Abstract
Coronavirus disease 2019 (COVID-19) is a contagious life-threatening infection caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Recent findings indicate an increased risk for acute kidney injury during COVID-19 infection. The pathophysiologic mechanisms leading to acute kidney injury in COVID-19 infection are unclear but may include direct cytopathic effects of the virus on kidney tubular and endothelial cells, indirect damage caused by virus-induced cytokine release, and kidney hypoperfusion due to a restrictive fluid strategy. In this report of 2 cases, we propose an additional pathophysiologic mechanism. We describe 2 cases in which patients with COVID-19 infection developed a decrease in kidney function due to kidney infarction. These patients did not have atrial fibrillation. One of these patients was treated with therapeutic doses of low-molecular-weight heparin, after which no further deterioration in kidney function was observed. Our findings implicate that the differential diagnosis of acute kidney injury in COVID-19–infected patients should include kidney infarction, which may have important preventive and therapeutic implications.
Index Words: Coronavirus disease 2019 (COVID-19), severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), acute kidney injury (AKI), kidney infarction, anticoagulation, renal complications of COVID-19, thrombotic events, arterial thrombi, computed tomography (CT), case report
Introduction
Although diffuse alveolar damage and acute respiratory failure are the main features of coronavirus disease 2019 (COVID-19) infection, recent findings indicate an increased risk for acute kidney injury during the course of COVID-19 infection.1, 2, 3 The pathophysiologic mechanisms leading to acute kidney injury in COVID-19 infection are yet not fully elucidated but may include direct cytopathic effects of the virus on kidney tubular and endothelial cells, indirect damage caused by virus-induced cytokine release, or kidney hypoperfusion due to a restrictive fluid strategy.4, 5, 6 In this report of 2 cases, we propose an additional pathophysiologic mechanism. We describe 2 cases of patients with COVID-19 infection who developed a decrease in kidney function due to kidney infarction.
Case Reports
Case 1
A 62-year-old man with a history of hypertension, Henoch–Schönlein glomerulonephritis (not biopsy proven), and living related kidney transplantation in 2016 presented with dry cough, fever, and worsening dyspnea during the last week. Maintenance immunosuppressive therapy consisted of mycophenolate mofetil, 500 mg, orally twice daily; tacrolimus (Advagraf; 2 mg orally once daily), and prednisolone, 5 mg, orally once daily. His other medications consisted of metoprolol, losartan, barnidipine, hydrochlorothiazide, omeprazole, and vitamin D. He did not take any anticoagulants. COVID-19 infection was diagnosed in late March 2020 based on a reverse transcriptase–polymerase chain reaction test that detected severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in a throat swab sample.
At presentation at the Queen Beatrix Hospital (Winterswijk, the Netherlands), the patient was tachypneic (respiratory rate, 40 breaths/min) with oxygen saturation of 89%. His body temperature was 38.3°C and blood pressure was 121/73 mm Hg, with a pulse rate of 59 beats/min. There were no symptoms of emboli to the extremities. Laboratory findings were as follows: C-reactive protein, 7.0 mg/dL; leukocytes, 5.4 × 103/μL; lymphocytes, 0.8 × 103/μL; serum lactate dehydrogenase (LDH), 290 U/L; and serum creatinine (Scr), 1.28 mg/dL. An x-ray of the chest showed bilateral consolidations. An overview of demographic, clinical, and laboratory findings is shown in Table 1 . Initial treatment was supportive, with high-flow oxygen therapy and tapering of immunosuppression therapy. Thrombosis prophylaxis with dalteparin (2,500 units daily) was prescribed.
Table 1.
Demographics, Clinical Characteristics, and Laboratory Findings
| Case 1 | Case 2 | Reference Range | |
|---|---|---|---|
| Demographic Characteristics | |||
| Age, y | 62 | 58 | |
| Sex | Male | Male | |
| Clinical Findings | |||
| Medical history | HTN; Henoch–Schönlein GN; living-related KTx | Obstructive sleep apnea syndrome | |
| Symptoms at onset of disease | Dry cough, fever, dyspnea | Dry cough, fever, dyspnea, abdominal pain | |
| Imaging features at ICU admission | Bilateral consolidations | Bilateral consolidations | |
| Laboratory Findingsa | |||
| Hemoglobin, g/dL | 12.1 | 12.7 | 13.7-17.7 |
| Hematocrit | 37% | 39% | 40%-50% |
| Erythrocytes, ×106/μL | 4.1 | 4.2 | 4.5-5.8 |
| Leukocytes, ×103/μL | 19.9 | 20.5 | 4-10 |
| Thrombocytes, ×103/μL | 331 | 226 | 150-400 |
| Po2, mm Hgb | 60 | 83 | 75-100 |
| Pco2, mm Hg | 38 | 53 | 35-45 |
| pH | 7.44 | 7.35 | 7.35-7.45 |
| Bicarbonate, mEq/L | 25 | 28 | 21-27 |
| Lactate, mg/dL | 17 | 14 | 5.0-15 |
| Glucose, mg/dL | 162 | 204 | 80-100 |
| CRP, mg/dL | 11.9 | 48.6 | <0.5 |
| Sodium, mEq/L | 134 | 136 | 135-145 |
| Potassium, mEq/L | 5.2 | 4.4 | 3.5-5.0 |
| Ionized calcium, mEq/L | 2.4 | 2.2 | 2.3-2.58 |
| Aspartate aminotransferase, U/L | 105 | 78 | <35 |
| Alanine aminotransferase, U/L | 119 | 42 | <45 |
| Lactate dehydrogenase, U/L | 1,145 | 733 | <248 |
| γ-Glutamyltransferase, U/L | 292 | — | <55 |
| Alkaline phosphatase, U/L | 178 | — | <115 |
| Serum creatinine, mg/dL | 1.92 | 2.45 | 0.6-1.2 |
| eGFR,c mL/min/1.73 m2 | 37 | 28 | |
| Coagulation | |||
| D-Dimer, ng/mL | — | 28,186d | <500 |
| aPTT, s | 29e | 33 | 25-36 |
| PT, s | 14e | 15.7 | 10-13 |
| Fibrinogen, mg/dL | 616e | 920 | 200-400 |
| Urinary Analyses | |||
| Urinary dipstick | 2+ erythrocytes | — | |
| Urinary sodium, mEq/L | 60 | 46 | |
| Urinary protein, mg/dL | 37 | 102 | |
| Urinary creatinine, mg/dL | 45 | 111 | |
Note: The reference ranges are given for the Department of Laboratory Medicine of the University Medical Center Groningen. Conversion factors for units: hemoglobin in g/dL to mmol/L, ×0.6206; lactate in mg/dL to mmol/L, ×0.111; glucose in mg/dL to mmol/L, ×0.0555; ionized calcium in mEq/L to mmol/L, ×0.5; creatinine in mg/dL to μmol/L, ×88.4; fibrinogen in mg/dL to μmol/L, ×0.0294.
Abbreviations: aPTT, activated partial thromboplastin time; CRP, C-reactive protein; eGFR, estimated glomerular filtration rate; GN, glomerulonephritis; HTN, hypertension; ICU, intensive care unit; KTx, kidney transplantation; PT, prothrombin time.
At time of kidney function deterioration unless otherwise noted.
With oxygen supplementation through nonrebreather mask.
Calculated using the Chronic Kidney Disease Epidemiology Collaboration creatinine equation.
Not measured on the day of kidney function deterioration, but 5 days later.
Not measured on the day of kidney function deterioration, but 6 days earlier.
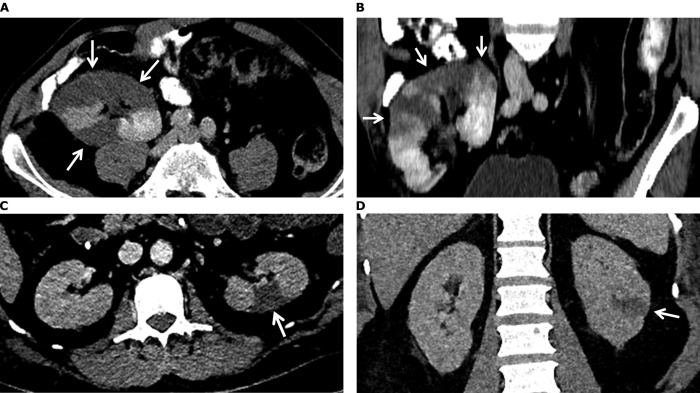
On day 9 after admission, the condition of the patient worsened. He became more tachypneic, and his temperature increased to 38.6°C. There were no episodes of hemodynamic instability and he did not experience abdominal pain, flank pain, nausea, vomiting, or worsening of previous arterial hypertension. He developed nonoliguric deterioration of kidney function, with an Scr level increase to 1.92 mg/dL and an LDH level increase to 1,145 U/L. C-reactive protein and leukocyte levels increased to 11.9 mg/dL and 19.9 ×103/μL, respectively. Urinalysis demonstrated erythrocytes, and urinary sodium, creatinine, and protein excretion were 60 mEq/L, 45 mg/dL, and 37 mg/dL, respectively. Fractional excretion of sodium was 1.9%. Computed tomography (CT) that was performed to rule out pulmonary embolism showed extensive consolidations and ground glass opacities on the pulmonary level, but no pulmonary emboli. An unexpected finding were multiple wedge-shaped perfusion defects in the kidney allograft (kidney infarcts), shown in Figure 1A and B. Electrocardiograms and physical examinations during admission had not shown atrial fibrillation. No signs of peripheral embolization of the hand or feet were noted. Blood cultures were negative. Therapeutic low-molecular-weight heparin (dalteparin, 15,000 units based on monitoring anti–factor Xa levels) was started, followed by acenocoumarol. Serum LDH and Scr increased to maximum levels of 2,130 U/L and 2.70 mg/dL, respectively.
Figure 1.
Computed tomography images from patients (A, B) 1 and (C-D) 2. (A) Axial and (B) coronal direction in the portal venous phase shows multiple perfusion defects (arrows) in the kidney allograft, most pronounced in the upper pole. (C) Axial and (D) coronal direction in the portal venous phase indicates a wedge-shaped perfusion defect dorsolateral in the interpolar area of the left kidney (arrows). Not shown, there were also multiple smaller perfusion defects visible in the upper and lower poles of both kidneys.
In the following period, the patient’s condition slowly improved, and at 20 days after admission, he was discharged. Mycophenolate therapy was still on hold. At the time of discharge, serum LDH and Scr levels had gradually decreased to 560 U/L and 1.79 mg/dL, respectively.
Case 2
A 58-year-old man with a history of obstructive sleep apnea presented to the emergency department with a 2-week history of fever, cough, rhinorrhea, abdominal pain, and increasing dyspnea. At presentation, the patient was tachypneic with an oxygen saturation of 90% with a nonrebreather mask with an oxygen flow rate of 15 L/min. His body temperature was 38.3°C and blood pressure was 137/78 mm Hg, with a pulse rate of 83 beats/min. Laboratory findings were as follows: C-reactive protein, 33.4 mg/dL; leukocytes, 17.9 ×103/μL; lymphocytes, 1.6 ×103/μL; serum LDH, 686 U/L; and Scr, 0.89 mg/dL. An x-ray of the chest showed diffuse bilateral infiltrates.
The patient was admitted to intensive care of the University Medical Center Groningen (Groningen, the Netherlands) and was intubated the same day. A nasopharyngeal swab underwent reverse transcriptase–polymerase chain reaction testing for SARS-CoV-2 and was positive. Thrombosis prophylaxis with nadroparin was started (5,700 units once daily). After 2 days, he developed oliguria and deterioration of kidney function, with Scr level rising from 0.89 to 2.45 mg/dL, which increased to 7.99 mg/dL over the next 2 days. Serum LDH level increased to 733 U/L. No sediment analysis was performed. Urinary sodium, creatinine, and albumin excretion were 46 mEq/L, 111 mg/dL, and 102 mg/dL, respectively. Fractional excretion of sodium was 1.5%. He did not have episodes of hemodynamic instability, new-onset hypertension, or atrial fibrillation in the period before acute kidney injury, but he developed cold feet and hands, with mottled skin and dry necrosis of 3 toes. Four days after admission, anuria led to the initiation of continuous venovenous hemofiltration. This treatment was complicated by frequent filter failure due to clotting, necessitating the addition of systemic anticoagulation therapy with heparin next to regular regional citrate anticoagulation therapy targeting a therapeutic activated partial thromboplastin time.
At day 10 after admission, the patient developed a bloated abdomen with absence of bowel sounds. CT suggested bowel ischemia and revealed multiple wedge-shaped kidney infarctions, demonstrated in Fig 1C and D. One meter of small bowel with transmural ischemia was resected. Anti-cardiolipin and β2-glycoprotein 1 antibody levels were within normal limits. Anticoagulation therapy was continued with nadroparin at a therapeutic dose with anti-factor Xa monitoring. In the following days, the patient showed gradual respiratory improvement and increasing diuresis. At day 28 after admission, kidney replacement therapy was stopped, although the patient was still dependent on mechanical ventilation. At 45 days after admission, the patient could be extubated. At 47 days after admission, he was discharged from the intensive care unit, and after 61 days, he was discharged from our hospital to a rehabilitation center. At the time of discharge, serum LDH and Scr levels had decreased to 190 U/L and 1.18 mg/dL, respectively.
Discussion
As knowledge regarding COVID-19 grows, studies are increasingly indicating that patients with COVID-19 are at risk for deep venous thrombosis and pulmonary embolisms.7 , 8 A recent case report found multiple cerebral infarcts in 3 separate patients with COVID-19, suggesting that the risk for thrombotic events may not be limited to the venous system.9 Our findings are in line with this and suggest kidney infarction as a potential cause of COVID-19–associated acute kidney injury.
The development of acute kidney injury despite hemodynamic stability in the presence of a mottled skin appearance and an increased LDH level are possible clues pointing toward kidney infarction. No extrarenal evidence of thromboembolism was detected in case 1 except for a sharp increase in serum LDH levels. However, in case 2, the patient developed intestinal ischemia and digital necrosis, as an indication of arterial thromboembolic disease. Contrast-enhanced CT led to the diagnosis in both patients and is the diagnostic method of choice for the detection of kidney infarction because ultrasound of the kidneys has much lower sensitivity.10 The logistic challenges of CT for patients with COVID-19 on mechanical ventilation and the possible risks of administering contrast agents in patients with acute kidney injury are possible limitations. At the time of writing, the University Medical Center Groningen had 101 intensive care unit admissions with COVID-19, of which 11 required kidney replacement therapy, but because contrast-enhanced CT of the abdomen was not routinely performed, we are unable to make statements regarding the incidence of kidney infarction in COVID-19 patients with acute kidney injury at our hospitals.
Thromboembolic events in patients with COVID-19 appear to involve both the arterial and venous system.7, 8, 9 However, the underlying mechanisms are currently largely unknown, but may include direct cytopathic effects of SARS-CoV-2 on endothelial cells leading to endothelial dysfunction,11 proinflammatory cytokines stimulating tissue factor expression,12 or the presence of antiphospholipid antibodies leading to thrombotic events.9 It should be noted that although we found large wedge-shaped arterial thrombi, there is also the possibility of microthrombi, which may remain undetected by radiologic examination. The possibility of microthrombus formation is supported by a recent post-mortem histopathologic analysis of 26 patients with COVID-19 in which segmental fibrin thrombus formation was found in the glomerular capillary loops of 3 of the patients.13
The course in our patients suggests that initiation of anticoagulant treatment may have contributed to the improvement in kidney function in both patients and underscores the possible benefit of a timely diagnosis of kidney infarction in COVID-19 patients with acute kidney injury. In addition, increased-dose prophylactic anticoagulation therapy may merit consideration in this population.
We acknowledge that the kidney infarctions may not have been the sole cause of acute kidney injury in these patients. Direct cytopathic effects of SARS-CoV-2 on kidney tubular and endothelial cells due to kidney tropism have been suggested and were not excluded in our patients.13 , 14 Additionally, severely ill patients with COVID-19 may also be prone to other risk factors for acute kidney injury, including hemodynamic instability and drug toxicity. However, both patients did not experience hemodynamic instability in the period before the development of acute kidney injury and the clear radiologic evidence of kidney infarction makes it probable that the infarctions at least contributed to the loss of kidney function in both patients.
In conclusion, we demonstrated 2 cases of patients with COVID-19 who developed an acute decrease in kidney function and who had radiologic signs of perfusion defects. These data suggest that the differential diagnosis of acute kidney injury in patients with COVID-19 infection should include kidney infarction, which may have important preventive and therapeutic implications.
Article Information
Authors’ Full Names and Academic Degrees
Adrian Post, PharmD, Edwin S.G. den Deurwaarder, MD, Stephan J.L. Bakker, MD, PhD, Robbert J. de Haas, MD, PhD, Matijs van Meurs, MD, PhD, Ron T. Gansevoort, MD, PhD, and Stefan P. Berger, MD, PhD.
Support
None.
Financial Disclosure
The authors declare that they have no relevant financial interests.
Patient Protections
The authors declare that they have obtained consent from each patient reported in this article for publication of the information about him that appears within this Case Report.
Peer Review
Received April 16, 2020. Evaluated by 3 external peer reviewers and a radiologist, with direct editorial input from the Education Editor and a Deputy Editor. Accepted in revised form May 23, 2020.
Footnotes
Complete author and article information provided before references.
References
- 1.Cheng Y., Luo R., Wang K. Kidney impairment is associated with in-hospital death of COVID-19 patients. Kidney Int. 2020;97(5):829–838. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Z., Wu M., Guo J. Caution on kidney dysfunctions of 2019-nCoV patients. Preprint at. http://medrxiv.org/content/early/2020/02/12/2020.02.08.20021212.abstract
- 4.Diao B., Feng Z., Wang C. Human kidney is a target for novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Preprint at. https://www.medrxiv.org/content/10.1101/2020.03.04.20031120v4 [DOI] [PMC free article] [PubMed]
- 5.Ronco C., Reis T. Kidney involvement in COVID-19 and rationale for extracorporeal therapies. Nat Rev Nephrol. 2020;16(6):308–310. doi: 10.1038/s41581-020-0284-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silversides J.A., Major E., Ferguson A.J. Conservative fluid management or deresuscitation for patients with sepsis or acute respiratory distress syndrome following the resuscitation phase of critical illness: a systematic review and meta-analysis. Intensive Care Med. 2017;43(2):155–170. doi: 10.1007/s00134-016-4573-3. [DOI] [PubMed] [Google Scholar]
- 7.Wang T., Chen R., Liu C. Attention should be paid to venous thromboembolism prophylaxis in the management of COVID-19. Lancet Haematol. 2020;7(5):e362–e363. doi: 10.1016/S2352-3026(20)30109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klok F.A., Kruip M., van der Meer N. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Y., Xiao M., Zhang S. Coagulopathy and antiphospholipid antibodies in patients with COVID-19. N Engl J Med. 2020;382(17):e38. doi: 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hazanov N., Somin M., Attali M. Acute renal embolism. Forty-four cases of renal infarction in patients with atrial fibrillation. Medicine (Baltimore) 2004;83(5):292–299. doi: 10.1097/01.md.0000141097.08000.99. [DOI] [PubMed] [Google Scholar]
- 11.Sardu C., Gambardella J., Morelli M.B., Wang X., Marfella R., Santulli G. Hypertension, thrombosis, kidney failure, and diabetes: is COVID-19 an endothelial disease? A comprehensive evaluation of clinical and basic evidence. J Clin Med. 2020;9(5):1417. doi: 10.3390/jcm9051417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Branchford B.R., Carpenter S.L. The role of inflammation in venous thromboembolism. Front Pediatr. 2018;6:142. doi: 10.3389/fped.2018.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Su H, Yang M, Wan C, et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020;98(1):219–227. [DOI] [PMC free article] [PubMed]
- 14.Puelles V.G., Lutgehetmann M., Lindenmeyer M.T. Multiorgan and renal tropism of SARS-CoV-2 [published online ahead of print May 13, 2020]. N Engl J Med. https://doi.org/10.1056/NEJMc2011400 [DOI] [PMC free article] [PubMed]