A genome-wide association study of asthma first identified SNPs on human chromosome 17 as a significant genetic risk factor underpinning the disease. The SNPs were consistently and strongly associated in cis with transcript levels of the gene ORMDL3 (P < 10−22) (1). This association has subsequently been replicated in numerous studies worldwide, including a recent multiancestry global meta-analysis of 23,948 asthma cases versus 118,538 controls (2). The locus is now recognized as the major predisposing factor for childhood-onset asthma. Early symptomatic human rhinovirus (RV) infection is a risk factor for subsequent asthma, and human RV infection accounts for nearly two-thirds of childhood asthma exacerbations. Significant increases in the number of wheezing illnesses have been observed in children with enhanced transcription genotypes at the 17q21 ORMDL3 locus (3). Recent investigation has shown that ORMDL3 has pleiotropic effects during cellular inflammation, and ORMDL3 knockdown in human epithelial cells was found to strongly reduce expression of the human RV receptor ICAM-1 (intercellular adhesion molecule-1) during the inflammatory response (4).
Human RVs are classified within the family Picornaviridae. There are more than 100 serotypes of RV; the diversities of serotypes are difficult for creating an effective vaccine against the major etiologic agent causing the common cold. The virus is a small, single-positive stranded RNA virus whose capsid contains four proteins. Three of these proteins, VP1, VP2, and VP3, are located on the surface of the capsid and are responsible for its antigenic diversity; the fourth, VP4, is located inside the virus and anchors the RNA core to the viral capsid. There are also seven nonstructural proteins—2A to 2C and 3A to 3D—of which 3D possesses RNA-dependent RNA polymerase function. Three genetically distinct RV species, RV-A, RV-B, and RV-C, have been described. RV-A and RV-B were distinguished from one another in the early 1990s on the basis of the activities of antiviral compounds. Presently, the RV-A class includes 77 recognized types and the RV-B includes 30 types (5). RV-C has only been recognized since 2009 and has at least 50 subtypes (6). RV-A and RV-B serotypes are classified into major (90% of viruses) and minor (10%) groups respectively, on the basis of cellular receptors. The majority of RV serotypes bind to ICAM-1 (also called CD54), whereas ∼10% bind to the LDLR (low-density lipoprotein receptor) (7). RV-C is also known to use CDHR3 (cadherin-related family member 3) for entry. More than 60% of the capacity for binding and fusion of the RV particle is mediated by attachment to ICAM-1 (8), which binds within a pocket groove of the VP-1 protein located on the viral capsid (9). The expression of ICAM-1 is also upregulated on infection both in vivo and in vitro to promote additional viral binding and infection spread in epithelial cells (10), leading to a vicious cycle of RV infection. As we previously reported, ORMDL3 is involved in this process (4).
In this issue of the Journal, Liu and colleagues (pp. 783–792) provide evidence that ORMDL3 is also involved in RV replication in epithelial cells, as knockdown of ORMDL3 inhibited the replication of RV-A16, which is the most commonly studied and validated human RV strain (11). In ORMDL3-silenced cells, the enhanced endoplasmic reticulum (ER) stress and IFN-β expression induced by RV infection significantly decreased. ER stress and the unfolded protein response induced by tunicamycin significantly increased IFN-β expression and inhibited RV-A replication, suggesting that different pathways exist between ORMDL3 and pharmacogenetic activators affecting RV replication. Myriocin, an inhibitor of SPT (serine palmitoyl-CoA transferase), the first and rate-limiting step in sphingolipid biosynthesis, increases RV-A16 replication.
These findings provide strong evidence that ORMDL3 is able to regulate RV replication in epithelial cells, although there could be more interesting experiments for this topic. Most of the experiments were performed in HeLa cells, a line derived from cervical cancer cells that are quite different from human airway cells. Expression of the RV receptor ICAM-1 may be different in these two cell types, which could have influenced their results. Under noninflammatory conditions, ICAM-1 expression is constitutively low in epithelial cells. IL-1β, TNF-α, IFN-γ, and other cytokines elicit increased expression in a cell- and cytokine-specific fashion (12). During ORMDL3 knockdown in lung epithelial cells, only the ICAM-1 expression levels were changed, while all other RV receptor levels remained unchanged (in-house data) after cytokine IL-1β stimulation. Viral replication initiates a cell inflammatory response, resulting in the expression of cytokines that feed back to promote ICAM-1 expression for more viral attachments.
Most Picornaviruses use the cytoplasmic surface of ER/Golgi membranes for genome replication. The viral nonstructural proteins 2B, 2C, and 3A are reported to associate with the ER and Golgi membranes and are believed to be important in remodeling through the recruitment of host proteins, such as Golgi proteins ACBD3 (GCP60) and PI3KIIIβ. In addition, RV is known to infect human epithelial cells via ceramide-enriched membrane platforms (13), suggesting that acid sphingomyelinase and ceramide may act as key molecules affecting the infection of human cells by RV. Human ORMDL3 encodes a transmembrane protein that is anchored in the ER. The ER is a site for protein folding, synthesis of lipids and the storage of free calcium. ER stress can reduce the capacity of the ER for protein folding and thereby influence cellular responses to inflammation. It interacts with the serine SPT enzyme complex in sphingolipid synthesis (14). ORMDL3 facilitates the unfolded protein response to cellular stress by influencing SERCA (sarcoplasmic/endoplasmic reticulum calcium ATPase) and ER-mediated Ca2+ flux (15). ORMDL3 also regulates ER stress, ceramide, and sphingosine 1-sulfate levels that regulate cytokine release as well as replication.
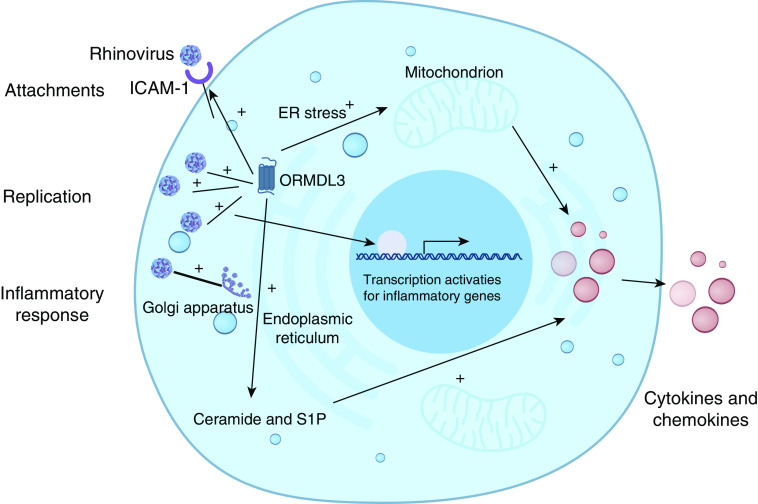
Thus, ORMDL3 can influence RV infection and associated inflammation in multiple ways: 1) it increases major RV receptor ICAM-1 expression in epithelial cells (and also ceramide-enriched membrane formation), thereby enhancing entry of RV; 2) it arguments RV replication through ER/Golgi membrane remodeling via unknown mechanisms; and 3) it regulates cell ER stress along with ceramide and sphingosine 1-sulfate levels in cells, causing expression and release of proinflammatory cytokines. Along with the epithelial cell damage caused by RV replication, RV infection-dependent activation of transcription of inflammatory genes contributes to the respiratory symptoms (Figure 1). The remaining questions need to be addressed in future research: 1) What is the mechanism that ORMDL3 regulates RV attachment, replication, budding, and cytokine response? and 2) What therapeutic targets can be identified that could be used for the clinical management of RV infection?
Figure 1.
Orosomucoid-like 3 (ORMDL3) and human rhinovirus infection. Rhinovirus (RV) uses ICAM-1 (intercellular adhesion molecule-1) receptor for cellular entry. RV then replicates by modifying the endoplasmic reticulum (ER)/Golgi complex. ORMDL3 can enhance RV entry by upregulating ICAM-1 expression in cells. Viral replication also initiates cell inflammatory response, leading to the generation of cytokines, resulting in further ICAM-1 expression for more viral attachments. ORMDL3 also regulates ER stress, ceramide, and sphingosine 1-sulfate (S1P) levels that regulate cytokine release as well as viral replication.
Supplementary Material
Footnotes
Originally Published in Press as DOI: 10.1165/rcmb.2020-0052ED on February 28, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Moffatt MF, Kabesch M, Liang L, Dixon AL, Strachan D, Heath S, et al. Genetic variants regulating ORMDL3 expression contribute to the risk of childhood asthma. Nature. 2007;448:470–473. doi: 10.1038/nature06014. [DOI] [PubMed] [Google Scholar]
- 2. Demenais F, Margaritte-Jeannin P, Barnes KC, Cookson WOC, Altmüller J, Ang W, et al. Australian Asthma Genetics Consortium (AAGC) collaborators. Multiancestry association study identifies new asthma risk loci that colocalize with immune-cell enhancer marks. Nat Genet. 2018;50:42–53. doi: 10.1038/s41588-017-0014-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Calışkan M, Bochkov YA, Kreiner-Møller E, Bønnelykke K, Stein MM, Du G, et al. Rhinovirus wheezing illness and genetic risk of childhood-onset asthma. N Engl J Med. 2013;368:1398–1407. doi: 10.1056/NEJMoa1211592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhang Y, Willis-Owen SAG, Spiegel S, Lloyd CM, Moffatt MF, Cookson WOCM. The ORMDL3 asthma gene regulates ICAM1 and has multiple effects on cellular inflammation. Am J Respir Crit Care Med. 2019;199:478–488. doi: 10.1164/rccm.201803-0438OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Palmenberg AC, Gern JE. Classification and evolution of human rhinoviruses. Methods Mol Biol. 2015;1221:1–10. doi: 10.1007/978-1-4939-1571-2_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jacobs SE, Lamson DM, St George K, Walsh TJ. Human rhinoviruses. Clin Microbiol Rev. 2013;26:135–162. doi: 10.1128/CMR.00077-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Casasnovas JM. The dynamics of receptor recognition by human rhinoviruses. Trends Microbiol. 2000;8:251–254. doi: 10.1016/s0966-842x(00)01749-2. [DOI] [PubMed] [Google Scholar]
- 8. Greve JM, Davis G, Meyer AM, Forte CP, Yost SC, Marlor CW, et al. The major human rhinovirus receptor is ICAM-1. Cell. 1989;56:839–847. doi: 10.1016/0092-8674(89)90688-0. [DOI] [PubMed] [Google Scholar]
- 9. Xiao C, Tuthill TJ, Bator Kelly CM, Challinor LJ, Chipman PR, Killington RA, et al. Discrimination among rhinovirus serotypes for a variant ICAM-1 receptor molecule. J Virol. 2004;78:10034–10044. doi: 10.1128/JVI.78.18.10034-10044.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Papi A, Papadopoulos NG, Stanciu LA, Degitz K, Holgate ST, Johnston SL. Effect of desloratadine and loratadine on rhinovirus-induced intercellular adhesion molecule 1 upregulation and promoter activation in respiratory epithelial cells. J Allergy Clin Immunol. 2001;108:221–228. doi: 10.1067/mai.2001.116861. [DOI] [PubMed] [Google Scholar]
- 11. Liu Y, Bochkov YA, Eickhoff JC, Hu T, Zumwalde NA, Tan JW, et al. Orosomucoid-like 3 supports rhinovirus replication in human epithelial cells. Am J Respir Cell Mol Biol. 2020;62:783–792. doi: 10.1165/rcmb.2019-0237OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pober JS, Gimbrone MA, Jr, Lapierre LA, Mendrick DL, Fiers W, Rothlein R, et al. Overlapping patterns of activation of human endothelial cells by interleukin 1, tumor necrosis factor, and immune interferon. J Immunol. 1986;137:1893–1896. [PubMed] [Google Scholar]
- 13. Grassmé H, Riehle A, Wilker B, Gulbins E. Rhinoviruses infect human epithelial cells via ceramide-enriched membrane platforms. J Biol Chem. 2005;280:26256–26262. doi: 10.1074/jbc.M500835200. [DOI] [PubMed] [Google Scholar]
- 14. Breslow DK, Collins SR, Bodenmiller B, Aebersold R, Simons K, Shevchenko A, et al. Orm family proteins mediate sphingolipid homeostasis. Nature. 2010;463:1048–1053. doi: 10.1038/nature08787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cantero-Recasens G, Fandos C, Rubio-Moscardo F, Valverde MA, Vicente R. The asthma-associated ORMDL3 gene product regulates endoplasmic reticulum-mediated calcium signaling and cellular stress. Hum Mol Genet. 2010;19:111–121. doi: 10.1093/hmg/ddp471. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.