.W., Blomkalns A.L. Characteristics of emergency department patients with COVID-19 at a single site in Northern California: clinical observations and public health implications. Acad Emerg Med. 2020 Apr 28 doi: 10.1111/acem.14003. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fadini G.P., Morieri M.L., Longato E., Avogaro A. Prevalence and impact of diabetes among people infected with SARS-CoV-2. J Endocrinol Invest. 2020 doi: 10.1007/s40618-020-01236-2. Mar 28. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yang J., Zheng Y., Gou X., Pu K., Chen Z., Guo Q. Prevalence of comorbidities and its effects in coronavirus disease 2019 patients: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Huang I., Lim M.A., Pranata R. Diabetes mellitus is associated with increased mortality and severity of disease in COVID-19 pneumonia - a systematic review, meta-analysis, and meta-regression. Diabetes, Metab Syndrome. 2020;14:395–403. doi: 10.1016/j.dsx.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fu L., Wang B., Yuan T., Chen X., Ao Y., Fitzpatrick T. Clinical characteristics of coronavirus disease 2019 (COVID-19) in China: a systematic review and meta-analysis. J Infect. 2020 Apr 10;(20):30170–30175. doi: 10.1016/j.jinf.2020.03.041. pii: S0163-4453. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang H., Penninger J.M., Li Y., Zhong N., Slutsky A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46:586–590. doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 1. Funnel plots of standard error by logit-transformed prevalence rate of established diabetes (panel A, n = 83 eligible studies); by log-odds ratio for risk of severe COVID-19 (panel B, n = 22 studies); and by log-odds ratio for risk of in-hospital mortality (panel C, n = 15 studies) among confirmed COVID-19 cases with and without established diabetes.