Abstract
During the ongoing COVID-19 pandemic due to the SARS-CoV-2 virus of which evidence-based medical paradigms cannot be easily applied; difficult clinical decisions shall be required particularly in the 'difficult-to-treat' cases of high risk group with associated comorbidities. Convalescent immune plasma therapy is a promising option as a sort of 'rescue' treatment in COVID-19 immune syndrome, where miraculous antiviral drugs are not available yet. In this report, we aim to convey our experience of multi-task treatment approach with convalescent immune plasma and anti-cytokine drug combination in a COVID-19 patient with extremely challenging comorbidities including active myeloid malignancy, disseminated tuberculosis and kidney failure.
Keywords: COVID-19, Convalescent, Plasmapheresis, Myelodysplastic, Tuberculosis
1. Introduction
COVID-19 immune syndrome is a multisystemic disorder following the infection of the SARS-CoV-2 virus [1]. The syndrome mainly affects the lungs but other systems including the myocardium [2], central nervous system [3], liver [4], kidneys [5], bone marrow [6], and cutaneous tissues [7] are also under attack. SARS-CoV-2 virus-induced immune suppression and the impaired exaggerated pathological immune multisystemic attacks with macrophage activation are the hallmarks of the COVID-19 immune syndrome [8]. There are not sufficient randomized controlled trials for the treatment of COVID-19 since it is not easy to establish a classical evidence-based medicine approach due to the emergency conditions caused by the pandemic. Case reports and case series are the currently available clinical evidence particularly for the passive immunity transfer namely convalescent immune plasma therapy. Given the absence of effective miracle antiviral drugs, convalescent plasma therapy is one of a few promising treatments for the specific COVID-19 management [9].
Among the studied COVID-19 subpopulations, patients with comorbidites like maligancy and kidney disease constitute the high-risk group for the poor clinical outcomes [10]. In this critical subpopulation, there is no data regarding the treatment with immune plasma products. We, herein, report an immunocompromised patient due to myelodysplastic syndrome (MDS/RAEB1 FAB subtype), complicated by recently disseminated tuberculosis with associated kidney disease, and attacked by SARS-CoV-2 leading to COVID-19 syndrome, which was successfully managed via the administration of double convalescent immune plasma therapies.
Elucidation of the exact administration schedule of convalescent immune plasma in COVID-19 immune syndrome is important for the true management of that difficult-to-treat disease states, which have a potential of morbidity and mortality.
2. Case presentation
A 55-year-old male with a history of MDS with FAB refractory anemia excess blasts-1 subtype (MDS/ RAEB-1) complicated by disseminated systemic tuberculosis and associated kidney disease was admitted to our hospital with the complaints of ongoing high fever and persistent cough lasting for about three days. He had recently been discharged from the hospital after a follow-up of two months for the disseminated tuberculosis infection and still was on the classic four-drug regimen (isoniazid, rifampin, pyrazinamide, ethambutol). The diagnosis of MDS was reached approximately
two and a half years ago in another clinic, of which he had been followed at three-months of intervals without any specific MDS-directed therapeutic intervention.
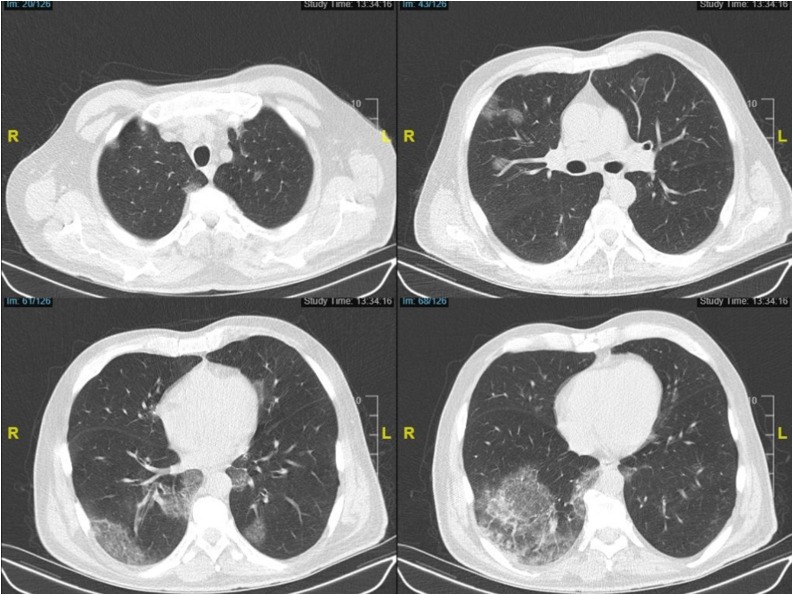
Upon the admission, physical examination revealed that he had a fever of 38.8 °C, tachycardia (121 bpm), and tachypnea (24 breaths per min). The oxygen saturation was 95 % in the room air. Low-dose chest computed tomography disclosed bilateral multiple peripheral multifocal ground-glass opacities, which indicated COVID-19 pneumonia (Fig. 1 ). Just after obtaining the nasopharyngeal swab sample, which was later found to be positive for COVID-19 infection, he was urgently hospitalized. Complete blood counts revealed leukocytosis, lymphopenia, neutrophilia and eosinophilia, with a high ferritin, C-reactive protein (CRP), D-dimer and LDH. The values of the laboratory tests are given in Table 1 . Basal corrected QT interval (QTc) was calculated as 488 milliseconds in the electrocardiogram (ECG). Based on those examinations, an antiviral drug, favipiravir was prescribed solely. The hydroxychloroquine/ azithromycin combination could not be administered due to the prolonged basal QTc. 4-drug regimen for tuberculosis was continued as well. Since he had a high neutrophil count and a procalcitonin level, complicating bacterial infection could not be ruled out and meropenem was added to the treatment scheme on the second day of antiviral treatment after obtaining the blood, urine and sputum cultures. On the fifth day of the symptom-onset, the patient complained of severe dyspnea and became tachypneic (26–28 breaths per min). He still had a fever of 39.5 °C. Taking his significant comorbidities and immunocompromised state into consideration, previously-stored 200 mL of convalescent plasma product was transfused to our patient by following universal infusion safety protocols without any adverse reaction or complication. The convalescent plasma product was collected using Trima Accel® Automated Blood Collection System from a donor who had previously recovered from COVID-19 disease and met universal donation criteria. The anti-SARS-CoV-2 IgG semi-quantitative titer of the donor’s plasma studied by the EUROIMMUN ELISA kit (Order no EI 2606-9601 G. Produced by EUROIMMUN AG, Seekamp 31, 23560 Luebeck, Germany) was found to be positive (Titer 6.6; <0.8 negative, ≥0.8 to <1.1 borderline, ≥ 1.1 positive) before collection.
Fig. 1.
Computed Tomography sections on admission are consistent with moderate COVID-19 pneumonia.
Table 1.
Significant laboratory values at the time of admission and discharge.
| Test | Admission (Day 0) | Discharge (Day 11) |
|---|---|---|
| Leukocyte Count (x103/ μL) | 15.2 | 14.2 |
| Neutrophile Count (x103/ μL) | 12.83 | 9.19 |
| Eosinophile Count (x103/ μL) | 1.3 | 2.04 |
| Lymphocyte Count (x103/ μL) | 1.01 | 1.58 |
| hsCRP (mg/dL) | 18.812 | 3.367 |
| Procalcitonin (ng/mL) | 2.57 | 0.93 |
| Ferritin (μg/L) | 1868 | 1943 |
| Creatinine (mg/dL) eGFR (mL/min/1.73m²) |
3.36 19.49 |
1.61 47.43 |
| D-dimer (mg/L) | 2.5 | 1.84 |
| LDH (U/L) | 477 | 316 |
During the follow-up period, he complained of progressive dyspnea again. Tachypnea (30–32 breaths per min) and fever did not resolve as well. The oxygen saturation was 90 % at the room air, hence oxygen supplementation of two L/min with nasal cannula was initiated on the fourth day of the antiviral treatment. Taking the clinical deterioration into consideration, the patient was transferred to the intensive care unit (ICU) and another 200 mL of convalescent immune plasma which was obtained from the same donor was transfused again. Since the acute phase reactants namely CRP, D- dimer, and ferritin remained high, lymphocyte count began to further decrease (0.89 × 103/μL) and persistent fever was evident; macrophage activation syndrome (MAS) was suspected. H-score for the reactive hemophagocytic syndrome was calculated as 169 points indicating the probability of MAS as 40–54 %. Serum IL-6 level was also high (72.2 pg/mL; normal range: 0–5.9). Therefore, tocilizumab 400 mg single dose was also given to manage the MAS status on the fourth day of the antiviral
treatment. Since the patient had already been under the effective treatment for tuberculosis for more than one month, the administration of tocilizumab was considered safe in this situation. However, as there was still a risk of tuberculosis reactivation, written informed consent was also obtained from the patient before the administration of the tocilizumab.
On the following days of admission, significant improvement in the general health status of the patient was observed via the administration of those multi-task clinical management approaches. Furthermore, anti-SARS-CoV-2 IgG test, which was studied by the previous same method was found to be positive as the titer of 5.1. His dyspnea improved and no febrile values were recorded. His respiratory rate was 24–26 breaths per minute and the oxygen saturation was above 95 % in the room air. Lymphocyte count increased gradually (up to 1.58 × 103/μL) and CRP values decreased (down to
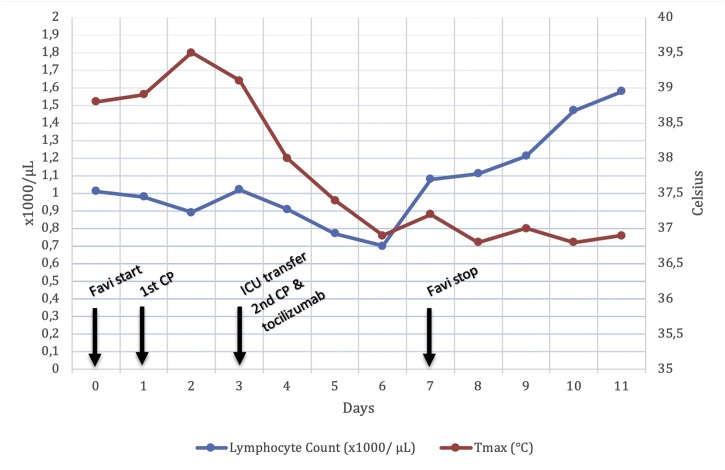
1.03 mg/dL). Favipiravir treatment was terminated on the seventh day. Since Klebsiella pneumoniae was also detected in the sputum culture, meropenem treatment was continued up to seven days as well. The patient was transferred back to the standard care ward from the ICU. The second PCR for SARS-CoV-2 was negative. After two days of the follow-up, he was discharged from the hospital with full recovery from the COVID-19 immune syndrome. Alterations of the absolute lymphocyte count and maximum body temperature throughout the clinical follow-up are shown in Fig. 2 .
Fig. 2.
Change in lymphocyte count and maximum body temperature throughout the days. Important clinical events have been marked.
3. Discussion
The ultimate growing hypothesis of this case report is that immune plasma treatment together with other multi-task clinical approach could be useful for the management of COVID-19 immune syndrome within ‘difficult-to-treat’ patients due to cancer, kidney failure and concurrent specific infection such as tuberculosis. The safety profile of this clinical approach is also acceptable since adverse events, intolerances, or toxicities were not observed regarding the double-administration of immune plasma treatment combined with anti-cytokine drug. Our COVID-19 patient truly represents a quite difficult subgroup to treat [10], since he already had acquired immunodeficiency due to active MDS/RAEB-1 myeloid neoplastic disease with disseminated tuberculosis infection together with renal injury complicated by multi-organ involvement.
This is just a single case report and those positive results inherently cannot be generalized to all immunocompromised patients with COVID-19. Nevertheless, in this period when treatment results with strong evidence cannot be obtained, it’s hard to find satisfactory options [11] and such examples are encouraging for the convalescent immune plasma therapies.
Another confounding clinical superimposed picture is the macrophage activation syndrome (MAS), which is linked to the severe acute phase reaction with IL-6 increment [8]. In order to break this link of the counterproductive chain, we preferred to administer the quite risky drug of tocilizumab in this already acquired immunodeficient person [12]. Meanwhile, significant improvements in both clinical findings and laboratory values of the patient took place just after the combination of double
immune plasma infusion and tocilizumab. Of course, it may not be easy to distinguish which of the main positive effect depends on those ‘paradoxical’ treatment choices (passive immune transfer versus immunosuppression). As he was a difficult patient to treat, we clinically decided to use the available options combined. The use of tocilizumab in immunocompromised patients is also a quite difficult decision, especially when there is a recent history of disseminated tuberculosis under active drug-combination treatment. In everyday medical practice particularly during the ongoing COVID-19 pandemic, sometimes it may be necessary to make difficult decisions to treat complicated patients with the infection. Hypothetically, the use of the immune plasma products may have also served as a buffering factor for the potential adverse effects of immunosuppressive medications such as anti- cytokine biological drugs. Of course, it is not possible to present this speculation objectively and further research is clearly needed. In all of the present expert opinion-based clinical decisions, the combined multi-task clinical approach was applied to our present patient and the results were positive on this single case basis. The contribution of the combined treatment of anti-cytokine treatments and convalescent immune plasma to COVID-19 management needs to be confirmed by future controlled studies.
CRediT authorship contribution statement
Olgu Erkin Çınar: Writing - original draft, Conceptualization. Başak Sayınalp: Writing - original draft. Elifcan Aladağ Karakulak: Investigation. Ayşe Avşar Karataş: Investigation. Mustafa Velet: Investigation. Ahmet Çağkan İnkaya: Investigation. Nazmiye Ebru Ersoy Ortaç: Investigation. Serpil Öcal: Investigation. Salih Aksu: Resources, Supervision. İbrahim Celalettin Haznedaroğlu: Methodology, Writing - review & editing. Nilgün Sayınalp: Conceptualization, Supervision. Osman İlhami Özcebe: Project administration, Supervision.
Declaration of Competing Interest
The authors declared no conflict of interest.
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Lai C.C. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents. 2020;55(3):105924. doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tavazzi G. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur J Heart Fail. 2020;22(5) doi: 10.1002/ejhf.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asadi-Pooya A.A., Simani L. Central nervous system manifestations of COVID-19: a systematic review. J Neurol Sci. 2020;413:116832. doi: 10.1016/j.jns.2020.116832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang C., Shi L., Wang F.S. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5(5):428–430. doi: 10.1016/S2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ronco C., Reis T. Kidney involvement in COVID-19 and rationale for extracorporeal therapies. Nat Rev Nephrol. 2020:1–3. doi: 10.1038/s41581-020-0284-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zini, G., et al., Morphological anomalies of circulating blood cells in COVID-19. American Journal of Hematology. n/a(n/a). [DOI] [PMC free article] [PubMed]
- 7.Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol. 2020;34(5) doi: 10.1111/jdv.16387. [DOI] [PubMed] [Google Scholar]
- 8.McGonagle D. The role of cytokines including Interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun Rev. 2020:102537. doi: 10.1016/j.autrev.2020.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duan K. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci U S A. 2020;117(17):9490–9496. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Emami A. Prevalence of underlying diseases in hospitalized patients with COVID- 19: a systematic review and meta-analysis. Arch Acad Emerg Med. 2020;8(1):e35. [PMC free article] [PubMed] [Google Scholar]
- 11.Şimşek Yavuz S., Ünal S. Antiviral treatment of COVID-19. Turk J Med Sci. 2020;50(Si-1):611–619. doi: 10.3906/sag-2004-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scott L.J. Tocilizumab: a review in rheumatoid arthritis. Drugs. 2017;77(17):1865–1879. doi: 10.1007/s40265-017-0829-7. [DOI] [PMC free article] [PubMed] [Google Scholar]