Abstract
Chronic hypoxia (CH) augments depolarization-induced pulmonary vasoconstriction through superoxide-dependent, Rho kinase–mediated Ca2+ sensitization. Nicotinamide adenine dinucleotide phosphate oxidase and EGFR (epidermal growth factor receptor) signaling contributes to this response. Caveolin-1 regulates the activity of a variety of proteins, including EGFR and nicotinamide adenine dinucleotide phosphate oxidase, and membrane cholesterol is an important regulator of caveolin-1 protein interactions. We hypothesized that derangement of these membrane lipid domain components augments depolarization-induced Ca2+ sensitization and resultant vasoconstriction after CH. Although exposure of rats to CH (4 wk, ∼380 mm Hg) did not alter caveolin-1 expression in intrapulmonary arteries or the incidence of caveolae in arterial smooth muscle, CH markedly reduced smooth muscle membrane cholesterol content as assessed by filipin fluorescence. Effects of CH on vasoreactivity and superoxide generation were examined using pressurized, Ca2+-permeabilized, endothelium-disrupted pulmonary arteries (∼150 μm inner diameter) from CH and control rats. Depolarizing concentrations of KCl evoked greater constriction in arteries from CH rats than in those obtained from control rats, and increased superoxide production as assessed by dihydroethidium fluorescence only in arteries from CH rats. Both cholesterol supplementation and the caveolin-1 scaffolding domain peptide antennapedia-Cav prevented these effects of CH, with each treatment restoring membrane cholesterol in CH arteries to control levels. Enhanced EGF-dependent vasoconstriction after CH similarly required reduced membrane cholesterol. However, these responses to CH were not associated with changes in EGFR expression or activity, suggesting that cholesterol regulates this signaling pathway downstream of EGFR. We conclude that alterations in membrane lipid domain signaling resulting from reduced cholesterol content facilitate enhanced depolarization- and EGF-induced pulmonary vasoconstriction after CH.
Keywords: caveolae, caveolin-1, membrane cholesterol, pulmonary hypertension
Plasma membrane lipid domains regulate a wide range of cellular responses. Caveolae, one such domain, are small plasmalemmal invaginations that compartmentalize a variety of signaling molecules that regulate signal transduction (1–3) and lipid transport (4). Cav-1 (Caveolin-1) is a protein that is required for the formation of caveolae (5), and the scaffolding domain of Cav-1 regulates many membrane-associated proteins, including Src family tyrosine kinases, small GTPases, and endothelial nitric oxide synthase (6–8). Cav-1 also directly binds cholesterol (9), serves as a cholesterol transporter (4, 10), and can regulate the cholesterol content in lipid microdomains such as caveolae (11).
Pulmonary hypertension (PH) is associated with changes to a variety of signaling mechanisms. Data suggest that altered lipid domains contribute to these responses. For example, Cav-1 expression is decreased in humans with idiopathic PH and in several animal models of PH (12–15), and this alteration causes endothelial dysfunction mediated in part through decreased Ca2+ entry after chronic hypoxia (CH) (16). Cav-1 knockout mice also demonstrate increased pulmonary vascular resistance and PH (15, 17). Hypoxia additionally reduces cholesterol levels (18), which are a key regulator of lipid domains such as caveolae (9, 19).
Although these changes have been demonstrated largely in endothelial cells, the physiological role of Cav-1 and cholesterol in smooth muscle cells is less well defined. Previously, we have demonstrated enhanced depolarization-induced vasoconstriction involving EGFR (epidermal growth factor receptor) and NADPH oxidase 2 (NOX2) in smooth muscle of pulmonary arteries (20). This EGFR and NOX2 activation leads to superoxide (O2−)-dependent, ROK (Rho kinase)-mediated Ca2+ sensitization (21). Interestingly, studies have shown that Cav-1/caveolae can regulate both EGFR and NOX (13, 22, 23), and other studies indicate that cholesterol can directly regulate EGFR and NOX activation (24, 25). Therefore, our aim in this study was to determine the contribution of the lipid domain components Cav-1 and cholesterol to enhanced depolarization-induced vasoconstriction, Ca2+ sensitization, and O2− production after CH. We addressed this issue by examining the effect of the Cav-1 scaffolding domain and manipulation of membrane cholesterol on pulmonary vasoconstriction and O2− production in pressurized pulmonary arteries from CH and control rats. Our findings reveal that alterations in lipid domains facilitate enhanced vasoconstriction and Ca2+ sensitization after CH.
Methods
Animals and CH Exposure
All protocols and procedures in this study were reviewed and approved by the Institutional Animal Care and Use Committee of the University of New Mexico Health Sciences Center. Male Sprague-Dawley rats (250–350 g, age 3–4 mo; Harlan Industries) were used for all experiments. The rats were housed in a hypobaric chamber for 4 weeks with barometric pressure at ∼380 mm Hg. Age-matched control rats were housed in similar cages at ambient atmospheric pressure (∼630 mm Hg). We have previously demonstrated that this 4-week CH exposure protocol results in PH, arterial remodeling, and right ventricular hypertrophy (26–29). An expanded Methods section detailing the protocols and statistics used is available in the data supplement.
Results
Caveolae Number and Cav-1 Expression Are Unaltered after CH
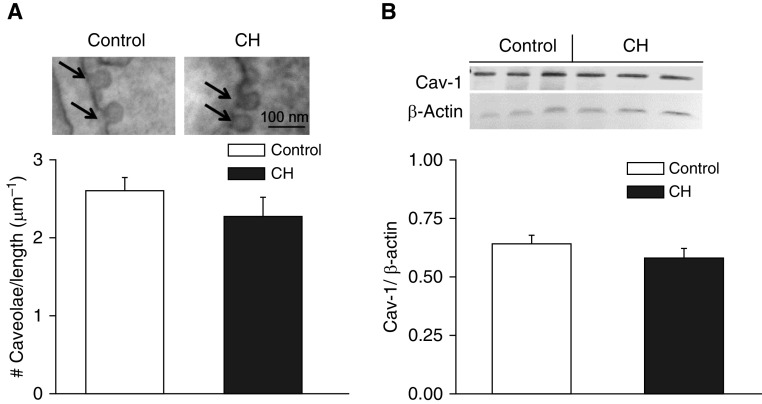
Effects of CH on the incidence of caveolae were assessed by transmission EM in pulmonary arterial vascular smooth muscle (VSM) cells from CH and control rats. We observed no difference in the number of caveolae normalized to cell membrane length between groups (Figure 1A). Levels of Cav-1 in intrapulmonary arterial homogenates also did not differ between CH and control arteries as assessed by Western blotting (Figure 1B).
Figure 1.
Chronic hypoxia (CH) does not alter pulmonary arterial smooth muscle cell caveolae number or Cav-1 (caveolin-1) expression. (A) Sample electron micrographs and mean caveolae number per cell membrane length (μm) in pulmonary arterial smooth muscle cells from control (n = 34 images from three rats) and CH (n = 25 images from three rats) animals. Arrows indicate individual caveolae. Data are means ± SE. Scale bar: 100 nm. (B) Representative Western blots and mean densitometric data for Cav-1 normalized to β-actin in intrapulmonary arteries from CH and control rats. Values are means ± SE of n = 4 rats/group. There are no significant differences between groups.
CH Exposure Decreases Smooth Muscle Cell Membrane Cholesterol
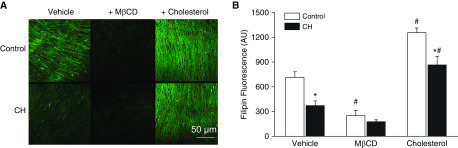
To determine whether CH alters pulmonary VSM cholesterol content, we measured the fluorescence intensity of the cholesterol-binding probe filipin (30) in the medial layer of small pulmonary arteries from control and CH rats by confocal microscopy. CH decreased the VSM cholesterol content, as indicated by lower filipin fluorescence compared with that in vessels from control rats (Figure 2). Methyl-β-cyclodextrin (MβCD, 10 mM) alone, used to deplete cholesterol, significantly reduced cholesterol in control arteries, and had a similar but nonsignificant effect (P = 0.058) in the CH group. Cholesterol supplementation increased cholesterol levels in arteries from both control and CH animals as expected, although cholesterol content remained lower in the CH group than in the control group. Nevertheless, this method of cholesterol supplementation served to normalize the cholesterol content in CH arteries to that in vehicle-treated control arteries, thus providing a useful approach to evaluate the role of reduced cholesterol in enhanced vasoconstrictor sensitivity after CH in subsequent experiments.
Figure 2.
CH decreases pulmonary arterial smooth muscle membrane cholesterol. (A) Representative images of filipin fluorescence (arbitrary units [AU]) as a marker of membrane cholesterol content in pulmonary arteries from control and CH rats after treatment with vehicle, methyl-β-cyclodextrin (MβCD) (10 mM), or supplemental cholesterol. Scale bar: 50 μm. (B) Mean filipin fluorescence in control and CH arteries under vehicle, MβCD, and cholesterol repletion conditions. Values are means ± SE of n = 5–7 animals/group. *P < 0.05 versus respective control. #P < 0.05 versus respective vehicle.
Decreased Membrane Cholesterol Facilitates Enhanced Depolarization-induced Vasoconstriction and O2− Generation after CH
The vasoreactivity protocols we used in this study assessed responses to depolarizing concentrations of KCl using a pressurized rat pulmonary artery preparation that permits simultaneous measurement of vessel inner diameter and vessel wall [Ca2+]i. All vessels were endothelium disrupted to evaluate the effects of CH on VSM reactivity independently of endothelial influences, and loaded with fura-2-acetoxymethyl ester to monitor vessel wall [Ca2+]i. Arteries from control and CH rats were additionally Ca2+-permeabilized to clamp [Ca2+]i, allowing direct assessment of depolarization-induced VSM Ca2+ sensitization independently of voltage-gated Ca2+ entry. In prior studies using this preparation (20, 21), we found that CH enhances depolarization-induced vasoconstriction that is independent of L-type Ca2+ channels or changes in [Ca2+]i, and instead is mediated by O2−-induced ROK activation.
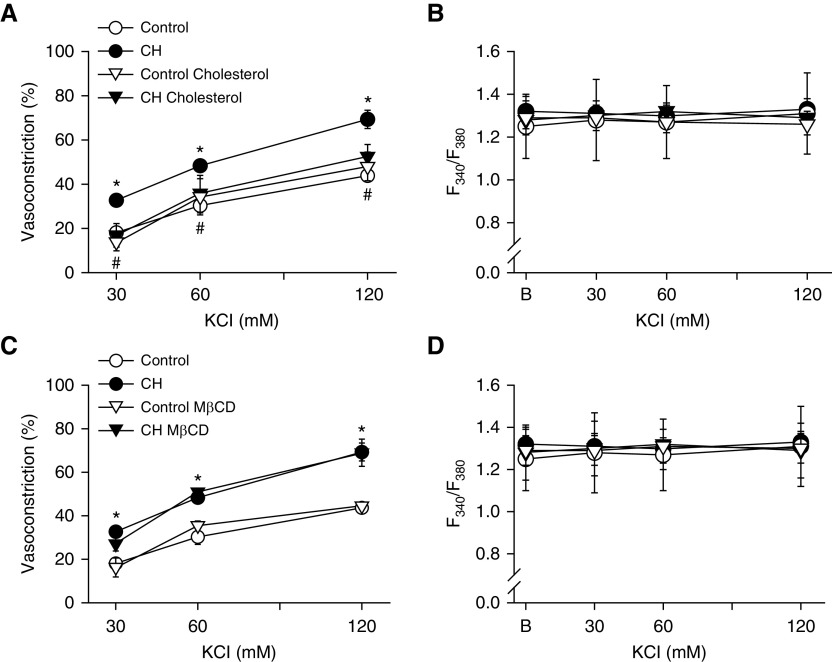
To evaluate the importance of reduced membrane cholesterol for enhanced vasoconstrictor sensitivity after CH, we examined the effects of cholesterol supplementation or depletion on KCl-induced vasoconstriction in arteries from control and CH rats. Cholesterol repletion prevented the enhanced vasoconstriction to KCl observed in Ca2+-permeabilized CH arteries and normalized responses between groups (Figure 3A). Cholesterol depletion with MβCD, in contrast, had no effect on vasoconstriction in either group (Figure 3C), indicating that this pathway cannot be evoked in control arteries by merely removing cholesterol. As expected, neither CH exposure nor cholesterol modification significantly altered vessel wall [Ca2+]i in Ca2+-clamped arteries (Figures 3B and 3D). There was no difference in baseline diameter between CH (162 ± 2 μm; n = 35) and control (160 ± 1 μm; n = 28) arteries.
Figure 3.
Augmented depolarization-dependent pulmonary arterial constriction after CH is prevented by cholesterol repletion. (A–D) Vasoconstriction in response to KCl (% baseline inner diameter) and vessel wall [Ca2+]i (F340/F380) in pressurized, Ca2+-permeabilized, endothelium-disrupted arteries from CH and control rats under conditions of cholesterol repletion (A and B), cholesterol depletion with MβCD (C and D), or after administration of their respective vehicles. Vessel wall [Ca2+]i (F340/F380) was not different between groups/treatments and was unaltered by depolarizing concentrations of KCl after Ca2+ permeabilization, confirming Ca2+-clamp conditions. Values are means ± SE of n = 4–5 rats/group. *P < 0.05 CH vehicle or CH MβCD versus the control group. #P < 0.05 CH cholesterol versus CH vehicle. B = baseline fura-2 (F340/F380) fluorescence.
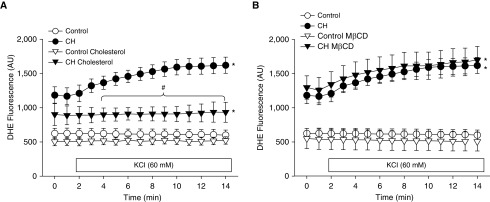
Using the same vessel preparation, we observed elevated basal and KCl-induced O2− production (measured by dihydroethidium [DHE] fluorescence) after CH in vehicle-treated arteries (Figures 4A and 4B), similar to what was previously described (20, 21). In contrast, KCl did not alter O2− production in arteries from control rats pretreated with vehicle. O2− production in response to KCl in CH arteries was prevented by cholesterol supplementation (Figure 4A), whereas MβCD had no effect on either CH or control arteries (Figure 4B). Although cholesterol had a tendency to reduce basal O2− generation in arteries from CH rats (Figure 4A), this result was not significant (P = 0.065). We have previously documented the specificity of DHE for detection of O2− in this and similar preparations (21, 31, 32).
Figure 4.
Cholesterol repletion prevents KCl-induced O2− production in pulmonary arteries from CH rats. (A and B) Dihydroethidium (DHE) fluorescence (AU) in pressurized, Ca2+-permeabilized, endothelium-disrupted arteries from control and CH rats under conditions of cholesterol repletion (A) or cholesterol depletion with MβCD (B). KCl (60 mM) was administered 1 minute into the recording. Values are means ± SE of n = 4–5 rats/group. *P < 0.05 versus the respective control group. #P < 0.05 CH cholesterol versus CH vehicle.
AP-Cav Prevents Enhanced Depolarization-Dependent Vasoconstriction and O2− Production after CH
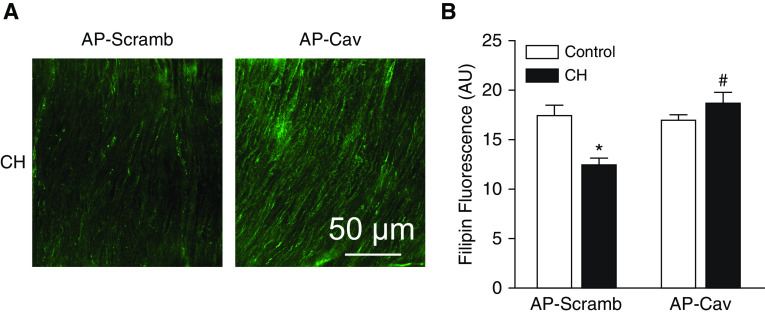
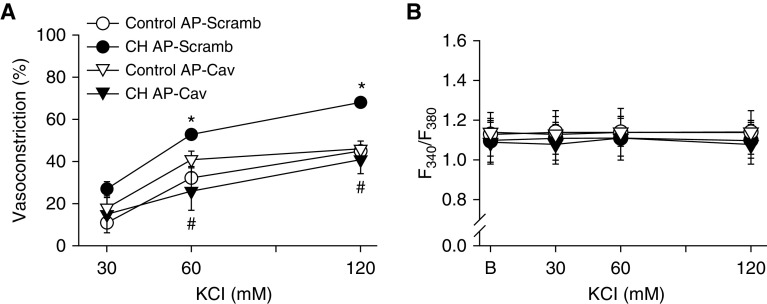
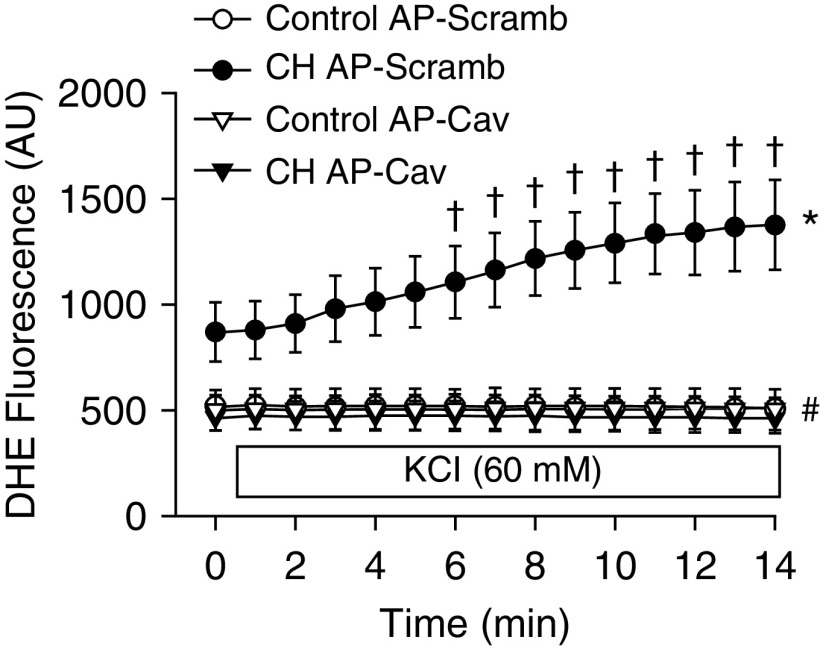
In addition to regulating the activity of various membrane proteins (6–8), Cav-1 can modulate cholesterol trafficking to the cell membrane (4, 10). Consistently, we observed that the membrane-permeable Cav-1 scaffolding domain peptide antennapedia (AP)-Cav (10 μM; also known as Cavtratin), restored membrane cholesterol in CH arteries to the level in controls (Figure 5). Similar to the effects of cholesterol repletion, AP-Cav prevented enhanced vasoconstriction to KCl in arteries from CH rats (Figure 6A) and normalized responses between groups. KCl did not increase vessel wall Ca2+, confirming Ca2+ clamp by ionomycin (Figure 6B), and vessel wall Ca2+ levels under basal conditions and after KCl administration were not different between treatments. In agreement with these findings, AP-Cav lowered basal DHE fluorescence in pulmonary arteries from CH rats to the level in controls, and prevented the effect of KCl to increase O2− production (Figure 7).
Figure 5.
The caveolin-1 scaffolding domain, antennapedia (AP)-Cav, restores membrane cholesterol in pulmonary arteries from CH rats. (A) Representative images of filipin fluorescence (AU) depicting membrane cholesterol in arteries from CH rats after pretreatment with AP-Cav or its scrambled control peptide (AP-Scramb) (10 μM each). Scale bar: 50 μm. (B) Mean filipin fluorescence in control and CH arteries after AP-Cav or AP-Scramb. Values are means ± SE of n = 8–11 animals/group. *P < 0.05 versus the respective control. #P < 0.05 versus CH AP-Scramb.
Figure 6.
AP-Cav prevents enhanced pulmonary arterial constriction in response to KCl after CH. (A) Vasoconstrictor (% baseline inner diameter) and (B) vessel wall [Ca2+]i (fura-2, F340/F380) responses to KCl in pressurized, Ca2+-permeabilized, endothelium-disrupted pulmonary arteries from CH and control rats in the presence of AP-Cav or AP-Scramb (10 μM each). Vessel wall [Ca2+]i was not different between groups or treatments, confirming Ca2+-clamp conditions. Values are means ± SE of n = 5–6 rats/group. *P < 0.05 versus control AP-Scramb. #P < 0.05 CH AP-Cav versus CH AP-Scramb.
Figure 7.
CH-induced increases in basal and KCl-stimulated O2− production in pressurized pulmonary arteries are prevented by AP-Cav. DHE fluorescence (AU) under basal conditions and after administration of KCl (60 mM beginning at 1 min) in pressurized, Ca2+-permeabilized, endothelium-disrupted pulmonary arteries from control and CH rats. Values are means ± SE of n = 4–5 rats/group. *P < 0.05 versus the respective control group at all time points. #P < 0.05 CH AP-Cav versus CH AP-Scramb at all time points. †P < 0.05 versus CH AP-Scramb before KCl administration.
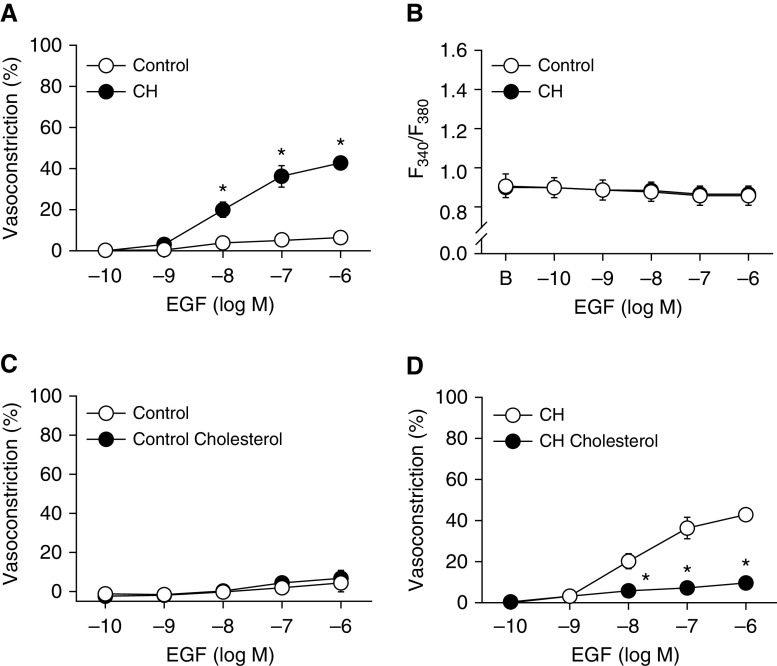
Enhanced EGF-Dependent Vasoconstriction after CH Requires Reduced Membrane Cholesterol
We have previously established that EGFR is required for enhanced depolarization-induced vasoconstriction after CH (20) and that EGF induces vasoconstriction through ROK-mediated Ca2+ sensitization in pulmonary arteries from CH, but not control, rats (26). Therefore, we sought to examine the role of cholesterol in regulating EGFR and its downstream signaling by stimulation with EGF. EGF (0.1 nM to 1 μM) caused a concentration-dependent vasoconstriction in nonpermeabilized CH arteries (Figure 8A) but elicited only minimal constriction in control arteries. This constriction in CH vessels was not associated with a significant increase in vessel wall Ca2+, indicating involvement of a Ca2+ sensitization mechanism (Figure 8B) that we have previously demonstrated to be EGFR-, NOX2-, and ROK-dependent (20, 21). Whereas supplemental cholesterol had no significant effect on vasoconstriction in response to EGF in control arteries, responses to EGF were nearly abolished by cholesterol in vessels from CH rats (Figures 8C and 8D). Because the responses to EGF in controls were minimal, we validated the viability of the preparation by assessing vasoconstriction in response to uridine 5′-triphosphate (10 μM) at the conclusion of each experiment. As predicted, cholesterol supplementation had no effect on responsiveness to uridine 5′-triphosphate in cholesterol-replete vessels from control rats (vehicle: % constriction = 68 ± 13, ΔF340/F380 = 0.43 ± 0.13; cholesterol: % constriction = 75 ± 8, ΔF340/F380 = 0.55 ± 0.15).
Figure 8.
Loss of membrane cholesterol facilitates EGF (epidermal growth factor)-dependent vasoconstriction after CH. (A) Vasoconstrictor and (B) vessel wall [Ca2+]i (F340/F380) responses to EGF in pressurized, endothelium-disrupted, nonpermeabilized pulmonary arteries from CH and control rats. (C and D) Vasoconstrictor responses to EGF in arteries from control (C) and CH rats (D) in the presence of supplemental cholesterol or vehicle. Values are means ± SE of n = 4–5/group. *P < 0.05 versus control or vehicle.
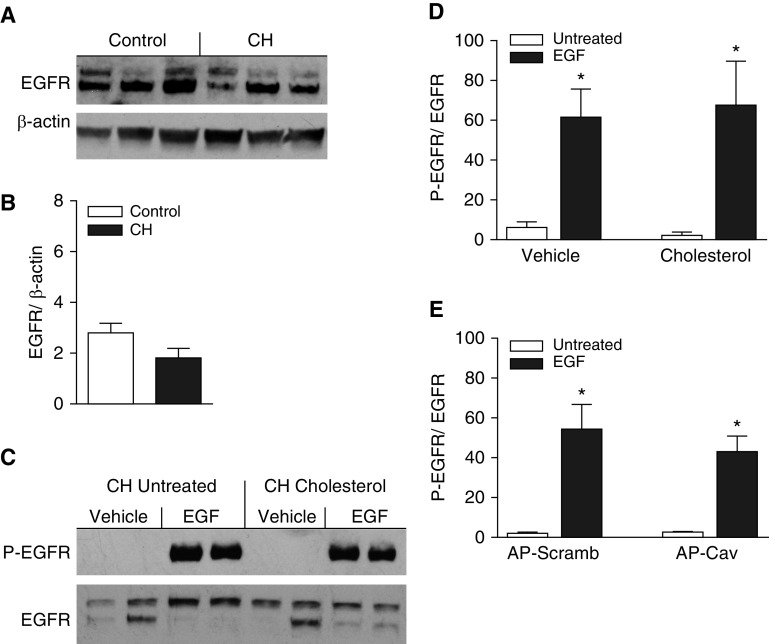
Pulmonary arterial EGFR expression and EGF-induced EGFR phosphorylation were assessed in intrapulmonary arteries from each group by Western blotting to determine whether enhanced EGFR-dependent vasoconstriction after CH is associated with increased EGFR expression or activity. However, CH did not significantly alter EGFR expression (Figures 9A and 9B), and levels of basal phosphorylated EGFR were nearly undetectable in both groups (e.g., see Figure 9C for CH arteries). Surprisingly, despite a lack of vasoconstriction in response to EGF in control arteries, EGF (100 nM) led to robust increases in EGFR phosphorylation in both control and CH arteries, resulting in similar levels of phosphorylated EGFR in the two groups as assessed by the ratio of phosphorylated EGFR/total EGFR (control: 2.16 ± 0.36, n = 6; CH: 2.49 ± 0.45, n = 8). Furthermore, neither supplemental cholesterol (Figure 9D) nor AP-Cav (Figure 9E) significantly altered EGF-induced EGFR phosphorylation in CH arteries.
Figure 9.
CH does not alter pulmonary arterial EGFR (EGF receptor) expression, and EGF-induced activation of EGFR is unaffected by supplemental cholesterol or AP-Cav in arteries from CH rats. (A) Representative Western blots and (B) mean densitometric data for total EGFR normalized to β-actin in intrapulmonary arteries from control and CH rats. (C) Representative Western blots for phosphorylated EGFR (P-EGFR) and total EGFR in arteries from CH rats with and without cholesterol supplementation. Arteries were treated with EGF (100 nM) or vehicle to evaluate the effects of cholesterol supplementation on EGFR activation. (D and E) Mean densitometric data for P-EGFR normalized to total EGFR in arteries from CH rats treated with cholesterol or vehicle (D), or AP-Cav or AP-Scramb (E), in the presence or absence of EGF. Values are means ± SE of n = 5–8 rats/group. *P < 0.05 versus untreated.
Discussion
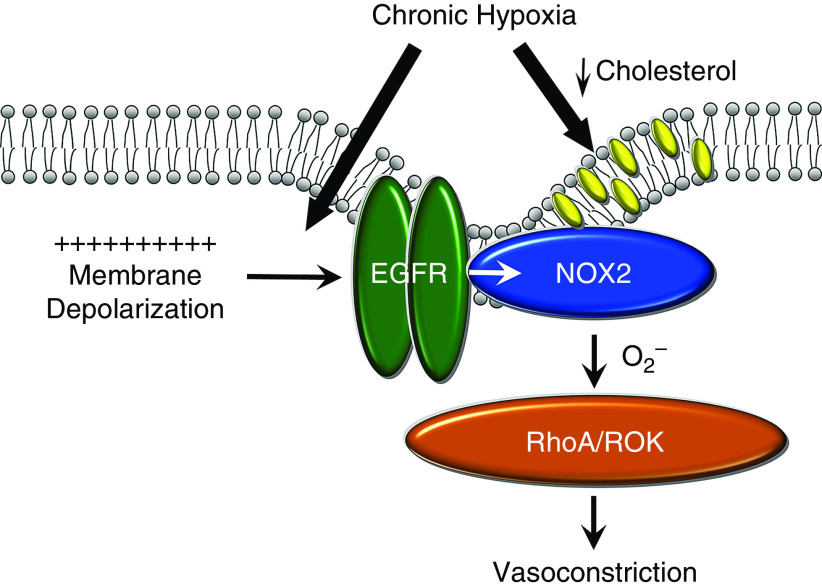
In the current study we examined the contribution of Cav-1 and membrane cholesterol content to enhanced depolarization-induced pulmonary arterial Ca2+ sensitization and constriction after CH. The major findings of this study are as follows: 1) CH exposure in rats did not alter Cav-1 expression in pulmonary arteries or caveolar number in smooth muscle, but reduced VSM cell membrane cholesterol content; 2) the Cav-1 scaffolding domain peptide AP-Cav restored VSM membrane cholesterol levels in CH rats to the levels observed in controls; 3) both cholesterol supplementation and AP-Cav prevented enhanced depolarization-induced vasoconstriction and O2− production after CH; 4) greater EGFR-mediated constriction in pulmonary arteries from CH rats was similarly prevented by cholesterol supplementation; and 5) CH did not alter pulmonary arterial EGFR expression or EGF-induced EGFR phosphorylation. We conclude that alterations in membrane lipid domains resulting from reduced cholesterol content in pulmonary VSM cells facilitate augmented depolarization-induced and EGFR-mediated Ca2+ sensitization and vasoconstriction after CH. However, these responses to CH are not dependent on changes in EGFR expression or activity (Figure 10).
Figure 10.
Proposed mechanism by which CH augments depolarization-induced pulmonary vasoconstriction. CH augments depolarization-induced activation of an EGFR/NOX2/ROK signaling axis and resultant vasoconstriction by coupling membrane depolarization to EGFR stimulation, and by reducing membrane cholesterol content. Cholesterol inhibits this signaling pathway downstream of EGFR. NOX2 = NADPH oxidase 2; ROK = RHO-associated protein kinase.
CH Reduces Membrane Cholesterol but Does Not Alter the Incidence of Caveolae
The effects of CH on the membrane lipid components caveolae and cholesterol have not previously been investigated in pulmonary VSM. CH had no effect on either caveolae number or Cav-1 expression (Figures 1A and 1B). However, CH significantly reduced levels of cholesterol in untreated pulmonary arteries. This is in agreement with findings in rat pulmonary arterial endothelial cells (33) and cultured rabbit fibroblasts (34). In CHO-7 cells, hypoxia impairs cholesterol synthesis via degradation of 3-hydroxy-3-methylglutaryl-CoA reductase, the rate-controlling enzyme in cholesterol synthesis (18). In contrast to our findings, others have reported elevations in Cav-1 in monocrotaline-induced PH (35, 36). Clinical findings also show increased VSM expression of Cav-1 in idiopathic pulmonary arterial hypertension (37) and patients with chronic obstructive pulmonary disease (38). However, other studies have demonstrated reduced Cav-1 expression in PH (12–15). The reasons for these discrepancies are unknown, but they could reflect differences in the species examined, disease etiology, or the model of PH studied.
Previous studies from our laboratory demonstrated an effect of CH to lower membrane cholesterol content in pulmonary endothelial cells. This reduction in endothelial membrane cholesterol contributes to endothelial dysfunction by limiting a variety of Ca2+ entry pathways (33), thereby promoting an imbalance that may favor production of vasoconstrictor and mitogenic factors that contribute to PH. Consequently, reduced membrane cholesterol content in multiple vascular wall cell types may function synergistically to augment vasoconstrictor sensitivity and arterial remodeling in the hypertensive pulmonary circulation.
Effects of Altered Lipid Domains on Pulmonary Vasoreactivity
Our laboratory has previously demonstrated enhanced ROK-dependent Ca2+ sensitization in pulmonary arteries from CH rats (21, 39, 40). Depolarization-induced ROK-mediated Ca2+ sensitization and vasoconstriction after CH are mediated by EGFR-dependent NOX2 activation and O2− production (20). The present study indicates a unique role for lipid domain dysfunction in facilitating this response. Decreased membrane cholesterol is required for augmented vasoconstriction to KCl because cholesterol repletion (validated by filipin fluorescence) normalized the response in Ca2+-permeabilized arteries from CH animals to the level observed in controls (Figure 3). Similarly, O2− production evoked by KCl was prevented by cholesterol supplementation in the CH group. After depletion of membrane cholesterol with MβCD, vasoconstriction and O2− generation in normoxic control arteries remained normal, indicating that reduced cholesterol alone is not adequate to evoke enhanced vasoreactivity after CH. This suggests that PH is associated with changes in signaling components other than lipid domains, which are required to evoke augmented depolarization-induced vasoconstriction, and yet reductions in membrane cholesterol are necessary to observe this phenotype.
Although our data suggest a direct role for reduced membrane cholesterol in augmenting vasoconstriction in CH arteries, these findings certainly do not preclude a role for caveolae/Cav-1 in facilitating this response, as Cav-1 expression is cholesterol dependent and cholesterol is believed to be rate limiting for Cav-1 trafficking to the plasma membrane, which is necessary for caveolae formation (19). Although reductions in membrane cholesterol associated with CH exposure did not change Cav-1 expression or caveolae number, it may be that diminished cholesterol or Cav-1 within caveolae alters the properties of these signaling domains, thereby coupling membrane depolarization to EGFR- and NOX2-mediated constriction. Cholesterol oxidation, potentially through increases in oxidative stress, can also result in Cav-1 dysfunction (41). Therefore, as opposed to a decrease in total membrane cholesterol, it is possible that increased oxidized cholesterol (not detected by filipin fluorescence) facilitates activation of EGFR and NOX2 in arteries from animals with PH. Future studies will be necessary to investigate this possibility, as well as the mechanism by which depolarization mediates EGFR stimulation independently of L-type voltage-gated Ca2+ channels, which may include G protein–coupled receptors (42) and voltage-sensitive phosphatases (43, 44).
In addition to the scaffolding domain of Cav-1, AP-Cav (amino acids 82–101) contains amino acids associated with cholesterol binding (45, 46). Remarkably, AP-Cav restored membrane cholesterol in CH arteries to the level in controls (Figure 5), and prevented both the augmented vasoconstrictor reactivity to KCl and O2− production associated with CH exposure in Ca2+-permeabilized arteries (Figures 6 and 7). Although the effects of AP-Cav may be mediated by increased cholesterol trafficking to the membrane, it is also possible that AP-Cav interacts with signaling components required for enhanced ROK-mediated Ca2+ sensitization after CH. Findings that NOX1 colocalizes with Cav-1 in VSM (47) suggest a direct role of Cav-1 in regulating NOX function. Indeed, Cav-1 can negatively regulate NOX activation in endothelial cells (13). However, Cav-1 also inhibits EGFR activation by angiotensin II in aortic VSM (48), whereas Cav-1 phosphorylation is linked to EGFR activation in renal mesangial cells (23). Although little is known about the effects of Cav-1 in regulation of VSM contraction in pulmonary arteries, Cav-1 and caveolae play an important role in contraction in response to agonists such as serotonin, bradykinin, and histamine in airway smooth muscle cells (49, 50).
Although supplemental cholesterol diminished vasoconstriction in response to KCl in Ca2+-permeabilized pulmonary arteries from CH rats (Figure 3), we wished to further evaluate the role of cholesterol in regulating downstream signaling mechanisms evoked by membrane depolarization. EGFR mediates enhanced depolarization-induced Ca2+ sensitization and vasoconstriction after CH (20), and EGFR phosphorylation is increased in arteries from animals with PH (20, 51). Direct regulation of EGFR by cholesterol occurs in human epithelial type 2 cells, where removal of cholesterol facilitates EGFR phosphorylation and activation (24). Therefore, we used EGF to directly activate EGFR. Interestingly, EGF resulted in vasoconstriction only in CH arteries. This response occurred without a change in vessel wall [Ca2+]i, further supporting that CH selectively couples EGFR to myofilament Ca2+ sensitization through NOX2 and ROK (26). Remarkably, cholesterol repletion prevented EGF-induced vasoconstriction in CH arteries, suggesting that cholesterol prevents activation of the EGFR/NOX2 signaling axis, and that reductions in membrane cholesterol after CH facilitate enhanced pulmonary arterial constriction and Ca2+ sensitization.
In agreement with our previous findings (20), basal EGFR and levels of phosphorylated-EGFR did not differ significantly between CH and control arteries (Figure 9). Surprisingly, EGF led to a robust increase in EGFR phosphorylation in arteries from both CH and control rats. This finding contrasts with evidence that EGFR phosphorylation is increased by membrane depolarization in CH, but not control, arteries (20), indicating that coupling of membrane depolarization to EGFR is only present in animals with PH. In addition, there were no effects of AP-Cav or cholesterol on EGF-induced EGFR phosphorylation, suggesting that lipid domains regulate enhanced depolarization-induced vasoconstriction downstream of EGFR. Because cholesterol can inhibit the activation of NOX2 (25), and KCl-dependent ROS production was inhibited by cholesterol (Figure 4), NOX2 is a possible site of this regulation.
Decreases in cholesterol after CH may additionally impact signaling pathways beyond those evoked by depolarizing stimuli. Because of the ability of cholesterol to regulate membrane stiffness, reduced cholesterol may change the activity of stretch-activated proteins in the cell membrane. This may play a role in the development of pressure-dependent tone, which occurs only in CH rats (26, 39). Cholesterol can also directly interact with a variety of ion channels, regulating their activity (52–55). Therefore, changes in channel activity may contribute to the depolarized resting membrane potential observed in CH arteries (20, 56), and to elevated basal VSM [Ca2+]i (57, 58). In addition, cholesterol causes apoptosis in VSM cells (59), and therefore reductions in cholesterol in CH arteries may facilitate the proliferation observed during PH.
Conclusions
Our goal in this study was to investigate the role that lipid domains play in mediating the effects of CH to enhance depolarization-induced vasoconstriction through a previously described EGFR/NOX2/ROK signaling pathway in pulmonary VSM. We show that changes in VSM membrane lipid domain function resulting from reduced cholesterol content are required for both augmented depolarization-induced and EGF-mediated Ca2+ sensitization and vasoconstriction after CH. However, reductions in cholesterol alone are not sufficient to activate this pathway, indicating that CH additionally links membrane depolarization to EGFR activation through an unknown mechanism. These findings provide an improved mechanistic basis for the vasoconstrictor component of CH-induced PH, and underscore the multiple levels of regulation that control physiological signaling associated with PH. In addition, these studies may provide a basis for new therapeutic approaches to treat PH that promote the maintenance of normal membrane cholesterol levels. Given the importance of ROK activation in the arterial remodeling response to chronic hypoxia (60, 61), evaluating the role(s) of altered lipid domains in VSM proliferation and migration represents an attractive avenue of future investigation.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank Dr. Bojun Zhang, Tamara Howard, and Minerva Murphy for technical assistance.
Footnotes
Supported by U.S. National Institutes of Health grants R01 HL132883, R01 HL088192, and T32 HL007736 (T.C.R.), R01 HL095640 (B.R.W.), and K12 GM088021 (Principal Investigator: A. Wandinger-Ness), and American Heart Association grant 13PRE14580015 (C.E.N.).
Author Contributions: C.E.N. contributed to study conception and design, performed experiments, analyzed and interpreted data, and drafted and revised the manuscript. L.W.-C., R.A., and S.Y. collected and interpreted data and revised the manuscript. N.L.J., M.L.P., and J.S.N. contributed to study design, interpreted data, and revised the manuscript. B.R.W. and T.C.R. contributed to study conception and design, supervised study execution, interpreted data, and revised the manuscript.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2018-0318OC on January 16, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Lisanti MP, Scherer PE, Vidugiriene J, Tang Z, Hermanowski-Vosatka A, Tu YH, et al. Characterization of caveolin-rich membrane domains isolated from an endothelial-rich source: implications for human disease. J Cell Biol. 1994;126:111–126. doi: 10.1083/jcb.126.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smart EJ, Graf GA, McNiven MA, Sessa WC, Engelman JA, Scherer PE, et al. Caveolins, liquid-ordered domains, and signal transduction. Mol Cell Biol. 1999;19:7289–7304. doi: 10.1128/mcb.19.11.7289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson RGW. The caveolae membrane system. Annu Rev Biochem. 1998;67:199–225. doi: 10.1146/annurev.biochem.67.1.199. [DOI] [PubMed] [Google Scholar]
- 4.Fielding CJ, Fielding PE. Intracellular cholesterol transport. J Lipid Res. 1997;38:1503–1521. [PubMed] [Google Scholar]
- 5.Li S, Song KS, Koh SS, Kikuchi A, Lisanti MP. Baculovirus-based expression of mammalian caveolin in Sf21 insect cells: a model system for the biochemical and morphological study of caveolae biogenesis. J Biol Chem. 1996;271:28647–28654. doi: 10.1074/jbc.271.45.28647. [DOI] [PubMed] [Google Scholar]
- 6.Ju H, Zou R, Venema VJ, Venema RC. Direct interaction of endothelial nitric-oxide synthase and caveolin-1 inhibits synthase activity. J Biol Chem. 1997;272:18522–18525. doi: 10.1074/jbc.272.30.18522. [DOI] [PubMed] [Google Scholar]
- 7.Tiruppathi C, Song W, Bergenfeldt M, Sass P, Malik AB. Gp60 activation mediates albumin transcytosis in endothelial cells by tyrosine kinase-dependent pathway. J Biol Chem. 1997;272:25968–25975. doi: 10.1074/jbc.272.41.25968. [DOI] [PubMed] [Google Scholar]
- 8.Chatterjee S, Cao S, Peterson TE, Simari RD, Shah V. Inhibition of GTP-dependent vesicle trafficking impairs internalization of plasmalemmal eNOS and cellular nitric oxide production. J Cell Sci. 2003;116:3645–3655. doi: 10.1242/jcs.00664. [DOI] [PubMed] [Google Scholar]
- 9.Murata M, Peränen J, Schreiner R, Wieland F, Kurzchalia TV, Simons K. VIP21/caveolin is a cholesterol-binding protein. Proc Natl Acad Sci USA. 1995;92:10339–10343. doi: 10.1073/pnas.92.22.10339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smart EJ, Ying Ys, Donzell WC, Anderson RGW. A role for caveolin in transport of cholesterol from endoplasmic reticulum to plasma membrane. J Biol Chem. 1996;271:29427–29435. doi: 10.1074/jbc.271.46.29427. [DOI] [PubMed] [Google Scholar]
- 11.Schroeder F, Huang H, McIntosh AL, Atshaves BP, Martin GG, Kier AB. Caveolin, sterol carrier protein-2, membrane cholesterol-rich microdomains and intracellular cholesterol trafficking. Subcell Biochem. 2010;51:279–318. doi: 10.1007/978-90-481-8622-8_10. [DOI] [PubMed] [Google Scholar]
- 12.Austin ED, Ma L, LeDuc C, Berman Rosenzweig E, Borczuk A, Phillips JA, III, et al. Whole exome sequencing to identify a novel gene (caveolin-1) associated with human pulmonary arterial hypertension. Circ Cardiovasc Genet. 2012;5:336–343. doi: 10.1161/CIRCGENETICS.111.961888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen F, Barman S, Yu Y, Haigh S, Wang Y, Black SM, et al. Caveolin-1 is a negative regulator of NADPH oxidase-derived reactive oxygen species. Free Radic Biol Med. 2014;73:201–213. doi: 10.1016/j.freeradbiomed.2014.04.029. [Published erratum appears in Free Radic Biol Med 81:184.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jasmin JF, Mercier I, Dupuis J, Tanowitz HB, Lisanti MP. Short-term administration of a cell-permeable caveolin-1 peptide prevents the development of monocrotaline-induced pulmonary hypertension and right ventricular hypertrophy. Circulation. 2006;114:912–920. doi: 10.1161/CIRCULATIONAHA.106.634709. [DOI] [PubMed] [Google Scholar]
- 15.Zhao YY, Liu Y, Stan RV, Fan L, Gu Y, Dalton N, et al. Defects in caveolin-1 cause dilated cardiomyopathy and pulmonary hypertension in knockout mice. Proc Natl Acad Sci USA. 2002;99:11375–11380. doi: 10.1073/pnas.172360799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paffett ML, Naik JS, Riddle MA, Menicucci SD, Gonzales AJ, Resta TC, et al. Altered membrane lipid domains limit pulmonary endothelial calcium entry following chronic hypoxia. Am J Physiol Heart Circ Physiol. 2011;301:H1331–H1340. doi: 10.1152/ajpheart.00980.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maniatis NA, Shinin V, Schraufnagel DE, Okada S, Vogel SM, Malik AB, et al. Increased pulmonary vascular resistance and defective pulmonary artery filling in caveolin-1−/− mice. Am J Physiol Lung Cell Mol Physiol. 2008;294:L865–L873. doi: 10.1152/ajplung.00079.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nguyen AD, McDonald JG, Bruick RK, DeBose-Boyd RA. Hypoxia stimulates degradation of 3-hydroxy-3-methylglutaryl-coenzyme A reductase through accumulation of lanosterol and hypoxia-inducible factor-mediated induction of insigs. J Biol Chem. 2007;282:27436–27446. doi: 10.1074/jbc.M704976200. [DOI] [PubMed] [Google Scholar]
- 19.Hailstones D, Sleer LS, Parton RG, Stanley KK. Regulation of caveolin and caveolae by cholesterol in MDCK cells. J Lipid Res. 1998;39:369–379. [PubMed] [Google Scholar]
- 20.Norton CE, Broughton BR, Jernigan NL, Walker BR, Resta TC. Enhanced depolarization-induced pulmonary vasoconstriction following chronic hypoxia requires EGFR-dependent activation of NAD(P)H oxidase 2. Antioxid Redox Signal. 2013;18:1777–1788. doi: 10.1089/ars.2012.4836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Broughton BR, Jernigan NL, Norton CE, Walker BR, Resta TC. Chronic hypoxia augments depolarization-induced Ca2+ sensitization in pulmonary vascular smooth muscle through superoxide-dependent stimulation of RhoA. Am J Physiol Lung Cell Mol Physiol. 2010;298:L232–L242. doi: 10.1152/ajplung.00276.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y, Peng F, Gao B, Ingram AJ, Krepinsky JC. Mechanical strain-induced RhoA activation requires NADPH oxidase-mediated ROS generation in caveolae. Antioxid Redox Signal. 2010;13:959–973. doi: 10.1089/ars.2009.2908. [DOI] [PubMed] [Google Scholar]
- 23.Zhang B, Peng F, Wu D, Ingram AJ, Gao B, Krepinsky JC. Caveolin-1 phosphorylation is required for stretch-induced EGFR and Akt activation in mesangial cells. Cell Signal. 2007;19:1690–1700. doi: 10.1016/j.cellsig.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 24.Ringerike T, Blystad FD, Levy FO, Madshus IH, Stang E. Cholesterol is important in control of EGF receptor kinase activity but EGF receptors are not concentrated in caveolae. J Cell Sci. 2002;115:1331–1340. doi: 10.1242/jcs.115.6.1331. [DOI] [PubMed] [Google Scholar]
- 25.Masoud R, Bizouarn T, Houée-Levin C. Cholesterol: a modulator of the phagocyte NADPH oxidase activity. A cell-free study. Redox Biol. 2014;3:16–24. doi: 10.1016/j.redox.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Norton CE, Sheak JR, Yan S, Weise-Cross L, Jernigan NL, Walker BR, et al. Augmented pulmonary vasoconstrictor reactivity after chronic hypoxia requires Src kinase and epidermal growth factor receptor signaling. Am J Respir Cell Mol Biol. 2020;62:61–73. doi: 10.1165/rcmb.2018-0106OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Resta TC, Chicoine LG, Omdahl JL, Walker BR. Maintained upregulation of pulmonary eNOS gene and protein expression during recovery from chronic hypoxia. Am J Physiol. 1999;276:H699–H708. doi: 10.1152/ajpheart.1999.276.2.H699. [DOI] [PubMed] [Google Scholar]
- 28.Resta TC, Kanagy NL, Walker BR. Estradiol-induced attenuation of pulmonary hypertension is not associated with altered eNOS expression. Am J Physiol Lung Cell Mol Physiol. 2001;280:L88–L97. doi: 10.1152/ajplung.2001.280.1.L88. [DOI] [PubMed] [Google Scholar]
- 29.Snow JB, Kitzis V, Norton CE, Torres SN, Johnson KD, Kanagy NL, et al. Differential effects of chronic hypoxia and intermittent hypocapnic and eucapnic hypoxia on pulmonary vasoreactivity. J Appl Physiol (1985) 2008;104:110–118. doi: 10.1152/japplphysiol.00698.2005. [DOI] [PubMed] [Google Scholar]
- 30.Norman AW, Demel RA, de Kruyff B, van Deenen LL. Studies on the biological properties of polyene antibiotics: evidence for the direct interaction of filipin with cholesterol. J Biol Chem. 1972;247:1918–1929. [PubMed] [Google Scholar]
- 31.Jernigan NL, Naik JS, Weise-Cross L, Detweiler ND, Herbert LM, Yellowhair TR, et al. Contribution of reactive oxygen species to the pathogenesis of pulmonary arterial hypertension. PLoS One. 2017;12:e0180455. doi: 10.1371/journal.pone.0180455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Plomaritas DR, Herbert LM, Yellowhair TR, Resta TC, Gonzalez Bosc LV, Walker BR, et al. Chronic hypoxia limits H2O2-induced inhibition of ASIC1-dependent store-operated calcium entry in pulmonary arterial smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2014;307:L419–L430. doi: 10.1152/ajplung.00095.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang B, Naik JS, Jernigan NL, Walker BR, Resta TC. Reduced membrane cholesterol limits pulmonary endothelial Ca2+ entry after chronic hypoxia. Am J Physiol Heart Circ Physiol. 2017;312:H1176–H1184. doi: 10.1152/ajpheart.00097.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mukodani J, Ishikawa Y, Fukuzaki H. Effects of hypoxia on sterol synthesis, acyl-CoA:cholesterol acyltransferase activity, and efflux of cholesterol in cultured rabbit skin fibroblasts. Arteriosclerosis. 1990;10:106–110. doi: 10.1161/01.atv.10.1.106. [DOI] [PubMed] [Google Scholar]
- 35.Huang J, Wolk JH, Gewitz MH, Loyd JE, West J, Austin ED, et al. Enhanced caveolin-1 expression in smooth muscle cells: possible prelude to neointima formation. World J Cardiol. 2015;7:671–684. doi: 10.4330/wjc.v7.i10.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jiao HX, Mu YP, Gui LX, Yan FR, Lin DC, Sham JS, et al. Increase in caveolae and caveolin-1 expression modulates agonist-induced contraction and store- and receptor-operated Ca(2+) entry in pulmonary arteries of pulmonary hypertensive rats. Vascul Pharmacol. 2016;84:55–66. doi: 10.1016/j.vph.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 37.Patel HH, Zhang S, Murray F, Suda RY, Head BP, Yokoyama U, et al. Increased smooth muscle cell expression of caveolin-1 and caveolae contribute to the pathophysiology of idiopathic pulmonary arterial hypertension. FASEB J. 2007;21:2970–2979. doi: 10.1096/fj.07-8424com. [DOI] [PubMed] [Google Scholar]
- 38.Huber LC, Soltermann A, Fischler M, Gay S, Weder W, Russi EW, et al. Caveolin-1 expression and hemodynamics in COPD patients. Open Respir Med J. 2009;3:73–78. doi: 10.2174/1874306400903010073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Broughton BR, Walker BR, Resta TC. Chronic hypoxia induces Rho kinase-dependent myogenic tone in small pulmonary arteries. Am J Physiol Lung Cell Mol Physiol. 2008;294:L797–L806. doi: 10.1152/ajplung.00253.2007. [DOI] [PubMed] [Google Scholar]
- 40.Jernigan NL, Walker BR, Resta TC. Reactive oxygen species mediate RhoA/Rho kinase-induced Ca2+ sensitization in pulmonary vascular smooth muscle following chronic hypoxia. Am J Physiol Lung Cell Mol Physiol. 2008;295:L515–L529. doi: 10.1152/ajplung.00355.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu P, Wang P, Michaely P, Zhu M, Anderson RG. Presence of oxidized cholesterol in caveolae uncouples active platelet-derived growth factor receptors from tyrosine kinase substrates. J Biol Chem. 2000;275:31648–31654. doi: 10.1074/jbc.M004599200. [DOI] [PubMed] [Google Scholar]
- 42.Liu QH, Zheng YM, Korde AS, Yadav VR, Rathore R, Wess J, et al. Membrane depolarization causes a direct activation of G protein-coupled receptors leading to local Ca2+ release in smooth muscle. Proc Natl Acad Sci USA. 2009;106:11418–11423. doi: 10.1073/pnas.0813307106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murata Y, Iwasaki H, Sasaki M, Inaba K, Okamura Y. Phosphoinositide phosphatase activity coupled to an intrinsic voltage sensor. Nature. 2005;435:1239–1243. doi: 10.1038/nature03650. [DOI] [PubMed] [Google Scholar]
- 44.Murata Y, Okamura Y. Depolarization activates the phosphoinositide phosphatase Ci-VSP, as detected in Xenopus oocytes coexpressing sensors of PIP2. J Physiol. 2007;583:875–889. doi: 10.1113/jphysiol.2007.134775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang G, Xu H, Li Z, Li F. Interactions of caveolin-1 scaffolding and intramembrane regions containing a CRAC motif with cholesterol in lipid bilayers. Biochim Biophys Acta. 2014;1838:2588–2599. doi: 10.1016/j.bbamem.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 46.Arbuzova A, Wang L, Wang J, Hangyás-Mihályné G, Murray D, Honig B, et al. Membrane binding of peptides containing both basic and aromatic residues: experimental studies with peptides corresponding to the scaffolding region of caveolin and the effector region of MARCKS. Biochemistry. 2000;39:10330–10339. doi: 10.1021/bi001039j. [DOI] [PubMed] [Google Scholar]
- 47.Hilenski LL, Clempus RE, Quinn MT, Lambeth JD, Griendling KK. Distinct subcellular localizations of Nox1 and Nox4 in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2004;24:677–683. doi: 10.1161/01.ATV.0000112024.13727.2c. [DOI] [PubMed] [Google Scholar]
- 48.Takaguri A, Shirai H, Kimura K, Hinoki A, Eguchi K, Carlile-Klusacek M, et al. Caveolin-1 negatively regulates a metalloprotease-dependent epidermal growth factor receptor transactivation by angiotensin II. J Mol Cell Cardiol. 2011;50:545–551. doi: 10.1016/j.yjmcc.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Keshayarz M, Schwarz H, Hartmann P, Weigland S, Skill M, Althaus M, et al. Caveolin-1: functional insights into its role in muscarine- and serotonin-induced smooth muscle constriction in murine airways. Front Physiol. 2017;8:295. doi: 10.3389/fphys.2017.00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Prakash YS, Thompson MA, Vaa B, Matabdin I, Peterson TE, He T, et al. Caveolins and intracellular calcium regulation in human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2007;293:L1118–L1126. doi: 10.1152/ajplung.00136.2007. [DOI] [PubMed] [Google Scholar]
- 51.Rafikova O, Rafikov R, Kangath A, Qu N, Aggarwal S, Sharma S, et al. Redox regulation of epidermal growth factor receptor signaling during the development of pulmonary hypertension. Free Radic Biol Med. 2016;95:96–111. doi: 10.1016/j.freeradbiomed.2016.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lin MW, Wu AZ, Ting WH, Li CL, Cheng KS, Wu SN. Changes in membrane cholesterol of pituitary tumor (GH3) cells regulate the activity of large-conductance Ca2+-activated K+ channels. Chin J Physiol. 2006;49:1–13. [PubMed] [Google Scholar]
- 53.Kwiatek AM, Minshall RD, Cool DR, Skidgel RA, Malik AB, Tiruppathi C. Caveolin-1 regulates store-operated Ca2+ influx by binding of its scaffolding domain to transient receptor potential channel-1 in endothelial cells. Mol Pharmacol. 2006;70:1174–1183. doi: 10.1124/mol.105.021741. [DOI] [PubMed] [Google Scholar]
- 54.Graziani A, Rosker C, Kohlwein SD, Zhu MX, Romanin C, Sattler W, et al. Cellular cholesterol controls TRPC3 function: evidence from a novel dominant-negative knockdown strategy. Biochem J. 2006;396:147–155. doi: 10.1042/BJ20051246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bergdahl A, Gomez MF, Dreja K, Xu SZ, Adner M, Beech DJ, et al. Cholesterol depletion impairs vascular reactivity to endothelin-1 by reducing store-operated Ca2+ entry dependent on TRPC1. Circ Res. 2003;93:839–847. doi: 10.1161/01.RES.0000100367.45446.A3. [DOI] [PubMed] [Google Scholar]
- 56.Naik JS, Earley S, Resta TC, Walker BR. Pressure-induced smooth muscle cell depolarization in pulmonary arteries from control and chronically hypoxic rats does not cause myogenic vasoconstriction. J Appl Physiol (1985) 2005;98:1119–1124. doi: 10.1152/japplphysiol.00819.2004. [DOI] [PubMed] [Google Scholar]
- 57.Golovina VA, Platoshyn O, Bailey CL, Wang J, Limsuwan A, Sweeney M, et al. Upregulated TRP and enhanced capacitative Ca(2+) entry in human pulmonary artery myocytes during proliferation. Am J Physiol Heart Circ Physiol. 2001;280:H746–H755. doi: 10.1152/ajpheart.2001.280.2.H746. [DOI] [PubMed] [Google Scholar]
- 58.Yuan JX, Aldinger AM, Juhaszova M, Wang J, Conte JV, Jr, Gaine SP, et al. Dysfunctional voltage-gated K+ channels in pulmonary artery smooth muscle cells of patients with primary pulmonary hypertension. Circulation. 1998;98:1400–1406. doi: 10.1161/01.cir.98.14.1400. [DOI] [PubMed] [Google Scholar]
- 59.Yin J, Chaufour X, McLachlan C, McGuire M, White G, King N, et al. Apoptosis of vascular smooth muscle cells induced by cholesterol and its oxides in vitro and in vivo. Atherosclerosis. 2000;148:365–374. doi: 10.1016/s0021-9150(99)00286-5. [DOI] [PubMed] [Google Scholar]
- 60.Oka M, Fagan KA, Jones PL, McMurtry IF. Therapeutic potential of RhoA/Rho kinase inhibitors in pulmonary hypertension. Br J Pharmacol. 2008;155:444–454. doi: 10.1038/bjp.2008.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Duong-Quy S, Bei Y, Liu Z, Dinh-Xuan AT. Role of Rho-kinase and its inhibitors in pulmonary hypertension. Pharmacol Ther. 2013;137:352–364. doi: 10.1016/j.pharmthera.2012.12.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.