Abstract
This chapter provides an overview of the natural history, anatomy, physiology, clinical examination, common diseases, and treatment of rats (Rattus norvegicus) and mice (Mus musculus) in the context of veterinary medicine. Guidelines for the care and feeding of rats and mice can provide owners with information to help prevent disease in their pets. Useful techniques for restraint, clinical examination, and diagnostic sample collection are provided to aid veterinarians in thorough evaluation of these small rodents. Common diseases and treatments are discussed separately for each species, organized by organ system. Zoonotic diseases are also discussed to provide guidance for rat and mouse pet owners.
Keywords: biology, disease, mouse, Rat, rodent, veterinary medicine
Taxonomy and Natural History
The word rodent is derived from the Latin verb rodere, which means “to gnaw.” This refers to the most distinguishing feature of this group, the elodont (continuously erupting) incisors possessed by all members of this order.11 Although body size varies greatly, in general rodents possess a uniform body structure with various adaptations. Although the medical approach to the many small rodents species commonly kept as pets is similar, unusual species are sometimes encountered in practice, and more specific information about these species can be found elsewhere.41 The chapter will focus on the biology and diseases of the two of most iconic species of rodents: rats and mice.
Mice
The mouse belongs to the family Muridae, subfamily Murinae. Most pet mice are domesticated from the wild house mouse (Mus musculus). Originally, house mice were bred by mouse fanciers for special coat colors and eventually were used for laboratory research.27 Wild Mus musculus live in close proximity to humans, which has likely contributed to their success as a research model.65 Wild mice are socially gregarious and associate in family groups called demes.28 Each deme is composed of a dominant breeding male, a hierarchy of females, subordinate males, and juveniles. Because of this natural behavior, female mice usually do well when housed together, but intact adult males should not be kept together, because aggression, injury, and death can result. Mice are omnivorous and nocturnal, although they are easily roused during daylight hours. Adult mice weigh approximately 30 g. Mice make good pets for older children (10 years of age and up) because they move more quickly compared with rats, so younger children may not be able to handle them. Female mice or castrated male mice are recommended over intact males because of the strong odor of the latter.44
Other, less common species, including a variety of African spiny mice (Heteromys species), are kept as pets, but their smaller size makes them more difficult for children to handle. Characterized by their dorsal, inflexible spine-like hairs, African spiny mice are likely more closely related to gerbils and are omnivorous.15
Rats
Rats also belong to the family Muridae, subfamily Murinae. Pet rats are derived from the brown rat or Norway rat (Rattus norvegicus). Domestication of this species was most likely begun in the 1800s, as a means to provide enough rats for the popular sport of rat baiting.28 Wild rats are social, burrowing animals, and domestic rats will also burrow if given the opportunity. Rats are omnivorous and nocturnal, similar to mice. Unlike mice, rats are communal and do well housed in mixed groups of several males and females.33
Rats are common pets and are considered one of the better rodent pets because of their larger size and calm nature. Rats are most popular in the pet market in hooded color varieties, in which the coat color is present only over the head and shoulders. Rats are generally hardy as young animals but may suffer from obesity, chronic respiratory disease, and mammary tumors when older. Rats are large enough to be easily grasped by children, and they rarely bite. Some may be excitable and run when removed from their cages; however, rats have been known to return to their cages after “escaping.”33 Strange animals can be introduced successfully on neutral territory. Rats are relatively intelligent and can be trained to perform a variety of tasks for a treat.
Anatomy and Physiology
General Characteristics
Mice and rats possess a generic rodent body structure: a long body that tapers toward the head, short legs with four front toes and five hind toes, and a long tail. Rodents do not pant and have no sweat glands; therefore, their ability to withstand high temperatures is limited. Heat dissipation occurs through the ears and tails.
Sensory Organs
Both mice and rats have bulging eyes and may appear exophthalmic. Because of this feature, take care to keep the eyes moist while the rodent is under anesthesia. The Harderian gland, which lies behind the eyeball, produces lipid- and porphyrin-containing secretions that aid ocular lubrication and may play a role in pheromone-mediated behavior.64 These secretions impart a red tinge to the tears and fluoresce under ultraviolet light. Normally, the lacrimal secretions are spread over the pelage during daily grooming. However, in stressful situations and in certain disease conditions, tears may overflow; this can be inaccurately diagnosed as bleeding from the eyes and nose.
The vibrissae, or sensory whiskers, are a very important sensory organ of touch for detecting objects and helping the animal navigate in dark environments.34 This is especially true for albino rats, whose eyesight is poor relative to their pigmented counterparts.
Integument
Both mice and rats come in many different coat colors, bred specifically by fanciers. Generally, mice and rats are fastidious self-groomers and will keep their coats clean without baths. The white hair coat of rats often yellows with age, and the tail becomes drier and scaly. Aged male rats develop brown, granular sebaceous secretions at the base of their hair shafts, which some owners may mistake for ectoparasitism.
Gastrointestinal
Mice and rats have a common dental formula: 2 (I 1/1, C 0/0, M 3/3). The four prominent, incisors are elodont, or continuously growing throughout life, whereas cheek teeth are anelodont and do not grow after eruption. The enamel of most common rodents is white; however, some species may have enamel that is orange to yellow. The crowns of the mandibular incisors are longer than the maxillary incisors and may be mistakenly assumed to be overgrown. In general, the crown/length ratio for the upper to lower incisors is approximately 1:3.
Mice and rats are monogastric, with an aglandular forestomach that is separated from the glandular stomach by a limiting ridge (margot plicatus). It was thought that this limiting ridge was the reason that mice and rats do not vomit; however, other factors, such as the pressure and strength of the esophageal sphincter and crural sling and the innervation of the diaphragm, may also play a role.60 , 66 It has also been suggested that rodents probably lack the critical brainstem circuitry required to generate an emetic response.38 Because of their inability to vomit and high metabolic rate, mice and rats should not be fasted before anesthesia. The cecum is relatively large in these species, with an elongated colon. Most rodents practice some degree of coprophagy in which ingested fecal pellets presumably provide nutrients, such as B vitamins, produced by the colonic bacteria. Rats do not have a gallbladder.27
Urogenital
The urinary and reproductive tracts terminate in separate urethral and vaginal orifices in female rats and mice. Females are spontaneous ovulators and polyestrous, with stages of the estrous cycle that can be determined with vaginal cytology. Mammary tissue is extensive in rodents and ranges from over the shoulders to the perianal region. Mammary tumors can develop anywhere along this tract. Most female rats have six pairs of nipples, whereas mice have five; however, variations in numbers can be seen.
Like most other male rodents, mice and rats have open inguinal canals, an os penis, and a complex urogenital system that contains several prominent accessory glands. Male mice produce a characteristic musty odor. Pheromones play an important role in mouse behavior and are mediated through tissues such as the vomeronasal (Jacobson’s) organ, which is located in the floor of the nasal cavity. Estrus is suppressed in female mice housed in large groups (the Whitten effect). Recently bred mice that are exposed to a strange male may have impaired implantation (the Bruce effect).27, 28 Normal physiologic, reproductive, and growth reference values are presented in TABLE 25.1, TABLE 25.2 .
TABLE 25.1.
Normal Physiologic Reference Values for Mice and Ratsa
| Value | Mouse | Rat |
|---|---|---|
| Average life span, months | 12–36 | 26–40 |
| Maximum reported life span, months | 48 | 56 |
| Average adult weight (male), g | 20–40 | 267–500 |
| Average adult weight (female), g | 22–63 | 225–325 |
| Heart rate, beats per minute | 450–800 | 300–500 |
| Respiratory rate, breaths per minute | 106–7230 | 71–146 |
| Tidal volume, mL | 0.15–0.29 | 0.6–1.5 |
| Minute volume, mL | 24 | 220 |
| Rectal temperature, °C | 37.1 | 37.7 |
| Approximate daily diet consumption of adult, g | 3–5 | 15–20 |
| Approximate daily water consumption of adult, mL | 5–8 | 22–33 |
| Approximate daily fecal production, g | 1–1.5 | 9–15 |
| Recommended environmental temperature, °C | 24–25 | 21–24 |
| Recommended environmental relative humidity, % | 45–55 | 45–55 |
| Total blood volume, mL/kg | 70–80 | 50–65 |
Average reference values from data given in references 7, 28, 32, 36, 40, and 50. Note that the ranges should be considered as guides; values are likely to vary between groups of animals according to such variables as strain, age, sex, fasted, and methodology.
TABLE 25.2.
Normal Reproduction and Growth Reference Values for Mice and Ratsa
| Value | Mouse | Rat |
|---|---|---|
| Estrogen cycle length, days | 4–5 | 4–5 |
| Estrus (heat) duration, hours | 9–20 | 9–20 |
| Length of gestation, days | 19–21 | 21–23 |
| Pups per litter | 7–11 | 6–13 |
| Weight at birth, g | 1–1.5 | 4–6 |
| Eyes open, days | 12–14 | 12–15 |
| Ears open, days | 10 | 2.5–3.5 |
| Hair coat starts, days | 10 | 7–10 |
| Start to eat dry food, days | 12 | 14 |
| Optimal weaning age, days | 18–21 | 21 |
| Age of maturation of male, weeks | 6 | 4–5 |
| Age of maturation of female, weeks | 6 | 4–5 |
| Recommended minimum breeding age, weeks | 8 | 9 |
| Chromosome number, diploid | 40 | 42 |
Average reference values from data given in references 7, 8, 28, 50 and 80. Note that the reference values may not represent the mean or range for certain populations or strains of animals; the values should be interpreted as approximations.
Sexing
Determining the sex of most rodents is easy in mature animals but can be more challenging in very young ones. In general, the distance between the anus and the genital papilla is a reliable method of determining the sex of young animals. The anogenital distance is greater in males than in females, and the genital papilla is usually more prominent and has a round opening in the male (Fig. 25.1 ). Examining multiple young animals to make a comparison is helpful. The testes of mature males are well developed, especially in rats. Holding the rodent vertically or applying gentle pressure directed caudally on the abdomen allows the testes to pass from the abdomen through the inguinal canal into the scrotum. Nipples are present only on the females of these species and can be seen at around 10 days of age.
Fig. 25.1.
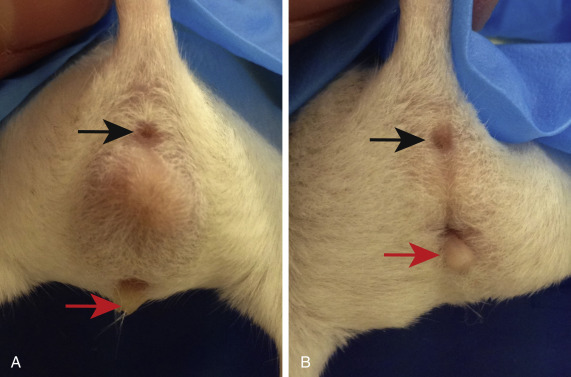
Sexing mice based on anogenital distance, which is the length between the anus (black arrow) and the genital papilla (red arrow). Males (A) have a greater anogenital distance compared with females (B).
HusbandRy
Housing
Suitable enclosures for rodents should be escape-proof and easy to clean. Although colorful and interesting, multilevel cages with tubes, wheels, and hide boxes may be so difficult to disassemble that basic cleaning is neglected. Manufacturers recommend that the entire cage be disassembled and washed thoroughly, which in reality is rarely done. Newer multilevel wire cages sit on a plastic base and can be separated to facilitate cleaning (Fig. 25.2 ). This type of cage provides the additional benefit of separating living space up and away from urine and feces. Features of optimal enclosures are slide-out or easy-to-remove bottoms for ease of cleaning, bottoms with high sides to contain bedding, adequate ventilation (aquariums are not ideal owing to poor ventilation), large doors for easy access to the pet, and a secure locking mechanism for each cage opening.
Fig. 25.2.

Ideal habitat for pet rats. The cage portion can be separated from the base for easy cleaning. Note the cloth and plastic “dens,” exercise wheel, and cardboard box filled with paper for enrichment.
Frequent cleaning of the cage is critical in the care of pet rodents. Failure to clean the cage results in the buildup of ammonia and contributes to stress and illness. In mice and rats, Mycoplasma pulmonis organisms multiply more rapidly in the presence of ammonia levels of 50 to 100 parts per million.71 Frequency of cage cleaning depends on the cage size and number of animals housed. Advise owners to notice the odor of the bedding; anything other than the scent of clean litter indicates that the cage should be cleaned. Provide food in heavy crocks or food dispensers so that the containers will not be tipped over.
Bedding choices include recycled paper, corncob, shredded paper, and wood shavings such as pine and various hardwoods. Much debate exists on the use hardwood and aromatic shavings such as cedar; anecdotally, their use is linked to skin and respiratory disease. Paper bedding is generally preferable, although these products are more expensive than wood shavings. Reclaimed wood pulp bedding is not recommended because of significantly increased intracage ammonia and nasal epithelium pathology seen in mice housed on this bedding material.24 When compared with 20 other types of rodent bedding, paper bedding had the lowest levels of endotoxin and coliform levels.86 In rats, the rate of sneezing and incidence of lung pathology was higher in animals housed on aspen shavings than in those housed on paper bedding.10 However, results of one study in laboratory mice found no difference in growth, food intake, oxygen consumption, immunoglobulin E antibody concentrations, or general appearance and behavior in mice kept on paper bedding, cedar shavings, or pine shavings over a 4-month period.6 Regardless of the bedding material chosen, emphasis should be placed on changing dirty bedding frequently to avoid development of disease.
Enrichment refers to providing mental stimulation and is appropriate for all captive animals. For rodents, enrichment usually includes such things as exercise wheels, hide boxes, materials to shred, treats wrapped in paper or hidden in toys, and time spent outside the enclosure interacting with the owner. Hide boxes can be commercially purchased or constructed from readily available materials such as polyvinyl chloride (PVC) pipe or cardboard boxes. Cardboard provides the additional advantage of a material that can be shredded, destroyed, and discarded when soiled. Smaller cat treat balls have been used successfully with larger rodents. Many small rodents enjoy exercise wheels, and plastic exercise wheels that are almost noise-free are available.
Diet and Feeding
Rodents naturally hoard food items, and exactly how much food is actually being consumed is difficult to gauge by the rate of disappearance from the feeder. Water can be provided in bowls, but good-quality water bottles are preferred because they will prevent bedding from soiling the water. Water bottles can malfunction over time, resulting in blockage or leakage. Educate owners to change water and test water bottles daily.
Most rodents are omnivorous, often eating grasses, seeds, grain, and occasionally invertebrates in the wild.44 Dietary requirements of species in laboratory settings are well established. In the pet environment, needs are best met with a formulated diet supplemented with small amounts of fresh foods and seeds for variety and interest. Although seed mixes are popular choices for rodents, these diets often lead to selective feeding and obesity. Animals usually consume high-calorie seeds (sunflower) and ignore formulated pellets, resulting in dietary imbalance. Although many rodents have such a short life span that dietary deficiency is rarely recognized, the most common adverse outcome in rats is obesity. Various studies have demonstrated increased longevity and reduction of certain diseases in rats maintained on a calorie-restricted diet.56
Protein requirements are 14% to 16% in mice and rats,44 and formulated diets should reflect these requirements. Diets for actively breeding animals should contain higher levels of protein.
Breeding and Neonatal Care
Both mice and rats are successful breeders in captivity, and dystocia is uncommon.79 The nesting instinct is strong in these species, with nest-building behavior occurring before parturition and even without a litter present (Fig. 25.3 ).79 Therefore providing materials that can be used for nest building (such as shreddable paper products) is important. After the litter is born, the nest should be minimally disturbed for at least 2 days postpartum because increased stress, especially in mice, may increase cannibalism of pups by the dam.33 Pups are born hairless with closed eyes and ear canals79 and without the ability to thermoregulate; thus an adequate nest helps to improve breeding success.29 Litter size can vary dramatically depending on the strain and age of the dam.33 Weaning typically occurs at around 21 days in both mice and rats, once the young can eat solid food.28 , 77
Fig. 25.3.
Nesting behavior in mice. Both mice and rats will naturally nest or burrow. Mice, in particular, have a strong drive to build a nest for thermoregulation and comfort, even in the absence of a litter of pups.
Restraint and Examination
The handling and restraint of mice and rats can be challenging. The key is to maximize diagnostic information while maintaining safety for both the examiner and the patient. If at any time the animal appears to be in distress, release it immediately and plan an alternative technique.
Many owners bring pet rodents to the clinic in their normal enclosures, which is helpful in terms of observing husbandry conditions and examining fecal output. However, attempting to retrieve a rodent from colorful caging tubes is time-consuming, can be stressful for the animal, and is best avoided. Therefore request that owners confine the pet to a separate carrier or a small travel cage within the home enclosure to facilitate capture and restraint.
The first step to rodent examination is close observation. Watch the rodent in its cage for quality of respirations, activity, condition of grooming, and the presence of a head tilt or discharges. If dyspnea or depression is observed, be extremely careful when handling the animal, as it is probably very sick and could die from the stress of a physical examination. At the same time, warn the owner of your guarded prognosis.
In general, restraint can be accomplished by three methods: (1) examining the animal as it is loose on the table; (2) restraining the animal with small towels or cloths; or (3) manual restraint. In some cases, combinations of the above methods are used. Scruffing is effective for mice, but rats do not tolerate this form of restraint. To scruff a mouse, gently grasp the base of the tail with one hand, and then grip the loose skin from the lateral aspects of the neck and over the shoulders with thumb and forefinger of the other hand (Fig. 25.4 ). Failure to incorporate enough skin allows the animal to rotate and bite the restrainer’s fingers. For rats, the author’s preferred method of manual restraint is by first gently grasping the base of the tail, and then restraining the shoulders and thorax using “the claw” or “baseball” method (Fig. 25.5 ). Place the index and third fingers on either side of the head, then gently wrap the thumb and remaining fingers around the thorax under the axillae, taking care not to squeeze too firmly on the thorax, which could restrict breathing.
Fig. 25.4.
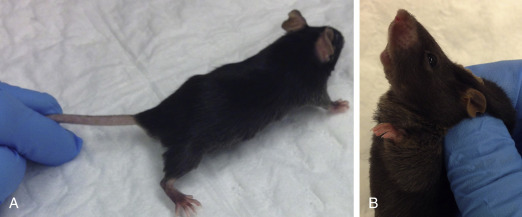
Restraint method for mice. First gently grasp the tail near the base (A), then scruff the skin around the dorsal neck (B). Be sure to include enough skin in the scruff so the mouse cannot turn its head to bite the handler.
Fig. 25.5.
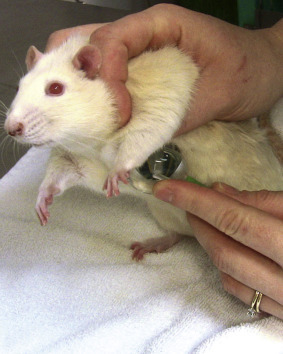
Restraint method for rats. Gently grasp the rat around the thorax with the index and middle fingers on either side of the head and the thumb under the axilla.
Courtesy Angela Lennox, DVM.
Extremely fractious or distressed rodents benefit from mild sedation for examination or diagnostic testing, but use caution when sedating sick or debilitated patients. In general, if the animal is alert enough to defend itself vigorously, it will tolerate sedation. Induction with gas anesthesia in a small induction box or large facemask is the least stressful for both veterinarian and patient (Fig. 25.6 ). For further details on sedation and anesthesia for rats and mice, see Chapter 37.
Fig. 25.6.
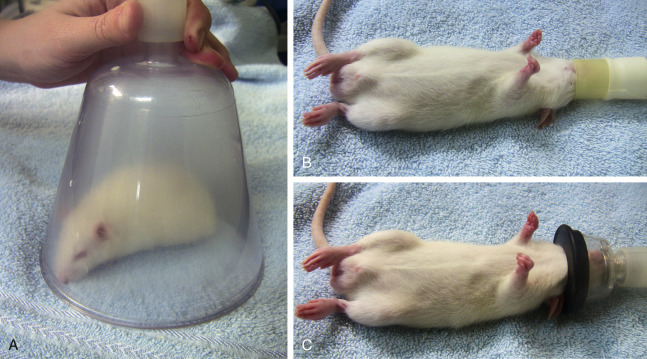
Induction of general anesthesia in a sedated rat by using a standard small animal mask as an induction chamber (A). Once the animal has lost its righting reflex, it can be taken out of the induction chamber and anesthesia can be continued by placing the nose into the gas inflow adapter (B) or in an extra small face mask (C).
Courtesy Angela Lennox, DVM.
Weight measurement is essential for calculating appropriate doses of medications and provides an opportunity for gauging the rodent’s temperament before the actual physical examination begins. Rodents are easily weighed in metal or plastic containers placed on a small digital gram scale. The carrier in which the rodent is presented can sometimes also be used as the weigh basket.
The physical examination itself in rodents is similar to that in other species. However, special tools such as a transilluminator, binocular loupe, bivalve nasal speculum, and otoscope can improve visualization in small patients. An otoscope or bivalve nasal speculum can be used to examine the mouth and ears of rats. However, general anesthesia is usually required for a thorough dental examination. Lymph nodes and glands of the head can be observed for size and palpated for consistency. Assessment of the head is probably the most time-consuming part of the examination. Take care during abdominal palpation, because overzealous palpation can result in visceral rupture. In rodents, it is especially important to examine the anogenital region for discharges and staining of the fur or skin. When a rodent is picked up, it generally urinates and defecates. Have a dipstick ready to perform an immediate urinalysis or a syringe handy to aspirate urine off a clean surface for urinalysis; feces can be caught in a small tube and examined later if required. Palpate the limbs for tenderness or fractures and pay special attention to the paws, noting the length of the nails and the state of the footpads. Rodents are fastidious groomers and therefore can groom away evidence of underlying disease easily. Observe the medial aspect of the front legs for crusts, debris, alopecia, or porphyrin staining, which may occur from excessive grooming associated with nasal discharge. Also, keep in mind that some cage mates are aggressive groomers and can remove any evidence of illness by keeping their mates well groomed.
Respirations and heart rate are difficult to measure in rodents because they are rapid in healthy animals; instead, look for signs of dyspnea. A sensitive pediatric stethoscope is useful for auscultating larger patients. Some respiratory infections, such as mycoplasmosis, are clinically silent. These diseases can be better heard than seen; abnormal sounds called “chattering” in mice and “snuffling” in rats are noticeable without a stethoscope. It may be useful to put the rodent next to your ear and perform a few gentle chest compressions to evaluate audible respiratory excursions. Wheezing or snuffling may not be present at rest, but when chest compressions are performed to create deep respiration and exhalation, they may often become apparent.
The value of determining rectal temperature is questionable. Physical examination combined with attempts to measure rectal temperature causes stress, which can increase body temperature. Core body temperature in mice and rats varies daily from 96.5°F to 100.5°F (36°C–38°C) because of circadian variation, sex, and age.73 Rectal temperatures can be measured safely with the use of small semiflexible temperature probes connected to a digital clinical thermometer.
Clinical Techniques
Sample Collection
Diagnostic testing can provide the same benefits in rodents as it does in other species. Improved techniques and the ability to acquire meaningful results from small sample sizes make sample collection possible and practical in rodents. Reference intervals for hematologic and biochemical testing, serum protein electrophoresis, and urinalyses are presented in TABLE 25.3, TABLE 25.4, TABLE 25.5, TABLE 25.6, TABLE 25.7 . Reference values are often derived from laboratory populations and will vary with animal age, sex, strain, and husbandry, as well as with the method of sample collection and laboratory methodology used to derive the values. Therefore, these reference ranges should be used only as a guide, to be evaluated in conjunction with physical examination findings and other diagnostic tests to make a diagnosis.
TABLE 25.3.
Hematologic Data for Mice
| Value | Malea (n = 133) | Femalea (n = 133) | Mixed sexb |
|---|---|---|---|
| Hematocrit, % | 53 (39–74) | 51 (38–64) | 42–44 |
| Hemoglobin, g/dL | 14.4 (11.9–18.4) | 14.0 (11.2–17.8) | 13.4 (12.2–16.2) |
| Red blood cells, ×106/μL | 9.5 (7.3–12.3) | 9.1 (7.2–11.4) | 8.7–10.5 |
| White blood cells, ×103/μL | 8.6 (4.4–14.0) | 8.0 (4.6–16.2) | 8.4 (5.1–11.6) |
| Neutrophils, % | 23.5 (8.7–55.7) | 19.1 (7.3–41.8) | 17.9 (6.7–37.2) |
| Lymphocytes, % | 67.2 (37.5–85.0) | 71.4 (43.5–86.5) | 69 (63–75) |
| Monocytes, % | 6.8 (2.8–13.1) | 6.8 (3.4–13.4) | 1.2 (0.7–2.6) |
| Eosinophils, % | 2.6 (0.3–5.2) | 2.1 (0.2–6.6) | 2.1 (0.9–3.8) |
| Basophils, % | 0.4 (0.0–1.70) | 0.4 (0.0–1.6) | 0.5 (0–1.5) |
| Platelets, 103/μL | 1580 (736–2374) | 1494 (469–2364) | 600 (100–1000) |
| Mean corpuscular volume, fL | 55.8 (46.5–69.0) | 56.6 (47.5–66.7) | — |
| Mean corpuscular hemoglobin, pg | 15.2 (13.1–18.0) | 15.5 (12.9–18.1) | — |
| Mean corpuscular hemoglobin concentration, g/dL | 27.5 (21.3–33.9) | 27.5 (21.9–33.5) | — |
Values are given as mean with 95% interval low and high. Charles River outbred mice, Crl:CD-1 (ICR), 8–10 weeks, raised under optimal laboratory conditions. (Available at http://www.criver.com/files/pdfs/rms/cd1/rm_rm_r_cd1_mouse_clinical_pathology_data.aspx; information accessed on November 27, 2016.)
Values expressed as means with range or range, from reference 28.
TABLE 25.4.
Biochemical Data for Mice
| Analyte | Male (n ≅130)a | Female (n ≅ 130)a | Mixed sexb |
|---|---|---|---|
| Albumin, g/dL | 3.3 (3.2–3.9) | 3.4 (2.6–4.1) | 2.5–4.8 |
| Alkaline phosphatase, IU/L | 136 (101–207) | 156 (37–249) | 51–285 |
| Alanine aminotransferase, IU/L | 47 (28–64) | 42 (18–71) | 29–191 |
| Aspartate aminotransferase, IU/L | 78 (47–120) | 84 (45–182) | — |
| Bilirubin total, mg/dL | 0.3 (0.2–0.4) | 0.3 (0.1–0.4) | 0.1–0.9 |
| Blood urea nitrogen, mg/dL | 17 (16–22) | 16 (9–24) | 18–29 |
| Calcium, mg/dL | 11.0 (10.2–12.0) | 10.9 (9.9–12.4) | 8.7–10.1 |
| Chloride, mEq/L | 113 (106–114) | 116 (107–131) | — |
| Cholesterol, mg/dL | 185 (168–275) | 148 (85–244) | — |
| Creatinine, mg/dL | 0.3 (0.3–0.3) | 0.3 (0.0–0.4) | 0.1–0.4 |
| Glucose, mg/dL | 240 (147–361) | 218 (137–319) | 90–193 |
| GGT, IU/Lc | 3 (0–13) | 3 (0–19) | — |
| Phosphorus, mg/dL | 11.2 (9.3–11.4) | 10.6 (7.9–14.1) | 5.4–9.3 |
| Potassium, mEq/L | 10.0 (9.3–14.2) | 9.40 (7.84–11.34) | — |
| Sodium, mEq/L | 154 (148–155) | 154 (138–169) | — |
| Total protein, g/dL | 5.8 (5.4–6.4) | 5.7 (4.7–6.6) | 4.6–6.9 |
| Triglycerides, mg/dL | 213 (128–288) | 228 (75–449) | — |
GGT, Gamma-glutamyl transpeptidase.
Values are given as mean with 95% interval low and high. Charles River outbred mice, Crl:CD-1 (ICR), 8–10 weeks, raised under optimal laboratory conditions. (Available at http://www.criver.com/files/pdfs/rms/cd1/rm_rm_r_cd1_mouse_clinical_pathology_data.aspx; information accessed on November 27, 2016)
Values expressed as range. Serum samples courtesy Carolyn Cray, PhD, University of Miami Miller School of Medicine. Ortho (Kodak:Ektachem) 700XR.
The n value for GGT measurement was 30 for males and 29 for females.
TABLE 25.5.
Hematologic and Serum Biochemical Data for Rats
| Value/Analyte | Malea (n > 220) | Femalea (n > 220) | Mixed sexb (n = 50) |
|---|---|---|---|
| Hematologic Testing | |||
| Hematocrit, % | 43.3 (28.3–49.2) | 42.2 (39.6–45.9) | 33.0–47.0 |
| Hemoglobin, g/dL | 15.4 (14.7–16.6) | 14.6 (13.5–15.5) | 11.2–15.9 |
| Red blood cells, ×106/μL | 8.3 (7.9–8.9) | 7.7 (7.2–8.1) | 6.4–8.2 |
| White blood cells, ×103/μL | 11.1 (9.8–12.9) | 8.6 (7.0–10.7) | 4.7–9.4 |
| Neutrophils, % | 11.1 (9.0–13.6) | 9.7 (7.7–14.2) | 7.0–32.0 |
| Lymphocytes, % | 83 (80.1–87.1) | 85 (80.6–87.0) | 57.0–91.0 |
| Monocytes, % | 2.6 (1.1–4.1) | 2.3 (1.4–3.3) | 2.0–5.0 |
| Eosinophils, % | 1.3 (0.7–2.0) | 1.2 (0.6–1.6) | 0–4.0 |
| Basophils, % | 0.4 (0.3–0.5) | 0.4 (0.2–0.5) | 0–3.0 |
| Platelets, ×103/μL | 852 (765–1029) | 841 (787–1021) | 411–626 |
| Biochemical Analysis | |||
| Albumin, g/dL | 3.7 (3.3–4.6) | 4.2 (3.5–5.3) | 2.8–5.3 |
| Alkaline phosphatase, IU/L | 125 (104–160) | 83 (65–117) | 87–381 |
| Alanine aminotransferase, IU/L | 35 (27–46) | 32 (25–45) | 36–80 |
| Aspartate aminotransferase, IU/L | 91 (77–110) | 93 (72–116) | — |
| Gamma-glutamyl transpeptidase, IU/L | 0.5 (0.0–1.0) | 0.5 (0.0–1.0) | — |
| Bilirubin, total, mg/dL | 0.6 (0.2–1.0) | 1.1 (0.2–2.0) | 0.2–0.7 |
| Blood urea nitrogen, mg/dL | 13.4 (10.0–16.0) | 13.2 (11–17) | 11–23 |
| Calcium, mg/dL | 10.2 (9.6–10.9) | 10.3 (9.6–11.2) | 5.7–12.4 |
| Chloride, mEq/L | 104 (101–107) | 105 (101–108) | — |
| Cholesterol, mg/dL | 65 (55–89) | 78 (66–97) | — |
| Creatinine, mg/dL | 0.6 (0.4–0.6) | 0.6 (0.4–0.7) | 0.3–0.6 |
| Glucose, mg/dL | 157 (121–197) | 159 (120–186) | 50–135 |
| Phosphorus, mg/dL | 7.7 (7.0–9.5) | 6.6 (5.6–8.6) | 6.5–12.2 |
| Potassium, mEq/L | 5.3 (4.6–6.1) | 4.9 (4.3–5.9) | — |
| Protein, total, g/dL | 6.4 (6.0–7.1) | 6.7 (6.3–7.5) | 5.6–7.4 |
| Sodium, mEq/L | 144 (141–149) | 143 (141–148) | — |
| Triglycerides, mg/dL | 76 (62–92) | 61 (51–75) | — |
Values given as means with ranges. Charles River outbred Sprague-Dawley Crl:CD(SD) rats, 13–22 weeks of age, raised under optimal laboratory conditions. Bayer Technicon H1E and BMC Hitachi-Model 717 analyzers were used. (Available at http://www.criver.com/files/pdfs/rms/cd/rm_rm_r_clinical_parameters_cd_rat_06.aspx; accessed November 27, 2016.)
Values given as ranges. Data courtesy Carolyn Cray, PhD, University of Miami Miller School of Medicine. Ortho (Kodak:Ektachem) 700XR; Automated analyzer for cell count, hemoglobin, and hematocrit. Differential based on 100-cell count by using blood smear made at the time of sample acquisition.
TABLE 25.6.
Reference Intervals for Serum Protein Electrophoresis for Mice and Ratsa
| Analyte | Mouse | Rat |
|---|---|---|
| Albumin, g/dL | 2.1–3.4 | 3.4–4.1 |
| Alpha-1 globulin, g/dL | 0.3–0.5 | 0.4–0.6 |
| Alpha-2 globulin, g/dL | 0.8–1.1 | 0.2–0.6 |
| Beta globulin, g/dL | 1.4–1.7 | 1.3–1.7 |
| Gamma globulin, g/dL | 0.2–0.3 | 0.1–0.5 |
| A/G ratio | 0.7–1.1 | 1.1–1.6 |
Data courtesy Carolyn Cray, PhD, University of Miami Miller School of Medicine. Analytes measured by Beckman Paragon SPEP II gels in adult animals.
TABLE 25.7.
Urinalysis Reference Values for Mice and Ratsa
| Value | Mouse | Rat |
|---|---|---|
| Urine volume, mL/24 h | 0.5–2.5 | 13–23 |
| Specific gravity | 1.034 | 1.022-1.050 |
| Average pH | 5.0 | 5.0 -7.0 |
| Protein, mg/dL | Males proteinuric | <30 |
Average reference values from data given in references 7, 36, and 50. Note that the ranges should be considered as guides; values are likely to vary between groups of animals according to such variables as strain, age, sex, fasting, and methodology.
Blood Collection
Because of their small size, blood collection in mice and rats can be risky. Venipuncture should be attempted only in normovolemic, normothermic patients; therefore, blood collection is often delayed until the animal’s condition is stabilized. Before collecting a sample, know the sample volume required for the test in question and the maximum blood volume that can be safely acquired from the patient. Ascertain from the laboratory what type of sample (e.g., plasma or serum) and the minimum volume required for a given test. The blood volume of most rodents is approximately 6% to 7% of total body weight85; removing a maximum of 10% of the blood volume is generally safe (see Table 25.1). Make modifications to these guidelines according to overall patient condition and, in all cases, collect only the blood volume required for testing.
Recommended venipuncture sites in pet mice and rats are the lateral saphenous, femoral, jugular, and lateral tail veins, as well as the ventral tail artery.53 In rats, the dorsal tail vein and cranial vena cava may also be used.53 Collecting blood from the orbital plexus, facial vein, or by cardiac puncture is common in laboratory rodents; however, these are not recommended in pet rodents because of high complication rates and the availability of more suitable options.
Small-gauge needles (25- to 27-guage) with attached small syringes (1 mL or smaller) are recommended for blood collection. In very small patients or with vessels where negative pressure causes collapse of the vessel, consider puncturing the vessel with a clear-hubbed needle only and collecting blood with a heparinized hematocrit tube as it flows into the needle hub. Anesthesia or sedation may be necessary to obtain an adequate blood sample. Keep in mind that use of sedation or anesthesia, as well as different venipuncture sites, has been shown to alter the results of certain hematologic values.37 , 53
A technique for lateral saphenous venipuncture of laboratory rodents has been described that is minimally invasive, does not require anesthesia, can be performed by a single person when combined with the appropriate restraint, and can be repeated at the same location multiple times.36, 37 For use in a mouse, place the mouse head first in a large syringe case that has been punctured to provide several breathing holes near the end (Fig. 25.7 ). Extend an exposed hind limb by firmly grasping the skin just in front of or just behind the knee (Fig. 25.8 ). Shave the hair over the lateral tarsal area and apply sterile petroleum jelly or ophthalmic ointment to prevent blood from migrating into the hair. Puncture the vessel at a 90-degree angle to the skin with a needle of appropriate size (a 25-gauge needle is usually sufficient in the mouse). A drop of blood should immediately appear and can be collected into a microcapillary tube or a microtube container. After the required volume has been obtained, release the skin. This usually is sufficient to stop the bleeding; if it is not, apply gentle pressure to the puncture site. This technique may be too stressful for ill or debilitated animals.
Fig. 25.7.

Modified syringe case for mouse restraint. Air holes should be placed near the tip to allow for proper ventilation during restraint.
Fig. 25.8.
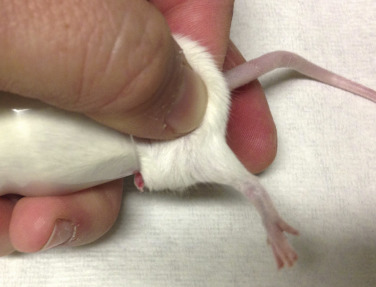
Restraint of a mouse for lateral saphenous vein blood collection. With the mouse placed headfirst into the modified syringe case, expose one of the rear legs and extend it by pinching the skin behind the stifle. The leg can then be shaved and prepared with sterile ointment for blood collection.
Other site options for collecting blood samples are the lateral tail vein, the ventral tail artery of the rat, and the tarsal veins of larger rodents.36 , 85 The lateral tail veins are located on both sides of the tail and are superficial; they can be seen easily in young animals and rats with nonpigmented tails. Warm the tail gently with a warm compress to increase blood flow and occlude the veins by placing a tourniquet around the base of the tail; a rubber band and mosquito hemostat are suitable for this purpose. With a needle of appropriate gauge for the species, enter the skin at a shallow angle at a point approximately one-third down the length of the tail (Fig. 25.9 ). If the initial attempt at collection is unsuccessful, try again at a site closer to the base of the tail. To avoid collapsing the vessel, use a small-volume syringe to withdraw the sample or collect the blood into a microhematocrit tube as it flows freely from the needle hub. A modified butterfly catheter (i.e., with all but the proximal 5 mm of tubing removed) may also be used.
Fig. 25.9.
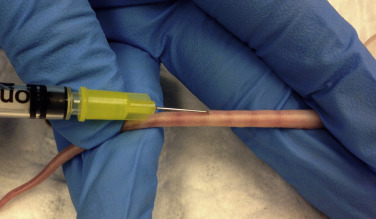
Collecting blood from the tail vein of a mouse. Restrain the mouse using a large syringe case with air holes. The tail can then be exposed and warmed to vasodilate the lateral tail veins. Note the shallow angle of the needle and the starting point at about ⅓ of the way down the tail.
The jugular vein can be used in both mice and rats, but this technique requires general anesthesia and usually needs two people to perform safely.63 Shave the area of the ventral neck and gently restrain the patient with the neck hyperextended. The jugular veins should be visible about 2–4 mm lateral to the sternoclavicular junction.37 , 63 The needle should be inserted directed toward the tail, and the syringe should be withdrawn slowly so as not to collapse the vein. Light digital pressure may be applied at the venipuncture site to allow for hemostasis after the needle is withdrawn.
The cranial vena cava is the largest vessel that is easily accessible in rodents, but it should only be used in rats that are sedated or general anesthesia. Place the animal in dorsal recumbency and prepare the area over the manubrium for venipuncture. Using a 25- to 27-gauge needle with a 1-mL or smaller syringe, approach the vessel from the right or left of the manubrium, angling slightly medially and dorsally (Fig. 25.10 ). As you advance the needle, apply gentle negative pressure. Entry of the cranial vena cava will result in a flash of blood. If blood does not flow freely, the needle may have passed through or oblique to the vessel or the bevel may be contacting the vessel wall. Rotate or redirect slightly. The vessel is just under the manubrium; therefore deep needle penetration is not needed or recommended. Potential complications include vessel rupture and exsanguination, but this appears to occur rarely and is likely associated with excessive patient movement.
Fig. 25.10.
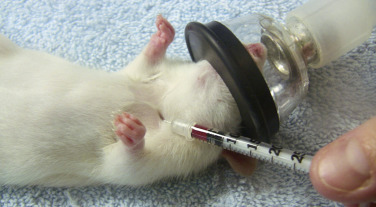
Collecting a blood sample from the cranial vena cava in an anesthetized rat by using an insulin syringe with a 29-gauge needle.
The ventral tail artery in the rat is another option for blood collection.7 The artery courses along the ventromedial aspect of the tail, although it is not as superficial as the lateral tail veins. Place the sedated or anesthetized animal in dorsal recumbency. Use a 22-gauge needle and a 3-mL syringe from which the plunger has been removed. Alternatively, some practitioners prefer to use a 23-gauge butterfly needle with short tubing connected to a 3-mL syringe. Make the first puncture attempt at a point one-third down the tail’s length. Enter the skin at a 20- to 30-degree angle to the tail, with the bevel of the needle facing upward. A perceptible “pop” usually indicates that the artery has been entered, and blood should quickly fill the syringe. The high blood pressure in this vessel negates the need for the negative pressure produced by withdrawal of the plunger. Indeed, the presence of the plunger within the syringe case impairs recognition of correct penetration of the vessel. After the required volume of blood has been collected, withdraw the needle and apply pressure to the puncture site. Note that more time is required to stop the bleeding from the tail artery than from the tail vein.
Urine and Fecal Collection
Many rodents will void during handling, sedation, and induction of anesthesia; thus, it can be difficult to collect an adequate sample volume for urinalysis.85 Nonsterile samples may be collected by simply picking up the animal and holding it over a clean petri dish or other collection device.49 Gently expressing the bladder and collecting the sample from the cleansed urinary orifice into a hematocrit tube can also yield enough urine for a dipstick or specific gravity. If neither of these methods is successful, another noninvasive method is lining an enclosed container (such as a plastic transport cage) with clean plastic wrap and allowing the animal time to urinate, then aspirating the sample from the bottom of the container.49
For sterile urine samples, cystocentesis may be attempted, although this may not be feasible in small patients. Deep sedation or full anesthesia is recommended. The bladder of all but the most obese rodents is readily palpable. With the animal in dorsal recumbency, use a very small–gauge needle and direct the needle caudally at an oblique angle through the abdominal wall into the bladder. Alternatively, use ultrasonography to locate the bladder for cystocentesis.
Collecting a fecal sample is simple in most rodents because of the large number of fecal pellets produced. Usually, most mice and rats will defecate when picked up or when the tail is lifted gently from the base. Avoid introducing fecal collection loops or devices into the rectum because of the fragile mucosa of the rodent colon.
Bone Marrow Collection
Collecting a bone marrow sample is possible, even in small rodents. Use the intraosseous catheterization technique described in the section below (Therapeutic Techniques) for the femur or tibia. Once the bone is catheterized, an empty syringe may be attached to the needle and aspirated to collect bone marrow. Indications are the same as for any other species and include nonregenerative anemia of uncertain origin. For optimal quality, prepare samples immediately after collection.
Miscellaneous Sample Collection
Many types of samples can be collected safely in rodents, including skin scrapings, hair samples, fine-needle aspirates, biopsy samples, and thoracic fluid samples. For each patient, carefully consider the type of restraint (manual vs chemical), anatomic variations, and the size of collection equipment (needles and syringes). Use the equipment that is least invasive and as small as possible to collect diagnostic samples.
Diagnostic Imaging Procedures
Radiology and ultrasonography are commonly performed in small pet rodents (see Chapter 38). In brief, diagnostic-quality images require optimal films and settings and an immobilized, well-positioned patient,12 which usually requires sedation or anesthesia. The increased availability of computed tomography and magnetic resonance imaging has allowed diagnostic imaging to become a useful ancillary examination.74 Two excellent books designed to provide clinicians with normal anatomic and abnormal comparative diagnostic images are Diagnostic Imaging of Exotic Pets: Birds, Small Mammals, Reptiles 47 and Radiology of Rodents, Rabbits and Ferrets: An Atlas of Normal Anatomy and Positioning.76
Advanced Techniques
Technological advances have made possible electrocardiography and accurate and sensitive recordings of heart rate, respiratory rate, and blood pressure in research rodents.46 The cost of the equipment for performing these measurements and the invasive procedures that are often necessary for achieving the recordings prohibit routine use of these testing modalities in most veterinary practices.
Therapeutic Techniques
As in most exotic species, therapeutic agents are not licensed for use in rodents, and such use is considered off label. Some medications may be too concentrated for small rodents and will require dilution or further compounding.77
Fluids can be administered by the subcutaneous, intravenous, intraosseous, and intraperitoneal routes; the method depends on the overall patient condition (see Chapter 41 Emergency and Critical Care).18 Intravenous or intraosseous administration is most suitable for unstable, severely dehydrated patients. Placement of an intravenous catheter is difficult in most rodent patients; therefore, intraosseous catheterization is a better option.52 Studies in multiple species have shown that drug and fluid administration by the intraosseous route results in similar onset of action and peak blood levels as use of the intravenous route.83 Needles manufactured specifically for the purpose of intraosseous catheterization are available; however, in most small rodents, simple injection needles are appropriate (22- to 27-gauge, depending on patient size). The most commonly used site is the tibia; however, use of the femur has been described as well. Insertion of an intraosseous catheter is uncomfortable for the animal, hence, sedation with a local block or full anesthesia is required. To place a catheter into the tibia, infuse the skin, subcutaneous tissues, and periosteum with lidocaine at 1 to 2 mg/kg and wait for at least 10 minutes. Introduce the catheter at the tibial crest at the insertion site of the patellar ligament to avoid penetrating into the joint (Fig. 25.11 ). The size and shape of the proximal tibia determine the exact angle of placement, and correct placement is best confirmed by radiography in two views.52
Fig. 25.11.
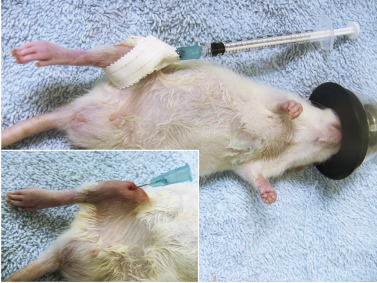
Placement of a 25-gauge needle in the bone marrow cavity of the tibia of a young anesthetized rat. The site is prepared aseptically. The needle is taped in place and can be fitted with a standard injection cap or a small-gauge syringe for intermittent infusion.
Courtesy Angela Lennox, DVM.
Fluid needs for rodent patients that are stable but mildly dehydrated can be met with subcutaneous or oral fluid administration. Administer subcutaneous fluids in the loose skin between the shoulder blades (Fig. 25.12 ).18
Fig. 25.12.
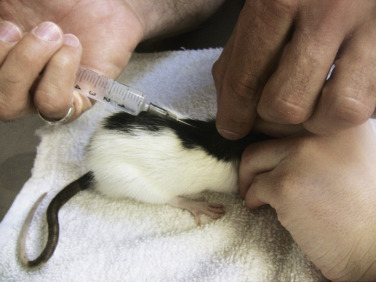
Administering warmed subcutaneous fluids in a rat by using manual restraint.
Intramuscular injections should be given in the caudal aspect of the rear legs or epaxial musculature. Injecting into the lateral leg muscles carries a risk of damage to the sciatic nerve, which can result in lameness or self-mutilation.18 Use a needle size as small as possible to allow passage of medication and penetration of the skin. Intraperitoneal administration of therapeutics is not advised because of the availability of other sites and potential risk of damage to abdominal structures or inadvertent injection into the gastrointestinal tract (especially the cecum).18 Indications for intravenous administration of medications are rare; however, intravenous administration is possible in a larger sedated or anesthetized patient if done carefully. An alternative to intravenous injection is injection via an intraosseous catheter.
Oral administration of medications is generally uncomplicated in most rodents and accomplished by carefully restraining the animal and then introducing a syringe into its mouth. Because the volume of medication is often small, insulin syringes with the needle removed allow accurate measurement and ease of delivery. Compounding medication with sweet-tasting bases often increases compliance.
Administering medications in water or food is problematic because of the tendency of rodents to distrust unfamiliar tastes and to cache food, as well as the overall inability to control volume and frequency of administration.18 Adding medications to water may reduce water intake. For these reasons, direct administration of medications is preferred.
Exercise caution in administering antibiotic therapy to rodents. Streptomycin and procaine are toxic in mice and nitrofurantoin causes neuropathologic lesions in rats. Commonly used antibiotics that are safe to use in rats and mice include amoxicillin/clavulanic acid, enrofloxacin, doxycycline, and trimethoprim-sulfa combinations. A comprehensive list of safe antibiotics and dosages can be found in the Exotic Animal Formulary.13
Because of the high metabolic rate of rodents, hand feeding is very important in anorectic animals. Hand feeding is generally easily accomplished with the use of a food supplement that can be fed through a small syringe. Hand-feeding products for herbivores, omnivores, or a mixture of both (Critical Care, Oxbow Animal Health, Murdock, NE; EmerAid Omnivore/Herbivore, EmerAid LLC, Cornell, IL) are nutritionally balanced and convenient for use. Strained vegetable baby foods such as mixed greens or sweet potato can be added to improve palatability if needed.
Hospitalization
Rodents are frequently hospitalized for advanced care or diagnostic testing. The hospital room should be quiet and free of visual or auditory signs of potential predators. Enclosures in the hospital must be escape-proof, even if the patient appears unable to attempt escape. Most rodents can be housed in clear square plastic pet carriers with snap-on tops. These carriers can be placed into most commercial pet incubators for temperature regulation (Fig. 25.13 ). Ideal bedding is light-colored towels or paper to allow easy identification of urine, feces, and abnormal discharges such as blood. Hospitalized rodents benefit from a hide box, which can be quickly constructed from a small cardboard box or paper. Stock a variety of rodent foods for feeding hospitalized animals or request a supply from the pet’s owner before hospitalization.
Fig. 25.13.

Hospitalized rodents are adept at escape. This rat is housed in an escape-proof plastic pet container inside a standard cage.
Preventive Medicine
Prevention of disease in rodents is far more successful than treatment. Disease prevention is based on ensuring the purchase of healthy animals and maintaining good husbandry practices. Measures that can be taken to ensure good husbandry are as follows: (1) providing balanced fresh food that is appropriate in protein and caloric content, (2) providing clean fresh water, and (3) cleaning cages on a regular basis to prevent accumulation of excess urine and fecal material. Adequate shelter, including providing shade from direct sunlight, avoiding drafts and extreme changes in temperature or humidity, and providing areas in which to nest and hide are also helpful in preventing disease development. If housing multiple animals, isolating sick animals from the rest of the group and protecting vulnerable animals from more aggressive members of their group (e.g., young animals from older animals) can be crucial to maintaining the health of the group. Stress reduction via separating natural predators living in the same household (e.g., mice from cats) is also ideal. Other sound husbandry practices include housing different species separately to prevent interspecies disease transmission (e.g., rats carry Streptobacillus moniliformis, a cause of septicemia in mice, in their nasopharyngeal cavities) and reducing obesity by limiting food intake and providing cage accessories (e.g., exercise wheels, tunnels, and ramps) that allow play and exploration.
Unlike larger companion animals, pet rodents are not vaccinated. Off-label treatment with avermectins (e.g., ivermectin, selamectin, moxidectin) has allowed routine systemic treatment of pet rodents for pinworms, mites, and lice. For ectoparasite and endoparasite treatment recommendations, refer to Table 25.8 .
Diseases of Mice
Oral and Dental
The most common problem in the oral cavity of mice is incisor malocclusion, especially the lower incisors. This is usually a genetic problem seen in younger mice, although it can occur in older mice because of trauma of the developing tooth buds. Prevalence of malocclusion was 3% (females) to 9% (males) in outbred mice in chronic toxicology studies.54 Clinical signs are weight loss, anorexia, traumatic injury to the upper lip, ptyalism, moist dermatitis around the mouth and forequarters, and eventually death if not treated.33 Trimming of the incisors with small nail clippers or scissors will temporarily correct the problem, but this needs to be performed routinely, as the incisors grow throughout the life of the animal. If the animal is fractious or if the handler is unable to attain proper immobilization of the head, brief sedation or anesthesia is recommended for the procedure. For more detailed information on dentistry procedures, see Chapter 36.
Respiratory System
Diseases of the upper and lower respiratory tracts are common in pet mice and rats. Animals may be presented with sniffling, sneezing, chattering, and labored breathing. If dyspnea is suspected, do not handle the animal more than is necessary during clinical examination, because it may die. Collection of tracheal and nasal secretions is not recommended because swabbing is highly traumatic, and the cause of disease is generally a mixed infection. Antibiotic treatment is helpful but does not eliminate the disease.
The two most common causes of clinical respiratory disease in mice are Sendai virus and M. pulmonis. Sendai virus is associated with an acute respiratory infection in which mice display chattering and mild respiratory distress. Neonates and weanlings may die, whereas adults generally recover within 2 months. When the disease expression exceeds this pattern, the cause is most likely concurrent Mycoplasma infection. Mycoplasma pulmonis is the cause of chronic pneumonia, suppurative rhinitis, and occasionally otitis media. Chattering and dyspnea are caused by accumulations of purulent exudate in inflamed and thickened nasal passages. Survivors develop chronic bronchopneumonia and bronchiectasis and may develop pulmonary abscesses. Antibiotic therapy may alleviate clinical signs but does not eliminate the infection. Enrofloxacin (10 mg/kg) as an antimicrobial agent in combination with doxycycline hyclate (5 mg/kg) as an immunomodulator (not as an antimicrobial) given every 12 hours by mouth (PO) for 7 days is helpful. Additional treatments such as nebulization therapy, expectorants, and nonsteroidal anti-inflammatory drugs can be used to ameliorate the disease.
Gastrointestinal System
Endoparasites are relatively common in mice. However, only two parasites regularly encountered in the digestive tract, the protozoan parasites Spironucleus muris and Giardia muris, are considered pathogenic, even though they are not always associated with clinical signs in immune-competent animals. Diagnosis is based on demonstrating characteristic trophozoites in wet mounts of fresh intestinal contents or feces. Treatment for protozoa is metronidazole (see Table 25.8).
TABLE 25.8.
Drugs for Treating Endoparasites and Ectoparasites in Pet Rodents
| Active Substance | Dose | Application | Remarks |
|---|---|---|---|
| Ectoparasites | |||
| Chlorpyrifos | 6 g per 27 × 48-cm cage | Mix in bedding | For environmental control of ectoparasites. |
| Chlorpyrifos + Fenoxycarb | — | Spray | Used to treat environment against ectoparasites (insecticide + insect growth regulator) |
| Dichlorvos | 5 g/kg mix/intersperse | Environment | Mix granules in bedding or hang strip 15 cm above cage for 24 hours, then 2×per week for 3 weeks |
| Doramectin | 0.5 mg/kg | Subcutaneous | For fur mites, 3 times one week apart |
| Fipronil | ≈7.5 mg/kg topically = 1 to 2 sprays in gloved hand | Spray | For fleas and ticks, apply to the whole body, repeat after 7 to 10 days. |
| Imidacloprid | 10 mg/kg | Spot on | 0.1 mL/kg of 10% solution |
| Imidacloprid + Moxidectin | 10 + 1 to 2 mg/kg | Spot on | 0.1 mL/kg of 10% solution. Flea species are used as an intermediate host and vector for tapeworms |
| Imidacloprid + Permethrin | 10 + 50 mg/kg | Spot on | For fleas and ticks |
| Ivermectin | 0.2 to 0.4 mg/kg | Subcutaneous | For fur mites and lice, every 7 to 10 days for 3 treatments |
| 10% solution (propylene glycol) | Topical | For fur mites and lice, 1 drop behind the ear | |
| Moxidectin | 0.5% solution at 5 mg/kg | Topical | For fur mite Myocoptes musculinus, repeat in 10 days. |
| 0.5% solution at 2 mg/kg | Oral | For fur mite Radfordia affinis, repeat in 15 days. | |
| Permethrin | Dependent on size of animal | Topical | Powder: cover body with powder or cotton ball soaked in 5% solution |
| Permethrin + Methopren | As needed for environmental treatment | Spray | Used to treat environment against ectoparasites (insecticide + insect growth regulator) |
| Pyrethrins | As needed for environmental treatment | Spray | Use “kitten-safe” product. Used to treat environment |
| Topical | Use 0.05% shampoo every 7 days for four treatments | ||
| Selamectin | 15 mg/kg | Spot on | For fleas and lice, repeat in 10 days. Strictly topical |
| Endoparasites | |||
| Doramectin | 0.2 mg/kg | Subcutaneous | For Syphacia muris (pinworm), every 7-14 days for 2-3 treatments |
| Fenbendazole | 20 mg/kg | Oral | For pinworms, once daily for 5 days |
| Ivermectin | 2 mg/kg | Oral or topical | For pinworms, once or twice 7-10 days apart |
| Metronidazole | 10 to 40 mg/kg | Oral | For protozoa, twice 5 days apart |
| Praziquantel | 25 mg/kg | Oral | For cestodes, trematodes, repeat twice every 10 to 14 days for three treatments. Stings if given SC |
| Toltrazuril | 0.5% solution at 10 to 20 mg/kg | Oral | For coccidia, give daily for 2 to 3 days, pause 5 days and then repeat |
Pinworms are ubiquitous, considered nonpathogenic, and found frequently in mice purchased from a pet store.16 Two species are commonly encountered in mice: Syphacia obvelata and Aspicularis tetraptera. Usually, pinworm infections are subclinical, although heavy infections may result in rectal prolapse, intussusception, enteritis, or fecal impaction.28 Adult S. obvelata females deposit their ova on the fur around the anus. A quick way to diagnose S. obvelata infections is to make a clear cellophane tape impression of the perianal skin. Characteristic banana-shaped eggs will readily be seen under a light microscope (Fig. 25.14 ). Aspicularis tetraptera cannot be diagnosed this way because ova are deposited directly into the large intestine; therefore, fecal smear or flotation is required to confirm A. tetraptera infection. Fenbendazole, doramectin, or ivermectin treatments can all be used to eliminate pinworms (see Table 25.8). Importantly, in addition to treating the animal(s), environmental decontamination is recommended because pinworm eggs have been shown to persist in the environment.55
Fig. 25.14.
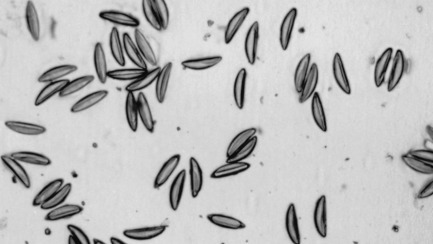
Ova of mouse pinworm (Syphacia obvelata) as seen on a perianal tape test. Note the characteristic banana shape.
From Hendrix CM, Robinson E. Diagnostic parasitology for veterinary technicians. 3rd ed. St. Louis: Elsevier; 2006.
Another commonly isolated bacterial organism from pet mice is Helicobacter species.16 Generally, mice infected with Helicobacter do not exhibit clinical signs; however, if an animal presents with rectal prolapse, Helicobacter should be a top differential diagnosis. Because this organism is difficult to grow in culture, the recommended test for detection is polymerase chain reaction (PCR) testing of fecal pellets. Recommended treatment is triple therapy with amoxicillin (1.5 mg/30 g mouse), metronidazole (0.69 mg/30 g), and bismuth (0.185 mg/30 g) PO every 8 hours for 2 weeks.26
Urinary System
Obstruction of the urethra in male mice has been described as resulting from infections of the preputial glands with Staphylococcus aureus and of the bulbourethral glands with Pasteurella pneumotropica. Accessory sex gland secretions and, rarely, urolithiasis have also been implicated. Mice are often presented to the veterinarian because they mutilate their penises as a result. In addition, occasional injury of the penis is seen in young males from aggressive breeding activity and abrasion on the cage. Treatment involves isolating the affected mouse, cleaning and debriding the affected areas, and treating the animal with antibiotics. A newly identified parvovirus, mouse kidney parvovirus, has recently been identified as the cause of a spontaneous nephropathy in mice.72 This disease, previously known as “inclusion body nephritis,” had puzzled pathologists for many years. Although incidence of viral infection is believed to be widespread in laboratory mice, only severely immunocompromised mice develop clinically significant disease.72
Reproductive System
Mice experience relatively few complications associated with parturition. Occasionally, vaginal and uterine prolapse occurs after parturition. The prolapse can be reduced by placing a small, lubricated 20-gauge Teflon intravenous catheter (without needle) into the lumen of the uterus and vagina, and manually manipulating the tissue back into the proper anatomic position.14 A purse-string suture using small-gauge (4-0 or smaller), absorbable suture material should be placed around the vaginal orifice before removing the catheter.
Integument
Many problems seen in pet mice are associated with the skin. In one survey from a large diagnostic laboratory that housed research animals, skin disease in mice represented 25% of all diagnostic cases (for all species) submitted.48 Four groups of skin problems are categorized in mice: behavioral disorders, husbandry-related problems, microbiologic and parasitic infections, and idiopathic conditions.
Behavioral skin issues mainly stem from the hierarchical nature of the mouse’s natural behavior. Mice naturally establish social dominance within a group, which can manifest as barbering and fighting. Barbering is commonly seen in group-housed mice, more often in females, where the dominant mouse nibbles off the whiskers and hair around the muzzles and eyes of cage mates. No other lesions are present, and only one mouse (the dominant one) retains all of its fur. Removal of the dominant mouse stops barbering; however, another mouse usually then assumes the dominant role. Barbering may also be seen associated with sexual overgrooming as a form of stress-evoked behavior, and lactating mice may display “maternal” barbering, produced in the process of suckling pups, in which alopecia is seen from tail to chin.42 Barbering has a genetic-based behavioral background, because aggressive inbred strains of mice do not use barbering in their behavior.42 Male mice, except littermates raised together from birth, are more likely to fight and can inflict severe bite wounds on one another, especially over the rump, tail, and shoulders. Recommended treatment involves isolating the aggressor and treating the wounds on injured mice. Bandages generally do not stay on mice, but small amounts of topical medications, such as triple antibiotic ointment or silver sulfadiazine, may be applied to provide antimicrobial activity and keep the wound moist.
Environmental causes of skin issues are hazardous cage equipment and poor husbandry conditions. Small patches of alopecia on the lateral surfaces of the muzzle are usually a result of chaffing on metal feeders, poorly constructed watering device openings, and metal cage tops. Unlike barbering, dermatitis may also be associated with the alopecic area. Individually housed mice can display aberrant stereotypic behaviors such as polydipsia and bar chewing, which results in mechanical abrasion and alopecia. Ringtail is a condition seen in young mice and rats under conditions of low humidity, in which annular constrictions can be seen around the tail and occasionally the feet or digits.28 In severe cases, amputation may be required. In all of these cases, treatment consists of replacing poor equipment, ensuring proper humidity and husbandry, and providing environmental enrichment.
Many infectious causes of alopecia and dermatitis are associated with fur mites. Ectoparasites are common in mice purchased from pet stores.70 In affected animals, the hair is generally thin, especially on difficult-to-groom areas such as the head and trunk. The coat often has a greasy appearance; in cases of heavy infestation, noticeable pruritus and self-inflicted dermal ulceration may occur. Three mites are commonly seen: Myobia musculi, Myocoptes musculinus, and Radfordia affinis.28 Mites are spread by direct contact with infected mice or infested bedding. Diagnosis is based on the identifying adult mites, nymphs, or eggs on hair shafts with the use of a hand lens or a stereoscopic microscope (Fig. 25.15 ). Adults and nymphs appear pearly white and elongated (being about twice as long as they are wide); eggs are oval and are seen attached to the base of hairs or inside mature females. Treat mite infestations with avermectins (e.g., ivermectin, selamectin) or milbemycins (moxidectin).13 , 68, 69 Refer to Table 25.8 for dosages. Ringworm, caused by Trichophyton mentagrophytes, is uncommon in pet mice. Lesions, when present, are most often on the face, head, neck, and tail. The lesions have a scurfy appearance with patchy areas of alopecia and variable degrees of erythema and crusting. Pruritus is usually minimal to absent, and the lesions do not fluoresce under a Wood’s lamp.21
Fig. 25.15.
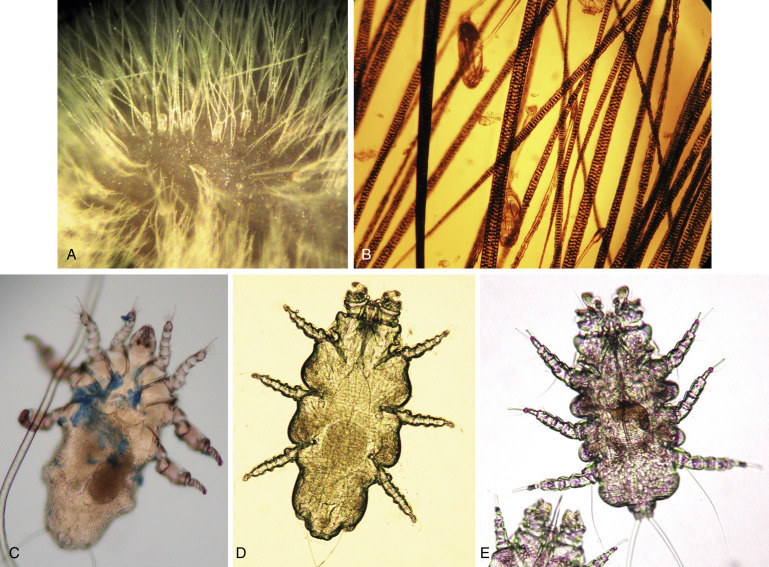
Adult fur mites and ova. The ova are pearly white, oval, and attached to the hair base (A). Diagnosis of fur mite infestation can be made by visualization of ova attached to the hairs under a microscope (B). The adults of three species of fur mites that can infest mice: Myocoptes musculinus (C), Radfordia affinis (D), and Myobia musculi (E).
Photos courtesy Cheryl Yost
Subcutaneous abscesses can also occur in mice, and fine-needle aspiration is recommended to aid in diagnosis and treatment. Three opportunistic bacterial pathogens—S. aureus, P. pneumotropica, and Streptococcus pyogenes—are often isolated from abscesses3 and can cause abscesses in other organs (e.g., P. pneumotropica is sometimes associated with conjunctivitis, panophthalmitis, and swollen eye abscesses). Antibiotic therapy with penicillins or cephalosporins, concurrent with drainage and debridement of the abscess, is effective.
Idiopathic ulcerative dermatitis is a well-recognized disease in black laboratory mice on the C57BL/6 strain background with a characteristic distribution on the thorax and head (Fig. 25.16 ). The disease is often very pruritic, causing mice to inflict more damage to the skin from scratching. The cause of idiopathic ulcerative dermatitis is an underlying vasculitis attributed to immune complex deposition on dermal vessels.43 Dietary factors and dysregulated fatty acid metabolism have been implicated in the development of the disease, and the severity appears to be modulated by dietary fat and vitamin E content. Gavaging affected mice with omega-3 fatty acids was associated with regressed lesions and resolved pruritus in a small sample of affected mice.51 The ulcers may heal with fibrosis and resulting skin contracture or progress to a Staphylococcus xylosus secondary bacterial infection.43 , 80 A recent study showed that nail trimming, added to topical treatment (Vetericyn [Innovacyn, Rialto, CA], Tresaderm [Merial, Duluth, GA], or triple antibiotic ointment), greatly improves healing compared with topical treatments alone.2 Additionally, 0.005% sodium hypochlorite solution was more effective for wound healing than povidone-iodine/silver sulfadiazine or triple antibiotic ointment.58 The author recommends using a multipronged approach for treatment of idiopathic ulcerative dermatitis, including toenail trimming, topical therapy, and omega-3 fatty acid supplementation (0.1–0.2 mL PO every 24 hours).
Fig. 25.16.
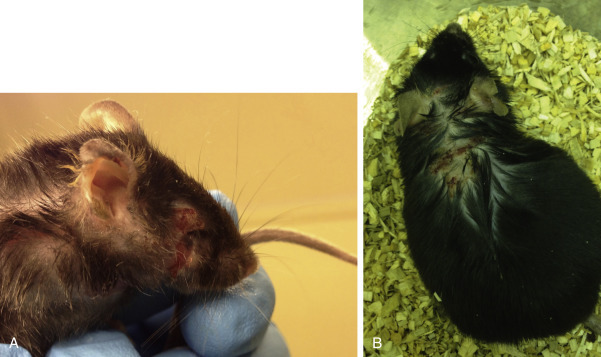
Idiopathic ulcerative dermatitis in mice. This disease usually occurs in black mice with a C57BL/6 background strain. The lesions can occur anywhere, but are most often on the neck, back of head, and face. Note the excoriations on the face (A), likely worsened by scratching because of the pruritic nature of the disease. The appearance of a typical healing ulcer (B). Treatments include trimming toenails, topical medication, and fatty acid supplementation.
Neoplasia
Neoplasia, both spontaneous and induced, has been exhaustively studied in laboratory mice. Three types of cancer that occur frequently in pet mice are mammary tumors, lymphoma, and primary lung tumors. The most common mammary tumor is adenocarcinoma, followed by fibrosarcoma. The incidence of mammary tumors varies by mouse strain and the presence or absence of mouse mammary tumor viruses; the incidence is as high as 70% in some strains.78 In wild and outbred mice, the incidence of fibrosarcoma ranges from 1% to 6%.35 Subcutaneous tumors are nearly always malignant and have often ulcerated by the time a diagnosis is made. Tumors can be treated by surgical excision, but the chance of recurrence is high, and the prognosis is poor. Attempts to treat these tumors in pet mice by radiation or chemotherapy have not been reported.
Lymphoma of various types occurs spontaneously in multiple strains of mice, both inbred and outbred.9 Because mice are used extensively in cancer research, many treatments for lymphoma are described in experimental studies; however, no treatments in pet mice have been reported.
Primary respiratory tumors are uncommon in other species but relatively common in mice.9 , 28 About 95% of these are pulmonary adenomas, and these are prone to invade the pulmonary parenchyma and metastasize.28 These cases are generally subclinical until the animal can no longer compensate for decreased lung capacity. Similar to lymphoma, no treatment of pulmonary adenomas in pet mice has been reported.
Zoonoses
Mice have the potential of carrying zoonotic diseases that can be transmitted to their owners. The diseases discussed are some of the more important zoonotic diseases of mice that the exotic pet veterinarian should be aware of.
Lymphocytic choriomeningitis virus is a virus that has been associated with many reports of human infection when in contact with laboratory or pet rodents, especially mice and hamsters.61 The wild house mouse is the principal host for lymphocytic choriomeningitis virus, and it is widely distributed throughout the world. Transmission occurs when people are exposed to live mice or their excreta, or to mouse carcasses. Clinical signs in people range from asymptomatic to mild flu-like symptoms, to more severe signs of meningitis such as drowsiness, confusion, and motor abnormalities. Death has been reported in several cases where the central nervous system has become involved.61 Owners of pet rodents that develop these symptoms are urged to contact their physicians immediately to seek proper diagnosis and treatment.
Salmonellosis is an important, worldwide zoonotic disease that is widespread in wild animals. Transmission is fecal-oral, and usually infection in pet rodents is associated with poor sanitation or contamination of food, bedding, or water by wild rodents.61 The Centers for Disease Control and Prevention provides guidelines for small pet owners to help keep themselves and their families healthy: https://www.cdc.gov/healthypets/resources/safety-around-small-pets-H.pdf. Clinical symptoms of salmonellosis in people are gastroenteritis, abdominal pain, diarrhea, nausea, and fever.61
Leptospirosis is another zoonotic disease with a reservoir in wild rodents. Rodents often do not show clinical signs but can shed leptospires throughout their life span.61 Leptospires are shed in urine, so people can become infected by handling animals or contaminated bedding or through aerosol exposure during cage cleaning. Clinical signs in people range from asymptomatic to severe infection and even death, with potential renal, hepatic, pulmonary, and gastrointestinal involvement.61 Refer to Chapter 42 for further information.
Diseases of Rats
Ocular
Chromodacryorrhea, or red tears, is a condition commonly seen in rats that present to the veterinarian. Owners will often claim that their pet rats are “bleeding” from the eyes or nose when they are actually seeing chromodacryorrhea. The red coloration of these tears is due to porphyrin pigments, which are produced by the Harderian glands located behind the eyes. Chromodacryorrhea is usually a response to stress and disease, and dried tears around the eyes and external nares can resemble dry crusts of blood (Fig. 25.17 ).19 Because porphyrin will fluoresce under ultraviolet light, porphyrins can be differentiated from blood by using a Wood’s lamp (Fig. 25.18 ). Red tears are often an indication of a chronic underlying disease, and their presence warrants thorough evaluation of the affected animal.
Fig. 25.17.
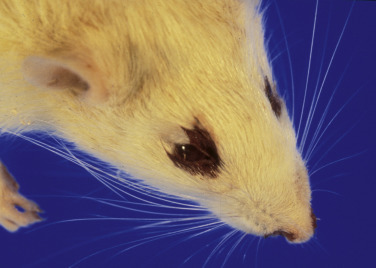
Chromodacryorrhea, or red tears, in a rat. The color results from porphyrin pigments in the Harderian gland secretions, which are visible around the eyes and occasionally the nares.
Fig. 25.18.

Chromodacryorrhea in a rat viewed under an ultraviolet light. The porphyrins in the Harderian gland secretions around the eyes and nares readily fluoresce under ultraviolet light and can be distinguished from blood.
Oral and Dental
As with mice, the most common abnormality of the mouth in rats is incisor malocclusion. Treatment is the same as for mice; however, older rats have thicker incisors that may fracture when trimming with regular nail clippers. Instead, incisors should be trimmed using a hand-held drill (e.g., Dremel Rotary Tool; Dremel Co, Racine, WI) or a dental drill with diamond-disc attachment. Sedation or anesthesia is recommended to prevent injury to the patient. Place a wooden tongue depressor behind the incisors during trimming to protect the tongue and other soft tissues of the mouth. Refer to Chapter 36. for more details regarding small mammal dentistry.
Respiratory System
Respiratory disease caused by infectious agents is the most common health problem in rats. Three major respiratory pathogens cause overt clinical disease: M. pulmonis, Streptococcus pneumoniae, and Corynebacterium kutscheri. Other organisms such as Sendai virus (also called murine respirovirus; a paramyxovirus), pneumonia virus of mice (a paramyxovirus), Pneumocystis carinii (a fungus) cilia-associated respiratory bacillus, and Haemophilus species are minor respiratory pathogens that rarely cause overt clinical disease by themselves. However, the minor respiratory pathogens interact synergistically as copathogens with the major respiratory pathogens to produce two major clinical syndromes: chronic respiratory disease (CRD) and bacterial pneumonia.
The best-understood multifactorial respiratory infection in rats is CRD. The major component of CRD is M. pulmonis, and the disease is also known as murine respiratory mycoplasmosis (MRM). Rats may live 2 to 3 years with CRD. A survey of 28 pet ratteries in the United States found that all were positive for M. pulmonis.17 Clinical signs are highly variable: in many cases no signs are present even though significant pulmonary lesions may exist. The prevalence and severity of signs typically increase with the age of the rat and the presence of environmental stressors. Initial infection commonly occurs without any clinical signs; early signs involve both the upper and the lower respiratory tracts and may include snuffling, nasal discharge, polypnea, weight loss, hunched posture, ruffled coat, head tilt, and red tears.84 The most important aspect of CRD for clinicians is that respiratory mycoplasmosis varies greatly in disease expression because of the different factors that influence the host–pathogen relationship. Examples of such factors include intracage ammonia levels, concurrent infection with other respiratory pathogens, genetic susceptibility of the host, virulence of the Mycoplasma strain, and vitamin A or E deficiency.84 Auscultation is insensitive in determining the severity of respiratory disease, and radiographs are often unremarkable. However, a computed tomography scan often reveals significant pulmonary disease. Serology is the preferred choice for diagnostic testing, as mycoplasma are difficult to grow in a laboratory. Younger rats naturally exposed to M. pulmonis may be seronegative for up to 4 months after exposure.20
The primary lesion of CRD is subacute and chronic bronchitis, a chronic inflammatory condition resulting in respiratory epithelial dysfunction.45 The underlying airway inflammation and clinical signs result from damage and remodeling of airway epithelium, colonization of the airways with secondary infections, and infiltration and activation of neutrophils, macrophages, and lymphocytes. Bronchodilators are the primary treatment for subacute and chronic bronchitis. Inhaled nonselective muscarinic antagonists (e.g., ipratropium bromide) and beta-2 adrenergic agonists (e.g., albuterol, salmeterol), as well as oral theophylline provide significant although modest efficacy. Frequent treatment with broad-spectrum antibiotics is necessary, because microbial colonization is a common finding. Tetracycline antibiotics such as doxycycline are efficacious in CRD. Treating with doxycycline (5–10 mg/kg PO every 12 hours) or a long-acting depot such as doxycycline (70–100 mg/kg subcutaneously or intramuscularly every 7 days; Vibravenos; Pfizer Animal Health, Parsippany-Troy Hills, NJ), often helps affected rats.
Bacterial pneumonia is nearly always caused by S. pneumoniae (Fig. 25.19 ), but usually in combination with M. pulmonis (Fig. 25.20 ), Sendai virus, or cilia-associated respiratory bacillus.84 Pneumonia caused by S. pneumoniae can occur acutely. Young rats are more severely affected than are older ones, and often the only sign they exhibit is sudden death. Mature rats may demonstrate dyspnea, snuffling, and abdominal breathing. A purulent exudate may be seen around the nares and on the front paws (from wiping of the nostrils). A tentative diagnosis is based on identifying numerous gram-positive diplococci on a Gram’s stain of the exudate or in a sample submitted for cytologic examination. Severe bacteremia is an important consequence of advanced disease and results in multiorgan abscesses and infarction. Treatment must be aggressive, and the use of beta-lactamase-resistant penicillins such as cloxacillin, oxacillin, and dicloxacillin (all of which can be administered orally) is recommended. The first-choice antibiotic treatment for S. pneumoniae pneumonia is amoxicillin/clavulanic acid (20 mg/kg PO every 12 hours).
Fig. 25.19.
Fibrinopurulent pneumonia (arrow) caused by Streptococcus pneumoniae in a rat.
Fig. 25.20.
Gross appearance of the lungs in a rat with pneumonia caused by Mycoplasma pulmonis. Compare the appearance of these lungs with that of the lungs in Fig. 25.19. Rats in the later stages of M. pulmonis chronic respiratory disease may show pulmonary abscesses (arrows).
Pneumonia caused by C. kutscheri occurs rarely, and only in conjunction with debilitation or immunosuppression.84 In pet rats, immune suppression can result from diabetes, neoplasia, or dietary deficiencies.
Gastrointestinal System
Sialodacryoadenitis virus, a coronavirus, causes inflammation and edema of the cervical salivary glands. Owners of infected rats often describe their pets as having mumps. Sialodacryoadenitis virus is highly contagious and initially causes rhinitis, followed by epithelial necrosis and inflammatory swelling of the salivary and lacrimal glands. Cervical lymph nodes also become enlarged. There is no treatment for this disease. Glandular healing follows within 7 to 10 days, and clinical signs subside within 30 days, with few residual lesions remaining. During acute inflammation, affected rats are at high risk for anesthesia-related death because of the decreased diameter of the upper respiratory tract lumen; also, ocular lesions such as conjunctivitis, keratitis, corneal ulcers, synechiae, and hyphema can develop as a result of lacrimal dysfunction. The eye lesions usually resolve but occasionally progress to chronic keratitis and megaglobus.
Rats, like mice, are susceptible to pinworm infections. They may be infected with the rat pinworm (Syphacia muris), as well as the two common mouse pinworm species (S. obvelata and A. tetrapetra). Generally infections are asymptomatic in rats; however, heavy infections may cause failure to gain weight.4 Various treatments have been shown to be successful, including ivermectin, fenbendazole, and levamisole (see Table 25.8 for dosages). However, topical selamectin and oral moxidectin have been shown to be ineffective.28 , 30 Decontamination of the environment is also important to eliminate ova that can persist and remain infectious.
Urinary System
Chronic progressive nephrosis (CPN) is the best known of age-related diseases in rats. In CPN, the kidneys are enlarged and pale and have a pitted, mottled surface that often contains pinpoint cysts. Lesions consist of a progressive glomerulosclerosis and myriad tubulointerstitial disease primarily involving the convoluted proximal tubule.31 The most striking change in renal function is proteinuria exceeding 10 mg/day that increases in severity progressively with age. The features of CPN are qualitatively similar among different strains of laboratory rats, but the onset, incidence, and severity of the disease vary considerably. The disease occurs earlier and is of greater severity in males than in females: urinary protein excretion averaging 137 mg/day has been documented in 18-month-old male Sprague-Dawley rats, whereas excretion averaging 76 mg/day was reported in female rats of the same age.75 Dietary factors appear to have an important role in the progression of CPN. Caloric restriction, the feeding of low-protein diets (4%–7%), and limiting the source of dietary protein reduce the incidence and severity of CPN. Feeding soybean protein (as opposed to casein) and caloric restriction contribute substantially to reducing the incidence and severity of CPN; low-calorie diets that contain high protein levels do not decrease the incidence or severity. Drugs and exposure to chemicals can also exacerbate CPN. Diagnosis is based on clinical signs (polyuria/polydipsia), plasma biochemical results, and proteinuria. If only a small amount of blood is obtained, blood urea nitrogen concentration can be estimated with the use of a blood dipstick. Treatment is supportive and involves feeding a low-protein diet and administering anabolic steroids.
Uroliths and renal pelvic calculi are relatively infrequent in rats, especially compared with guinea pigs, rabbits, and chinchillas. Most uroliths that have been described were composed of struvite.62
Musculoskeletal and Peripheral Nervous System
Clinically, old rats show disturbances in motor function, posterior paresis and paralysis, loss of tail control, incontinence, and weight loss. Distinguishing between age-related peripheral or central nervous system changes, neurogenic muscular atrophy, and primary age changes in muscles is very difficult. Posterior paresis is commonly caused by spinal nerve root degeneration (also known as radiculoneuropathy, polyradiculoneuropathy, and degenerative myelopathy).87 Spontaneous tumors of the peripheral nervous system in rats are very rare; only isolated cases are reported in extensive tumor incidence surveys.1 Allowing young rats to stand on their hind legs frequently is a predisposing factor in the development of avascular necrosis of the femoral head (Legg-Calve-Perthes disease).59
Integument
Ectoparasitic infestation is more common in rats than in mice. The most common fur mites that infest rats are Radfordia ensifera, although mouse fur mites (R. affinis and M. musculi) can sometimes be found.28 Although R. ensifera infestation produces few ill effects, heavy infestation may lead to self-traumatization and ulcerative dermatitis. Other mites, including Demodex species, have been described in rats maintained in laboratories; however, they are seldom seen, and no contemporary reports of infestations in pet rats appear in the literature.82 The tropical rat mite (Ornithonyssus bacoti) is an important opportunistic ectoparasite often found on pet mice and rats (Fig. 25.21 ). The mites spend a relatively short time on a host and penetrate the skin for feeding only; therefore cleaning the environment is essential for clearing up an infestation. Severe infestations can cause anemia, debilitation, and death in rodents. When an animal host is not available, humans can become the victims of mite infestation. Detect adult parasites by macroscopic examination of the host; the mites are orange red. Rats with ectoparasites can be treated with selamectin (15 mg/kg as topical spot on, repeat in 10 days). The environment can be decontaminated with either synthetic pyrethroids or fipronil (see Table 25.8).5
Fig. 25.21.
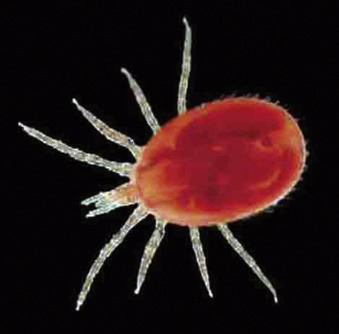
The tropical rat mite (Ornithonyssus bacoti). Engorged with the host’s blood, the mite appears red. It is an opportunistic ectoparasite often found on pet rodents including rats and mice. When an animal host is not available, humans can become victims of mite infestation. The mite spends only a short time on the host, penetrating the skin only for feeding. Severe infestations can resemble a rodent covered in fine sawdust; the sawdust is thousands of mites. Affected rodents can experience anemia, debilitation, and even death.
Ringtail is a pathologic condition of the tail of young rats that is typically characterized by dry skin and the formation of annular constrictions. In severe cases, blood vessels distal to the constrictions thrombose, resulting in pain, necrosis, and even autoamputation in some cases. This lesion is highly photogenic and probably for this reason is always described in textbooks and articles on diseases of rats. It occurs primarily during the preweaning period in rats aged 2 to 19 days and occasionally in young mice. Low environmental relative humidity (less than 40%) appears to be the cause, and it is more often seen in rats housed in hanging cages; it is rarely seen in pet rats. If ringtail is diagnosed, treatment options involve adding unsaturated fatty acids to the diet (e.g., 5% corn oil or an essential fatty acid supplement containing omega-3 fatty acids) or topical application of lanolin.25 , 81 If avascular necrosis has occurred, amputate the tail proximal to the necrotic annular constriction.
Ulcerative dermatitis caused by S. aureus infection results from self-traumatization associated with fur mite infestation or, more commonly, from scratching of the skin over an inflamed salivary gland (see sialodacryoadenitis virus under “Gastrointestinal System,” above). Rats have a remarkable ability to resist infection with S. aureus.22 Treatment consists of clipping the toenails of the hind paws, cleaning the ulcerated skin, and applying a topical antibiotic. Systemic treatment is rarely necessary.
Neoplasia
The most common subcutaneous tumor in the rat is fibroadenoma of the mammary glands. The distribution of the mammary tissue is extensive, and the tumors can occur anywhere from the neck to inguinal region (Fig. 25.22 ). Tumors can reach 8 to 10 cm in diameter and occur in both males and females. Adenocarcinomas are less common and represent fewer than 15% of mammary tumors in pet rats. In a study of 105 subcutaneous masses in 100 pet rats, 53% were benign mammary fibroadenomas, and 12% were mammary carcinomas.88 In the same study, 14 of 35 rats with mammary fibroadenomas that were necropsied had concomitant reproductive tract abnormalities, such as endometrial polyps, hyperplasia, paraovarian cysts, and suppurative metritis.
Fig. 25.22.
Mammary fibroadenoma in the inguinal region of a female rat. The rat weighed 355 g before surgery, and the excised tumor weighed 40 g, representing 11% of the rat’s body weight.
Prevention and treatment of mammary neoplasia involves surgery and medical treatment. The surgical technique for mammary tumor removal ranges from straightforward to complicated (for high cervical or inguinal tumors) depending on the location and size of the tumor (see Chapter 33). However, recurrence of fibroadenomas is common in uninvolved mammary tissue, and repeat surgeries are often required. Although tumors do not metastasize, death is often caused by large tumors that ulcerate and become secondarily infected. The incidence of mammary tumors is significantly lower in early (<3 months) ovariectomized versus sexually intact rats due to influence of hormones.39 Spaying sexually mature females (4–7 months old) has been shown to reduce mammary tumor formation67; efficacy of spaying rats older than 7 months to prevent mammary gland tumors is not known. It has been recommended that female rats be spayed when undergoing surgery for tumor removal. However, there are little data on efficacy of spaying at tumor removal in preventing tumor recurrence, and more tumors can develop in these cases.88 In cases where an abdominal surgery cannot be performed or is not advised, subcutaneous deslorelin implants can be placed to suppress hormone production, although the effectiveness of this treatment at the time of tumor occurence is unknown and debated in practice. As with spaying, age may be a factor in efficacy of hormonal implants in prevention of mammary tumors. Placement of a deslorelin implant at the time of tumor removal has not been effective in preventing tumor recurrence in some cases.88
Another common neoplasm in rats is pituitary adenoma. Incidence increases with age, and many of these tumors secrete prolactin, which can cause spontaneous lactation.8 Prevalence of pituitary adenomas does not differ significantly between rats with or without mammary fibroadenomas, and a causal role of adenomas in mammary fibroadenoma development is unknown.88 Often older rats with pituitary adenomas will present with neurologic signs such as hind limb paresis or paralysis, changes in vision, etc. Advanced imaging, such as computed tomography or magnetic resonance imaging, is recommended instead of radiographs to make the diagnosis. Treatment with cabergoline (0.6 mg/kg PO every 72 hours) can decrease the size of the mass and even eliminate clinical signs.57 However, this does not eliminate the tumor entirely, and regrowth can occur.57 Surgery is not possible because of the location of the tumor.
Zymbal’s gland tumor is a unique neoplasm that occurs in the specialized sebaceous glands of rats. The Zymbal’s gland is an auditory sebaceous gland that lies ventral to the orifice of the external ear.8 Most Zymbal’s gland tumors are carcinomas and usually have abundant keratin production and papillary projections of epithelial cells. Many of these tumors can be mistaken for squamous cell carcinomas on histologic study.8 The invasive, malignant nature of these tumors and location close to vital structures makes surgical removal difficult or impossible.
Zoonoses
One of the more important zoonotic diseases of rats is S. moniliformis, also known as “rat bite fever.” Streptobacillus moniliformis is a gram-negative bacteria that can reside in the nasopharyngeal cavity of asymptomatic rats but can also cause otitis media.23 Transmission to people occurs most commonly through a bite wound, so educating clients about gentle and appropriate handling of pet rats is very important. If a bite does occur, urge clients to seek medical attention. Symptoms of S. moniliformis infection in people are fever, malaise, headache, sore throat, lymphadenitis, myalgia, and petechial rash.23 If one or more large joints are involved, septic arthritis can develop.23 Screening PCR tests to detect the presence of S. moniliformis in pet rats are available.
As mentioned above, the tropical rat mite (O. bacoti) can bite humans opportunistically. This mite can also be a vector for transmission of hantavirus.23 People generally develop pruritic dermatitis and a rash after exposure to this mite. Clients who develop these symptoms should treat their pet rodents for mite infestation (see Table 25.8). Emphasis should also be placed on proper cleaning and disinfection of the environment (nest, bedding materials, cage accessories, etc.), because these mites spend most of their time in the environment and not on the host.
Two other potential zoonotic diseases in rats, salmonellosis and leptospirosis, are also seen in mice. Refer to the previous section on zoonoses in mice for more information on these infectious agents. For further information on zoonotic diseases, see Chapter 42.
References
- 1.Abbott D.P. Malignant schwannoma of the dorsal spinal nerve root in a laboratory rat. Lab Anim. 1982;16:265–266. doi: 10.1258/002367782780891750. [DOI] [PubMed] [Google Scholar]
- 2.Adams S.C., Garner J.P., Felt S.A., et al. A “pedi” cures all: toenail trimming and the treatment of ulcerative dermatitis in mice. PLoS One. 2016;11(1):e0144871. doi: 10.1371/journal.pone.0144871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baker D.G. Natural pathogens of laboratory mice, rats, and rabbits and their effects on research. Clin Microbiol Rev. 1998;11:231–266. doi: 10.1128/cmr.11.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baker D.G. In: The Laboratory Rat. 2nd ed. Suckow M.A., Weisbroth S.H., Franklin C.L., editors. Academic Press; Boston: 2006. Parasitic diseases. [Google Scholar]
- 5.Beck W. Common endo- and ectoparasitic diseases in small mammals—clinical feature, diagnosis and treatment. A review of the literature and own experiences. [German] Haufige Endo- und Ektoparasitosen bei kleinen Heimsaugern - Klinik, Diagnostik und Therapie Literaturubersicht und eigene Erfahrungen. Tierarztl Prax Ausg K Klientiere Heimtiere. 2004;32:311–321. [Google Scholar]
- 6.Becker C.E., Mathur C.F., Rehnberg B.G. The effects of chronic exposure to common bedding materials on the metabolic rate and overall health of male CD-1 mice. J Appl Anim Welf Sci. 2010;13(1):46–55. doi: 10.1080/10888700903372010. [DOI] [PubMed] [Google Scholar]
- 7.Bober R. Technical review: drawing blood from the tail artery of a rat. Lab Anim. 1988;17:33–34. [Google Scholar]
- 8.Boorman G.A., Everitt J.I. In: The Laboratory Rat. 2nd ed. Suckow M.A., Weisbroth S.H., Franklin C.L., editors. Academic Press; Boston: 2006. Neoplastic disease. [Google Scholar]
- 9.Brayton C. In: Fox J.G., Barthold S.W., Davisson M.T., et al., editors. Vol. 2. Academic Press; Burlington: 2007. Spontaneous diseases in commonly used mouse strains. (The Mouse in Biomedical Research). [Google Scholar]
- 10.Burn C.C., Peters A., Day M.J., et al. Long term effects of cage-cleaning frequency and bedding type on laboratory rat health, welfare and handleability: a cross-laboratory study. Lab Anim. 2006;40(4):353–370. doi: 10.1258/002367706778476460. [DOI] [PubMed] [Google Scholar]
- 11.Capello V., Gracis M., Lennox A.M., editors. Rabbit and Rodent Dentistry Handbook. Wiley-Blackwell; Ames: 2005. [Google Scholar]
- 12.Capello V., Lennox A.M. Wiley-Blackwell; Ames: 2008. Radiology of Exotic Companion Mammals. [Google Scholar]
- 13.Carpenter J.W., editor. Exotic Animal Formulary. 5th ed. Elsevier; St. Louis: 2018. pp. 459–493. [Google Scholar]
- 14.Castro P.A., Sohn C.S., Roman L. Nonsurgical correction for vaginal/uterine prolapse in mice [P27] Proc 61st Annu Meet Am Assoc Lab Anim Sci. 2010;150 [Google Scholar]
- 15.Chevret P., Denys C., Jaeger J., et al. Molecular evidence that the spiny mouse (Acomys) is more closely related to gerbils (Gerbillinae) than to true mice (Murinae) Proc Nat Acad Sci USA. 1993;90(8):3433–3436. doi: 10.1073/pnas.90.8.3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dammann P., Hilken G., Hueber B., et al. Infectious microorganisms in mice (Mus musculus) purchased from commercial pet shops in Germany. Lab Anim. 2011;45:271–275. doi: 10.1258/la.2011.010183. [DOI] [PubMed] [Google Scholar]
- 17.Deeb B. Respiratory disease in pet rats. Exot DVM. 2005;7:31–33. [Google Scholar]
- 18.de Matos R. In: BSAVA Manual of Rodents and Ferrets. Keeble E., Meredith A., editors. British Small Animal Veterinary Association; Gloucester, UK: 2009. Rodents: therapeutics; pp. 52–62. [Google Scholar]
- 19.Donnelly T.M. What’s your diagnosis? Blood-caked staining around the eyes in sprague-dawley rats. Lab Anim. 1997;26:17–18. [Google Scholar]
- 20.Donnelly T.M. Application of laboratory animal immunoassays to exotic pet practice. Exot DVM. 2006;8:19–26. [Google Scholar]
- 21.Donnelly T.M., Rush E.M., Lackner P.A. Ringworm in small exotic pets. Sem Avian Exot Pet Med. 2000;9:82–93. [Google Scholar]
- 22.Donnelly T.M., Stark D.M. Susceptibility of laboratory rats, hamsters, and mice to wound infection with Staphylococcus aureus. Am J Vet Res. 1985;46:2634–2638. [PubMed] [Google Scholar]
- 23.Feldman S.H., Easton D.N. In: The Laboratory Rat. 2nd ed. Suckow M.A., Weisbroth S.H., Franklin C.L., editors. Academic Press; Boston: 2006. Occupational health and safety. [Google Scholar]
- 24.Ferrecchia C.E., Jensen K., Van Andel R. Intracage ammonia levels in static and individually ventilated cages housing C57BL/6 mice on 4 bedding substrates. J Am Assoc Lab Anim Sci. 2014;53(2):146–151. [PMC free article] [PubMed] [Google Scholar]
- 25.Flynn R.J. In: Husbandry of Laboratory Animals. Conalty M.L., editor. Academic Press; London & New York: 1967. Notes on ringtail in rats; pp. 285–288. [Google Scholar]
- 26.Foltz C.J., Fox J.G., Yan L., Shames B. Evaluation of antibiotic therapies for eradication of Helicobacter hepaticus. Antimicrob Agents Chemother. 1995;39(6):1292–1294. doi: 10.1128/aac.39.6.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fox J.G., Cohen B.J., Loew F.M., editors. Laboratory Animal Medicine. Academic Press; Orlando: 1984. [Google Scholar]
- 28.Fox J.G., Andersen L.C., Otto G.M., et al., editors. Laboratory Animal Medicine. 3rd ed. Academic Press; San Diego: 2015. [Google Scholar]
- 29.Gaskill B.N., Pritchett-Corning K.R., Gordon C.J., et al. Energy reallocation to breeding performance through improved nest building in laboratory mice. PLoS One. 2013;8(9):e74153. doi: 10.1371/journal.pone.0074153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gonenc B., Sarimehmetoglu H.O., Ica A., et al. Efficacy of selamectin against mites (Myobia musculi, Mycoptes musculinus and Radfordia ensifera) and nematodes (Aspiculuris tetraptera and Syphacia obvelata) in mice. Lab Anim. 2006;40:210–213. doi: 10.1258/002367706776319105. [DOI] [PubMed] [Google Scholar]
- 31.Gray J.E. In: Urinary System. Jones T.C., Mohr U., Hunt R.D., editors. Springer-Verlag; Berlin: 1986. Chronic progressive nephrosis, rat; pp. 174–178. [Google Scholar]
- 32.Harkness J.E., Turner P.V., VandeWoude S., et al. 5th ed. Wiley-Blackwell; Ames: 2010. Harkness and Wagner’s Biology and Medicine of Rabbits and Rodents. [Google Scholar]
- 33.Harkness J.E., Wagner J.E. 4th ed. Williams & Wilkins; Baltimore: 1995. The Biology and Medicine of Rabbits and Rodents. [Google Scholar]
- 34.Hartmann M.J.Z. A night in the life of a rat: vibrissal mechanics and tactile exploration. Ann N Y Acad Sci. 2011;1225:110–118. doi: 10.1111/j.1749-6632.2011.06007.x. [DOI] [PubMed] [Google Scholar]
- 35.Heider K., Eustis S.L. In: Tumours of the Mouse. Turusov V.S., Mohr U., editors. International Agency for Research on Cancer; Lyon: 1994. Tumors of the soft tissues; pp. 611–631. [Google Scholar]
- 36.Hem A., Smith A.J., Solberg P. Saphenous vein puncture for blood sampling of the mouse, rat, hamster, gerbil, guinea pig, ferret, and mink. Lab Anim. 1998;32:364–368. doi: 10.1258/002367798780599866. [DOI] [PubMed] [Google Scholar]
- 37.Hoff J. Methods of blood collection in the mouse. Lab Anim. 2000;29(10):47–53. [Google Scholar]
- 38.Horn C.C., Kimball B.A., Wang H., et al. Why can’t rodents vomit? A comparative behavioral, anatomical, and physiological study. PLoS One. 2013;8(4):e60537. doi: 10.1371/journal.pone.0060537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hotchkiss C.E. Effect of surgical removal of subcutaneous tumors on survival of rats. J Am Vet Med Assoc. 1995;206:1575–1579. [PubMed] [Google Scholar]
- 40.Hoyt R.F., Hawkins J.V., St Clair M.B., Kennett M.J. In: Fox J.G., Barthold S.W., Davisson M.T., et al., editors. Vol. 3. Academic Press; Burlington: 2007. Mouse physiology. (The Mouse in Biomedical Research). [Google Scholar]
- 41.Johnson-Delaney C. In: BSAVA Manual of Rodents and Ferrets. Keeble E., Meredith A., editors. British Small Animal Veterinary Association; Gloucester, UK: 2009. Rodents: biology, husbandry and clinical techniques in more unusual pet species; pp. 96–106. [Google Scholar]
- 42.Kalueff A.V., Minasyan A., Keisala T., et al. Hair barbering in mice: implications for neurobehavioural research. Behav Processes. 2006;71:8–15. doi: 10.1016/j.beproc.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 43.Kastenmayer R.J., Fain M.A., Perdue K.A. A retrospective study of idiopathic ulcerative dermatitis in mice with a C57BL/6 background. J Am Assoc Lab Anim Sci. 2006;45:8–12. [PubMed] [Google Scholar]
- 44.Keeble E. In: BSAVA Manual of Rodents and Ferrets. Keeble E., Meredith A., editors. British Small Animal Veterinary Association; Gloucester, UK: 2009. Rodents: biology and husbandry; pp. 1–17. [Google Scholar]
- 45.Kerstjens H.A., Postma D., ten Hacken N. 2008. COPD. Clin Evid (Online) p. 1502. [PMC free article] [PubMed] [Google Scholar]
- 46.Kramer K., Kinter L.B. Evaluation and applications of radiotelemetry in small laboratory animals. Physiol Genomics. 2003;13:197–205. doi: 10.1152/physiolgenomics.00164.2002. [DOI] [PubMed] [Google Scholar]
- 47.Krautwald-Junghanns M.E., Pees M., Reese S., et al. Schlutersche; Hannover: 2010. Diagnostic Imaging of Exotic Pets: Birds, Small Mammals, Reptiles. [Google Scholar]
- 48.Krogstad A.P., Franklin C.L., Besch-Williford C.L. An epidemiological and diagnostic approach to murine skin lesions [meeting abstract] Contemp Top Lab Anim Sci. 2001;40:82–83. [Google Scholar]
- 49.Kurien B.T., Everds N.E., Scofield R.H. Experimental animal urine collection: a review. Lab Anim. 2004;38:333–361. doi: 10.1258/0023677041958945. [DOI] [PubMed] [Google Scholar]
- 50.Laber-Laird K., Swindle M.M., Flecknell P., editors. Handbook of Rodent and Rabbit Medicine. Elsevier Science; Oxford, UK: 1996. [Google Scholar]
- 51.Lawson G.W., Sato A., Fairbanks L.A., et al. Vitamin E as a treatment for ulcerative dermatitis in C57BL/6 mice and strains with a C57BL/6 background. Contemp Top Lab Anim Sci. 2005;44:18–21. [PubMed] [Google Scholar]
- 52.Lennox A.M. Intraosseous catheterization of exotic animals. J Exot Pet Med. 2008;17(4):300–306. [Google Scholar]
- 53.Lindstrom N.M., Moore D.M., Zimmerman K., Smith S.A. Hematologic assessment in pet rats, mice, hamsters and gerbils: Blood sample collection and blood cell identification. Vet Clin Exot Anim. 2015;18:21–32. doi: 10.1016/j.cvex.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 54.Losco P.E. Dental dysplasia in rats and mice. Toxicol Pathol. 1995;23:677–688. doi: 10.1177/019262339502300605. [DOI] [PubMed] [Google Scholar]
- 55.Lytvynets A., Langrova I., Lachout J., Vadlejch J. Detection of pinworm eggs in the dust of laboratory animals breeding facility, in the cages and on the hands of the technicians. Lab Anim. 2013;47(1):71–73. doi: 10.1258/la.2012.012060. [DOI] [PubMed] [Google Scholar]
- 56.Masoro E.J. Caloric restriction-induced life extension of rats and mice: a critique of proposed mechanisms. Biochim Biophys Acta. 2009;1790(10):1040–1048. doi: 10.1016/j.bbagen.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 57.Mayer J., Sato A., Kiupel M., et al. Extralabel use of cabergoline in the treatment of a pituitary adenoma in a rat. J Am Vet Med Assoc. 2011;239(5):656–660. doi: 10.2460/javma.239.5.656. [DOI] [PubMed] [Google Scholar]
- 58.Michaud C.R., Qin J., Elkins W.R., Gozalo A.S. Comparison of 3 topical treatments against ulcerative dermatitis in mice with a C57BL/6 background. Comp Med. 2016;66(2):100–104. [PMC free article] [PubMed] [Google Scholar]
- 59.Mihara K., Hirano T. Standing is a causative factor in osteonecrosis of the femoral head in growing rats. J Pediatr Orthop. 1998;18:665–669. doi: 10.1097/00004694-199809000-00022. [DOI] [PubMed] [Google Scholar]
- 60.Montedonico S., Godoy J., Mate A., et al. Muscular architecture and manometric image of gastroesophageal barrier in the rat. Dig Dis Sci. 1999;44(12):2449–2455. doi: 10.1023/a:1026678820384. [DOI] [PubMed] [Google Scholar]
- 61.Newcomer C.E., Fox J.G. In: Fox J.G., Barthold S.W., Davisson M.T., et al., editors. Vol. 2. Academic Press; Burlington: 2007. Zoonoses and other human health hazards. (The Mouse in Biomedical Research). [Google Scholar]
- 62.Osborne C.A., Albasan H., Lulich J.P., et al. Quantitative analysis of 4468 uroliths retrieved from farm animals, exotic species, and wildlife submitted to the Minnesota Urolith Center: 1981 to 2007. Vet Clin North Am Small Anim Pract. 2009;39:65–78. doi: 10.1016/j.cvsm.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 63.Parasuraman S., Raveendran R., Kesavan R. Blood sample collection in small laboratory animals. J Pharmacol Pharmacother. 2010;1(2):87–93. doi: 10.4103/0976-500X.72350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Payne A.P. The harderian gland: a tercentennial review. J Anat. 1994;185:1–49. [PMC free article] [PubMed] [Google Scholar]
- 65.Phifer-Rixey M., Nachman M.W. Insights into mammalian biology from the wild house mouse Mus musculus. eLife. 2015;4:e05959. doi: 10.7554/eLife.05959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pickering M., Jones J.F.X. The diaphragm: two physiological muscles in one. J Anat. 2002;201(4):305–312. doi: 10.1046/j.1469-7580.2002.00095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Planas-Silva M.D., Rutherford T.M., Stone M.C. Prevention of age-related spontaneous mammary tumors in outbred rats by late ovariectomy. Cancer Detect Prev. 2008;32(1):65–71. doi: 10.1016/j.cdp.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 68.Pollicino P., Rossi L., Rambozzi L., et al. Oral administration of moxidectin for treatment of murine acariosis due to Radfordia affinis. Vet Parasitol. 2008;151:355–357. doi: 10.1016/j.vetpar.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 69.Pullium J.K., Brooks W.J., Langley A.D., et al. A single dose of topical moxidectin as an effective treatment for murine acariasis due to Myocoptes musculinus. Contemp Top Lab Anim Sci. 2005;44:26–28. [PubMed] [Google Scholar]
- 70.Reeves W.K., Cobb K.D. Ectoparasites of house mice (Mus musculus) from pet stores in South Carolina, U.S.A. Comp Parasitol. 2005;72:193–195. [Google Scholar]
- 71.Roediger B., Lee Q., Tikoo S., et al. An atypical parvovirus drives chronic tubulointerstitial nephropathy and kidney fibrosis. Cell. 2018:530–543. doi: 10.1016/j.cell.2018.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Saito M., Nakayama K., Muto T., et al. Effect of gaseous ammonia on Mycoplasma pulmonis infection in mice and rats. Jikken dobutsu. 1982;31(3):203–206. doi: 10.1538/expanim1978.31.3_203. [DOI] [PubMed] [Google Scholar]
- 73.Sanchez-Alavez M., Alboni S., Conti B. Sex- and age-specific differences in core body temperature of C57Bl/6 mice. Age (Dordr) 2011;33:89–99. doi: 10.1007/s11357-010-9164-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sandhu G.S., Solorio L., Broome A.M., et al. Whole animal imaging. Wiley Interdiscip Rev Syst Biol Med. 2010;2:398–421. doi: 10.1002/wsbm.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Short B.G., Goldstein R.S. In: Pathobiology of the Aging Rat. Mohr U., Dungworth D.L., Capen C.C., editors. International Life Sciences Institute Press; Washington: 1992. Nonneoplastic lesions in the kidney; pp. 211–225. [Google Scholar]
- 76.Silverman S., Tell L. WB Saunders; Philadelphia: 2005. Radiology of Rodents, Rabbits and Ferrets: An Atlas of Normal Anatomy and Positioning. [Google Scholar]
- 77.Spenser E.L. Compounding, extra label drug use and other pharmaceutical quagmires in avian and exotics practice. J Exot Pet Med. 2004;13(1):16–24. [Google Scholar]
- 78.Squartini F., Pingitore R. In: Tumours of the Mouse. Turusov V.S., Mohr U., editors. International Agency for Research on Cancer; Lyon: 1994. Tumors of the mammary gland; pp. 47–100. [Google Scholar]
- 79.Suckow M.A., Weisbroth S.H., Franklin C.L., editors. The Laboratory Rat. 2nd ed. Academic Press; Boston: 2006. [Google Scholar]
- 80.Sundberg J.P., Brown K.S., McMahon W.M. In: Handbook of Mouse Mutations With Skin and Hair Abnormalities. Sundberg J.P., editor. CRC Press; Boca Raton: 1994. Chronic ulcerative dermatitis in black mice; pp. 485–492. [Google Scholar]
- 81.Taylor D.K., Rogers M.M., Hankenson F.C. Lanolin as a treatment option for ringtail in transgenic rats. J Am Assoc Lab Anim Sci. 2006;45:83–87. [PubMed] [Google Scholar]
- 82.Walberg J.A., Stark D.M., Desch C., et al. Demodicidosis in laboratory rats (Rattus norvegicus) Lab Anim Sci. 1981;31:60–62. [PubMed] [Google Scholar]
- 83.Waren D.W., Kissoon N., Mattar A., et al. Pharmacokinetics from multiple intraosseous and peripheral intravenous site injections in normovolemic and hypovolemic pigs. Crit Care Med. 1994;22(5):838–843. doi: 10.1097/00003246-199405000-00021. [DOI] [PubMed] [Google Scholar]
- 84.Weisbroth S.H., Kohn D.F., Boot R. In: The Laboratory Rat. 2nd ed. Suckow M.A., Weisbroth S.H., Franklin C.L., editors. Elsevier Academic Press; Burlington: 2006. Bacterial, mycoplasmal and mycotic infections; pp. 339–421. [Google Scholar]
- 85.Wesche P. In: BSAVA Manual of Rodents and Ferrets. Keeble E., Meredith A., editors. British Small Animal Veterinary Association; Gloucester, UK: 2009. Rodents: clinical pathology; pp. 42–51. [Google Scholar]
- 86.Whiteside T.E., Thigpen J.E., Kissling G.E., et al. Endotoxin, coliform, and dust levels in various types of rodent bedding. J Am Assoc Lab Anim Sci. 2010;49(2):184–189. [PMC free article] [PubMed] [Google Scholar]
- 87.Witt C.J., Johnson L.K. Diagnostic exercise: rear limb ataxia in a rat [radiculoneuropathy] Lab Anim Sci. 1990;40:528–529. [PubMed] [Google Scholar]
- 88.Vergneau-Grosset C., Keel M.K., Goldsmith D., et al. Description of the prevalence, histologic characteristics, concomitant abnormalities, and outcomes of mammary gland tumors in companion rats (Rattus norvegicus): 100 cases (1990-2015) J Am Vet Med Assoc. 2016;249(10):1170–1179. doi: 10.2460/javma.249.10.1170. [DOI] [PubMed] [Google Scholar]


