Sporadic lymphangioleiomyomatosis (s-LAM) is a rare multisystem, neoplastic disease that almost exclusively affects premenopausal women (1). A second form of LAM occurs in up to 30–40% of women and ∼12% of men with tuberous sclerosis complex (TSC-LAM) (2). Women with s-LAM present with progressive dyspnea upon exertion, recurrent pneumothorax, chylous thoracic and abdominal effusions, and thoracic and abdominal tumors (including lymphangioleiomyomas and angiomyolipomas) (3). Progressive respiratory failure leading to death occurs if the disease is not treated.
LAM cells, the histopathological hallmark of the disease, arise from an unknown extrapulmonary source and spread to the lung and other organs via the circulation and lymphatics. LAM cells express lymphangiogenic growth factors (vascular endothelial growth factor [VEGF]-C and VEGF-D) that induce disordered lymphatic channel formation in the lung, which, along with high-level expression of proteinases by LAM cells, likely contributes to lung remodeling and cystic lung destruction (4, 5). LAM pulmonary nodules contain inner spindle-shaped α-actin–expressing smooth muscle–like cells and are surrounded by epithelioid polygonal cells that express high amounts of melanocyte markers, including gp100, which is a transmembrane glycoprotein (5, 6).
LAM is caused by loss-of-function mutations in one of two tumor suppressor genes, TSC1 (hamartin) and TSC2 (tuberin) (2, 7). TSC1 and TSC2 form a complex with TBC1D7 (Tre2-Bub2-Cdc16 [TBC]-1 domain family member 7), which inhibits activation of mTOR. Loss-of-function mutations in the TSC1 or TSC2 gene lead to uncontrolled activation of mTOR signaling, which induces the proliferation of tumor-like LAM cells (Figure 1) (2). The mTOR inhibitor sirolimus (rapamycin) is approved to treat patients with LAM in the United States. In clinical trials, sirolimus stabilized lung function and improved the quality of life and functional capacity of patients with LAM (8). However, sirolimus is associated with significant toxicities, and long-term safety and efficacy data are lacking. Thus, there is an unmet need for more safe and effective therapies for LAM.
Figure 1.
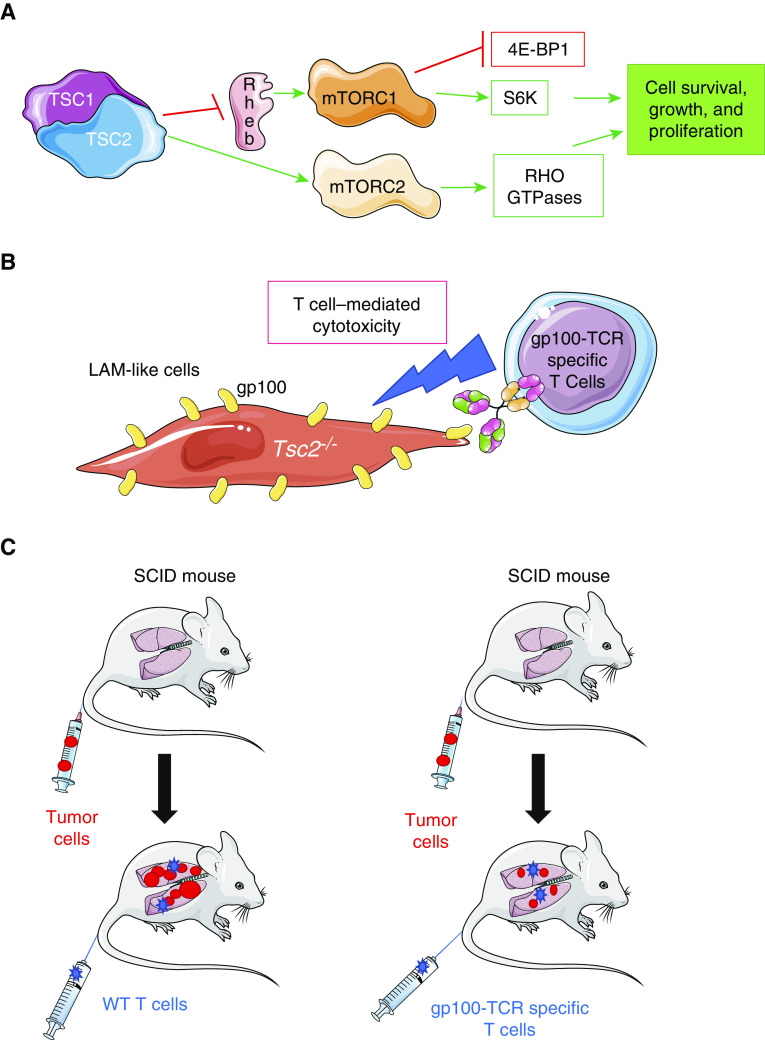
(A) mTOR is an important homeostatic factor that controls different cellular functions depending on how it is coupled. mTORC1 (mTOR complex 1) is composed of mTOR, raptor (a regulatory protein), and other complementary proteins. mTORC1 promotes cellular proliferation and growth by regulating the activity of other proteins, including 1) inhibiting eukaryotic translation initiation factor 4E-BP1 (4E-binding protein 1), which is a repressor of protein translation; and 2) activating S6K (S6 kinase), a serine/threonine-protein kinase that promotes cell proliferation and growth, and cell cycle progression. mTORC2 is composed of mTOR and rictor (a rapamycin-insensitive compound), and this complex promotes cell proliferation and migration, and cytoskeletal remodeling, in part by activating RHO GTPases. TSC (tuberous sclerosis complex) functions as an inhibitor of mTORC1. Loss-of-function mutations in the genes encoding two components of TSC (TSC1 [hamartin] and TSC2 [tuberin]) result in excessive activation of mTOR, the formation of lymphangioleiomyomatosis (LAM) cells, and the seeding and growth of these cells within the lungs and other organs, where they form tumors. (B) Han and colleagues transduced Tsc2-deficient kidney tumor cells with a lentiviral vector to induce high-level and stable expression of gp100 by the cells to generate LAM-like cells in vitro (Tsc2−/− gp100+ cells) (9). T cells expressing a TCR specific for gp100 protein were isolated from pmel-1 transgenic mice, and these T cells were shown to have cytotoxic activity against Tsc2−/− gp100+ LAM-like cells in vitro. (C) Severe combined immunodeficiency (SCID/beige) mice (which lack an adaptive immune response) were injected via the intravenous route with Tsc2−/− gp100+ LAM-like cells, and LAM-like tumors developed in the lungs of the mice over 1–2 weeks. One group of mice was then treated with a single dose of wild-type (WT) T cells, and another group of mice received gp100-TCR–specific T cells by the intravenous route. The group that received the gp100-TCR–specific T cells developed fewer and smaller lung lesions 3 weeks later than the group that was treated with WT T cells. gp100 = glycoprotein 100; Rheb = Ras homolog enriched in brain.
In a study reported in this issue of the Journal, Han and colleagues (pp. 793–804) tackled this issue by evaluating a highly innovative immunotherapy approach (9). The rationale for this strategy was based on the fact that LAM cells are susceptible to T-cell–mediated toxicity in vitro (10, 11), but LAM tumors are devoid of infiltrating T cells and express immune checkpoint inhibitors such as PD-L1 (programmed cell death ligand-1) (12). GP100 was selected as the target antigen because epithelioid cells in LAM tumors express high levels of this protein (which is only expressed by melanocytes in healthy subjects). The authors used a multistage strategy. First, they developed a LAM-like tumor cell by engineering Tsc2-deficient cells to express gp100. They transduced kidney tumor cells originating from aged Tsc2−/− mice with a lentiviral vector in vitro to induce stable expression of gp100 by the cells (Tsc2−/−gp100+ LAM-like cells). T cells isolated from transgenic mice with gp100-TCR–specific T cells (TCR transgenic pmel-1 mice) were shown to efficiently kill these LAM-like cells in vitro (Figure 1). The authors then developed an animal model of pulmonary LAM by injecting the gp100-expressing LAM-like cells into the circulation of mice that were genetically deficient in T and B cells (severe combined immunodeficiency [SCID]/beige mice), and confirmed that the LAM-like cells seeded in the lungs and formed pulmonary tumors over 1–2 weeks. The authors then injected gp100-TCR–specific T cells isolated from major histocompatibility complex–matched pmel-1 transgenic mice (or T cells from wild-type [WT] mice as a control) into the circulation of the SCID/beige mice with Tsc2−/−gp100+ LAM-like pulmonary tumors, and evaluated tumor growth. T cells were identified within the LAM-like pulmonary tumors, and the gp100-TCR–specific T cells (but not WT T cells) reduced both the size and number of the pulmonary tumors in the mice (Figure 1). However, the tumors were not completely eliminated. Next, to determine whether the incomplete efficacy of this immunotherapeutic approach was due to exhaustion of the transferred T cells, Han and colleagues measured the levels of PD-1 (programmed cell death protein-1) on tumor-infiltrating T cells and PD-L1 on tumor cells using immunostaining methods. PD-1 expression was lower on infiltrating gp100-TCR–specific T cells than on WT T cells, and PD-L1 expression by tumor cells was inversely related to tumor size, suggesting that PD-1–induced T cell exhaustion was not responsible to the lack of complete responsiveness of the tumors to the immunotherapy immune checkpoint activation. To further test this hypothesis, the authors added an anti–PD-1 antibody to the adoptive transfer of gp100-TCR–specific T cells versus WT T cells in SCID/beige mice with LAM-like tumors. This combined immunotherapeutic approach led to greater elimination of pulmonary tumors only in the mice that received WT T cells.
A strength of this study is that it is the first to report that a targeted immunotherapy has efficacy in an animal model of LAM. In addition, this approach has the potential to attack LAM cells in multiple organs while minimizing on-target toxicity (because gp100 is normally expressed only by melanocytes). A limitation of this work is the small sample sizes that were studied. Also, the authors only treated mice with early-stage disease (allowing tumors to grow for only 1–2 wk) before a single T-cell treatment was given. They also evaluated treatment efficacy only at 3 weeks after the single injection. It is not clear how effective the combined therapy would be if it were initiated in late-stage disease with large tumors, or whether multiple treatments would be needed in later-stage disease. Furthermore, high-level expression of gp100 only occurs in a subset of LAM cells (4); in particular, the spindle cells with high proliferative potential in LAM lesions have low levels of gp100 expression (13). Thus, targeting only the gp100 antigen in human LAM lesions may not kill sufficient numbers of tumor cells to effectively treat LAM. In addition, patients with vitiligo have T cells that are reactive to gp100, so it remains to be determined whether skin depigmentation would be a side effect of immunotherapy targeting gp100.
Nevertheless, the results of Han and colleagues are exciting because they suggest that antigen-targeted immunotherapy either alone or in combination with immune checkpoint inhibitors may be efficacious in s-LAM. However, additional studies are needed to confirm these findings and evaluate whether immunotherapy is efficacious in late-stage disease in animal models before this approach can advance to clinical trials.
Supplementary Material
Footnotes
Supported by Public Health Service, National Institute of Allergy and Infectious Diseases grant AI111475-01, Flight Attendants Medical Research Institute grant CIA123046, and Department of Defense (Congressionally Directed Medical Research Programs) grant PR152060.
Originally Published in Press as DOI: 10.1165/rcmb.2020-0049ED on February 26, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Johnson SR. Lymphangioleiomyomatosis. Eur Respir J. 2006;27:1056–1065. doi: 10.1183/09031936.06.00113303. [DOI] [PubMed] [Google Scholar]
- 2.Carsillo T, Astrinidis A, Henske EP. Mutations in the tuberous sclerosis complex gene TSC2 are a cause of sporadic pulmonary lymphangioleiomyomatosis. Proc Natl Acad Sci USA. 2000;97:6085–6090. doi: 10.1073/pnas.97.11.6085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Henske EP, McCormack FX. Lymphangioleiomyomatosis—a wolf in sheep’s clothing. J Clin Invest. 2012;122:3807–3816. doi: 10.1172/JCI58709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glasgow CG, El-Chemaly S, Moss J. Lymphatics in lymphangioleiomyomatosis and idiopathic pulmonary fibrosis. Eur Respir Rev. 2012;21:196–206. doi: 10.1183/09059180.00009311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krymskaya VP, McCormack FX. Lymphangioleiomyomatosis: a monogenic model of malignancy. Annu Rev Med. 2017;68:69–83. doi: 10.1146/annurev-med-050715-104245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krymskaya VP. Smooth muscle-like cells in pulmonary lymphangioleiomyomatosis. Proc Am Thorac Soc. 2008;5:119–126. doi: 10.1513/pats.200705-061VS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strizheva GD, Carsillo T, Kruger WD, Sullivan EJ, Ryu JH, Henske EP. The spectrum of mutations in TSC1 and TSC2 in women with tuberous sclerosis and lymphangiomyomatosis. Am J Respir Crit Care Med. 2001;163:253–258. doi: 10.1164/ajrccm.163.1.2005004. [DOI] [PubMed] [Google Scholar]
- 8.McCormack FX, Inoue Y, Moss J, Singer LG, Strange C, Nakata K, et al. National Institutes of Health Rare Lung Diseases Consortium; MILES Trial Group. Efficacy and safety of sirolimus in lymphangioleiomyomatosis. N Engl J Med. 2011;364:1595–1606. doi: 10.1056/NEJMoa1100391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han F, Dellacecca ER, Barse LW, Cosgrove C, Henning SW, Ankney CM, et al. Adoptive T-cell transfer to treat lymphangioleiomyomatosis. Am J Respir Cell Mol Biol. 2020;62:793–804. doi: 10.1165/rcmb.2019-0117OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klarquist J, Barfuss A, Kandala S, Reust MJ, Braun RK, Hu J, et al. Melanoma-associated antigen expression in lymphangioleiomyomatosis renders tumor cells susceptible to cytotoxic T cells. Am J Pathol. 2009;175:2463–2472. doi: 10.2353/ajpath.2009.090525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boorjian SA, Sheinin Y, Crispen PL, Lohse CM, Leibovich BC, Kwon ED. T-cell co-regulatory molecule expression in renal angiomyolipoma and pulmonary lymphangioleiomyomatosis. Urology. 2009;74:1359–1364. doi: 10.1016/j.urology.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maisel K, Merrilees MJ, Atochina-Vasserman EN, Lian L, Obraztsova K, Rue R, et al. Immune checkpoint ligand PD-L1 is upregulated in pulmonary lymphangioleiomyomatosis. Am J Respir Cell Mol Biol. 2018;59:723–732. doi: 10.1165/rcmb.2018-0123OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Juvet SC, McCormack FX, Kwiatkowski DJ, Downey GP. Molecular pathogenesis of lymphangioleiomyomatosis: lessons learned from orphans. Am J Respir Cell Mol Biol. 2007;36:398–408. doi: 10.1165/rcmb.2006-0372TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.